Abstract
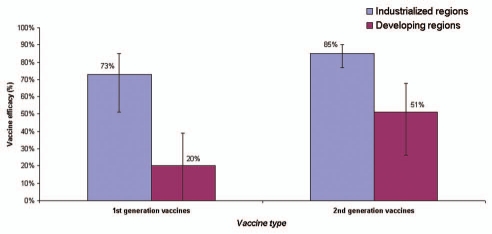
The World Health Organization estimates that rotavirus diarrhea results in approximately half a million deaths and approximately 2.4 million hospitalizations in developing countries each year. Two live oral rotavirus vaccines, RotaTeq® (RV 5; Merck) and Rotarix® (RV 1; GlaxoSmithKline) with good efficacy against severe rotavirus disease and a reassuring safety profile could substantially impact the burden of rotavirus disease. In April 2009, WHO provided a recommendation for global introduction of these vaccines in national immunization programs of developing countries worldwide. In this article, we review published data on previous candidate rotavirus vaccines and vaccines in current use, with emphasis on their performance in developed versus developing countries. In developed countries, both first and second generation rotavirus vaccines have demonstrated high efficacy against severe rotavirus disease (pooled efficacy = 73% and 85%, respectively). In developing countries, small early trials for the first generation vaccines failed to provide protection against rotavirus disease (pooled efficacy = 20%), however, trials of the second generation vaccines yielded substantial improvements in efficacy in developing countries (pooled efficacy of 51%), leading to a global recommendation for rotavirus vaccine introduction by WHO. Future efforts for these vaccines should focus on optimizing the efficacy and delivery of these vaccines in challenging target populations of Asia and Africa with the greatest burden of severe rotavirus disease.
Key words: rotavirus, vaccines, immunization, vaccination, diarrhea, gastroenteritis
Introduction
Rotavirus diarrhea causes over 500,000 deaths and 2.4 million hospitalizations globally each year among children less than 5 years of age.1–3 Because of the tremendous global burden of rotavirus, the World Health Organization (WHO) has prioritized vaccine development and introduction to control this disease. In recent years, clinical trials of two live oral rotavirus vaccines, RotaTeq® (RV5; Merck Vaccines, Whitehouse Station, NJ) and Rotarix® (RV1; GlaxoSmithKline Biologicals, Rixensart, Belgium) have demonstrated good efficacy against severe rotavirus disease and a reassuring safety profile in developed and middle income countries.4–7 On the basis of existing data on the performance of these vaccines, WHO recommends the global introduction of these vaccines in national immunization programs worldwide.8 In 2006, WHO strongly recommended the inclusion of these new rotavirus vaccines into national immunization programs of countries in the Americas and Europe on the basis of pivotal clinical trials from these regions.9 In April 2009, WHO extended this recommendation to all regions of the world after review of clinical trial data from Africa and Asia, and postlicensure data from the Americas.8
The success of these vaccines will in part depend on their efficacy in the poorest settings with the highest burden of severe rotavirus disease. In this review, we summarize the experience of the current and previous candidate rotavirus vaccines, with emphasis on an examination of their performance in developed versus developing countries. Our primary interest was to understand if variations in vaccine efficacy exist between the two populations that are independent of vaccine type, study design, and outcome measures of efficacy. For rotavirus vaccines to have an optimal public health impact, sustained protection should be attained through first 2 to 3 years of life, when a majority of the severe rotavirus disease occurs worldwide.10 Thus, as a secondary objective we assessed for potential waning of immunity by restricting analysis to efficacy and effectiveness studies assessing duration of protection after rotavirus vaccination.
General Background on Rotaviruses: Factors Relevant to Vaccine Development and Introduction
Human rotavirus was first isolated by Ruth Bishop in 1973 from epithelial cells of the small intestine of children with diarrhea.11 Rotavirus has an 11-segmented double-stranded RNA genome that is surrounded by three protein shells: a core, an inner capsid, and outer capsid.12 Each RNA gene segment encodes a structural (VP1-VP4, VP6, VP7) or nonstructural protein(s) (NSP1-NSP4), except for gene 11 which codes for both NSP5 and NSP6. Two structural proteins, VP7 (a glycoprotein—G protein) and VP4 (a protease-cleaved protein—P protein), forming the outer shell of the rotaviruses are used for rotavirus strain characterization. The two gene segments that encode VP4 and VP7 can segregate independently in vivo and in vitro, and theoretically lead to more than 100 different G and P protein combinations, however four combinations (P[8]G1, P[4]G2, P[8]G3, P[8]G4) are responsible for >80% of the global burden of severe rotavirus disease.13 However, strains can vary from year to year, and by region; in addition, periodic emergence of novel strains (G9 and G12) have also been reported.
The mechanisms and duration of protection against rotavirus infection are incompletely understood, however, it is suspected that clinical protection likely involves mucosal (intestinal) and systemic antibody response as well as cell-mediated immunity.14 Evidence suggests that intestinal (jejunal) IgA is possibly one of the more important mechanisms for long-term protection against rotavirus infection.14 With regard to potential protection from systemic antibodies, immune response has been hypothesized to partly depend on neutralizing epitopes VP7 and VP4 on the viral capsid.14,15 As such, the antigen composition of the current and candidate rotavirus vaccines are typically described on the basis of these VP7 (G-type) and VP4 (P-type) proteins on the vaccine strain. Does natural rotavirus infection with one strain confer cross-protection (i.e., heterotypic) against other antigens? If neutralizing epitopes VP7 and VP4 were the predominant modulators of immunity, for heterotypic protection to occur, strains that cause initial infections would need to share epitopes with strains that they will eventually protect against. The presence of a few predominant circulating strains globally, often over many years, argues against the serotype-specific neutralizing antibodies as the exclusive mechanism of protection—that is, under exclusive serotype-specific protection, selective pressure would have lead to an apparent antigenic drift and frequent changes in serotype prevalence. In addition, many observational studies and vaccine trials support the presence of heterotypic protection after primary infection.10,14 However, it has been observed that the first infection with rotavirus elicits a predominantly homotypic, serum-neutralizing antibody response to the virus, and subsequent infections (asymptomatic and symptomatic) elicit a broader, heterotypic response.16,17
The current and previous candidate rotavirus vaccines can be categorized in two groups on the basis of their development (Table 1):18,19
first generation vaccines: these early rotavirus vaccines were single-strain animal strains that were naturally attenuated in that they did not cause clinical disease in humans but conferred protection against subsequent infection with human rotavirus strains;
second generation vaccines: these rotavirus vaccines developed subsequent to the first generation vaccines include two subgroups: (a) human-animal reassortants: these reassortants contain the backbone of an animal strain which incorporate additional genes from human strains by capitalizing on the viruses' ability to reassort in vitro. The development process involves combining naturally attenuated animal strains in cell culture systems with human rotavirus strains. Subsequently, reaassortant vaccine strains are recovered that contain either the VP7 or VP4 gene from a human strain, and the remaining 10 rotavirus genes from the naturally attenuated animal virus strain. (b) human strains: these are vaccines developed through attenuation of human rotavirus strains.
