Abstract
The focus in antioxidant research is on enzyme derivative investigations. Extracellular superoxide dismutase (EC-SOD) is of particular interest, as it demonstrates in vivo the protective action against development of atherosclerosis, hypertension, heart failure, diabetes mellitus. The reliable association of coronary artery disease with decreased level of heparin-released EC-SOD was established in clinical research. To create a base for and to develop antioxidant therapy, various SOD isozymes, catalase (CAT), methods of gene therapy, and combined applications of enzymes are used. Covalent bienzyme SOD-CHS-CAT conjugate (CHS, chondroitin sulphate) showed high efficacy and safety as the drug candidate. There is an evident trend to use the components of glycocalyx and extra-cellular matrix for target delivery of medical substances. Development of new enzyme antioxidants for therapeutic application is closely connected with progress in medical biotechnology, the pharmaceutical industry, and the bioeconomy.
Key words: reactive oxygen species, enzyme antioxidants, oxidative stress, cardiovascular diseases, vascular wall injury, endothelial glycocalyx, extracellular superoxide dismutase, catalase, novel bienzyme superoxide dismutase - chondroitin sulphate - catalase conjugate, antioxidant therapy.
Introduction
The high prevalence of cardiovascular diseases has made them a constant focus of medical research. Reperfusion therapy has become the efficient means of response to acute and threatening manifestations of cardiovascular disorders. It is based on application of thrombolytic effect and stent and/or balloon therapy in the damaged part of the vascular bed. Such interventions proved to be very efficient in restricting the size of acute myocardial infarction (AMI), one of the most serious lesions of the cardiovascular system. The size of myocardial infarction is the principal prognostic factor for patients with AMI. Reperfusion therapy itself can restrict the size of AMI by up to 50% of the ischemic region.1 One in 4 patients with AMI has a lesion covering over 75% of the risk zone. It is thought that left ventricle lesion that exceeds 20% reliably confirms a prognosis of vulnerability in favor of severe morbidity and mortality. This requires medical resources to reduce the size of AMI from the fatal 75% of the ischemic region to 40% and under1 especially important in the most serious cases. The known limitation of reserves of reperfusion therapy justifies the development of adjacent treatment2 to help restrict the size of AMI and raise the survival index from the estimated rate of 0.25 (typical for use of reperfusion therapy only) to 0.60 and over.1
The role of oxidative stress in the development of cardiovascular injuries has been reliably established.3,4 One of the objectives of adjacent (additional) therapy is to block it. Oxidative stress contributes to the aggravation of disorders during thrombolysis for the patency of the infarct-related artery (reperfusion injury)5,6 and during vascular angioplasty procedures (development of restenosis).7,8 That is why it is important to search for highly efficient resources to ensure antioxidant protection of the cardiovascular system.
Experimental and clinical antioxidant research
Efficiency of antioxidant use in response to the destructive action of oxidative stress was shown in numerous experimental studies on animals and human cells.9 Nevertheless, the clinical trial data did not confirm their universal efficiency to be established in experimental research.10,11 Inconsistency of the clinical results of testing antioxidants was explained by the difference between the models of lesions used on animals and cells from the human body environment,1,5 by an incorrect choice of the tested antioxidant, monitoring parameters of its activity, amount of the derivative doses injected, the duration of trials, and patient selection criteria.9,11 New clinical trials are, therefore, needed and strict protocols should be applied with a wider use of new antioxidant derivatives. Given the well-known lack of financial, organizational, scientific, and medical resources, the future development of antioxidant therapy could take two directions. The first is related to the accurate, step-by-step experimental and clinical research of various antioxidants to gradually collect the critical mass of data on efficacy of antioxidant therapy in response to the accumulated scepticism of clinicians. The second is based on obtaining clear and evident data of the medical efficiency of antioxidants to promote a breakthrough in practice methods and means of antioxidant therapy.
