Abstract
Heart failure, a major symptom in the progression of cardiac hypertrophy, is a critical risk factor for cardiac death. A large body of research has investigated cardioprotective mechanisms that prevent or minimize hypertrophy, identifying a variety of specific peptide hormones, growth factors, and cytokines with cardioprotective properties. Recent investigation of the downstream effector pathways for these growth factors has identified molecules involved in the progression of cardiac hypertrophy and heart failure, including phosphoinositide 3-kinase (PI3K), Akt and mammalian target of rapamycin (mTOR). Using genetically modified transgenic or knockout mice and adenoviral targeting to manipulate expression or function in experimental models of heart failure, several investigators have demonstrated that the PI3K-Akt pathway regulates cardiomyocyte size, survival, angiogenesis, and inflammation in both physiological and pathological cardiac hypertrophy. In this review, we discuss the reciprocal regulation of PI3K, Akt and mTOR in cardiomyocytes and their association with cardiac disease.
Keywords: PI3K, Akt, mTOR, cardiac hypertrophy, heart failure
INTRODUCTION
Despite advances in the treatment of cardiac diseases, a cohort study in 2004 revealed that there had been no substantial reduction in the incidence of heart failure in over 2 decades [1]. Cardiac hypertrophy, a major risk factor for heart failure [2], is observed in a wide variety of clinical conditions, including hypertension and aortic stenosis [3, 4]. While cardiac hypertrophy has long been thought to play an adaptive role early in these pathological conditions by reducing wall stress, it is not clear whether it is a necessary protective response [5]. In fact, progressive hypertrophy ultimately becomes maladaptive, transitioning to ventricular dilatation and dysfunction [3, 4]. The functional outcomes classify cardiac hypertrophy as physiological and pathological hypertrophy [6-8]. Physiological hypertrophy resulting from exercise shows adaptive cardiac growth [9], which is also observed during normal postnatal development and pregnancy. Physiological hypertrophy is characterized by preserved contractile function and lack of interstitial fibrosis [6]. On the other hand, while pressure overload initially induces adaptive hypertrophy [10, 11], sustained pressure overload in the left ventricle (LV) eventually results in maladaptive hypertrophy, which is classified as pathological hypertrophy [3, 7, 8]. Pathological hypertrophy is associated with fibrosis and cardiac dysfunction [3, 8]. This hypertrophic response is comprised not only of an increase in cell size and protein content, but also complex alterations in gene transcription and translation [7, 8]. Understanding the mechanisms responsible for each of these hypertrophic reactions may help identify novel therapeutic targets to prevent heart failure.
Many years of research dedicated into cardioprotection, has identified a variety of specific peptide hormones, growth factors, and cytokines such as insulin, gp130-dependent cytokines, and insulin-like growth factor (IGF)-I, that can protect the heart from ischemic injury, cardiac hypertrophy, and heart failure [12, 13]. Activation of phosphoinositide 3-kinase (PI3K) and its downstream serine-threonine kinase, Akt (or Protein Kinase B) plays an important role in the cardioprotective effect of the tyrosine kinase receptor signaling pathways [6, 14]. Among the downstream effectors of Akt, mammalian target of rapamycin (mTOR) is particularly well-characterized. Inhibition of mTOR with rapamycin can prevent cardiac hypertrophy, suggesting that mTOR and its downstream molecules regulate development of cardiac hypertrophy [15]. Recent reports revealed that in addition to the rapamycin-sensitive mTOR complex (mTOR complex 1, mTORC1), there is a rapamycin-insensitive complex (mTOR complex 2, mTORC2) [16]. Here, we present an overview of the role of PI3K-Akt-mTOR signaling pathways in the regulation of cell morphology, cell survival, angiogenesis and inflammation in cardiomyocytes in settings of experimental and clinical heart failure.