Table 1.
Past or current rotavirus vaccines with efficacy data from clinical trials
| Acronym (trade name) | Backbone | Valency | Antigens | Concept | |
| First generation (animal backbone) | |||||
| RIT 4237 | Bovine | single-strain | G6, P6 | derived from the Nebraska calf diarrhea virus strain | |
| RRV-MMU | Rhesus | single-strain | G3, P3 | isolated from a rhesus monkey | |
| WC3 (non-reassortant) | Bovine | single-strain | G6, P5 | low-passage bovine strain from calf in Pennsylvania | |
| Second generation (animal-human reassortants) | |||||
| RRV-TV (Rotashield) | Rhesus | tetravalent | G1, G2, G3, G4, P3 | rhesus-human reassortant with G3 rhesus backbone | |
| Reassortant WC3 (RV 5; RotaTeq) | Bovine | pentavalent | G1, G2, G3, G4, P8 | bovine-human reassortant with G6 bovine backbone | |
| RI X-4414 (RV1; Rotarix) | Human | single strain | G1, P8 | attenuated human strain | |
Results
Summary of first generation rotavirus vaccines—Jennerian approach.
The first generation of rotavirus vaccines were developed on the basis of the Jennerian approach where single-strain animal rotaviruses were used as human vaccines—these animal strains were naturally attenuated in that they did not cause clinical disease in humans but conferred protection against subsequent infection with human rotavirus strains.18,19 In industrialized countries, clinical trials for three Jennerian vaccines using attenuated animal strains demonstrated good efficacy against severe rotavirus disease (pooled efficacy = 73%: 95% confidence interval [CI] = 51 to 85) (Table 2)(Figure 1).20–27 In contrast, these vaccines failed to provide protection in challenging impoverished settings (pooled efficacy = 20%; 95% CI = <0 to 39) where the vaccine would be most critical to saving lives.28–33 That said, the encouraging data from developed countries for these early vaccines confirmed that rotavirus vaccines held great promise; moreover, the trials identified early in the course of vaccine development that improvements in performance would be necessary for success of these vaccines in developing country settings. It is important to note that substantial heterogeneity existed among the trials for the first generation vaccines with regard to number of doses administered, age at vaccine administration, outcome measures of severity, and time of follow-up. The development and testing of these first generation Jennerian vaccines is reviewed below.
Table 2.
Review of clinical trials of efficacy for first generation rotavirus vaccines
| Ref# | Vaccine | Location | Age of vaccinees | # Doses | Titer | Time of follow-up | Vaccine | Placebo | Vaccine efficacy | Severity classification* | |||
| n | N | n | N | % | (95% CI) | ||||||||
| Industrialized regions [Pooled analysis] | 73 | 51 to 85 | |||||||||||
| 20 | RI T-4237 | Finland | 8–11 mo | 1 | 108.1 | 5 months | 2 | 86 | 18 | 92 | 88 | 56 to 97 | “Clinically significant” |
| 21 | RIT-4237 | Finland | 6–12 mo | 2 | 108.1 | 5 months | 5 | 168 | 26 | 160 | 82 | 55 to 93 | “Clinically significant” |
| 22 | RIT-4237 | Finland | <1 week | 1 | 108.3 | 24–32 mo | 15 | 519† | 35 | 481† | 60 | 28 to 78 | Severity >= 11 |
| 23 | RI T-4237 | Finland | birth, 7 mos | 2 | 108.3 | 28 months | 1 | 124 | 9 | 128 | 89 | 32 to 98 | Severity >= 11 |
| 24 | WC3‡ | US | 2–8 mo | 3 | 107.3 | 1 season | 4 | 207 | 18 | 118 | 87 | 63 to 96 | Severity > 8 |
| 25 | RRV-MMU | US | 4–26 weeks | 1 | 104 | 4 months | 11 | 85 | 13 | 88 | 12 | <0 to 58 | WHO criteria for severity |
| 26 | RRV-MMU | Finland | 2–5 mo | 1 | 104 | 2 seasons | 3 | 100 | 6 | 100 | 50 | <0 to 87 | Severity >= 9 |
| 27 | RRV-MMU | Venezuela | 1–10 mo | 1 | 1054 | 1 year | 1 | 151 | 10 | 151 | 90 | 33 to 99 | Severity >= 9 |
| Developing regions [Pooled analysis] | 20 | <0 to 39 | |||||||||||
| 28 | RIT-4237 | Rwanda | 3–8 mo | 1 | - | 4–6 weeks | 6 | 122 | 6 | 123 | 0 | 0 | No severity information |
| 29 | RIT-4237 | Gambia | 10 weeks | 3 | 107.8 | 3 months | 40 | 170 | 21 | 83 | 7 | <0 to 41 | Clinical definition of severity |
| 30 | RIT-4237 | Peru | 2–18 mo | 3 | 108.3 | 18 months | 2 | 99 | 8 | 100 | 75 | <0 to 94 | Severe = health center visits |
| 31 | RIT-4237 | US Native Indians | 2–5 mo | 1 | 108.3 | 17 months | 3 | 106 | 0 | 107 | 0 | <0 to 20 | Severe = hospitalization |
| 31 | RRV-MMU | US Native Indians | 2–5 mo | 1 | 104 | 18 months | 2 | 108 | 0 | 107 | 0 | <0 to 48 | Severe = hospitalization |
| 32 | RRV-MMU | Peru | 2 mos | 1 | 104 | ∼2 years | 31 | 338 | 40 | 339 | 22 | <0 to 50 | Severity = health service use |
| 33 | WC3 (RV 5)‡ | Cent. African Rep | 3–4 mo | 2 | 107 | 9 months | 30 | 237 | 43 | 235 | 31 | <0 to 55 | Severity > 9 on 24 point scale |
denotes score on a 20-point Vesikari scale or hospitalization when available; else, we report the parameter of severity as selected by authors.
denominators for this study were person-years, obtained from a previous review (REF).
in this table, WC3 denotes the single-strain bovine vaccine (non-reassortant).