Wide scope of antioxidant research
Continuing study through clinical trials seems to be the most attractive of the vast range of ongoing antioxidant research. Therefore, edaravone (3-methyl-1-phenyl-2-pyrazoline-5-one) was used (intravenous infusion 30 mg during 10 min within 6 h after manifestation of lesion symptoms) during stenting of the arteries of patients with AMI confirmed by angiography.12 It appeared that edaravone (compared to the control group/physiological solution) inhibited almost all the manifestations of reperfusion injury, such as reperfusion arrhythmia, myocardium stunning, fatal reperfusion injury. Edaravone administration before reperfusion ensured a smaller area of AMI by enzyme index (area under the curve of level of isozymes of creatine kinase, e.g. CK-MB isoenzyme of the heart in serum) and better clinical data.12 It should be mentioned that just by inhibiting the reactive oxygen forms which deplete the level of nitric oxide (NO), edaravone increased the blood-flow in the forearm of smokers, acting on endothelium-related vascular dilatation.13 This was also the target of co-enzyme Q10 action. It ameliorated bioenergetic parameters, increased the activity of extracellular superoxide-dismutase (EC-SOD) and endothelium-related dilatation after one month of oral dose (300 mg per day) in patients with coronary artery disease.14 Clinical trials of food (diet) antioxidants (vitamins E, C, polyphenols, carotenoids/lycopine, beta-carotene, coenzyme Q10) are ongoing, aimed at the prevention of atherogenic oxidation of low-density lipoproteins and oxidative damage of endothelium. This research is focused on evaluating the influence on activity of transcription factors (with definition of polymorphisms of the organism) and the development of individual diets based on dietary antioxidants.15 Although a huge amount of research into low-molecular antioxidants still continues to be published, the volume of literature is decreasing. On such a background, our attention is drawn to the appearance of interesting publications dedicated to the curative effects of enzyme antioxidants.
Extracellular superoxide dismutase
An increased amount of superoxide radical (O−2) was noted in the arteries of spontaneously hypertensive rats; in this case genetic transfer of EC-SOD ameliorated endothelium function and decreased the arterial pressure.16 Thus, interaction of O−2 with NO should have first place in extracellular space.17 Among all antioxidant enzymes, only EC-SOD is localized on the surface of the vascular lumen, interacting with heparan sulphate proteoglycan, and also with collagen18 and fibulin-5,19 by its heparan-binding domain.10,17 EC-SOD is probably located along the whole depth of the vascular wall, including the area between endothelium and vessel muscle.20 Heparin injection (in concentrations used for patients) led to the EC-SOD bounded earlier by the endothelium cells and other tissues to be released to the bloodflow.17,21 The antioxidant effect of EC-SOD appears mainly on/into the vascular wall, not inside the volume of the bloodflow.10,17 It was established that human coronary artery diseases are reliably related to the decreased level of heparin-released EC-SOD (131.0±42.8 ng/mL, P=0.0003 in the atherosclerosis group, n=189, and 156.9±66.2 ng/mL in control group, n=63).22,23 Its positive correlation with the level of high-density lipoprotein cholesterol and age was noted.23 The protective effect of EC-SOD is explained by defence of vascular dilatator NO that is diffusing from the endothelium cells to guanylatcyclase of the smooth muscle cells.10,17,24 This was confirmed by the data received from the model of high-volume hypertension in mice; the 1-kidney-1-clamp model markedly induced EC-SOD expression, whereas its activity was increased by only 25%, suggesting a partial inactivation of EC-SOD in high-volume hypertension.25 A decline in dilatation connected to endothelium, increased arterial pressure and oxidative stress of the vessels are noted among wild-type and EC-SOD knock-out mice. Recombinant EC-SOD decreased the arterial pressure and ameliorated bioavailability of NO in the aorta of wild-type mice