STRUCTURE AND ACTIVATION OF PI3K, Akt and mTOR
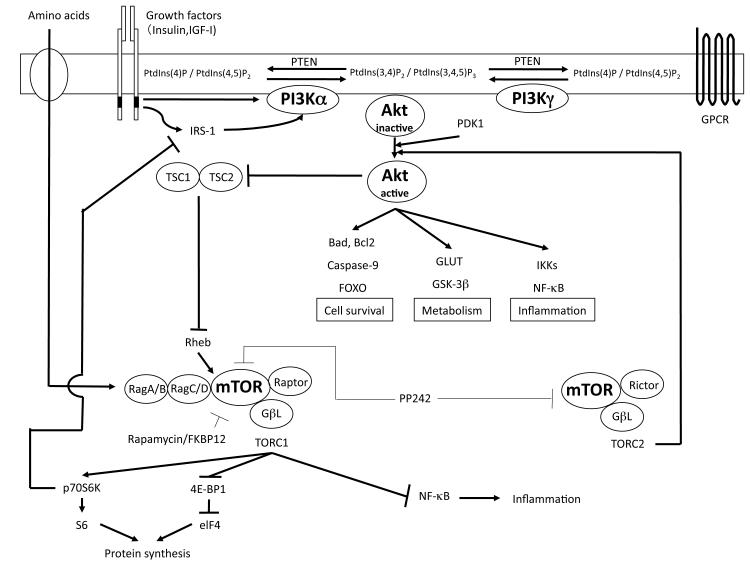
When insulin or IGF-1 binds and activates tyrosine kinase receptors, heterodimers of PI3K are recruited by the SH2 domain in regulatory subunit of PI3K which has high affinity for phosphotyrosine in the receptors [17] (Figure 1). PI3Ks are divided into three classes based on the combination of isoforms of catalytic and regulatory subunits. Class IA PI3K complexes are heterodimers of the α, β or δ isoforms of p110 catalytic domain with the isoforms of the regulatory domain p85 (α, β), p55 (α. γ) or p50α [17, 18]. Class IB PI3K, also referred to as “PI3Kγ”, is a heterodimer of p110γ and p101, and is activated by G-protein coupled receptors (GPCR) [18] (Figure 1). All of the class I PI3K phosphorylate PtdIns(4, 5)P2 to generate PtdIns(3, 4, 5)P3. Actions of class I PI3Ks is antagonized by tumor suppressor phosphatase and tensin homolog deleted on chromosome ten (PTEN), which dephosphorylates both PtdIns(3, 4)P2 and PtdIns(3, 4, 5)P3 [19-21] (Figure 1). Among the class I PI3Ks expressed in the heart, p110α and p110γ expression is higher compared to p110β and p110δ expression, which is relatively low [22]. Class II PI3K consists of large proteins, 170-210 kDa, which phosphorylate PtdIns and PtdIns4P, however the physiological role of the Class II enzymes remains unclear [23]. Class III PI3K phosphorylates only PtdIns and acts in agonist-independent membrane trafficking processes [17].
Figure 1. Signaling pathway of PI 3-kinase, Akt and mTOR.
Signaling pathways are depicted schematically. FKBP: FK506-binding protein, GLUT: glucose transporter, GPCR: G protein-coupled receptor, GSK: Glycogen synthase kinase, IGF: Insulin-like growth factor, PDK: phosphoinositide-dependent kinase, PI3K: phosphoinositide 3-kinase, PtdIns: phosphatidylinositol, PTEN: phosphatase and tensin homolog deleted on chromosome ten, p70S6K: p70S6 kinase, TSC: tuberous sclerosis complex, eIF4: eukaryotic initiation factor 4.
Akt contains three isoforms: Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ). Akt1 and Akt2 are expressed ubiquitously, with high levels seen especially in the brain, heart and lungs [24]. Expression of Akt2 is also observed in brown adipose tissue [25]. Akt3 expression is higher in brain as compared to in the skeletal muscle and liver [26]. PtdIns(3, 4)P2 and PtdIns(3, 4, 5)P3 stimulated by growth factors or GPCR in cellular membrane recruit Akt to the plasma membrane from the cytosol, where it is phosphorylated at Thr308 by phosphoinositide-dependent kinase (PDK1) to generate its active form [27, 28] (Figure 1). For full activation of Akt, phosphorylation of Ser473 in a C-terminal regulatory domain is required [29]. Although the mechanism of phosphorylation of Ser473 took many years to reveal, recent reports strongly indicate that a mTOR complex regulates the kinase reaction as discussed below. Activated Akt phosphorylates downstream molecules such as Glucose transporter (GLUT), Glycogen synthase kinase (GSK)-3, and mTOR [14].