Figure 1.
Pooled estimates of efficacy against severe rotavirus disease by income settings for first and second generation rotavirus vaccines. These estimates are the pooled estimates and 95% confidence limits are generated from studies outlined in Tables 2 and 3 (refer to Methods).
RIT 4237. The first rotavirus vaccine tested was a bovine strain belonging to serotype G6 (P type 6 specificity), Nebraska calf diarrhea virus, that underwent 147 passages in bovine embryonic kidney cells and seven in African green monkey kidney cells.34 Efficacy of this vaccine, designated RIT 4237, was 60%–89% against severe rotavirus disease in clinical trials in industrialized settings (Finland and US).20–23 In contrast, RIT 4237 provided no protection in poor settings of Rwanda (efficacy = 0%),28 the Gambia (7%),29 and US Native Americans (0%).31 In Peru, however, vaccine efficacy was 58%–75% against the more severe rotavirus illnesses.30 The studies also highlighted that the vaccine did not induce sufficient protection against milder rotavirus disease in either developed or developing country settings, which was consistent with the observation that under natural conditions, milder re-infections are not uncommon. Moreover, because RIT 4237 was derived from serotypes G6 and P6, a strain that does not cause human disease, proof of concept was established that a heterologous virus could indeed provide heterotypic protection against severe rotavirus disease.
RRV-MMU. The RRV-MMU vaccine was a G3 strain that was originally isolated from a 3 month-old rhesus monkey with acute diarrhea.35 RRV-MMU was developed as an alternative to RIT 4237 on the premise that it shared the G3 epitope with one of the four strains causing most of the human rotavirus disease worldwide. The RRV-MMU strain underwent 9 passages in monkey kidney cell cultures and 7 passages in diploid rhesus cells.34 Clinical trials for the vaccine in industrialized countries resulted in variable efficacy ranging from 12% to 90%.25–27 No protection was observed after one dose of RRV-MMU in an impoverished Native American population in the US.31 Several findings from these trials were of interest for future vaccine development. First, in the one trial from Venezuela where high efficacy (90%) was observed against severe disease, the predominant circulating strain (G3) was similar to the rhesus vaccine strain, indicating that serotype-specific protection might be of importance for the animal-based rotavirus vaccines, and prompted the addition of more antigens in future modifications of animal-based vaccines.27 Second, the vaccine provided greater protection against severe illness compared to mild and moderate disease. Third, a small immunogenicity trial from Japan indicated that additional booster doses of RRV-MMU may enhance protection against heterotypic strains.36
WC3 (non-reassortant). This is a vaccine similar to RIT 4237 in that it was derived from a G6 serotype bovine strain (P type 5) that was isolated from a calf with diarrhea in Pennsylvania.37 The vaccine underwent 12 cell-culture passages prior to evaluation in two efficacy trials. In a trial in the US, three doses of WC3 conferred 87% protection against severe rotavirus disease;24 in contrast, two doses of WC3 provided substantially lower protection (31%) in a trial conducted in the Central African Republic.33
Summary of second generation vaccines—modified Jennerian approach.
The second generation of vaccines, including currently licensed or candidate rotavirus vaccines, use the modified Jennerian approach where the vaccines are either: (1) reassortant vaccines with the backbone of an animal strain (e.g., RRV-TV, WC3) which incorporate one or more human VP7 or VP4 genes; or (2) attenuated human rotavirus strains such as the common G1 strain or uncommon neonatal strains.18,19 Of these second generation vaccines, three have undergone phase III efficacy trials in various developing and developed country settings: RRV-TV (RotaShield); RV1 (Rotarix); and RV5 (RotaTeq).10 In general, less heterogeneity in study design was observed across these trials because of lessons learned from trials of first generation vaccines with regard to number of doses administered, vaccine titer, age at immunization, outcome measure, and time of follow-up (Table 3).
Table 3.