and of EC-SOD knock-out mice. This enzyme antioxidant did not decrease the arterial pressure of knock-out mice with blocked endothelium NO-synthase, and of wild-type mice receiving NO-synthase inhibitor. These results provided evidence of the link between the vascular effect of recombinant EC-SOD and the NO-protection.25 In line with other data,26–28 the results also proved the important role of this biocatalyst in cases of hypertension. Similarly to the cases of atherosclerosis10,17 and hypertension, the oxidative stress and enzyme antioxidants play an important role in the development of diabetes mellitus and heart failure.17 The adhesive properties of EC-SOD were used to obtain recombinant SOD2/3.29 This SOD2/3 consisted of the mature human mitochondrial SOD-2 /Mn-SOD/ and C-terminal 26-amino acid heparin-binding tail from EC-SOD /SOD-3/. The SOD2/3 was fully active and its tail was responsible for enzyme affinity for endothelial surface. Because of this, the SOD2/3 had anti-inflammatory action against lung injury and limb edema in rats, and ischemia/reperfusion in rabbits.29,30
Experimental study of antioxidant gene therapy
The notable interest of researchers in EC-SOD,16,17,20–28 the tetramer of Cu,Zn-SOD with a high affinity to heparan sulphate31–33 in mammals, or in its dimer form of Cu,Zn-SOD with low affinity to heparan sulphate,16,31,33 in rats led to the development of gene therapy approaches aimed at ensuring antioxidant activity of the organism due to transfer of the appropriate genes.10,34 Antioxidant gene therapy proved to be successful in experimental models of restenosis (rabbits, rats, pigs, etc.) with an increase in expression of EC-SOD, cytosolic dimeric Cu,Zn-SOD (SOD-1), catalase (CAT), and hemoxygenase-1. This latter is the enzyme induced by stress; it transforms heme in biliverdin (for its further metabolic transformation in bilirubin), CO and ions of iron.34,35 The difficulties in the clinical use of gene therapy are caused by the limited duration of transgenic expression, but cardiovascular disorders (e.g., atherosclerosis) develop over the decades, or, visa versa, occur spontaneously (plaque disruption, thrombosis). These factors make patient selection criteria and the appropriate moment to start treatment unclear. Development of efficient methods of delivery of the gene material (limited transduction of cardiovascular cells), immunogenecity of the used delivery systems, and distortion of redox status of cells required for normal signaling34 are also important problems. Given these limitations, local delivery of gene constructions seems to be the most efficient. Endothelium denudation of rabbits with placement of stent in the damaged area, with simultaneous catheter injection of adenovirus with EC-SOD code (treatment group) or of beta-galactosidase (control group) made it possible to establish (according to monitoring vascular histology, the level of reactive oxygen species and expression) that EC-SOD significantly accelerated endothelium restoration and decreased the size of neointimal thickening.36 Such information certainly represents local gene therapy as the promising strategy of response to the vessel lesions caused by artery stenting. Moreover, EC-SOD protein concentration in the hypertensive and normotensive subjects are indistinguishable, whereas hypertensive patients have significantly reduced plasma EC-SOD activity.37 These data suggest that this decrease in EC-SOD activity is not due to a downregulation of the SOD-3 gene. Meanwhile it should be also noted that polymorphism of the EC-SOD gene (for hypertension development in human) may also have an important role in patient classification and possible gene therapy in future.
Superoxide dismutase and catalase activity