mTOR is a large (290 kDa) serine-threonine protein kinase belonging to the phosphatidylinositol kinase-related kinase (PIKK) family [16]. Akt regulates mTOR activation via tuberous sclerosis complex (TSC)-1/2 and Rheb [30, 31]. Phosphorylation of TSC2 by Akt inhibits TSC2 function, disinhibits Rheb, which results in mTOR activation [31]. mTOR exists in two different complex forms usually classified as mTORC1 and mTORC2 [16]. mTORC1, consists of mTOR, Raptor, mLST8/GβL, and PRAS40, and stimulates protein synthesis through p70S6 kinase and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) in protein translation [32]. Not only does mTORC1 phosphorylate p70S6 kinase, but it also activates elF4E, a translation initiator. Phosphorylation of p70S6 kinase drives the phosphorylation and activation of S6 ribosomal protein [32], whereas activation of e1F4E leads to phosphorlyation and inactivation of 4E-BP1 [33]. mTOR-induced acceleration of protein translation can enhance cell growth and mass [16, 33-35], as seen in osteosarcoma cells, which are enlarged following stimulation by mTOR [36]. mTORC1 is stimulated by amino acid-activated Rag GTPases associated with Rheb [37, 38], and inhibited by rapamycin, a known immunosuppressant. Rapamycin binds with FK506-binding protein (FKBP) 12, and this complex binds specifically to mTORC1 and inhibits its activity [32, 39]. mTOR forms a complex with Rictor, mLST8/GβL and Sin1 to form mTORC2, which stimulates phosphorylation of Akt (Ser473) [16, 40]. PP242, a newly introduced mTORC1 & 2 inhibitor and a member of the family “TORKinibs” (for TOR kinase domain inhibitors), inhibits both mTOR complexes by blocking the ATP-binding site [41, 42].
MECHANISMS OF CARDIAC HYPERTROPHY AND HEART FAILURE
The shift from adaptive (physiological) to maladaptive (pathological) hypertrophy is characterized by multiple pathological changes, including alterations in cell size and survival, angiogenesis and inflammation. The mechanisms underlying each type of hypertrophy and the transition between them are complex and are just beginning to be defined [6]. For studies of the PI3K-Akt signaling pathway in the heart, many gene-targeted and transgenic models have been generated and characterized by multiples groups [43].
CARDIOMYOCYTE MORPHOLOGY
Cardiac hypertrophy is induced by increased myocyte volume which is accompanied by new sarcomere formation in both physiological and pathological forms [6]. The sarcomere is the contractile unit of cardiac muscle [44]. Myocyte enlargement involves acceleration of transcription and translation of mRNA, as well as reduced protein turnover [45]. The ribosomal S6-p70S6 kinase pathway and the S6-4E-BP1-elf4E pathway are important regulators of translation during the development of hypertrophy, and the activity of these pathways is increased in both physiological and pathological hypertrophy [35, 46] As described above, both p70S6 kinase and 4E-BP1 are downstream effectors of mTOR [33, 35], suggesting that mTORC1 activity plays a crucial role in regulation of cell size.
In fact, inhibition of mTOR through systemic rapamycin treatment attenuates the hypertrophic response to pressure overload [15, 46]. However, whether long-term treatment with rapamycin is effective for preserving cardiac function in pathological hypertrophy remain undefined. Several groups have reported that thyroid hormone stimulates cardiac hypertrophy in both animals and humans [47, 48]. Tri-iodo-L-thyronine (T3), one of the thyroid hormones, stimulates mTOR activity by activating thyroid hormone receptor, which is co-localized with the p85α subunit of PI3K and stimulates its activity in cardiomyocytes [49]. Recent reports have shown that administration of thyroid hormone can prevent cardiac dysfunction [50, 51]. These findings imply that the effects of thyroid hormone via mTOR, may play an important role in both cardiac hypertrophy and cardioprotection.
CARDIOMYOCYTE SURVIVAL
Cell death of cardiomyocytes is a major factor in pathological hypertrophy which does not occur in physiological hypertrophy [6, 52]. There are three different types of cell death - necrosis, apoptosis, and autophagy [53]. All three types of cell death are present in heart failure [52, 54]. In necrotic cell death, organelles such as mitochondria swell due to the opening of plasma membrane channels that increase influx of extracellular fluid, eventually resulting in failure of the plasma membrane accompanied by inflammation [53]. Apoptosis, which is programmed cell death, involves nuclear atrophy, shrinkage of the cytosol, and loss of mitochondrial membrane potential. Macrophages eliminate cells showing apoptotic shrinkage by phagocytosis without any traces of inflammation [53]. A significant increase in apoptosis is an indication of heart failure [52, 55]. In animals lacking caspase-8 activity, a major mediator of apoptosis, there were fewer apoptotic cells in experimental models of heart failure [56]. Autophagy, which serves to recycle aged organelles and proteins regulated by autophagy-regulated genes (ATG), is also increased in heart failure patients and animal models [53, 57-59] Autophagy suppresses apoptosis and this mechanism is mediated by mTOR in cardiomyocytes [60]. In addition, several reports have proposed that up regulation of autophagy has cardioprotective effects in heart failure [61, 62]. However, it is still unclear whether the role of autophagy in heart failure is protective or dysfunctional. Recent technical advances may soon elucidate the role of autophagy. LC3, one of the ATGs, acts to select protein for degradation and promote fusion of membrane [63]. Transgenic mice bearing a green fluorescent protein (GFP)-LC3 gene should enable visualization of autophagosomes, making it a useful tool for investigating the mechanisms and effects of autophagy in hypertrophy [64].
