Abstract
Gallium-68 is a generator-produced radionuclide for positron emission tomography (PET) that is being increasingly used for radiolabeling of tumor-targeting peptides. Compounds [68Ga]3 and [68Ga]6 are high-affinity, urea-based inhibitors of the prostate-specific membrane antigen (PSMA) that were synthesized in decay-uncorrected yields ranging from 60 – 70% and radiochemical purities of more than 99%. Compound [68Ga]3 demonstrated 3.78 ± 0.90 percent injected dose per gram of tissue (%ID/g) within PSMA+ PIP tumor at 30 min post-injection, while [68Ga]6 showed a two hour PSMA+ PIP tumor uptake value of 3.29 ± 0.77%ID/g. Target (PSMA+ PIP) to non-target (PSMA− flu) ratios were 4.6 and 18.3, respectively, at those time points. Both compounds delineated tumor clearly by small animal PET. The urea series of imaging agents for PSMA can be radiolabeled with 68Ga, a cyclotron-free isotope useful for clinical PET studies, with maintenance of target specificity.
Keywords: gallium, molecular imaging, positron emission tomography, prostate-specific membrane antigen, radiopharmaceutical
Introduction
Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer-related death in men in the United States.1 In 2009, approximately 192,000 men were diagnosed with prostate cancer with 27,000 succumbing to the disease. The integral membrane protein prostate-specific membrane antigen (PSMA) is becoming increasingly recognized as a viable target for imaging and therapy of prostate and other forms of cancer.2, 3
Because of its similarity to Fe(III), Ga(III) complexes are emerging as an interesting alternative to Pt-based anticancer agents.4–6 From a diagnostic standpoint, positron-emitting versions of Ga(III) can be used for tumor imaging.7–9 Recently, the application of 68Ga-labeled peptides has attracted considerable interest for cancer imaging because of the physical characteristics of 68Ga.10 68Ga is available from an in-house 68Ge/68Ga generator (68Ge, t1/2 = 270.8 day), which renders it independent of an onsite cyclotron. Therefore, 68Ga-based PET agents possess significant commercial potential and serve as a convenient alternative to cyclotron-based isotopes for positron emission tomography (PET), such as 18F or 124I. 68Ga has a high positron-emitting fraction (89% of its total decay). The maximum positron energy of 68Ga (max. energy = 1.92 MeV, mean = 0.89 MeV) is higher than that of 18F (max = 0.63 MeV, mean = 0.25 MeV). However, a study of spatial resolution using Monte Carlo analysis revealed that under the assumption of 3 mm spatial resolution for most PET detectors, the full-width-at-half-maximum (FWHM) of 18F and 68Ga are indistinguishable in soft tissue (3.01 mm vs. 3.09 mm).9 That finding implies that with the standard spatial resolution of 5 to 7 mm for current clinical scanners, image quality using 68Ga-based radiotracers will likely be indistinguishable from that of 18F-based agents, stimulating interest in the development of 68Ga-labeled compounds for medical imaging.7–9 With a physical half-life of 68 min, 68Ga is also matched nicely to the pharmacokinetics of many peptides used for imaging. Furthermore, 68Ga is introduced to biomolecules through macrocyclic chelators, which allows possible kit formulation and wide availability of the corresponding imaging agents.
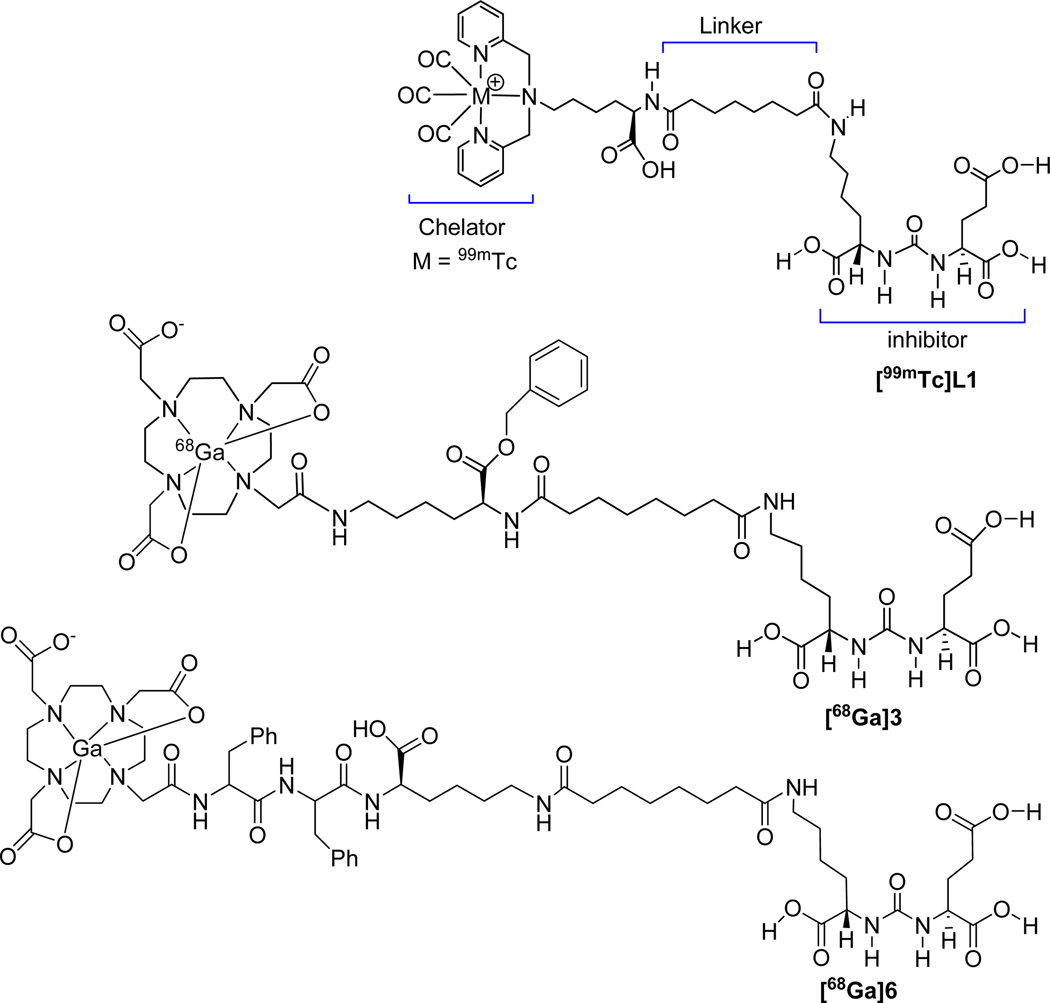
We and others have previously demonstrated the ability to image PSMA-expressing prostate tumor xenografts with radiohalogenated, urea-based, low molecular weight inhibitors of PSMA.11, 12, 13, 14, 15 Recently, we have extended that work to include the radiometal 99mTc via a coordinated, 99mTc tricarbonyl moiety.16 To retain the binding affinity of those inhibitors to PSMA a linker moiety was introduced between the amino functionalized PSMA urea and the metal chelator (Figure 1). A similar approach by Kularatne et al. produced 99mTc-oxo labeled inhibitors.14, 15 We have now extended this work further to include 68Ga for PET imaging. Gallium(III) ion forms a stable complex (formation constant, logKML = 21.33) with the commercially available, widely used multidentate chelating agent, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA).17 This report describes the synthesis and in vitro binding of two new 68Ga-labeled, conjugated PSMA inhibitors, [68Ga]3 and [68Ga]6 (Figure 1), as well the biodistribution and in vivo imaging studies of these compounds. The chelating agent we have employed is the triacetic acid mono-amide of DOTA. Few 68Ga-labeled, mechanism-based radiotracers for prostate cancer have been reported previously, and none for PSMA or that approach such low molecular weights as these.
Figure 1.
Urea-based PSMA radioligands 99mTcL1, [68Ga]3 and [68Ga]6.