Review of clinical trials of efficacy for second generation rotavirus vaccines
| Ref# | Vaccine | Location | Age of vaccinees | # Doses | Titer | Time of follow-up | Vaccine | Placebo | Vaccine efficacy | Severity classification* | |||
| n | N | n | N | % | (95% CI) | ||||||||
| Industrialized regions [Pooled analysis] | 85 | 77 to 90 | |||||||||||
| 38 | RRV-TTV | US | 4–26 weeks | 3 | 104 | 2 seasons | 6 | 305 | 27 | 296 | 78 | 38 to 93 | Severity = medical visits |
| 39 | RRV-TTV | US | 5–25 weeks | 3 | 4 × 105 | 6 months | 7 | 398 | 34 | 404 | 80 | 56 to 91 | Severity > 14 |
| 40 | RRV-TTV | Finland | 3–5 mo | 3 | 4 × 105 | 2 seasons | 8 | 1128 | 92 | 1145 | 91 | 82 to 96 | Vesikari >= 11 |
| 41 | RRV-TTV | Brazil | 1–5 | 3 | 4 × 104 | 2 years | 21 | 270 | 39 | 270 | 46 | 12 to 67 | Severity >= 9 |
| 42 | RRV-TTV | Venezuela | 8–18 weeks | 3 | 105 | 19–20 mo | 10 | 1247 | 33 | 1233 | 70 | 40 to 85 | Admission |
| 43 | RIX-4414 | Finland | 2–4 mos | 2 | 104.7 | 2 seasons | 3 | 245 | 10 | 123 | 85 | 42 to 97 | Vesikari >= 11 |
| 44 | RIX-4414 | Brazil, Mexico, Venezuela | 2–4 | 2 | 105.8 | 1 year | 5 | 464 | 34 | 454 | 86 | 63 to 96 | Vesikari >= 11 |
| 5 | RI X-4414 | Latin America and Finland | 2–4 mos | 2 | 106.5 | 1 year | 12 | 9009 | 77 | 8858 | 85 | 72 to 92 | Admission or rehydration |
| 45 | RIX-4414 | Belem, Brazil | 2–4 mos | 2 | 105.8 | 1 year | 4 | 170 | 19 | 149 | 82 | 44 to 95 | Vesikari >= 11 |
| 4 | RIX-4414 | Latin America | 2–4 mos | 2 | 106.5 | 2 years | 32 | 7205 | 161 | 7081 | 80 | 71 to 87 | Vesikari >= 11 |
| 6 | RIX-4414 | Europe | 2–4 mos | 2 | 106.5 | 2 years | 24 | 2572 | 127 | 1302 | 90 | 85 to 94 | Vesikari >= 11 |
| 46 | WC3† | US | 2–6 mos | 3 | 107 | 1 year | 0 | 218 | 8 | 229 | 100 | 44 to 100 | Severity > 16 on 24 point scale |
| 7 | WC3† | US, Finland | 2–6 mos | 3 | 107 | 2 years | 1 | 2834 | 57 | 2839 | 98 | 88 to 100 | Vesikari >= 11 |
| 47 | WC3† | US, Finland, Latin America | 2–8 mos | 3 | 107.3 | 1 year | 20 | 28646 | 369 | 28488 | 94 | 91 to 97 | Hospitalization and ED visits |
| Developing regions [Pooled analysis] | 51 | 26 to 68 | |||||||||||
| 50 | RRV-TTV | Peru | 2–4 mos | 3 | 104 | ∼2 years | 26 | 209 | 38 | 219 | 30 | <0 to 55 | Severity >= 9 |
| 49 | RRV-TTV | US Native Indians | 6–24 weeks | 3 | 4 × 105 | 2 years | 12 | 396 | 33 | 391 | 64 | 32 to 81 | Severity > 15 |
| 48 | RIX-4414 | South Africa & Malawi | 2–4 mos | 2 | 106.5 | 1 year | 30 | 1496 | 70 | 1443 | 59 | 36 to 74 | Vesikari >= 11 |
denotes score on a 20-point Vesikari scale or hospitalization when available; else, we report the parameter of severity as selected by authors.
in this table, WC3 denotes the pentavalent bovine reassortant vaccine.
Similar to the first generation vaccines, in developed countries, the second-generation vaccines demonstrated high efficacy against severe rotavirus disease (pooled efficacy = 85%; 95% CI = 77 to 90).4–7,38–47 In addition, substantial improvements were noted compared with the first generation vaccines with regard to vaccine efficacy in developing countries where a pooled efficacy of 51% (95% CI = 26 to 68) was observed.32,48,49 While efficacy of the second generation vaccines was still lower in developing countries than efficacy in industrialized settings, the higher burden of severe disease in developing countries translated into a greater absolute reduction in severe rotavirus disease in the poorest settings.48
Rhesus-human reassortant (RRV-TV; Rotashield). The human-rhesus reassortant vaccine resulted from two strategic improvements to the first generation single-strain RRV-MMU vaccine. Initially, a single reassortant preparation was developed by combining 10 genes from the naturally attenuated RRV-MMU (G3 strain) with a single VP7 gene from commonly encountered human rotaviruses (i.e., G1, G2 or G4).34 The reassortants were recovered after coinfection of monkey kidney cell cultures with the RRV-MMU strain with the human rotavirus strains. Successful development of a reassortant vaccine with broad protection against diverse, epidemiologically important human strains was sought by combining the individual reassortants into a tetravalent RRV (RRV-TV) formulation, with G1, G2 and G4 human strains and G3 rhesus component.
RRV-TV was evaluated in both developed and developing country settings. In the early trials, a lower titer (4 × 104 plaque forming units)38,41,50 was used compared with subsequent trials of RRV-TV (4 × 105).39,40,42 The trials using lower RRV-TV titers yielded good protection (efficacy = 78%)38 in the US, but efficacy was lower in Brazil (46%)41 and Peru (30%),50 a middle and low-middle income country, respectively. However, in a reanalysis of data from the Brazil and Peru studies, greater protection was attained against the most severe rotavirus disease.51
In subsequent studies of RRV-TV, a higher concentration (4 × 105) was administered in multiple doses to improve vaccine performance. These trials yielded good efficacy against severe rotavirus disease in the US (80%)39 and Finland (91%),40 leading to the introduction of RRV-TV in the routine childhood immunization schedule in the US. In addition, the higher titer of RRV-TV also proved to be efficacious in an impoverished setting in Venezuela, where the vaccine prevented 88% of the severe rotavirus disease.42 Efficacy of the higher titer RRV-TV against severe rotavirus disease was somewhat lower (69%) among a low socioeconomic American Indian population in the US during the first year.49
Due to these encouraging results, RRV-TV was licensed and introduced in the US; however, RRV-TV was subsequently withdrawn by the manufacturer from the US after a postlicensure association was uncovered between the vaccine and a rare adverse event, intussusception.52,53
In summary, substantial improvements were attained in vaccine performance in developing and developed countries for RRV-TV compared with the first generation vaccines. Morever, the trials and postlicensure experience for RRV-TV imparted crucial information to the development and testing of the two licensed vaccines that are currently in use.
Bovine-human reassortants (WC3; RotaTeq). RotaTeq (RV5) is a pentavalent vaccine with five separate reassortant viruses, each one that incorporates either a VP7 (G1, G2, G3, G4) or VP4 (P8) gene from a human rotavirus strain on the RNA backbone of the previously discussed WC3 bovine rotavirus strain.37 Thus, the naturally attenuated WC3 bovine virus was altered to provide cross-protection against epidemiologically important human rotavirus strains.