CARDIAC HYPERTROPHY AND ANGIOGENESIS
It has been reported that physiological cardiac hypertrophy is accompanied with an increase in myocardial capillary density [65]. Conversely, pathological hypertrophy is associated with a decrease in myocardial capillaries in part by concomitant fibrosis [66]. Decreased myocardial perfusion results in decreased availability of oxygen and nutrients, which leads to an increased risk of heart failure [67]. Therefore, a decrease in myocardial capillaries promotes pathological hypertrophy, leading to heart failure.
Recent studies have suggested that vascular endothelial growth factor (VEGF) secreted from cardiomyocytes plays an important role in cardiac hypertrophy and myocardial infarction [68-71]. It has been reported that Akt and mTOR contributes to angiogenesis by increasing the expression of VEGF and angiopoietin (Ang)-2 [68, 72]. This may be mediated by hypoxia inducible factor (HIF)-1α, an oxygen-sensing protein that stimulates transcription of VEGF and Ang-2, which can be induced by mTOR [73, 74]. These findings indicate that Akt and mTOR play a crucial role in angiogenesis during physiological cardiac hypertrophy, and suggest that disruption of these signals may promote pathological hypertrophy. However, further studies are required to confirm their role in cardiac angiogenesis and their progression towards pathological hypertrophy.
INFLAMMATION AND CARDIAC FIBROSIS
Although the effects of proinflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor alpha (TNF) α, and IL-1β are complex [75], increased circulating and intracardiac levels of these cytokines followed by fibrosis are known to be key features of pathological heart failure [76, 77]. The mechanisms underlying the onset of inflammation in human heart failure and animal models such as transverse aortic constriction (TAC) have not been clearly established [78]. TNFα is produced not only by immune cells, but also by cardiac structural cells during pressure overload and ischemia-reperfusion injury [79, 80]. Increased TNFα is believed to have a cardioprotective effect due to activation of manganese superoxide dismutase (MnSOD) and heat-shock proteins (HSPs), however it also contributes to maladaptive responses, including dysfunctional contraction, cardiac hypertrophy, induction of apoptosis, and extracellular matrix (ECM) remodeling [75, 81-85]. Gp130, a component of the IL-6 receptor, suppresses apoptosis and stimulates hypertrophy in cardiomyocytes [86]. However, long-term exposure to IL-6 decreases cardiac contractility [87]. Treatment with IL-1 ameliorates ischemia-reperfusion injury [88] and increases cell size in cardiomyocytes [89]. IL-1 suppresses cardiac contractility in accordance with TNFα and also induces apoptosis [90]. Furthermore, IL-1 regulates ECM remodeling by controlling the proliferation and migration of fibroblasts and collagen synthesis [91].
In addition to these cytokines, several reports have shown association of reactive oxygen species (ROS) with heart failure [92-96]. ROS production is increased in TAC-induced cardiac hypertrophy [92-94]. Furthermore, activity of NADPH oxidase, which produces ROS, is increased in human heart failure [95], suggesting that ROS levels are tightly associated with the inflammatory response during progression of heart failure [96].