Results
Chemical and Radiochemical Syntheses
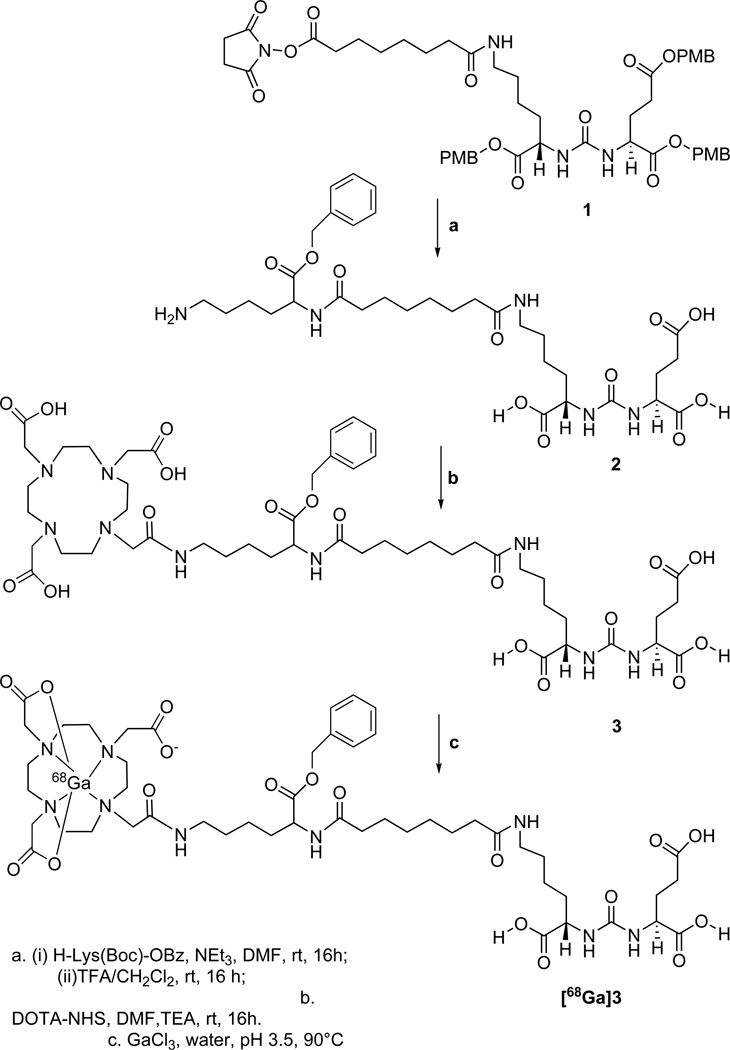
DOTA-conjugated urea inhibitors
Key N-hydroxysuccinimide (NHS) ester intermediate 1, (Scheme 1) was prepared following our previous report.16 Compound 1 was then conjugated with the α-amine of H-Lys(Boc)-OBz18 followed by simultaneous removal of Boc and PMB (p-methoxybenzyl) groups using a solution of trifluoracetic acid (TFA)/CH2Cl2 at 25°C to produce 2. The primary amine of 2 was then conjugated with DOTA-NHS (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono(N-hydroxysuccinimide ester)) to obtain 3, in ~ 40% yield after purification by high-performance liquid chromatography (HPLC). NMR and mass spectrometry (MS) were used to confirm the identity of 3.
Scheme 1.
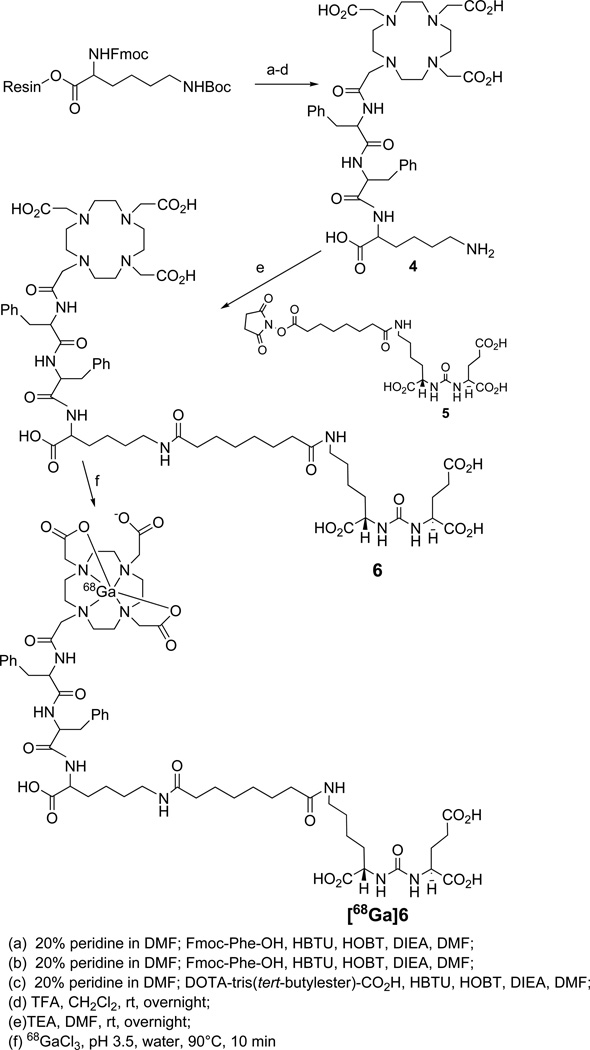
Synthesis of DOTA conjugated PSMA inhibitor 6 was performed by using standard fluorenylmethoxycarbonyl (Fmoc) solid phase peptide synthesis (SPPS) starting from Fmoc-Lys(Boc)-Wang resin according to Scheme 2. After conjugating two phenylalanine residues with the resin bound lysine, DOTA was conjugated at the N-terminal of the second phenylalanine residue after which the compound was cleaved from the resin by a 1:1 mixture of TFA:CH2Cl2 to produce 4. The free ε-amine of lysine was then conjugated with 5,19 which was prepared from 1 by PMB cleavage, to produce 6. Compound 6 was characterized through standard spectroscopic techniques.
Scheme 2.
A stable gallium isotope (69,71Ga) was introduced to the urea-DOTA conjugate by incubation of 3 or 6 with an aqueous solution of GaCl3 at 95°C for 10 min. Compounds [69,71Ga]3 and [69,71Ga]6, were characterized by standard spectroscopic analyses. The proposed structures of compounds [69,71Ga]3 and [69,71Ga]6, as shown in Schemes 1 and 2 and Figure 1, were based on the reported X-ray crystal structures of gallium-DOTA compounds described by Maecke et al20, 21 and Doyle et al.22 The mass spectra of the Ga compounds showed the expected isotope distribution pattern for natural gallium, which is a mixture 69Ga (60.11%) and 71Ga (39.89%).23 The stable gallium-labeled conjugates were used as authentic reference material for the chromatographic analysis of the radiolabeling reactions to identify the corresponding 68Ga-labeled compounds.
Radiochemistry
The 68Ga(III) was eluted from the 68Ge/68Ga generator using ~ 6 – 7 mL of a solution of 0.1 N hydrochloric acid (HCl). To achieve high specificity radioactivity, radioactive material was preconcentrated and purified on a cation exchange resin following a literature method.24 The 68Ga(III) was eluted from the resin with 400 µL of an 97.6% acetone/0.05 N HCl mixture (pH, 2.30 ± 0.05) and was used immediately for aqueous radiolabeling of 3 and 6. No buffer solution was added. The radiolabeling was performed at 90 – 95°C for 10 min with decay-uncorrected yields ranging from 60 – 70% and radiochemical purities of more than 99%. On analysis of the reaction mixture by HPLC, the retention time of the radiolabeled compound was slightly longer than the corresponding free ligand. The specific radioactivity of purified [68Ga]3 and [68Ga]6 was between 3.0 and 6.0 MBq/nmol. The log Poctanol/water values for [68Ga]3 and [68Ga]6 were approximately −3.9 as determined by the shake-flask method.25 However, using an HPLC method, we found that the HPLC retention times for 6 (28 min) and [69/71Ga]6 (32 min) were longer than for 3 (19 min) and [69/71Ga]3 (24 min). It is evident that 6 and the corresponding gallium compound were more lipophilic than 3 and its gallium–labeled analog, which is reasonable in light of the presence of two phenylalanine residues in the long linker of 6, while 3 has only one lysine residue protected as the benzyl ester. Interestingly, our previous lead compound, 99mTcL1,16 was found to be much more lipophilic than either of these gallium compounds, with log Poctanol/water~ −3.1, possibly because of its organometallic tricarbonyl core as well as the presence of the lipophilic bispyridyl chelating agent.
Biology
Cell binding assay
Ki values for 3, [69, 71Ga]3, 6 and [69, 71Ga]6 were determined using a fluorescence-based PSMA inhibition assay.26 All compounds were found to be potent inhibitors of PSMA, as we reported earlier for 99mTcL1 and related compounds.16 Compounds 3 and [69,71Ga]3 had inhibitory capacities of 2.9 nM and 29 nM, respectively. For 6 and [69, 71Ga]6, values were 1.23 nM and 0.44 nM, respectively.
Biodistribution
Compound [68Ga]3 was assessed for its pharmacokinetics ex vivo in severe-combined immunodeficient (SCID) mice bearing both PSMA+ PC3-PIP and PSMA− PC3-flu xenografts.27 Table 1 shows the percent injected dose per gram (%ID/g) of radiotracer in selected organs for [68Ga]3. Compound [68Ga]3 showed clear PSMA-dependent binding in PSMA+ PC3 PIP xenografts, reaching a maximum uptake of 3.78 ± 0.90 (SEM) %ID/g at 30 min post-injection (pi). The blood, spleen and kidney displayed highest uptake at 30 min. By 60 min, the urinary bladder showed highest uptake, however, this uptake represents excretion at all time points. The high values noted in kidney are partially due to high expression of PSMA within proximal renal tubules.28, 29 Rapid clearance from the kidneys was demonstrated, decreasing from 97.19 ± 16.07 %ID/g at 30 min to 2.31 ± 0.11%ID/g at 3 h. The radioactivity in the PSMA+ PIP tumor cleared more slowly, from its aforementioned value at 30 min to 1.08 ± 0.19 %ID/g at 3 h.