The wide scope of research into antioxidant protection with superoxide dismutases17,21,31 also revealed the inactivation of endogenous enzyme by hydrogen peroxide.25 Prolonged CAT treatment in vivo (intravenous bolus injection of derivative of catalase-polyethylene glycol for 3 days) decreased the blood pressure of spontaneously hypertensive wild-type mice (but not knock-out mice with bloked EC-SOD) and ameliorated ex vivo function of aorta endothelium. These data clearly demonstrated the central role of hydrogen peroxide in inactivation of endogenous EC-SOD.25,38 The efficiency of the decrease in hydrogen peroxide level in cases of the oxidative stress was shown on the cell cultures. Overexpression of CAT protected endothelium of human aorta against apoptosis caused by the oxidized forms of low-density lipoproteins (oxLDL).39 Transfer (in the smooth muscle cells of human aorta) of SOD-1 and/or CAT genes inhibited the proliferation induced by oxLDL.40 Overexpression of CAT or CAT together with SOD-1 in mice lacking apolipoprotein E (ApoE−/−) inhibited development of atherosclerosis in this model (lesion area/aortic tree, % of ApoE−/− mice with overexpression: CAT 7.2±1.2, SOD-1 and CAT 5.3±0.7; without overexpression 21.0±3.4).41 Such results underlined the important role of hydrogen peroxide in atherogenesis and the need to protect against oxidative stress of the vessels by simultaneous SOD- and CAT-activity. Such properties were combined in EUK-8, the synthetic mimetic of SOD- and CAT-activity, containing Se and Mn. This derivative has shown the protective effect (intra-abdominal injections 25 mg/kg/day, three times a day for four weeks) against remodeling of the left ventricle and cardiac decompensation in mice with a model development of heart failure.42 In the future, these results may provide the key treatment for human heart failure; they confirm the efficacy of the combined effect of SOD- and CAT-activity in blocking the oxidative stress. Meanwhile, the conjugation of SOD or CAT with antibody to platelet-endothelial cell adhesion molecule-1 (PECAM-1) provided a versatile molecular tool for testing the role of reactive oxygen species in vascular pathology.43 Anti-PECAM/SOD, but not anti-PECAM/CAT, inhibited a vascular endothelial growth factor (VEGF)-induced increase in endothelial permeability. This has identified a key role for endogenous superoxide radical in VEGF-mediated regulation of endothelial barrier function. On the contrary, anti-PECAM/CAT, but not anti-PECAM/SOD, alleviated endothelial hyperpermeability, implicating primarily hydrogen peroxide in the disruption of the endothelial barrier. Targeting the antioxidant enzymes to endothelial cells offers a future perspective for the development of effective cardioprotective remedies.
Combination of superoxide dismutase with catalase
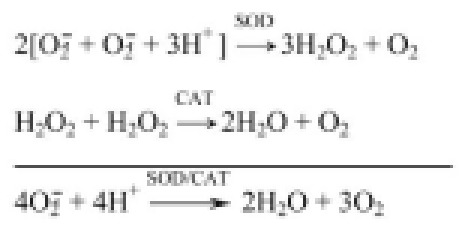
The combined use of native forms of SOD and CAT provided controversial data on antioxidant protection of the organism.10 The simultaneous action of SOD and CAT in the area of lesion development was required to demonstrate the reliable effect of treatment as compared to application of other compositions of native or CHS modified SOD and CAT. It was made possible by obtaining bienzyme conjugate, where SOD-1 was covalently bound via glycosaminoglycan of the vascular wall, chondroitin sulphate (CHS), with CAT (obtained adduct was SOD-CHS-CAT covalent conjugate) (Figure 1).44 Such conjugation contributed to likening of SOD-1 to SOD-3 (since EC-SOD is a glycoprotein),31–33 and the biochemically coupled SOD- and CAT-activity is rendered in the received bienzyme derivative (when the product of SOD reaction, hydrogen peroxide, is the substrate for the following CAT transformation in water and molecular oxygen being safe in such conditions. Chemical reactions of catalyzed superoxide dismutase and catalase are:
Figure 1.
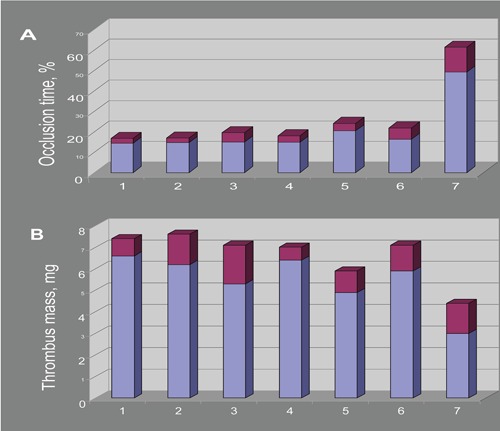
Antithrombotic effects of various combinations of superoxide dismutase (SOD) and catalase (CAT) derivatives and SOD-CHS-CAT conjugate44 on (A) occlusion time and (B) formed thrombus mass in rat model of arterial thrombosis (intervals of effects are shown as dark areas at the top of bars). 1, control; 2, SOD + CAT; 3, SOD + CHS + CAT; 4, SOD + (CAT-CHS); 5, (SOD-CHS) + CAT; 6, (SOD-CHS) + (CAT-CHS); 7, SOD-CHS-CAT conjugate. Each combination was injected at the same dose of SOD (37±3 U) and CAT (80±3 U) activity as the SOD-CHS-CAT conjugate. Each group of animals consisted of 6 rats. Error values of the specific activity determination did not exceed 2–4%.