One significant difference between physiological and pathological cardiac hypertrophy is fibrosis, which only occurs in pathological hypertrophy and is characterized by increased collagen deposition [4, 97, 98]. Transforming growth factor (TGF)β plays a central role in induction of fibrosis by stimulating the Smad system, which increases expression of collagen types I, III, and VI [97]. In addition to the Smad pathway, TGFβ accelerates production of ECM protein by activating TGFβ-activated kinase (TAK)-1 [97]. Recently, mouse models of cardiac hypertrophy have exhibited that activation of TGFβ signal in non-myocytes stimulates proliferation of non-myocytes and fibrosis in the heart [99]. Additionally, expression of matrix metalloproteinases (MMPs) is controlled by TGFβ and proinflammatory cytokines, and disruption of these mechanisms under conditions of pathological hypertrophy induces degradation of ECM [100]. Procollagen type I, the final precursor to collagen type I in the heart, is strongly associated with fibrosis [101] and increased by connective tissue growth factor (CTGF), which is regulated by TGFβ [102]. Angiotensin II increases TGFβ expression, which enhances fibrosis [103]. Interestingly, angiotensin II-mediated CTGF expression in the heart is enhanced by everolimus, an mTOR inhibitor [104], suggesting that mTOR might inhibit fibrosis by CTGF inhibition. On the other hand, Goraksha-Hicks et al. described PI3K-Akt-mTOR signaling pathway as the Smad-independent pathway of TGFβ-induced cellular hypertrophy [105]. Thus, further studies are required to assess the effects of cardiac mTOR on TGFβ signaling pathway.
ROLE OF PI3K, Akt and mTOR IN CARDIAC HYPERTROPHY
A number of studies have demonstrated that the PI3K-Akt signaling cascade is associated with cardiac hypertrophy. In dominant negative PI3K transgenic (dnPI3K) mice, exercise-induced physiological cardiac hypotrophy was attenuated compared to WT animals [106]. Interestingly, pathological cardiac hypertrophy induced by TAC in WT and dnPI3K mice had no significant difference[106]. PI3K (p110α)-dependent hypertrophy was protective in animal models of hypertrophic cardiomyopathy and heart failure [106, 107]. Thus, p110α plays a crucial role in physiological hypertrophy but not in pathological hypertrophy. Furthermore, inactivation of PTEN, which dephosphorylates PtdIns(3, 4, 5)P3 produced by PI3K, inhibits cardiac hypertrophy [6, 21, 22]. However, PI3K (p110γ) deficiency has been shown to protect against isoproterenol-induced heart failure, suggesting that p110γ mediates pathological cardiac hypertrophy [108]. Consistent with these data, overexpression of either IGF-1 or IGF receptor, activates PI3K (p110α), and induces physiological cardiac hypertrophy and myocardial infarction [109]. Therefore, results indicate that p110α stimulates physiological cardiac hypertrophy, while p110γ stimulates pathological cardiac hypertrophy.
Akt, a downstream molecule of PI3K, is also involved in cardiac hypertrophy. Even though Akt-deficient and WT mice showed similar heart weight and cardiac function as measured by echocardiography, exercise-induced cardiac hypertrophy was attenuated in Akt-KO mice. The pathological hypertrophic response to pressure-overload was intact, suggesting that Akt mediates physiological hypertrophy rather than pathological hypertrophy [110]. Furthermore, we and other groups previously demonstrated that mice with cardiac-specific overexpression of a constitutively active form of Akt showed significant cardiac hypertrophy, apparently due to increased cardiomyocyte size [111-113]. In transgenic mouse models, prolonged or excess expression of cardiac Akt caused deterioration of cardiac function in models of cardiac hypertrophy or ischemia [68, 114], while moderately activated Akt showed cardioprotection with anti-apoptotic effects against ischemia-reperfusion injury [112]. These findings suggest that Akt alone is not sufficient to protect hearts against cardiac injuries.
mTOR expression has also been found to be associated with the onset of heart failure. mTOR in mRNA and protein levels are elevated in cardiac tissue from heart failure patients compared to healthy subjects [115]. Inhibition of mTOR with rapamycin attenuates cardiac hypertrophy in humans and in animal models [15, 46, 116]. However, transgenic mice with cardiac-specific overexpression of either dominant negative or constitutively active mTOR showed cardiac hypertrophy similar to WT animals following physiological and pathological stimuli [117]. These results suggest that cardiac mTOR has little effect on hypertrophy. The role of mTOR in cardiac function during pathological hypertrophy was fully determined recently. A study in cardiac-specific mTOR deficient mice suggested that cardiac mTOR is necessary to preserve cardiac function under LV pressure overload [118]. The report also showed that 4E-BP1, a downstream effector of mTOR, plays an important role in cardiac function [118]. Recently, we generated cardiac-specific transgenic mice overexpressing wild-type mTOR and observed protection of cardiac function under LV pressure overload [115]. In these animals, TAC-induced interstitial fibrosis and increases in IL-1β and IL-6 were significantly attenuated compared to WT animals [115]. These data indicate that mTOR is protective against heart injury, and acts by suppressing the inflammatory response [115]. Previously, it was reported that rapamycin increased production of proinflammatory cytokines via NF-κB, and suppressed anti-inflammatory cytokines via STAT3 [119]. In addition, deletion of TSC2, inhibits mTOR, and suppresses the inflammatory response following lipopolysaccharide (LPS) - or bacteria-induced inflammation [39, 119, 120]. Consistent with a protective and anti-inflammatory role for mTOR, inhibition of mTOR increased mortality in the endotoxin shock model with an accompanying increase in plasma IL-1β [121]. Rapamycin was first identified as an immunosuppressor and used for the prevention of allograft rejection [122], suggesting that mTOR is pro-inflammatory. However, recent studies suggest that mTOR plays an important anti-inflammatory role based on the stimuli or the tissue.