In a large clinical trial conducted primarily in the US and Finland, three doses of RV5 showed an efficacy of 98% against severe rotavirus gastroenteritis.7 A substantial reduction (59%) in admissions from all-cause gastroenteritis was also noted in the trial. Protection was good against all G1–4 and G9 serotypes (range 88% to 100%).6,7
Clinical trials in developing countries of Asia and Africa have recently been completed. While a complete analysis is pending, preliminary results indicate efficacy estimates of RV5 in Africa and Asia are similar to those for RV1 in Africa (see below section). 54 RV5 has also been introduced in routine immunization programs of several countries.55–57 In the US, field effectiveness of the vaccine (88%–100%) was similar to that observed in the clinical trial. RV5 was also introduced in Nicaragua, a developing country in Central America, where the vaccine prevented ∼44% of the hospitalizations from rotavirus gastroenteritis.58
Human rotavirus vaccine (RIX-4414; rotarix). Rotarix (RV1) is a single-strain vaccine of P[8]G1 specificity derived from a human rotavirus strain (89-12) which was isolated from a child who was naturally infected with rotavirus gastroenteritis in 1989.59 This 89-12 strain was selected because children with infection from this P[8]G1 strain were fully protected against subsequent severe gastroenteritis from homotypic rotavirus strains. Attenuation of the virulent 89-12 strain was conducted by serial passages in monkey kidney cell cultures and plaque purification.
RV1 was subsequently evaluated in large clinical trials in middle and high income countries in Latin America, where the vaccine demonstrated efficacy of >80% against severe rotavirus disease and 42% against all diarrhea hospitalizations.4–6,43–45 In Europe, RV1 prevented 90% of the severe rotavirus gastroenteritis episodes and 72% of all-cause diarrhea hospitalizations, indicating the potential for substantial impact on childhood diarrhea morbidity from use of rotavirus vaccines.6
Subsequent trials of RV1 in poorer settings of Africa have also yielded reasonable efficacy, resulting in a 59% overall reduction in severe rotavirus disease.8,48 In South Africa, a middle income country, RV1 provided 72% protection against severe rotavirus disease; however, in the more impoverished low income country of Malawi, efficacy was ∼49% against severe rotavirus disease. Although overall efficacy was lower in Malawi compared with South Africa, the absolute reduction in severe rotavirus disease was significantly greater in Malawi, where 6.7 gastroenteritis episodes were prevented per 100 vaccinated infants compared with 4.2 episodes per 100 vaccinated infants in South Africa. In both settings, diverse homo- and heterotypic strains were observed during the clinical trial period, however, protection was similar against disease from both vaccine and non-vaccine serotypes.48,60
Many countries, predominantly in the Region of the Americas, have introduced RV1, and several postlicensure evaluations have been conducted (Table 4).61 In Brazil, RV1 effectiveness against rotavirus hospitalizations was 85% during the first year of life.62 Of note, the fully heterotypic (non-vaccine) P[4]G2 strain was responsible for all infections in this study, thus confirming the contention that RV1 provides sufficient cross-protection against a broad range of serotypes. Two studies from field settings in Australia also further support that cross-protection occurs after RV1 vaccination, demonstrating that RV1 provided good protection against severe disease from partially heterotypic P[8]G9 (84%) and fully heterotypic P[4]G2 (86%) strains.63,64 RV1 has also been introduced in the routine immunization program of El Salvador, a developing country in Central America, where the vaccine yielded 76% protection against severe rotavirus disease.8
Table 4.
Postlicensure studies assessing effectivenes of Rotarix (RI X-4414) and RotaTeq (WC3)
| Ref# | Location | Age of vaccinees | # Doses | Age of children | Severe diarrhea | Severty classification | Circulating strains | |||
| % | (95% CI) | |||||||||
| Industrialized regions [Pooled analysis] | 91 | 82 to 95 | ||||||||
| 62 | RIX-4414 | Brazil | 2–4 mos | 2 | <12 months | 85 | 54 to 95 | Hospitalization; | G2P[4] | |
| 64 | RIX-4414 | Australia | 2–4 mos | 2 | <12 months | 84 | 23 to 97 | Hospitalization; G9P[8] strain | G9P[8] | |
| 63 | RIX-4414 | Australia | 2–4 mos | 2 | <12 months | 86 | 24 to 98 | Hospitalization; G2P[4] strain | G2P[4] | |
| 87 | WC3 (RV 5) | United States | 2–8 mos | 3 | <24 months | 100 | 71 to 100 | Hospitalization | G1P[8] and G12P[8] were most common | |
| 88 | WC3 (RV 5) | United States | 2–8 mos | 3 | <24 months | 88 | 47 to 97 | Emergency and hospitalization | G3P[8] were G1P[8] most common (75%) | |
| Developing regions [Pooled analysis] | 66 | 52 to 77 | ||||||||
| 8 | RI X-4414 | El Salvador | 2–4 mos | 2 | <24 months | 76 | 63 to 84 | Hospitalization; G1P[8] strain | G1P[8] | |
| 55 | WC3 (RV 5) | Nicaragua | 2–8 mos | 3 | <18 months | 44 | 15 to 63 | Hospitalization; G2P[4] strain | G2P[4] | |
RI X4414 denotes Rotarix, the attenuated single-strain human vaccine; WC3 denotes RotaTeq, the pentavalent bovine reassortant vaccine.
Duration of protection: first and second generation vaccines.
Eight clinical trials and three post-licensure vaccine effectiveness studies have assessed duration of protection after vaccination with a second generation rotavirus vaccine (Table 5).4,6,7,38,40,41,43,49,58,62,65 To date, none of the published clinical trials have assessed duration of protection in developing countries. For all licensed second generation vaccines, efficacy in industrialized countries was sustained through the first two to three years of life. For RRV-TV, protection after the lower titer dosing in Brazil diminished from 57% during the first year to 12% during the second year of follow-up.41 However, the licensed higher titer dosing led to sustained protection for 2 years in large studies in the US and Finland.38,40 Interestingly, efficacy did decrease some from 69% to 44% in a low socioeconomic status American Indian population, although the difference was not statistically significant.49 In the large clinical efficacy trials for RV1 and RV5, efficacy was maintained (>80%) through 2 years of life in middle and high-income settings of Europe, US, and Latin America.4–7,66 In recently completed trials, a RV1 trail in Finland and a RV5 trial in industrialized countries of Asia showed sustained protection was for at least 3 years after immunization.67,68
Table 5.