On the model of arterial thrombosis in rats induced by treatment of the vessel with saturated solution of ferrous chloride, the bienzyme conjugate SOD-CHS-CAT has shown antithrombotic effect in doses for two orders less than for the mix of native SOD and CAT, and for one order less than for the mix of SOD and CAT modified by CHS. The SOD-CHS-CAT conjugate had the optimal dose of anti-thrombotic action (Figure 2). Cross-linking the proteins with CHS allows the bienzyme conjugate to be directed to the areas of vessel lesions. It is well-known that the areas of atherosclerotic lesions of the vascular wall are featured with the increased content of CHS.10 Early intimal thickening of the vascular wall in atherogenesis is also linked to accumulation of CHS.7
Figure 2.
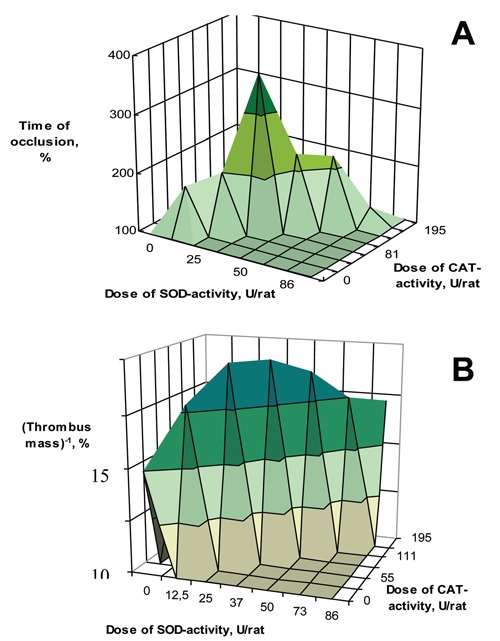
The influence of bienzyme SOD-CHS-CAT conjugate in rat model of arterial thrombosis10 (A) on occlusion time and (B) on obtained thrombus mass. Control rats (with the same arterial injury) were injected normal saline instead of the bienzyme conjugate. The control values were assume to be 100%. Reverse magnitude of the thrombus mass was used to provide a better demonstration (B). The SOD-CHS-CAT doses are given as units of enzyme activity injected per rat.
Chondroitin sulphate proteoglycan (CSPG) was observed in the artery sub-endothelium after interventional procedure and stent placement45 in atherosclerotic New Zealand white rabbits. CSPG was the target for binding the cationic liposomes (with prednisolone), contributing to the reliable depression of growth of intra-stent neointimae. These data provide evidence for the possibility and efficiency of using the components of vascular cell glycocalyx for targeted delivery of medical substances.46 Moreover, glycocalyx of erythrocytes may protect the tissue plasminogen activator (tPA) bound with such biotin modified cells against interaction with inhibitor of plasminogen activator of type 1 (PAI-1), destroying the local electrostatic interactions between tPA and PAI-1, not disturbing the binding of tPA with fibrin and plasminogen.47 Such stabilization by glycocalyx makes it possible to offer biotinilated components (tPA and erythrocytes), bounded via streptavidin, for thrombosis prophylaxis in patients with high risk of cerebral or vascular lesion.48,49 The noted effects confirm the importance of a network of distribution of coulomb charges during interaction with glycocalyx, and this may explain the high efficiency of antithrombotic action of bienzyme conjugate SOD-CHS-CAT. This derivative does not disturb the hemodynamic parameters of the rats and rabbits (blood pressure and heart rate) and helps normalize them after hydrogen peroxide administration in vivo;50 it has acceptable indexes of acute toxicity and evident antithrombotic potential.10 The accumulated data contribute to the promotion of the received derivative up to the status of drug candidate.