Table 1. Ex Vivo Tissue Biodistribution of [68Ga]3.
| 30 min | 60 min | 120 min | 180 min | |||||
|---|---|---|---|---|---|---|---|---|
| Blood | 2.19 ± 0.88 | 1.93 ± 0.71 | 0.80 ± 0.29 | 0.62 ± 0.34 | ||||
| heart | 0.69 ± 0.12 | 0.50 ± 0.07 | 0.21 ± 0.08 | 0.19 ± 0.02 | ||||
| lung | 2.36 ± 0.6 | 1.34 ± 0.23 | 0.45 ± 0.07 | 0.37 ± 0.08 | ||||
| liver | 0.84 ± 0.24 | 0.83 ± 0.09 | 0.42 ± 0.06 | 0.47 ± 0.03 | ||||
| stomach | 0.73 ± 0.13 | 0.75 ± 0.32 | 0.24 ± 0.06 | 0.24 ± 0.05 | ||||
| pancreas | 0.65 ± 0.06 | 1.66 ± 1.54 | 0.18 ± 0.04 | 0.24 ± 0.16 | ||||
| spleen | 4.90 ± 1.1 | 3.36 ± 1.16 | 0.43 ± 0.18 | 0.31 ± 0.13 | ||||
| fat | 0.63 ± 0.25 | 1.46 ± 1.31 | 0.069 ± 0.04 | 0.21 ± 0.27 | ||||
| kidney | 97.19 ± 16.07 | 64.67 ± 4.05 | 5.35 ± 2.10 | 2.12 ± 0.11 | ||||
| muscle | 0.45 ± 0.17 | 0.25 ± 0.07 | 0.075 ± 0.03 | 0.05 ± 0.00 | ||||
| small intestine | 0.79 ± 0.12 | 0.69 ± 0.32 | 0.26 ± 0.11 | 0.33 ± 0.20 | ||||
| large intestine | 0.76 ± 0.14 | 0.95 ± 0.48 | 0.34 ± 0.09 | 0.46 ± 0.10 | ||||
| bladder | 8.96 ± 5.3 | 25.28 ± 8.62 | 2.70 ± 4.01 | 5.39 ± 2.97 | ||||
| PC-3 PIP | 3.77 ± 0.88 | 3.32 ± 0.34 | 1.31 ± 0.06 | 1.08 ± 0.19 | ||||
| PC-3 flu | 0.82 ± 0.22 | 0.67 ± 0.08 | 0.40 ± 0.07 | 0.39 ± 0.02 | ||||
| PIP:flu | 4.60 | 4.92 | 3.24 | 2.77 | ||||
| Pip:muscle | 8.28 | 13.13 | 17.40 | 20.36 | ||||
| flu:muscle | 1.79 | 2.66 | 5.37 | 7.34 | ||||
N=4 for all time points
Compound [68Ga]6 was also investigated for its pharmacokinetic characteristics in tumor-bearing mice at 5 min, 1 h, 2 h and 3 h pi. Table 2 shows the %ID/g of radiotracer in selected organs for [68Ga]6. As for [68Ga]3, [68Ga]6 showed PSMA-dependent tumor uptake. After a peak, flow-related, uptake at 5 min pi of 6.61 ± 0.55%, [68Ga]6 demonstrated a 2 h tumor uptake value of 3.29 ± 0.77%, which dropped to 1.80 ± 0.16% at 3 h. Uptake in blood was high at 5 min and rapidly washed out within 1 h. Non-target organs such as kidney, spleen and lung showed high uptake at 5 min and rapidly washed out with time. With the exception of the kidneys and spleen, clearance from blood and normal organs was faster for [68Ga]6 than for [68Ga]3. Again, high kidney uptake is associated with high expression of PSMA within proximal renal tubules.28, 29 Similar to [68Ga]3, [68Ga]6 demonstrated faster clearance of radioactivity from kidney than from the PSMA+ tumor. However, the rate of clearance from kidney for [68Ga]6 was much slower than for [68Ga]3, i.e., 65 ± 12% at 5 min pi and 10 ± 1.22% at 3 h.
Table 2. Ex Vivo Tissue Biodistribution of [68Ga]6.
| 5 min | 60 min | 120 min | 180 min | |||||
|---|---|---|---|---|---|---|---|---|
| Blood | 6.28 ± 0.08 | 0.41 ± 0.05 | 0.15 ± 0.07 | 0.13 ± 0.01 | ||||
| heart | 2.01 ± 0.24 | 0.19 ± 0.07 | 0.05 ± 0.03 | 0.03 ± 0.01 | ||||
| lung | 4.59 ± 0.68 | 0.74 ± 0.54 | 0.20 ± 0.05 | 0.14 ± 0.03 | ||||
| liver | 1.57 ± 0.16 | 0.24 ± 0.09 | 0.19 ± 0.03 | 0.14 ± 0.02 | ||||
| stomach | 2.38 ± 0.35 | 0.38 ± 0.16 | 0.18 ± 0.02 | 0.04 ± 0.02 | ||||
| pancreas | 1.52 ± 0.19 | 0.25 ± 0.14 | 0.08 ± 0.03 | 0.04 ± 0.02 | ||||
| spleen | 5.17 ± 2.22 | 2.43 ± 1.07 | 0.78 ± 0.15 | 0.34 ± 0.09 | ||||
| fat | 1.03 ± 0.02 | 0.40 ± 0.04 | 0.08 ± 0.02 | 0.02 ± 0.01 | ||||
| kidney | 64.75 ± 12.00 | 26.57 ± 10.93 | 12.25 ± 1.79 | 10.04 ± 1.22 | ||||
| muscle | 1.58 ± 0.33 | 0.12 ± 0.08 | 0.03 ± 0.02 | 0.004 ± 0.009 | ||||
| small intestine | 2.04 ± 0.25 | 0.23 ± 0.05 | 0.09 ± 0.04 | 0.06 ± 0.03 | ||||
| large intestine | 2.02 ± 0.49 | 0.50 ± 0.70 | 0.12 ± 0.03 | 0.12 ± 0.03 | ||||
| bladder | 5.97 ± 1.50 | 7.65 ± 3.34 | 1.41 ± 1.17 | 0.75 ± 0.54 | ||||
| PC-3 PIP | 6.61 ± 0.55 | 2.80 ± 1.32 | 3.29 ± 0.77 | 1.80 ± 0.16 | ||||
| PC-3 flu | 2.63 ± 0.51 | 0.16 ± 0.08 | 0.18 ± 0.03 | 0.12 ± 0.03 | ||||
| PIP:flu | 2.50 | 17.30 | 18.28 | 15.20 | ||||
| Pip:muscle | 4.17 | 23.27 | 122.13 | 436.29 | ||||
| flu:muscle | 1.67 | 1.34 | 6.68 | 28.70 | ||||
N=4 for all time points
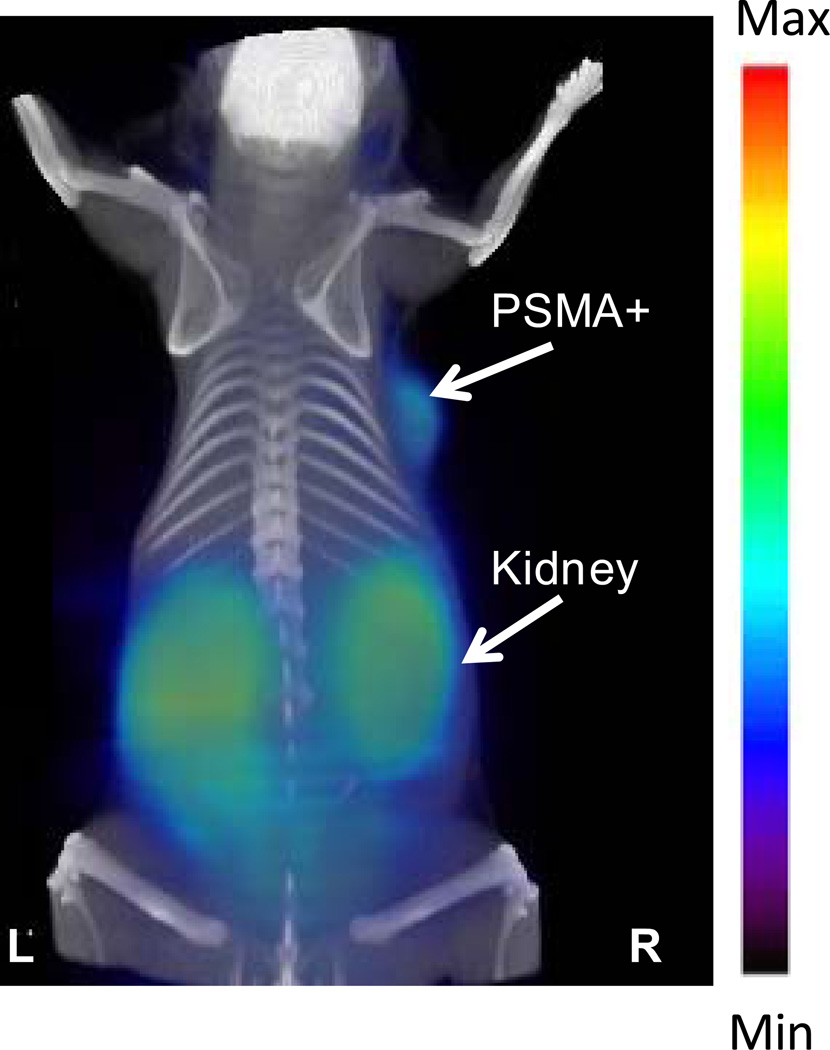
Small animal PET imaging
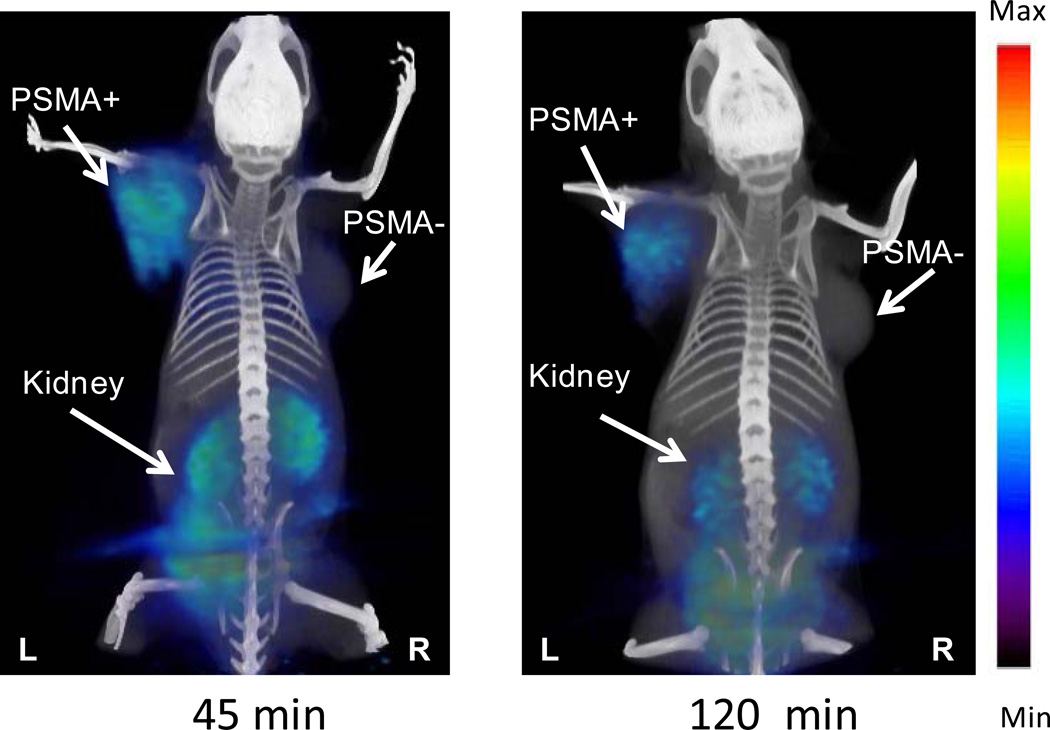
Intense radiotracer uptake was seen only in the kidneys and tumor for both [68Ga]3 (Figure 2) and [68Ga]6 (Figure 3). As noted above for the ex vivo study, the intense renal uptake was partially due to specific binding of the radiotracer to proximal renal tubules28, 29 as well as to excretion of this hydrophilic compound. Apart from the kidneys, only the PSMA+ tumor demonstrated significant radiotracer uptake.
Figure 2.
GE eXplore VISTA pseudodynamic PET image (co-registered with the corresponding CT image) of a PSMA+ LNCaP tumor-bearing mouse injected intravenously with 0.2 mCi (7.4 MBq) of [68Ga]3.
Figure 3.
GE eXplore VISTA PET image (co-registered with the corresponding CT image) of a PSMA+ PIP and PSMA− flu tumor-bearing mouse injected intravenously with 0.2 mCi (7.4 MBq) of [68Ga]6.
Discussion