Studies of rotavirus vaccines assessing duration of protection of second generation rotavirus vaccines against severe rotavirus diarrhea
| Vaccine efficacy | ||||||||
| Ref# | Vaccine | Location | First year | Second year | Severity | Study design | ||
| % | (95% CI) | % | (95% CI) | |||||
| Industrialized regions | ||||||||
| 41 | RRV-TTV | Brazil | 57 | - | 12 | - | Hospitalization | Randomized trial |
| 38 | RRV-TTV | US | 64 | 24 to 83 | 57 | 29 to 74 | All severity | Randomized trial |
| 40 | RRV-TTV | Finland | 95 | 63 to 99 | 90 | 79 to 95 | Severity > 11 | Randomized trial |
| 43 | RIX-4414 | Finland | 90 | 10 to 100 | 83 | 7 to 98 | Vesikari > 11 | Randomized trial |
| 4 | RIX-4414 | Latin America | 83 | 67 to 93 | 79 | 66 to 87 | Vesikari > 11 | Randomized trial |
| 6 | RIX-4414 | Europe | 96 | 90 to 99 | 86 | 76 to 92 | Vesikari > 11 | Randomized trial |
| 62 | RIX-4414 | Brazil | 85 | 54 to 95 | 5 | <0 to 69 | Hospitalization | Case-control |
| 7 | WC3 (RV 5)* | United States, Finland | 98 | 88 to 100 | 88 | 49 to 99 | Vesikari > 11 | Randomized trial |
| Developing regions | ||||||||
| 49 | RRV-TTV | US Native Indian Reservation | 69 | 29 to 88 | 44 | <0 to 88 | Severity > 15 | Randomized trial |
| 89 | RIX-4414 | El Salvador | 83 | 68 to 91 | 59 | 27 to 77 | Hospitalization | Case-control |
| 58 | WC3 (RV 5)* | Nicaragua | 69 | 24 to 83 | 32 | <0 to 68 | Hospitalization | Case-control |
in this table, WC3 denotes the pentavalent bovine reassortant vaccine.
Three postlicensure vaccine effectiveness studies from impoverished settings provide suggestive evidence for some decrease in protection during the second year of life after RV1 and RV5 vaccination. In a poor population in Brazil, RV1 effectiveness against the fully heterotypic P[4]G2 strains was 85% during the first year of life, but no protection was noted among children older than 12 months of life, although confidence bounds were wide (<0 to 69%).62 Similarly, in El Salvador, a significant decline (p < 0.05) in RV1 effectiveness was observed from 83% among children <12 months of age to 59% among children older than 12 months of age.8 In the only published study from a developing country, for RV5 effectiveness in Nicaragua decreased from 69% to 32% (p = 0.19) among children less or greater than 12 months of age, respectively.58
Clinical trials for RV5 and RV1 in Africa and Asia monitored efficacy through 2 years of life and these data when published in the near future should be useful indicators of duration of protection in the poorest settings, and how it compares with existing data from industrialized countries.
Discussion
Our review highlights that both first and second generation rotavirus vaccines have largely demonstrated high efficacy (∼73% and ∼85%, respectively) against severe rotavirus diarrhea in industrialized nations. In poor settings with the greatest rotavirus disease burden, however, the immune response and clinical protection from rotavirus vaccines has been fraught with challenges.18,19,69 Efficacy of the first generation rotavirus vaccines was poor (∼20%), however, substantial experience was gained from these early vaccines which led to strategic improvements in the development and application of second generation vaccines. These improvements and an aggressive research agenda led to success of rotavirus vaccines in developing country settings, where the second generation vaccines have shown to provide efficacy of ∼51% to 66% protection against severe rotavirus in clinical trials and postlicensure studies. Thus, while rotavirus vaccines still continue to be less effective in the most impoverished settings compared with rich populations, efficacy of the current vaccines has substantially improved in the past decade, possibly due to improvements in vaccine type, study design, age at vaccination, number of doses, or outcome measures studied. Moreover, despite the lower efficacy, the trials have unequivocally demonstrated that the absolute reduction in severe disease is greater in developing country settings than more affluent settings with higher efficacy.48
Immune responses to rotavirus vaccination also vary between infants in developed and industrialized settings, with a trend of reduced immunogenicity in countries with the lowest income level.70,71 While immunogenicity data do not directly correlate with efficacy, they may offer some further evidence of impaired immune response in the children from the poorest regions. For example, after immunization with RV1, infants in high income countries, where the pooled efficacy of second generation vaccines was 85%, had substantially higher concentrations of IgA antibodies (mean titers = 206 U/mL; seroconversion = 86%) compared with vaccinated infants in low-income countries (mean titers = 68 U/mL; serocoversion = 63%), where pooled efficacy was 51%.71 These variations in immune response are not surprising given the differences in epidemiology of rotavirus disease between the developing and developed countries, and could possibly reflect differences in force of infection, host characteristics (e.g., level of nourishment, breastfeeding), circulating strain patterns, and presence of other enteric pathogens.18 Rotavirus disease in poor tropical countries is often year round, likely reflecting the higher force of infection. Although immunogenecity may not perfectly correlate with efficacy, experience from clinical trials with first and second generation candidate rotavirus vaccines suggests that field performance of these vaccines is also less optimal in developing countries than in more affluent settings. Several findings from these efficacy studies in developing countries could shed light on future use of currently licensed vaccines in these settings. Our review highlights the difficulties in comparability of study results, particularly for the first generation vaccines, due to differences in study design, such as number of doses administered, age at vaccine administration, outcome measures of severity, and time of followup. While these differences in study design could have partly explained differences in observed efficacy between developed and developing regions, the consistency of the decrease in efficacy across multiple studies and oral vaccines supports the contention that host factors among children in developing countries are likely to play a role in lower efficacy.