Scheduled development of scientific and productive background
Scientific development of the new biopharmaceutical substances (biopharmaceuticals) implies the development of methods of medical biotechnology, slowly but inevitably, becoming the basis of the pharmaceutical industry. The development of the industry is based on progress in systems biology adding to the knowledge and understanding of health protection, and contributing targeted work-out of biopharmaceuticals. The development of the bioeconomy, the new model of economic activity, leads to the establishment of new types of enterprise, and to new life for the companies involved. The need to establish plants and factories manufacturing certified bioreactives (recombinant proteins, RNA, DNA, their fragments, blood proteins, enzymes, peptides, vaccines, etc.), toxicology centers, and the creation of medical organizations for clinical trials of bioderivatives, evidently limit the practical use of biopharmaceuticals, and enzyme antioxidants are of special interest. Such an approach requires significant, regular and adequate financial support. A balanced combination of the stages of scientific, pre-clinical, biotechnological, toxicological, clinical, and technological developments is the pre-condition for the effective production of the medical substances of the new generation.
Conclusion
Oxidative stress is the peculiarity of all cardiovascular diseases. Reactive oxygen species may contribute to initiation and development of the pathological process. Antioxidants which are capable of blocking it, were efficient in experimental works; nevertheless, data from clinical trials appeared much more modest. This led to scepticism about antioxidants on the part of clinicians. An analysis of the limitations in the clinical trials performed with them leaves two ways open for development of antioxidant therapy. The first is the accurate, step-by-step research of antioxidants according to strict, professionally designed trial protocols. Many reports of such trials are still being published; nevertheless their numbers are decreasing with time. The second is to find break-through data on the new antioxidant substances, thus accelerating the development of antioxidant therapy. The spate of recent publications about enzyme antioxidants encourages discussion about the next stage in the development of antioxidant treatment. EC-SOD is of important interest for researchers, as it appeared to be the efficient marker and corrector in cases of hypertension, heart failure, diabetes mellitus, and atherosclerosis. The research into antioxidant efficacy of the other enzymes with autonomic functions, such as SOD-1, SOD-2, and CAT, continue; co-factor, the reduced glutathione, is required for glutathione peroxidase activity, and its quantity in cases of oxidative stress is rather limited, thus reducing its importance for medical purposes. Methods of biological and chemical synthesis are used to obtain the enzymes with connected catalytic activity. Derivatives of this kind (such as conjugate SOD-CHS-CAT) offer great promise for future biopharmaceutical research. Nevertheless, the success of these derivatives is the subject of efforts of specialists in various disciplines, while the actual conditions of start-up and progress of medical biotechnology and the pharmaceutical industry are important steps for the bioeconomy. At the present time, the main focus in trials of enzyme antioxidants is on scientific and pre-clinical stages of this research.
Acknowledgments:
the authors sincerely thank and highly appreciate the support of research and efficient discussions with professors EI Chazov, VN Smirnov, AM Egorov, EV Arzamastcev, VI Kapelko, VZ Lankin, EK Ruuge, AK Tichaze. This work was performed thanks to the support of the grants of Russian Foundation for Basic Research 07-04-12057-ofi, 09-08-00023, Rosmedtechnologies Agency and Roszdravsozrazvitija Ministry of Russian Federation.