CLINICAL ASPECTS
There are many effective pharmacological therapies for heart failure, including angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), β-adrenergic receptor blocker, diuretics, antithrombotic agents and vasodilators such as nitric oxide (NO) donors [123, 124]. Numerous clinical studies and meta-analysis have demonstrated that mono therapy or combination therapy with these drugs significantly improve symptoms and increase survival [6, 123, 124]. According to Laughlin et al., the risk of cardiovascular disease is increased in patients showing lower plasma IGF levels [125]. Replacement therapy with IGF or administration of activators of this pathway may contribute to physiological hypertrophy, and provide protection against subsequent cardiac injury. The data we have summarized in this review strongly suggest that the PI3K-Akt-mTOR signaling pathway activated by IGF-1 is a potent therapeutic target for treatment of cardiac hypertrophy and heart failure. Activation of this pathway is likely to counteract fibrosis and cell death apoptosis, thus preserving cardiac function. On the other hand, mTOR inhibitor rapamycin was introduced as a potential therapeutic agent against cardiac hypertrophy [15, 46]. As discussed above, recent reports including ours suggest that inhibiting cardiac mTOR deteriorates cardiac function in pressure-overload induced cardiac hypertrophy [115, 118]. Therefore, further studies are required to assess the beneficial effects of rapamycin for patients with cardiac hypertrophy.
CONCLUSION
Therefore, accumulated evidence strongly suggests that the PI3K signaling pathway plays an important role in controlling hypertrophic change and preserving cardiac function during the progression of heart failure in cardiac hypertrophy. However, developing effective therapeutic approaches based on these findings is difficult, due to the diverse downstream effectors of PI3K signaling and the multiple pathophysiological features of pathological hypertrophy. Recent data with advanced pharmacological and molecular techniques has revealed critical factors underlying the pathogenesis of cardiac hypertrophy leading to heart failure, including cell death, angiogenesis and inflammation. Hence, it would take additional research to find effective strategies to target the pathogenesis. To find effective strategies to target these processes, we must evaluate optimal duration, timing and dose of pharmacological and molecular agents that control activation of cardiac PI3K signaling.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from the NIH (HL098423 and RR016453) to TM.
REFERECES
- [1].Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- [2].Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- [3].Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol. 2005;289:H8–H16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- [4].Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–9. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- [5].Esposito G, Rapacciuolo A, Prasad SV Naga, Takaoka H, Thomas SA, Koch WJ, et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- [6].Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- [7].Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–37. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- [9].Scheuer J, Malhotra A, Hirsch C, Capasso J, Schaible TF. Physiologic cardiac hypertrophy corrects contractile protein abnormalities associated with pathologic hypertrophy in rats. J Clin Invest. 1982;70:1300–5. doi: 10.1172/JCI110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gunther S, Grossman W. Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation. 1979;59:679–88. doi: 10.1161/01.cir.59.4.679. [DOI] [PubMed] [Google Scholar]
- [11].Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- [13].Franke TF, Cantley LC. Apoptosis. A Bad kinase makes good. Nature. 1997;390:116–7. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- [14].Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- [15].Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–70. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- [16].Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–81. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- [17].Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- [18].Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- [19].Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–8. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- [20].Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–8. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- [22].Crackower M, Oudit G, Kozieradzki I, Sarao R, Sun H, Sasaki T, et al. Regulation of Myocardial Contractility and Cell Size by Distinct PI3K-PTEN Signaling Pathways. Cell. 2002;110:737. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- [23].Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- [24].Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Altomare DA, Lyons GE, Mitsuuchi Y, Cheng JQ, Testa JR. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998;16:2407–11. doi: 10.1038/sj.onc.1201750. [DOI] [PubMed] [Google Scholar]