Several recent reviews have explored potential explanations for the impaired immune response in poor settings in hopes of identifying strategies to improve protection against severe rotavirus disease among children who would most benefit the vaccine.19,69–72 Factors that distinguish children in developing countries from industrialized nations have posed challenges to take of rotavirus vaccines in developing countries and oral vaccines for other diseases such as cholera,73–75 typhoid76 and polio,77,78 and thus closer attention is warranted to identify modifiable factors that could enhance the performance of these vaccines in challenging settings.71 If these barriers are inherent and cannot be bypassed, alternative strategies may warrant consideration including schedule modifications, increasing vaccine titers, increase in number of doses, and inclusion of broader antigens so that the full lifesaving potential of these interventions is realized. Ongoing clinical development of other live oral vaccines with different strains (i.e., neonatal strains, rhesus and bovine strain variants) as well as parenteral vaccines could also potentially fulfill the promise of vaccines that are more affordable. Whether these candidate vaccines perform better in poor settings remains to be seen.70,79,80
In both developing and developed countries, some proportion (30–40%) of severe rotavirus disease occurs between 12–36 months of age.10 Thus, for vaccines to have a substantial public health impact, they should ideally provide protection against severe rotavirus disease through 2–3 years of life. Our review indicates that in industrialized countries the second generation vaccines provide sustained protection against severe rotavirus disease through these important early years. However, clinical trial data on duration of protection in developing countries are yet to be published. The postlicensure evidence indicates a decrease in efficacy may very well be a possibility. The suggestion has been made that acquisition of immunity from natural infection may potentially explain decreasing efficacy with age. Two explanations argue against this theory. First, in a randomly vaccinated population, no biological reason exists to suspect a different rate of exposure (and acquisition of immunity) to natural infection between susceptible vaccinated and unvaccinated groups. Thus, incidence of rotavirus disease by increasing age should be similar between susceptible unvaccinated children and those who are vaccinated but do not mount immunity (i.e., susceptible vaccine failures). In absence of waning immunity, the reduction in disease incidence attributable to vaccination should be stable with age. Vaccine efficacy is computed on the basis of difference in disease incidence between unvaccinated and vaccinated, thus making it unlikely that efficacy estimates would decrease with age. Second, we would have observed reductions in efficacy during the second and third season after vaccination in trials from industrialized countries if, hypothetically, vaccine failures were less likely to mount immunity from natural infection compared with unvaccinated persons. Because immunity was sustained in these trials from industrialized countries for 3 years but diminished some in developing countries after 1 year suggests that the observed reduction in efficacy with age is likely to be related to waning of immunity.
In cohort studies following children with natural infection, although recurrent episodes of severe disease are unlikely, repeat infections do occur and suggests that natural protection may be short-lived or incomplete.81–84 This important observation and the finding that repeated infections are more likely to induce a heterotypic response have provided support for use of two or more doses of an attenuated vaccine.16,17 Clinical protection after rotavirus infection likely involves mucosal (intestinal) and systemic antibody responses as well as the cell-mediated immune system. Duration of intestinal immunity is shorter than systemic because intestinal antibodies decay at a rate faster than serum antibody titer decreases.14 Moreover, in developing countries, IgA titers are significantly lower than titers in industrialized countries.71 Although the immune correlates of protection from rotavirus infection and disease are not fully understood, taken together with the evidence that intestinal immunity is an important mechanism of protection,14 the lower systemic immune response in poor regions might provide one possible explanation for the shorter duration of protection in developing countries. The incomplete understanding of the mechanisms and duration of protection against rotavirus infection warrant further research so that effectiveness of efforts to reduce rotavirus illness can be maximized and development of other potential vaccine strategies can be realized.
We tried to minimize sources of heterogeneity related to differences in vaccine type and outcome measure in the different vaccine trials. This allowed us to better determine if inherent differences between children as well as the environmental conditions in industrialized and developing countries contribute to variation in vaccine performance between the two populations. It is certain that a gradient of poverty exists between countries we classified as “developing.” This gradient would also likely reflect in the efficacy estimates. Although a finer grouping of country classification would be optimum, the limited efficacy data by region did not allow us to further differentiate the developing countries. Such a distinction would be particularly useful for the large RV1 clinical trial from 10 Latin American countries.4 Of the 15,183 infants from 10 Latin American countries, 60% were from upper-middle income countries (Mexico, Panama, Venezuela, Brazil, Argentina and Chile) and 29% were low-middle income (Honduras, Colombia, and Dominican Republic) and 11% from a low income country (Nicaragua). The authors of this study presented pooled estimates from all 10 countries, and a breakdown of these efficacy estimates by country has not been published. Because a majority of the participants were from upper-middle income countries, we included this study in the industrialized region, as we surmised that the estimates are more likely to reflect the higher socioeconomic group rather than lowest socioeconomic group represented by the single country of Nicaragua. We suspect that a gradient of efficacy likely exists between countries in this region which would be useful to know for fully understanding the reasons for the location-dependent variation in protection.
In summary, substantial advancements towards the prevention of rotavirus disease have occurred in the three decades since the initial discovery of rotavirus. Through persistent research and advocacy, rotavirus is now accepted as a leading preventable cause of childhood morbidity and mortality worldwide. The path to vaccine development and implementation has been tortuous, with a major setback in 1999 due to an unexpected association between the highly effective RRV-TV vaccine and intussusception. However, an energetic research agenda and global public-private collaboration led to further development and testing of novel and safe strategies for rotavirus disease prevention. The current rotavirus vaccines have proven to be effective in a variety of developed and developing country settings, and have received a global recommendation for introduction by WHO. Even though vaccines have demonstrated somewhat lower efficacy and effectiveness in low income settings, they are still likely to have a substantial public health impact because of the higher background rates of severe rotavirus disease in these populations. Room for improvement in efficacy exists in the less affluent settings, and thus, to optimize the full life-saving potential of these vaccines, future efforts should focus on ways to improve immune response in challenging populations, and ensuring that demand and availability of vaccines is sufficient to reach those at the greatest risk of rotavirus disease.
Materials and Methods
Our review was restricted to clinical trials of vaccine efficacy against rotavirus disease and studies of postlicensure vaccine effectiveness.
To minimize differences in vaccine type, we categorized the current and previous candidate vaccines in two groups, as described above (Table 1). Uniformity in outcome measure is also an important consideration when assessing potential differences in vaccine performance by setting. Rotavirus vaccines are more efficient in preventing severe rotavirus disease than milder disease.18,19 Thus, to minimize variation in protection observed in clinical trials as a result of differences in outcome measured (i.e., severity of rotavirus disease), we restricted our analysis to efficacy against ‘severe rotavirus disease.’ To the extent possible, we report efficacy estimates against severe rotavirus disease, as defined by severity score ≥11 on 20-point Vesikari scale or overnight admission to the hospital. The Vesikari scale is a 20-point scoring system that rates severity of rotavirus diarrhea on the basis of clinical symptoms and treatment.85 When these outcome measures were not applied, we report efficacy estimates and the clinical parameter of severity presented by the authors.