References
- 1.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–13. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 2.Maksimenko AV. Thrombolysis research - new objectives after a shift of accent. Med Sci Monit. 2002;8:RA13–RA21. [PubMed] [Google Scholar]
- 3.Dubinina EE. Medicinal Press; Sankt-Petersburgh: 2006. Oxygen metabolism products in the functional activity of cells. [Google Scholar]
- 4.Mentschikova EB, Zenkov NK, Lankin VZ, et al. Pathological states and diseases. ARTA; Novosibirsk: 2008. Oxidative stress. [Google Scholar]
- 5.Dirksen MT, Laarman GT, Simoons ML, Duncker DJGM. Reperfusion injury in humans: a review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc Res. 2007;74:343–55. doi: 10.1016/j.cardiores.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Kloner RA. Does reperfusion injury exist in humans? J Am Coll Cardiol. 1993;21:537–45. doi: 10.1016/0735-1097(93)90700-b. [DOI] [PubMed] [Google Scholar]
- 7.Maksimenko AV. Effects of glycosaminoglycans in vascular events. Pharm Chemistry J. 2008;42:3–13. [Google Scholar]
- 8.Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key role for versican. Circ Res. 2004;94:1158–67. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 9.Mentschikova EB, Lankin VZ, Zenkov NK, et al. Prooxidants and antioxidants. Slovo; Moscow: 2006. Oxidative stress. [Google Scholar]
- 10.Maksimenko AV. Experimental antioxidant biotherapy for protection of the vascular wall by modified forms of superoxide dismutase and catalase. Curr Pharm Design. 2007;11:2007–16. doi: 10.2174/1381612054065756. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Pashkow FY. Oxidative stress and heart disease. Am J Cardiol. 2008;101:1D–86D. doi: 10.1016/j.amjcard.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Tsujita K, Shimomura H, Kaikita K, et al. Effect of edaravone on reperfusion injury in patients with acute myocardial infarction. Am J Cardiol. 2004;94:481–4. doi: 10.1016/j.amjcard.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Jitsuiki D, Higashi Y, Goto C, et al. Effect of edaravone, a novel free radical scavenger, on endothelium-dependent vasodilation in smokers. Am J Cardiol. 2004;94:1070–3. doi: 10.1016/j.amjcard.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 14.Tiano L, Belardinelli R, Carnevali P, et al. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J. 2007;28:2249–55. doi: 10.1093/eurheartj/ehm267. [DOI] [PubMed] [Google Scholar]
- 15.Kaliora AC, Dedoussis GVZ, Schmidt H. Dietary antioxidant in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Chu Y, Iida S, Lund DD, et al. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin binding domain. Circ Res. 2003;92:461–8. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 17.Heistad DD. Oxidative stress and vascular disease. Duff lecture. Arterioscler Thromb Vasc Biol. 2006;26:689–95. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- 18.Petersen SV, Oury TD, Ostergaard L, et al. Extracellular superoxide dismutase (EC-SOD) binds to type 1 collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–10. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen AD, Itoh S, Jeney V, et al. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95:1067–74. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- 20.Onry TD, Day BJ, Crapo JD. Extracellular superoxide dismutase: a regulator of nitric oxide bioavailability. Lab Invest. 1996;75:617–36. [PubMed] [Google Scholar]
- 21.Fukai T, Folz RZ, Landmesser U, Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res. 2002;55:239–49. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 22.Landmesser U, Merten R, Spiekermann S, et al. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease, relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–70. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 23.Tasaki H, Yamashita K, Tsutsui M, et al. Heparin-released extracellular superoxide dismutase is reduced in patients with coronary artery atherosclerosis. Atherosclerosis. 2006;187:131–8. doi: 10.1016/j.atherosclerosis.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–42. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 25.Jung O, Marklund SL, Xia N, et al. Inactivation of extracellular superoxide dismutase contributes to the development of high-volume hypertension. Arterioscler Thromb Vasc Biol. 2007;27:470–7. doi: 10.1161/01.ATV.0000254823.15843.1f. [DOI] [PubMed] [Google Scholar]