- [26].Brodbeck D, Cron P, Hemmings BA. A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 1999;274:9133–6. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- [27].Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–4. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- [28].Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- [29].Andjelkovic M, Maira SM, Cron P, Parker PJ, Hemmings BA. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol Cell Biol. 1999;19:5061–72. doi: 10.1128/mcb.19.7.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- [31].Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–66. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- [32].Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–72. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- [33].Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- [34].Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- [35].Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–16. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–87. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–26. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- [40].Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- [41].Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–13. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–60. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- [44].Wick M. Filament assembly properties of the sarcomeric myosin heavy chain. Poult Sci. 1999;78:735–42. doi: 10.1093/ps/78.5.735. [DOI] [PubMed] [Google Scholar]
- [45].Zak R, Rabinowitz M. Molecular aspects of cardiac hypertrophy. Annu Rev Physiol. 1979;41:539–52. doi: 10.1146/annurev.ph.41.030179.002543. [DOI] [PubMed] [Google Scholar]
- [46].McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–5. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- [47].Ching GW, Franklyn JA, Stallard TJ, Daykin J, Sheppard MC, Gammage MD. Cardiac hypertrophy as a result of long-term thyroxine therapy and thyrotoxicosis. Heart. 1996;75:363–8. doi: 10.1136/hrt.75.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bedotto JB, Gay RG, Graham SD, Morkin E, Goldman S. Cardiac hypertrophy induced by thyroid hormone is independent of loading conditions and beta adrenoceptor blockade. J Pharmacol Exp Ther. 1989;248:632–6. [PubMed] [Google Scholar]
- [49].Sui L, Wang J, Li BM. Administration of triiodo-L-thyronine into dorsal hippocampus alters phosphorylation of Akt, mammalian target of rapamycin, p70S6 kinase and 4E-BP1 in rats. Neurochem Res. 2008;33:1065–76. doi: 10.1007/s11064-007-9551-2. [DOI] [PubMed] [Google Scholar]
- [50].Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation. 2010;122:385–93. doi: 10.1161/CIRCULATIONAHA.109.917922. [DOI] [PubMed] [Google Scholar]
- [51].Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. 2006;281:20666–72. doi: 10.1074/jbc.M512671200. [DOI] [PubMed] [Google Scholar]
- [52].Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- [53].Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–83. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, et al. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–66. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- [55].Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–41. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- [56].Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–8. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- [58].Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–24. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- [59].Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- [61].Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–87. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–78. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- [64].Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–11. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- [66].Gavin JB, Maxwell L, Edgar SG. Microvascular involvement in cardiac pathology. J Mol Cell Cardiol. 1998;30:2531–40. doi: 10.1006/jmcc.1998.0824. [DOI] [PubMed] [Google Scholar]
- [67].De Boer RA, Pinto YM, Van Veldhuisen DJ. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation. 2003;10:113–26. doi: 10.1038/sj.mn.7800188. [DOI] [PubMed] [Google Scholar]
- [68].Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–8. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- [70].Tirziu D, Chorianopoulos E, Moodie KL, Palac RT, Zhuang ZW, Tjwa M, et al. Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice. J Clin Invest. 2007;117:3188–97. doi: 10.1172/JCI32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Heineke J, Auger-Messier M, Xu J, Oka T, Sargent MA, York A, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007;117:3198–210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–65. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- [73].Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5. [PubMed] [Google Scholar]
- [75].Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N. Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev. 2010;15:543–62. doi: 10.1007/s10741-010-9168-4. [DOI] [PubMed] [Google Scholar]
- [76].Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, et al. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res. 1997;81:664–71. doi: 10.1161/01.res.81.5.664. [DOI] [PubMed] [Google Scholar]
- [77].Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–81. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann Med. 2005;37:74–85. doi: 10.1080/07853890510007232. [DOI] [PubMed] [Google Scholar]
- [79].Baumgarten G, Knuefermann P, Kalra D, Gao F, Taffet GE, Michael L, et al. Load-dependent and -independent regulation of proinflammatory cytokine and cytokine receptor gene expression in the adult mammalian heart. Circulation. 2002;105:2192–7. doi: 10.1161/01.cir.0000015608.37608.18. [DOI] [PubMed] [Google Scholar]
- [80].Gurevitch J, Frolkis I, Yuhas Y, Paz Y, Matsa M, Mohr R, et al. Tumor necrosis factor-alpha is released from the isolated heart undergoing ischemia and reperfusion. J Am Coll Cardiol. 1996;28:247–52. doi: 10.1016/0735-1097(96)00105-2. [DOI] [PubMed] [Google Scholar]
- [81].Wong GH, Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–4. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- [82].Sharma HS, Stahl J, Weisensee D, Low-Friedrich I. Cytoprotective mechanisms in cultured cardiomyocytes. Mol Cell Biochem. 1996;160-161:217–24. doi: 10.1007/BF00240052. [DOI] [PubMed] [Google Scholar]