Because of its demonstrated clinical utility and the appearance of dual modality [PET/computed tomography (CT)] systems, clinical PET imaging has been accelerating worldwide and may soon become the dominant technique in nuclear medicine. PET isotopes tend to be short-lived and enable synthesis of “physiologic” radiotracers, namely, those that incorporate 15O, 13N or 11C, enabling precise conformity to the tracer principle. Being essentially isosteric to H, 18F enables nearly tracer-level studies, with important caveats, particularly for [18F]fluorodeoxyglucose (FDG), which is by far the most commonly used radiopharmaceutical for PET. But, in part because FDG does not accumulate well within many tumor types,30 including prostate cancer,30, 31 there has been a sustained effort in the development of radiometallated peptides, often employing 99mTc, that target G-protein coupled receptors. Gallium-68 provides a link between PET and single photon emission computed tomography (SPECT) since metal chelating methodology needed for 99mTc can also be applied to 68Ga. A further analogy is the convenience of a 68Ge/68Ga generator (PET), as with 99Mo/99mTc (SPECT), to provide readily available isotope, with no need for an in-house cyclotron. Although 18F-labeled, low molecular weight PSMA inhibitors have shown promise in preclinical imaging studies,13, 32 the ready availability of generator-produced 68Ga and the logical extension to PET from our published 99mTc-labeled series of PSMA-binding radiometallated imaging agents16 provide the rationale for this study.
As for the 99mTc-labeled agents, the strategy we employed involves a tripartite imaging agent containing a PSMA targeting moiety, a linker and a chelator for 68Ga. The linker is necessary to enable productive binding by directing the 68Ga-chelate complex through a 20Å tunnel away from the active site. Because of its ability to chelate metals with a +3 oxidation state and its clinical track record we used DOTA as the chelator for both [68Ga]3 and [68Ga]6.33–39 Both are also derived from a lysine-urea-glutamate construct that confers PSMA specificity. That still leaves significant structural differences, which are confined to the linker, between those two compounds. Those differences include two phenylalanines for [68Ga]6 relative to [68Ga]3, while the latter compound possesses one benzyl-protected lysine within the linker. Because of the strict structural requirements of the S1’ (pharmacophore) pocket in which the glutamate moiety resides,40 and the need for at least one additional carboxylate (derived from lysine), modification of the linker is the best option to enable pharmacokinetic optimization of this series. A careful balance is sought whereby sufficient localization of the radiotracer to the tumor is needed, favoring higher hydrophobicity, while washout from non-target sites such as liver and intestine is also desired, favoring higher hydrophilicity. The benzyl group was initially added to [68Ga]3 to provide a chromophore to facilitate purification, but the phenylalanines in [68Ga]6 were added to offset the high hydrophilicity of these compounds, potentially enabling longer and/or higher tumor sequestration, as originally proposed in a previous report.14 The need for long retention times, while not necessary when using 68Ga (physical half-life = 68 min), may be needed for longer lived isotopes, such as 111In, or for therapeutic radiometals. However, even addition of two phenylalanines was not able to provide a compound as lipophilic as our previously published SPECT agent, 99mTcL1.
Figures 2 and 3 demonstrate the high target selectivity of [68Ga]3 and [68Ga]6 by delineating the PSMA+ tumors as well as kidney, which is a PSMA+ structure. Because we anticipate that most metastatic foci, for which these compounds are designed, will be in bone or lymph nodes (particularly those in pelvis), we do not anticipate that renal uptake will provide a significant confound for these agents. Although a PSMA− control tumor was not included in Figure 2, a separate blocking study was performed for [68Ga]3, in which an animal pre-treated with 50 mg/kg of the known PSMA-binding ligand, 2-(phosphonomethyl)pentanedioic acid (2-PMPA),41 did not demonstrate PSMA+ tumor uptake (see Supplementary Information Available), attesting to the binding specificity of this compound. The more quantitative, ex vivo studies of [68Ga]3 and [68Ga]6 further support high PSMA target specificity, demonstrating target-to-nontarget (PIP/flu) ratios of approximately 5 and 18 at 1 h and 2 h pi, respectively. One hour and 2 h PSMA+ tumor uptake values for these compounds, 3.32 ± 0.33% and 3.29 ± 0.77%, respectively, for [68Ga]3 and [68Ga]6, are comparable to other radiometallated PSMA inhibitors we have developed.16 As shown in Figures 2 and 3 those values are sufficient for clear tumor imaging. Notably, PIP tumors contain about one order of magnitude lower PSMA than LNCaP tumors (data not shown), which are often employed to assess for binding specificity of PSMA-targeting agents. We generally prefer the PIP/flu comparison as both are derived from PC-3 cells, providing a more controlled study.
Conclusions
Compounds [68Ga]3 and [68Ga]6 demonstrate PSMA-specific tumor imaging in vivo. Because of higher target-to-nontarget ratios with comparable absolute uptake values to [68Ga]3, [68Ga]6 will be pursued in additional animal models and for toxicity testing en route to clinical translation. In this manner we hope to add this cyclotron-independent radiopharmaceutical to the array of emerging agents for imaging prostate cancer.