- 26.Gongora MC, Qin Z, Laude K, et al. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–81. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 27.Jung O, Marklund SL, Geiger H, et al. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from EC-SOD deficient mice. Circ Res. 2003;93:622–9. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 28.Welch WJ, Chabrashvili T, Solis G, et al. Role of extracellular superoxide dismutase in the mouse angiotensin slow press or response. Hypertension. 2006;48:934–41. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 29.Gao B, Flores SC, Leff JA, et al. Synthesis and anti-inflammatoryactivity of chimeric recombinant superoxide dismutase: SOD 2/3. Am J Physiol Lung Cell Mol Physiol. 2003;284:L917–25. doi: 10.1152/ajplung.00374.2002. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Saavedra D, Zhou H, McCord JM. Anti-inflammatory properties of a chimeric recombinant superoxide dismutase: SOD2/3. Biomed Pharmacother. 2005;59:204–8. doi: 10.1016/j.biopha.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson LM, Marklund SL, Edlund T. The rat extracellular superoxide dismutase dimer is converted to a tetramer by the exchange of a single amino acid. Proc Natl Acad Sci USA. 1996;93:5219–22. doi: 10.1073/pnas.93.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984;74:1398–403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol. 1995;15:2032–6. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 34.Levonen AL, Vahakangas E, Koponen JK, Yla-Herttuala S. Antioxidant gene therapy for cardiovascular disease. Current status and future perspectives. Circulation. 2008;117:2142–50. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 35.Stocker R, Pererella MA. Hem oxigenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–89. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 36.Bräsen JH, Leppädnen O, Inkala M, et al. Extracellular superoxide dismutase accelerates endothelial recovery and inhibits in-stent restenosis in stented atherosclerotic Watanabe heritable hyperlipidemic rabbit aorta. J Am Coll Cardiol. 2007;50:2249–53. doi: 10.1016/j.jacc.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Xiang W, Potts J, et al. Reduction in extracellular superoxide dismutase activity in African-American patients with hypertension. Free Radic Biol Med. 2006;41:1384–91. doi: 10.1016/j.freeradbiomed.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Fukai T. Extracellular SOD inactivation in high-volume hypertension: Role of hydrogen peroxide. Arterioscler Thromb Vasc Biol. 2007;27:442–4. doi: 10.1161/01.ATV.0000258920.36436.8e. [DOI] [PubMed] [Google Scholar]
- 39.Lin SJ, Shyue SK, Liu PL, et al. Adenovirus-mediated overexpression of catalase attenuates oxLDL-induced apoptosis in human aortic endothelial cells via AP-1 and C-Jun N-terminal kinase pathways. J Mol Cell Cardiol. 2004;36:129–39. doi: 10.1016/j.yjmcc.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Lin SJ, Shyue SK, Shih MC, et al. Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis. 2007;190:124–34. doi: 10.1016/j.atherosclerosis.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Roberts LJ, Shi MJ, et al. Retardation of atherosclerosis by overexpression of catalase or both Cu,Zn-SOD dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–81. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 42.Van Empel VP, Bertrand AT, van Oort RJ, et al. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the Harlequin mouse mutant. J Am Coll Cardiol. 2006;48:824–32. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 43.Han J, Shuvaev VV, Muzykantov VR. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J Parmacol Exp Ther. 2011;338:82–91. doi: 10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maksimenko AV, Golubykh VL, Tischenko EG. The combination of modified antioxidant enzymes for anti-thrombotic protection of the vascular wall: the significance of covalent connection of superoxide dismutase and catalase activities. J Pharmacy Pharmacol. 2004;56:1463–8. doi: 10.1211/0022357044544. [DOI] [PubMed] [Google Scholar]
- 45.Joner M, Morimoto K, Kasukawa H, et al. Site-specific targeting of nanoparticles prednisolone reduces instent restenosis in a rabbit model of established atheroma. Arterioscler Thromb Vasc Biol. 2008;28:1960–6. doi: 10.1161/ATVBAHA.108.170662. [DOI] [PubMed] [Google Scholar]
- 46.Sarembock IJ. From systemic shotgun to site-specific nanoparticle-targeted delivery: A new paradigm for drug delivery. Arterioscler Thromb Vasc Biol. 2008;28:1879–81. doi: 10.1161/ATVBAHA.108.175190. [DOI] [PubMed] [Google Scholar]
- 47.Ganguly K, Murciano JC, Westrick R, et al. The glycocalyx protects erythrocyte-bound tissue-type plasminogen activator from enzymatic inhibition. J Pharmacol Exp Ther. 2007;321:158–64. doi: 10.1124/jpet.106.114405. [DOI] [PubMed] [Google Scholar]
- 48.Danielyan K, Ganguly K, Ding BS, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118:1442–9. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider DJ, Sobel BE. A novel role for tissue-type plasminogen activator: Prevention of thromboembolic occlusion. Circulation. 2008;118:1408–9. doi: 10.1161/CIRCULATIONAHA.108.807586. [DOI] [PubMed] [Google Scholar]
- 50.Maksimenko AV, Vavaev AV, Bouryachkovskaya LI, et al. Biopharmacology of enzyme conjugates: vasoprotective activity of supramolecular superoxide dismutase-chondroitin sulfate-catalase derivative. Acta Naturae. 2010;2:82–94. [PMC free article] [PubMed] [Google Scholar]