- [83].Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, et al. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- [84].Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest. 2007;117:2692–701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–65. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- [86].Yamauchi-Takihara K, Kishimoto T. Cytokines and their receptors in cardiovascular diseases--role of gp130 signalling pathway in cardiac myocyte growth and maintenance. Int J Exp Pathol. 2000;81:1–16. doi: 10.1046/j.1365-2613.2000.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yu X, Kennedy RH, Liu SJ. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. J Biol Chem. 2003;278:16304–9. doi: 10.1074/jbc.M212321200. [DOI] [PubMed] [Google Scholar]
- [88].Brown JM, White CW, Terada LS, Grosso MA, Shanley PF, Mulvin DW, et al. Interleukin 1 pretreatment decreases ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 1990;87:5026–30. doi: 10.1073/pnas.87.13.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Palmer JN, Hartogensis WE, Patten M, Fortuin FD, Long CS. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J Clin Invest. 1995;95:2555–64. doi: 10.1172/JCI117956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric NH. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res. 1999;84:21–33. doi: 10.1161/01.res.84.1.21. [DOI] [PubMed] [Google Scholar]
- [91].Brown RD, Jones GM, Laird RE, Hudson P, Long CS. Cytokines regulate matrix metalloproteinases and migration in cardiac fibroblasts. Biochem Biophys Res Commun. 2007;362:200–5. doi: 10.1016/j.bbrc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–31. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–98. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–9. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- [95].Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–71. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- [96].Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, et al. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal. 2010;13:1033–49. doi: 10.1089/ars.2009.2930. [DOI] [PubMed] [Google Scholar]
- [97].Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–75. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 2010;120:3520–9. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hutchinson KR, Stewart JA, Jr., Lucchesi PA. Extracellular matrix remodeling during the progression of volume overload-induced heart failure. J Mol Cell Cardiol. 2010;48:564–9. doi: 10.1016/j.yjmcc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lopez B, Gonzalez A, Beaumont J, Querejeta R, Larman M, Diez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:859–67. doi: 10.1016/j.jacc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- [102].Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–19. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- [103].Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–69. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- [104].Finckenberg P, Inkinen K, Ahonen J, Merasto S, Louhelainen M, Vapaatalo H, et al. Angiotensin II induces connective tissue growth factor gene expression via calcineurin-dependent pathways. Am J Pathol. 2003;163:355–66. doi: 10.1016/S0002-9440(10)63659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Goraksha-Hicks P, Rathmell JC. TGF-beta: a new role for an old AktTOR. Dev Cell. 2009;17:6–8. doi: 10.1016/j.devcel.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–60. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, et al. Protective effects of exercise and phosphoinositide 3-kinase(p110{alpha}) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104:612–7. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, et al. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation. 2003;108:2147–52. doi: 10.1161/01.CIR.0000091403.62293.2B. [DOI] [PubMed] [Google Scholar]
- [109].McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–93. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- [110].DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- [111].Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, et al. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–8. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- [113].Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–38. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, et al. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–66. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, et al. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–7. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Shen WH, Chen Z, Shi S, Chen H, Zhu W, Penner A, et al. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mTOR) mutant impairs the mTOR-mediated signaling and cardiac function. J Biol Chem. 2008;283:13842–9. doi: 10.1074/jbc.M801510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–16. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–77. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- [120].Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–61. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- [121].Schmitz F, Heit A, Dreher S, Eisenacher K, Mages J, Haas T, et al. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol. 2008;38:2981–92. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- [122].Miller JL. Sirolimus approved with renal transplant indication. Am J Health Syst Pharm. 1999;56:2177–8. doi: 10.1093/ajhp/56.21.2177. [DOI] [PubMed] [Google Scholar]
- [123].House AA, Haapio M, Lassus J, Bellomo R, Ronco C. Therapeutic strategies for heart failure in cardiorenal syndromes. Am J Kidney Dis. 2010;56:759–73. doi: 10.1053/j.ajkd.2010.04.012. [DOI] [PubMed] [Google Scholar]
- [124].McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–89. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- [125].Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–20. doi: 10.1210/jc.2003-030967. [DOI] [PubMed] [Google Scholar]