Experimental Section
General Procedures
Solvents and chemicals obtained from commercial sources were of analytical grade or better and used without further purification. All experiments were performed in duplicate or triplicate to ensure reproducibility. Analytical thin-layer chromatography (TLC) was performed using Aldrich aluminum-backed 0.2 mm silica gel Z19, 329-1 plates and visualized by ultraviolet light (254 nm), I2 and 1% ninhydrin in EtOH. Flash chromatography was performed using silica gel purchased from Bodman (Aston PA), MP SiliTech 32–63 D 60Å. 1H NMR spectra were recorded on a Bruker ultrashield™ 400 MHz spectrometer. Chemical shifts (δ) are reported in ppm downfield by reference to proton resonances resulting from incomplete deuteration of the NMR solvent. Low resolution ESI mass spectra were obtained on a Bruker Daltonics Esquire 3000 Plus spectrometer. High resolution mass spectra were obtained at the University of Notre Dame Mass Spectrometry & Proteomics Facility, Notre Dame, IN using ESI either by direct infusion on a Bruker micrOTOF-II or by LC elution via an ultra-high pressure Dionex RSLC with a C18 column coupled with a Bruker micrOTOF-Q II. High-performance liquid chromatographic purification of new compounds, 3, [69/71Ga]3, 6, [69/71Ga]6 and [68Ga]3, was performed using a Phenomenex C18 Luna 10 × 250 mm2 column on a Waters 600E Delta LC system with a Waters 486 tunable absorbance UV/Vis detector, both controlled by Empower software. For purification of radiolabeled [68Ga]6, a Varian Microsorb-Mv C18 250 × 4.6 mm2 column was used. HPLC was performed using the following isocratic conditions: For Method 1, the mobile phase was 80% solvent A (0.1% TFA in water) and 20% solvent B (0.1% TFA in CH3CN), flow rate 4 mL/min; for Method 2, the mobile phase was 80% solvent A and 20% solvent B, flow rate 1 mL/min. Method 1 was used for purification of compounds 3, [69/71Ga]3, 6, [69/71Ga]6 and [68Ga]3. For purification of [68Ga]6, Method 2 was used. For radiosynthetic purification, HPLC was performed on a Varian Prostar System (Palo Alto, CA), equipped with a model 490 UV absorbance detector and a Bioscan NaI scintillation detector connected to a Bioscan Flow-count system. All final compounds were obtained in > 95% purity, as determined by HPLC.
2-{3-[5-(7-{1-Benzyloxycarbonyl-5-[2-(4,7,10-tris-carboxymethyl-1,4,7,10tetraazacyclododec-1-yl)-acetylamino]-pentylcarbamoyl}-heptanoylamino)-1-carboxy-pentyl]-ureido}-pentanedioic acid, 3
Compound 3 was prepared in three steps according to Scheme 1. Compound 1 was prepared according to a literature method.16 To a solution of 1 (100 mg, 0.11 mmol in 5 mL DMF) was added H-Lys(Boc)-OBz (36 mg, 0.11 mmol).18 The solution was stirred for 16 h at ambient temperature. The solvent was removed under vacuum. The solid residue thus obtained was dissolved in 10 mL ethyl acetate and extracted with 3 × 10 mL water. The organic layer was dried under vacuum to provide a colorless solid ESIMS: 1154 [M+1]+. This crude compound was dissolved in 3 mL CHCl3 followed by addition of 3 mL TFA at 0°C. The solution was allowed to stir overnight at ambient temperature. The volume of the solution was reduced under vacuum and the solid residue was washed with 3 × 5 mL CH2Cl2 to remove impurities. The colorless solid residue, 2, was dried under vacuum. The crude yield for 2 was 80 mg. Compound 2 was purified further by using a 2 g Sep Pak C18 cartridge with a solution of 85/15 water/acetonitrile (0.1% TFA in each). 1H NMR (D2O, δ): 7.5 (bm, 5H, Ph), 5.12 (s, 2H, PhCH2), 4.27 (m, 1H, HC(NH)CO2(Glu)), 4.16(m, 1H, HC(NH)CO2(Lys)), 3.99 (m, 1H, HC(NH)CO2(Lys-linker)), 3.08 (m, 4H, H2CNH(Lys), H2CNH(Lys-linker)), 2.39(t, 2H, H2CCO-linker), 2.21 (m, 2H, H2CCO2(Glu)), 2.19(t, 2H, H2CCO-linker), 1.89-1.57(m, 6H, H2CCH(Glu), H2CCH(Lys), H2CCH(Lys-linker)), 1.43-1.16 (m, 16H, (CH2)2(Lys), (CH2)2(Lys-linker), (CH2)4 (linker)). ESIMS: 694 [M+1]+.
To a solution of DOTA-mono-NHS (54 mg, 0.11 mmol in 5 mL DMF) was added 2 (80mg, 0.08 mmol) and TEA (60 µL, 0.43 mmol) and the solution was allowed to stir for 16 h at ambient temperature. Solvent was removed under vacuum and the crude solid, 3, was purified by HPLC Method 1, retention time 19 min. Yield: ~ 40%. 1H NMR (D2O) δ: 7.88 (m, 5H, Ph), 5.10 (s, 2H, H2CPh), 4.26 (m, 1H, HC(NH)CO2(Glu)), 4.16(m, 1H, HC(NH)CO2(Lys)), 4.06 (m, 1H, HC(NH)CO2(Lys-linker), 3.66 (m, 8H, H2CCO2), 3.18 (m, 20H, N(CH2)2N(DOTA), H2CNH(Lys), H2CNH(Lys-linker)), 2.39(t, 2H, H2CCO-linker), 2.15 (m, 2H, H2CCO2(Glu)), 2.07(t, 2H, H2CCONH-linker),1.85-1.55(m, 6H, H2CCH(Glu), H2CCH(Lys), H2CCH(Lys-linker)), 1.41-1.14 (m, 16H, (CH2)2(Lys), (CH2)2(Lys-linker), (CH2)4 (linker). 13C (D2O) δ: 177.8 (CO2H), 177.6 (CO2H), 177.5 (CO2H), 177.1 (CO2H), 176.3 (CO2H), 174.2(CO2CH2Ph), 173.9 (CONH), 159.8,(NHCONH), 135.5 (C, Ph), 128.9(CH, Ph), 128.5 (CH, Ph),128.1(CH, Ph), 67.3 (CH2Ph), 55.5 (CH2CO2H), 53.4 (CH, Glu), 53.2, 53.1(CH, Lys, Lys-linker), 52.5, 52.3 (CH2, DOTA), 39.0 (CH2NH, Lys), 38.9 (CH2NH, Lys-linker), 35.5 (CH2CO, linker), 35.4 (CH2CO, linker), 30.7(CH2CO, (Glu)), 28.0 (CH2CH (Glu)), 27.4, 27.3, 27.1, 26.4, 25.1 (CH2 (linker), (Lys), (Lys-linker)), 22.3, 22.2(CH2(Lys), CH2(Lys-linker)). ESIMS: 1080[M+1]+, HRESI+-MS: Calcd. For C49H77N9O18, 1080.5487 [M+H], found: 1080.5459.
2-{3-[5-(7-{1-Benzyloxycarbonyl-5-[2-(4,7,10-tris-carboxymethyl-1,4,7,10tetraazacyclododec-1-yl)-acetylamino]-pentylcarbamoyl}-heptanoylamino)-1-carboxy-pentyl]-ureido}-pentanedioic acid Gallium (III), [69/71Ga]3
To a solution of GaNO3 (5 mg, 20 µmol) in deionized water was added compound 3 (20 mg, 20 µmol) in 1 mL deionized water. The resulting solution was heated in boiling water for 10 min. The solvent was evaporated to dryness and the crude residue was purified by HPLC Method 1. Retention time for the product was at 24 min. Yield: ~ 35%. 1H NMR (D2O) δ: 7.87 (m, 5H, Ph), 5.21 (s, 2H, H2CPh), 4.26-4.1 (m, 3H, HC(NH)CO2(Glu), HC(NH)CO2(Lys), HC(NH)CO2(Lys-linker)), 3.45 -3.18 (bm, 28H, H2CCO2, N(CH2)2N(DOTA), H2CNH(Lys), H2CNH(Lys-linker)), 2.42(m, 2H, H2C-linker), 2.20 (m, 2H, H2CCO2(Glu), 2.06 (m, 3H, H2C-linker, H2CNH(Glu)), 1.85-1.18 (m, 21H, H2CNH(Glu), H2C(Lys), H2C(Lys-linker), (CH2)4 (linker)). 13C (D2O) δ: 178.2 (CO2H), 178.1 (CO2H), 177.9 (CO2H), 177.5 (CO2H), 177.4 (CO2H) 176.3 (CO2H), 174.5(CO2CH2Ph), 173.9, 173.4 (CONH), 160.1,(NHCONH), 135.6 (C, Ph), 129.1(CH, Ph), 128.9 (CH, Ph),128.1(CH, Ph), 67.3 (CH2Ph), 60.1, 59.6, 57.6, 57.3 (CH2CO2H), 53.4 (CH, Glu), 53.2, 53.1(CH, Lys, Lys-linker), 52.9, 52.8, 52.5 (CH2, DOTA), 39.0, 38.9(CH2NH, Lys, Lys-CH2), 35.7, 35.5 (CH2CO, linker), 31.1 (CH2CO, Glu), 27.9(CH2CH(Glu)), 27.7, 27.6, 27.5, 26.4, 25.1(linker, CH2(Lys), CH2(Lys-linker), 22.3, 22.2 (CH2(Lys), CH2(Lys-linker)). ESIMS m/Z: 1146[M+H]+, HRESI+-MS: Calcd. for C49H75GaN9O18, 1146.4486 [M+H], found: 1146.4480.
2-[3-(1-Carboxy-5-{7-[5-carboxy-5-(3-phenyl-2-{3-phenyl-2-[2-(4,7,10-tris-carboxymethyl-1,4,7,10tetraaza-cyclododec-1-yl)-acetylamino]-propionylamino}-propionylamino)-pentylcarbamoyl]-heptanoylamino}-pentyl)-ureido]-pentanedioic acid, 6
Fmoc-Lys(Boc)-Wang resin (100 mg, 0.43 mM) was allowed to swell with CH2Cl2 (3 mL) followed by DMF (3 mL). A solution of 20% piperidine in DMF (3 × 3 mL) was added to the resin that was then shaken gently on a mechanical shaker for 30 min at ambient temperature. The resin was washed with DMF (3 × 3 mL) and CH2Cl2 (3 × 3 mL). Formation of free amine was assessed by the Kaiser test.42 After swelling the resin in DMF, a solution of Fmoc-Phe-OH (3 eq), HBTU (3 eq), HOBt (3 eq), and DIPEA (4.0 eq) in DMF was added and gently shaken for 2 h. The resin was then washed with DMF (3 × 3 mL) and CH2Cl2 (3 × 3 mL). The coupling efficiency was assessed by the Kaiser Test. That aforementioned sequence was repeated for two more coupling steps with Fmoc-Phe-OH and DOTA-(t-butyl ester)3-CO2H. Final compound was cleaved from the resin using TFA:CH2Cl2 (1:1) and concentrated under vacuum to produce 4. The concentrated product was purified by using a C18 SepPak Vac 2g column. The product was eluted with a solution 70/30 water/acetonitrile (0.1% TFA in each). 1H NMR (D2O, δ): 7.14-7.00 (m, 10H, Ph), 4.51(m, 1H, HC(Phe)), 4.42 (m, 1H, HC(Phe)), 4.04(m, 1H, HC(Lys)), 3.16-2.4(bm, 30H, H2CCO2, N(CH2)2N(DOTA), H2CPh(Phe), H2CNH(Lys)), 1.61-1.39(m, 4H, H2C (Lys)), 1.16(m, 2H, H2C(Lys)). 13C (D2O) δ: 174.8 (CO2H), 172.24 (CONH), 172 (CONH), 136.5 (C, Phe), 135.8 (C, Phe), 129.3 (CH, Phe), 128.5 (CH, Phe), 126.9 (CH, Phe), 54.6 (CH2CO2), 53.07 (CH, Phe, Lys), 52.1-51.0 (CH2, DOTA), 39.06 (CH2NH2(Lys), 36.32 (CH2Ph), 29.61 (CH2, Lys), 26.0 (CH2, Lys), 21.73, (CH2, Lys). ESIMS:827 [M+1]+.
Lyophilized 4 (10 mg, 12 µmol in 2 mL DMF) was added to 519 (20 mg, 21.4 µmol in 1 mL DMF) followed by TEA (214 µmol, 30 µL) and then stirred at 25°C for 16 h. After solvent removal, solid residue was treated with 3 mL TFA:CH2Cl2 to remove the PMB group. The residue was washed 2 × 5 mL CH2Cl2 to remove impurities. The colorless solid residue, compound 6 thus obtained was purified by a C18 SepPak Vac 2g column using an eluent of 70/30 water/acetonitrile (0.1% TFA in each). The product was further purified using preparative RP-HPLC by Method 1, retention time 17 min. Yield: ~ 30%. 1H NMR (CD3CO2D) δ: 7.35-7.20 (m, 10H, Ph), 4.86 (bm, 2H, HC(Phe)), 4.57-4.46 (3H, HC(NH)CO2(Glu), HC(NH)CO(Lys), HC(NH)CO(Lys-linker)), 4.4-3.0 (m, 30H, H2CCO2, N(CH2)2N(DOTA), H2CPh(Phe), H2CNH(Lys), H2CNH(Lys-linker)), 2.8(m, 2H, H2CPh(Phe)), 2.6 (m, 2H, H2CCO2(Glu)), 2.3 (m, 5H, H2CCHNH(Glu), H2CCONH-linker)), 2.1-1.3 (m, 21H, H2CCHNH(Glu), (CH2)4-linker, (CH2)3(Lys), (CH2)3(Lys-linker)). 13C (CD3CO2D) δ: 178.71, (CO2H), 178.14 (CO2H), 177.72 (CO2H), 177.66 (CO2H), 177.06 (CO2H), 174.24 (CONH), 173.9(CONH), 161.3(NHCONH), 138.6(C, Ph) 137.7(C, Ph), 130.5 (CH, Ph), 129.5 (CH, Ph), 127.9 (CH, Ph), 127.7(CH, Ph), 56.72 (CH2CO2), 56.16 (CH, Phe), 54.6 (CH, Glu), 53.5 (CH, Lys, Lys-linker), 53.3 (CH2, DOTA), 40.8 (CH2NH (Lys)), 39.4 (CH2NH, (Lys-linker)), 37.5 (CH2Phe), 32.6 (CH2, (linker)) 31.8 (CH2, (linker)), 30.7, 29.42, 27.9, 26.53 (CH2 (linker), CH2(Lys)). ESIMS m/Z: 1284[M+H]+, HRESI+-MS: Calcd. for C68H90N11O20, 1284.6365 [M+H], found: 1284.6358.
2-[3-(1-Carboxy-5-{7-[5-carboxy-5-(3-phenyl-2-{3-phenyl-2-[2-(4,7,10-tris-carboxymethyl-1,4,7,10tetraaza-cyclododec-1-yl)-acetylamino]-propionylamino}-propionylamino)-pentylcarbamoyl]-heptanoylamino}-pentyl)-ureido]-pentanedioic acid Gallium (III), [69/71Ga]6
This compound was prepared according to the same general procedure as described for [69/71Ga]3. Compound [69/71Ga]6 was purified by Method 1, retention time 22 min. Yield: ~ 30%. 1H NMR (MeOD) δ: 7.30-7.20 (m, 10H, Ph), 4.76-4.67(bm, 2H, HC(Phe)), 4.36-4.27 (3H, HC(NH)CO2(Glu), HC(NH)CO2(Lys), HC(NH)CO(Lys-linker)), 4.0-3.35 (m, 24H, H2CCO2, N(CH2)2N(DOTA)), 3.29-3.1(m, 5H, H2CPh(Phe), H2CNH(Lys), H2CNH(Lys-linker)), 3.05(m, 1H, H2CNH(Lys)), 2.27(m, 2H, H2CPh(Phe)), 2.4 (m, 2H, H2CCONH-linker), 2.28-2.1 (m, 5H, H2CCO2(Glu), H2CCHNH(Glu), H2CCONH-linker)), 1.98-1.8(3H, H2CCHNH(Glu), CH2-linker), 1.8-1.3 (m, 18H, (CH2)4-linker, (CH2)3-Lys, (CH2)3-Lys-linker)). 13C (MeOD) δ: 175.71 (CO2H), 174.4 (CO2H), 174.2 (CO2H), 173.2 (CO2H), 171.9 (CO2H), 170.4(CONH), 170.3, 170.2, 169.9 (CONH), 169.5(CONH), 159.0(NHCONH), 137.3(C, Ph) 136.9(C, Ph), 129.3 (CH, Ph), 129.2 (CH, Ph), 128.3 (CH, Ph), 128.2(CH, Ph), 126.3, 126.2(CH, Ph), 61.8, 60.7, 59.4, 59.3 (CH2CO2), 57.6 (CH, (Glu)), 57.5(CH, (Lys), (Lys-linker)), 54.4, 54.3(CH(Phe)), 54.2, 54.1, 52.8, 52.5, 52.3 (CH2, DOTA), 37.5, 37.4 (CH2NH, (Lys-linker), Lys), 35.5 (CH2Phe), 35.4 (CH2Phe), 32.0 (CH2CO2, Glu), 30.8 (CH2CONH, linker), 29.7 (CH2CH, Glu), 29.42, 29.3, 29, 7, 27.9, 26.53, 22.5, 22.3 (CH2(linker), CH2(Lys), CH2(Lys-linker)). ESIMS m/Z: 1351[M+H]+, HRESI+-MS: Calcd. for C68H86GaN11NaO20, 1372.5204 [M+Na]+, found: 1372.5199.
Preparation of 68Ga
68Ga labeling of compounds [68Ga]3 and [68Ga]6 were performed according to a literature procedure.24 A detailed description for [68Ga]3 is given below.
Preconcentration of [68Ga(III)]
488 MBq (13 mCi) of 68GaCl3 in 7 mL of 0.1 N HCl were obtained from an 18-month-old 1,850 MBq (50 mCi) 68Ge/68Ga generator, Eckert-Ziegler (Berlin). The solution was transferred on a cation-exchange cartridge, Phenomenex Strata-X-C (33 µm strong cation exchange resin, part no. 8B-S029-TAK, 30 mg/1mL). The column was eluted with 5 mL of a solution of 20/80 of hydrochloric acid (0.10 N)/acetone. The eluent remaining on the cation exchanger was removed by passage of nitrogen. That process was performed to remove most of the remaining chemical and radiochemical impurities from the resin, whereas 68Ga(III) should remain on the column. The column was filled with 150 µL of a 2.4/97.6 HCl (0.05 N)/acetone solution. About 2 min standing appeared to be best for complete desorption of the 68Ga(III) from the resin into the liquid phase. An additional 250 µL of that 2.4/97.6 HCl (0.05 N)/acetone solution was applied, and the purified 68Ga(III) was obtained in a total volume of 400 µL.
General Radiolabeling Procedure
The 400 µL combined fractions of 68Ga(III) in HCl/acetone was used directly for the radiolabeling of 3/6. The concentrated radioactivity was added to 500 µL of deionized H2O in a standard glass reagent vial containing 100 µl (92 nmol, 1 mg/mL solution) of ligand. No buffer solution was added. The reaction vial was heated at 95°C for 10 min. The complexation was monitored by injecting aliquots of 100 µL (7.77 MBq) of the solution onto the HPLC. Product obtained = 5.92 MBq. For [68Ga]3, radiochemical yield: 76.2% (without decay correction) and the radiochemical purity was >99%. HPLC was performed by Method 1 as described in the General experimental section. Rt = 25 min for the desired product and Rt = 19 min for the free ligand. For [68Ga]6, radiochemical yield: 70% and radiochemical purity > 99%. HPLC was performed by Method 2 as mentioned in General experimental section. Rt = 22.5 min for the desired product and Rt = 16 min for the free ligand. The acidic eluate was neutralized with 100 µL 0.1M NaHCO3 solution and the volume of the eluate was reduced under vacuum to dryness. The solid residue was diluted with saline to the desired radioactivity concentration for biodistribution and imaging studies.
Lipophilicity Determination
Partition coefficients, logo/w (pH = 7.4) values were determined according to a literature procedure.25 Briefly, a solution of either [68Ga]3 or [68Ga]6 was added to a presaturated solution of 1-octanol (5 mL) mixed with phosphate buffered saline (PBS) (5 mL) in a 15 mL centrifuge tube. After vigorously shaking the mixture, it was centrifuged at 3,000 rpm for 5 min. Aliquots (100 µL) were removed from the two phases and the radioactivity was measured in a γ-counter, 1282 Compugamma CS (LKB, Wallac, Turku, Finland).
Cell Lines and Tumor Models
PC-3 PIP (PSMA+) and PC-3 flu (PSMA−) cell lines were obtained from Dr. Warren Heston (Cleveland Clinic) and were maintained as previously described.13 LNCaP cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and were maintained as per ATCC guidelines. All cells were grown to 80–90% confluence before trypsinization and formulation in Hank’s Balanced Salt Solution (HBSS, Sigma, St. Louis, MO) for implantation into mice.
Animal studies were undertaken in compliance with institutional guidelines related to the conduct of animal experiments. For biodistribution studies of [68Ga]3, and [68Ga]6 and imaging studies of [68Ga]3, male SCID mice (NCI) were implanted subcutaneously with 1 – 5 × 106 PSMA+ PC-3 PIP and PSMA− PC-3 flu cells behind either shoulder. For imaging studies of [68Ga]3, male SCID mice (NCI) were implanted subcutaneously with 5 × 106 LNCaP cells behind the right shoulder. Mice were imaged or used in biodistribution studies when the tumor xenografts reached 3 – 5 mm in diameter.
Biodistribution
PSMA+ PC-3 PIP and PSMA− PC-3 flu xenograft-bearing SCID mice were injected via the tail vein with 30 µCi (1.1 MBq) of [68Ga]3 or [68Ga]6. In case each four mice were sacrificed by cervical dislocation at 30, 60, 120, 180 min pi. for [68Ga]3 and at 5, 60, 120, 180 min pi for [68Ga]6. The heart, lungs, liver, stomach, pancreas, spleen, fat, kidney, muscle, small and large intestines, urinary bladder, and PC-3 PIP and flu tumors were quickly removed. A 0.1 mL sample of blood was also collected. Each organ was weighed, and the tissue radioactivity was measured with an automated gamma counter (1282 Compugamma CS, Pharmacia/LKB Nuclear, Inc., Gaithersburg, MD). The %ID/g was calculated by comparison with samples of a standard dilution of the initial dose. All measurements were corrected for decay.
PET and CT Imaging
A single SCID mouse implanted with a PSMA+ LNCaP xenograft was injected intravenously with 0.2 mCi (7.4 MBq) of [68Ga]3 in 200 µL 0.9% NaCl. At 0.5 h pi, the mouse was anesthetized with 3% isoflurane in oxygen for induction and maintained under 1.5% isoflurane in oxygen at a flow rate of 0.8 L/min. The mouse was positioned in the prone position on the gantry of a GE eXplore VISTA small animal PET scanner (GE Healthcare, Milwaukee, WI). Image acquisition was performed using the following protocol: The images were acquired as a pseudodynamic scan, i.e., a sequence of successive whole-body images were acquired in three bed positions for a total of 120 min. The dwell time at each position was 5 min, such that a given bed position (or mouse organ) was revisited every 15 min. An energy window of 250 – 700 keV was used. Images were reconstructed using the FORE/2D-OSEM method (two iterations, 16 subsets) and included correction for radioactive decay, scanner dead time, and scattered radiation. After PET imaging, the mobile mouse holder was placed on the gantry of an X-SPECT (Gamma Medica Ideas, Northridge, CA) small animal imaging device to acquire the corresponding CT. Animals were scanned over a 4.6 cm field-of-view using a 600 µA, 50 kV beam. The PET and CT data were then co-registered using Amira 5.2.0 software (Visage Imaging Inc., Carlsbad, CA).
Imaging studies and blocking studies of [68Ga]6 and [68Ga]3 were carried out on PSMA+ PC-3 PIP and PSMA− PC-3 flu xenograft-bearing SCID mice or PSMA+ PC-3 PIP (25.9 MBq in 100 µL NaCl) xenograft-bearing SCID mice. At 30 min, 1 h and 2 h pi the mice were anesthetized and whole-body images were obtained using the PET scanner as mentioned above, in two bed positions, 15 min at each position for a total of 30 min using the same energy window. Images were reconstructed and co-registered with the corresponding CT images using the same methods as described above.
Supplementary Material
Acknowledgments
We thank Dr. Richard L. Wahl for provision of the 68Ga generator. We also thank NIH R01 CA134675 and U24 CA92871 and the AdMeTech Foundation for financial support.
Abbreviations
- PSMA
prostate-specific membrane antigen
- DCFBC
N-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-(S)-4-fluorobenzyl-L-cysteine
- PMPA
2-(phosphonomethyl)pentanedioic acid
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- PET
positron emission tomography
Footnotes
Supporting Information Available
Additional figures demonstrating the HPLC traces for [68Ga]3 and [68Ga]6 as well as the high-resolution mass spectra for these compounds are provided. Also provided is a figure containing a PET blocking study for [68Ga]3. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2009. [Google Scholar]
- 2.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell Biochem. 2004;91:528–539. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 3.Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, Ross JS, Bander NH. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol. 2007;25:540–547. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 4.Kowol CR, Berger R, Eichinger R, Roller A, Jakupec MA, Schmidt PP, Arion VB, Keppler BK. Gallium(III) and iron(III) complexes of alpha-N-heterocyclic thiosemicarbazones: synthesis, characterization, cytotoxicity, and interaction with ribonucleotide reductase. J. Med. Chem. 2007;50:1254–1265. doi: 10.1021/jm0612618. [DOI] [PubMed] [Google Scholar]
- 5.Kowol CR, Trondl R, Heffeter P, Arion VB, Jakupec MA, Roller A, Galanski M, Berger W, Keppler BK. Impact of metal coordination on cytotoxicity of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (triapine) and novel insights into terminal dimethylation. J. Med. Chem. 2009:5032–5043. doi: 10.1021/jm900528d. [DOI] [PubMed] [Google Scholar]
- 6.Kaluderovic MR, Gomez-Ruiz S, Gallego B, Hey-Hawkins E, Paschke R, Kaluderovic GN. Anticancer activity of dinuclear gallium(III) carboxylate complexes. Eur. J. Med. Chem. 2010;45:519–525. doi: 10.1016/j.ejmech.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Fani M, Andre JP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging. 2008;3:67–77. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- 8.Khan MU, Khan S, El-Refaie S, Win Z, Rubello D, Al-Nahhas A. Clinical indications for Gallium-68 positron emission tomography imaging. Eur. J. Surg. Onco.l. 2009;35:561–567. doi: 10.1016/j.ejso.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Crespo A, Andreo P, Larsson SA. Positron flight in human tissues and its influence on PET image spatial resolution. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:44–51. doi: 10.1007/s00259-003-1330-y. [DOI] [PubMed] [Google Scholar]
- 10.Reubi JC, Maecke HR. Peptide-based probes for cancer imaging. J. Nucl. Med. 2008;49:1735–1738. doi: 10.2967/jnumed.108.053041. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Foss CA, Byun Y, Nimmagadda S, Pullambhatla M, Fox JJ, Castanares M, Lupold SE, Babich JW, Mease RC, Pomper MG. Radiohalogenated Prostate-Specific Membrane Antigen (PSMA)-Based Ureas as Imaging Agents for Prostate Cancer. J. Med. Chem. 2008;51:7933–7943. doi: 10.1021/jm801055h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foss CA, Mease RC, Fan H, Wang Y, Ravert HT, Dannals RF, Olszewski RT, Heston WD, Kozikowski AP, Pomper MG. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. 2005;11:4022–4028. doi: 10.1158/1078-0432.CCR-04-2690. [DOI] [PubMed] [Google Scholar]
- 13.Mease RC, Dusich CL, Foss CA, Ravert HT, Dannals RF, Seidel J, Prideaux A, Fox JJ, Sgouros G, Kozikowski AP, Pomper MG. N-[N-[(S)-1,3-Dicarboxypropyl]Carbamoyl]-4-[18F]Fluorobenzyl-L-Cysteine, [18F]DCFBC: A New Imaging Probe for Prostate Cancer. Clin. Cancer Re.s. 2008;14:3036–3043. doi: 10.1158/1078-0432.CCR-07-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kularatne SA, Zhou Z, Yang J, Post CB, Low PS. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted (99m)Tc-radioimaging agents. Mol. Pharm. 2009;6:790–800. doi: 10.1021/mp9000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kularatne SA, Wang K, Santhapuram H-K R, Low PS. Prostate-specific membrane antigen (PSMA)-targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol. Pharm. 2009;6:780–789. doi: 10.1021/mp900069d. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee SR, Foss CA, Castanares M, Mease RC, Byun Y, Fox JJ, Hilton J, Lupold SE, Kozikowski AP, Pomper MG. Synthesis and evaluation of technetium-99m- and rhenium-labeled inhibitors of the prostate-specific membrane antigen (PSMA) J Med Chem. 2008;51:4504–4517. doi: 10.1021/jm800111u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke ETM. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12-, 13-, and 14-membered tetraazamacrocycles. Inorg. Chim. acta. 1992;190:37–46. [Google Scholar]
- 18.Hamachi I I, Y Y, Matsugi T, Shinkai S. Single- or Dual-Mode Switching of Semisynthetic ribonuclease S' with an iminodiacetic acid moiety in response to the copper(II) concentration. Chem. Eur. J. 1999;5:1503–1511. [Google Scholar]
- 19.Chandran SS, Banerjee SR, Mease RC, Pomper MG, Denmeade SR. Characterization of a targeted nanoparticle functionalized with a urea-based inhibitor of prostate-specific membrane antigen (PSMA) Cancer Biol. Ther. 2008;7:974–982. doi: 10.4161/cbt.7.6.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heppeler A, Andre JP, Buschmann I, Wang X, Reubi J-C, Hennig M, Kaden TA, Maecke HR. Metal-ion-dependent biological properties of a chelator-derived somatostatin analogue for tumor targeting. Chem. Eur. J. 2008;14:3026–3034. doi: 10.1002/chem.200701264. [DOI] [PubMed] [Google Scholar]
- 21.Heppeler A, Froidevaux S, Maecke HR, Jermann E, Behe M, Powell P, Hennig M. Radiometal-labelled macrocyclic chelator-derivatized somatostatin analogue with superb tumour-targeting properties and potential for receptor-mediated internal radiotherapy. Chem. Eur. J. 1999;5:1974–1981. [Google Scholar]
- 22.Viola NA, Rarig RS, Ouellette W, Doyle RP. Synthesis, structure and thermal analysis of the gallium complex of 1,4,7,10-tetraazacyclododecane-N,N',N",N'"-tetraacetic acid (DOTA) Polyhedron. 2006;25:3457–3462. [Google Scholar]
- 23.Henderson W, Taylor MJ. An electrospray mass spectrometric investigation of gallium trihalide and indium trihalide solutions. Inorg Chim Acta. 1998;277:26–30. [Google Scholar]
- 24.Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, Jahn M, Jennewein M, Rosch F. Processing of generator-produced 68Ga for medical application. J. Nucl. Med. 2007;48:1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 25.Antunes P, Ginj M, Walter MA, Chen J, Reubi JC, Maecke HR. Influence of different spacers on the biological profile of a DOTA-somatostatin analogue. Bioconjug. Chem. 2007;18:84–92. doi: 10.1021/bc0601673. [DOI] [PubMed] [Google Scholar]
- 26.Kozikowski AP, Zhang J, Nan F, Petukhov PA, Grajkowska E, Wroblewski JT, Yamamoto T, Bzdega T, Wroblewska B, Neale JH. Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II: efficacy as analgesic agents. J. Med. Chem. 2004;47:1729–1738. doi: 10.1021/jm0306226. [DOI] [PubMed] [Google Scholar]
- 27.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 28.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 29.Slusher BS, Tsai G, Yoo G, Coyle JT. Immunocytochemical localization of the N-acetyl-aspartyl-glutamate (NAAG) hydrolyzing enzyme N-acetylated alpha-linked acidic dipeptidase (NAALADase) J. Comp. Neurol. 1992;315:217–229. doi: 10.1002/cne.903150208. [DOI] [PubMed] [Google Scholar]
- 30.Caroli P, Nanni C, Rubello D, Alavi A, Fanti S. Non-FDG PET in the practice of oncology. Indian J. Cancer. 47:120–125. doi: 10.4103/0019-509X.62998. [DOI] [PubMed] [Google Scholar]
- 31.Hao G, Zhou J, Guo Y, Long MA, Anthony T, Stanfield J, Hsieh JT, Sun X. A cell permeable peptide analog as a potential-specific PET imaging probe for prostate cancer detection. Amino Acids. 2010;38 doi: 10.1007/s00726-010-0515-5. ASAP. [DOI] [PubMed] [Google Scholar]
- 32.Lapi SE, Wahnishe H, Pham D, Wu LY, Nedrow-Byers JR, Liu T, Vejdani K, VanBrocklin HF, Berkman CE, Jones EF. Assessment of an 18F-labeled phosphoramidate peptidomimetic as a new prostate-specific membrane antigen-targeted imaging agent for prostate cancer. J. Nucl. Med. 2009;50:2042–2048. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimitrakopoulou-Strauss A, Hohenberger P, Haberkorn U, Macke HR, Eisenhut M, Strauss LG. 68Ga-labeled bombesin studies in patients with gastrointestinal stromal tumors: comparison with 18F-FDG. J. Nucl. Med. 2007;48:1245–1250. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- 34.Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, Macke HR, Eisenhut M, Debus J, Haberkorn U. Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J. Nucl. Med. 2005;46:763–769. [PubMed] [Google Scholar]
- 35.Henze M, Schuhmacher J, Dimitrakopoulou-Strauss A, Strauss LG, Macke HR, Eisenhut M, Haberkorn U. Exceptional increase in somatostatin receptor expression in pancreatic neuroendocrine tumour, visualised with (68)Ga-DOTATOC PET. Eur J Nucl Med Mol Imaging. 2004;31:466. doi: 10.1007/s00259-003-1436-2. [DOI] [PubMed] [Google Scholar]
- 36.Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging. 37:67–77. doi: 10.1007/s00259-009-1205-y. [DOI] [PubMed] [Google Scholar]
- 37.Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging. 54:61–67. [PubMed] [Google Scholar]
- 38.Putzer D, Gabriel M, Kendler D, Henninger B, Knoflach M, Kroiss A, Vonguggenberg E, Warwitz B, Virgolini IJ. Comparison of 68Ga-DOTA-Tyr3-octreotide and 18F-fluoro-L-dihydroxyphenylalanine positron emission tomography in neuroendocrine tumor patients. Q. J. Nucl. Med. Mol. Imaging. 2010;54:68–75. [PubMed] [Google Scholar]
- 39.van Essen M, Krenning EP, Kam BL, de Herder WW, Feelders RA, Kwekkeboom DJ. Salvage therapy with (177)Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2010;51:383–390. doi: 10.2967/jnumed.109.068957. [DOI] [PubMed] [Google Scholar]
- 40.Barinka C, Byun Y, Dusich CL, Banerjee SR, Chen Y, Castanares M, Kozikowski AP, Mease RC, Pomper MG, Lubkowski J. Interactions between human glutamate carboxypeptidase II and urea-based inhibitors: structural characterization. J. Med. Chem. 2008;51:7737–7743. doi: 10.1021/jm800765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson PF, Cole DC, Slusher BS, Stetz SL, Ross LE, Donzanti BA, Trainor DA. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase. J. Med. Chem. 1996;39:619–622. doi: 10.1021/jm950801q. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.