Because our primary outcome of interest was to determine efficacy estimates of rotavirus vaccine in poor versus industrialized settings, we stratified data in two geographical regions on basis of World Bank data on the per capita income for the country where the study was conducted86—countries with high and high-middle income economies were considered industrialized, and those with low- and low-middle income economies were considered developing. Studies from the US Native American Reservations were grouped in the low-income category because of the low-socioeconomic, rural nature of the population.19 For each group of vaccines, we calculated region specific pooled estimates and 95% confidence intervals (CI) for the vaccine efficacy parameters using random effects models, or fixed effects when appropriate (StatsDirect version 2.5.7, StatsDirect Ltd., Cheshire, England). For each of the randomized control trials, the relative risk of disease and 95% confidence intervals (CI) among vaccinated and unvaccinated study participants was calculated. To obtain region specific estimates of vaccine efficacy, estimates of risk ratios were pooled from all studies by vaccine type (first versus second generation) and region (developing versus industrialized). Heterogeneity was reduced by restricting pooling to vaccine type and region, and using severe disease as outcome of interest. To address additional variation across studies, the random effects model was applied using the DerSimonian and Laird method to estimate the pooled risk ratio for each strata of interest. For the postlicensure case-control studies of the second-generation vaccines, the odds ratio of vaccination between cases and controls and 95% CI for each study by region were used to estimate a pooled estimate using a fixed effects model. Stratum weights were calculated as the inverse of the variance and subsequently summed to calculate a pooled estimate using a weighted mean.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/11278
References
- 1.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200:9–15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 2.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerging infectious diseases. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerging infectious diseases. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 6.Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 7.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 8.WHO, author. Meeting of the immunization Strategic Advisory Group of Experts, April 2009—conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:220–236. [PubMed] [Google Scholar]
- 9.WHO, author. Rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285–295. [PubMed] [Google Scholar]
- 10.Patel MM, Parashar UD. Assessing the effectiveness and public health impact of rotavirus vaccines after introduction in immunization programs. J Infect Dis. 2009;200:291–299. doi: 10.1086/605059. [DOI] [PubMed] [Google Scholar]
- 11.Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. Lancet. 1974;1:149–151. doi: 10.1016/s0140-6736(74)92440-4. [DOI] [PubMed] [Google Scholar]
- 12.Estes MK, Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989;53:410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192:146–159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 14.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 15.Ward R. Mechanisms of protection against rotavirus infection and disease. Pediatr Infect Dis J. 2009;28:57–59. doi: 10.1097/INF.0b013e3181967c16. [DOI] [PubMed] [Google Scholar]
- 16.Green KY, Taniguchi K, Mackow ER, Kapikian AZ. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccinees: implications for vaccine development. J Infect Dis. 1990;161:667–679. doi: 10.1093/infdis/161.4.667. [DOI] [PubMed] [Google Scholar]
- 17.Rojas AM, Boher Y, Guntinas MJ, Perez-Schael I. Homotypic immune response to primary infection with rotavirus serotype G1. J Med Virol. 1995;47:404–409. doi: 10.1002/jmv.1890470418. [DOI] [PubMed] [Google Scholar]
- 18.Bresee JS, Glass RI, Ivanoff B, Gentsch JR. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine. 1999;17:2207–2222. doi: 10.1016/s0264-410x(98)00376-4. [DOI] [PubMed] [Google Scholar]
- 19.Bresee JS, Parashar UD, Widdowson MA, Gentsch JR, Steele AD, Glass RI. Update on rotavirus vaccines. Pediatr Infect Dis J. 2005;24:947–952. doi: 10.1097/01.inf.0000186295.18969.e6. [DOI] [PubMed] [Google Scholar]
- 20.Vesikari T, Isolauri E, D'Hondt E, Delem A, Andre FE, Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;1:977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- 21.Vesikari T, Isolauri E, Delem A, d'Hondt E, Andre FE, Beards GM, et al. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985;107:189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- 22.Ruuska T, Vesikari T, Delem A, Andre FE, Beards GM, Flewett TH. Evaluation of RIT 4237 bovine rotavirus vaccine in newborn infants: correlation of vaccine efficacy to season of birth in relation to rotavirus epidemic period. Scand J Infect Dis. 1990;22:269–278. doi: 10.3109/00365549009027047. [DOI] [PubMed] [Google Scholar]
- 23.Vesikari T, Ruuska T, Delem A, Andre FE, Beards GM, Flewett TH. Efficacy of two doses of RIT 4237 bovine rotavirus vaccine for prevention of rotavirus diarrhoea. Acta Paediatr Scand. 1991;80:173–180. doi: 10.1111/j.1651-2227.1991.tb11830.x. [DOI] [PubMed] [Google Scholar]
- 24.Treanor JJ, Clark HF, Pichichero M, Christy C, Gouvea V, Shrager D, et al. Evaluation of the protective efficacy of a serotype 1 bovine-human rotavirus reassortant vaccine in infants. Pediatr Infect Dis J. 1995;14:301–307. doi: 10.1097/00006454-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Christy C, Madore HP, Pichichero ME, Gala C, Pincus P, Vosefski D, et al. Field trial of rhesus rotavirus vaccine in infants. Pediatr Infect Dis J. 1988;7:645–650. doi: 10.1097/00006454-198809000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Vesikari T, Rautanen T, Varis T, Beards GM, Kapikian AZ. Rhesus Rotavirus candidate vaccine. Clinical trial in children vaccinated between 2 and 5 months of age. Am J Dis Child. 1990;144:285–289. [PubMed] [Google Scholar]
- 27.Perez-Schael I, Garcia D, Gonzalez M, Gonzalez R, Daoud N, Perez M, et al. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. J Med Virol. 1990;30:219–229. doi: 10.1002/jmv.1890300315. [DOI] [PubMed] [Google Scholar]
- 28.De Mol P, Zissis G, Butzler JP, Mutwewingabo A, Andre FE. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986;2:108–108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- 29.Hanlon P, Hanlon L, Marsh V, Byass P, Shenton F, Hassan-King M, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1:1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 30.Lanata CF, Black RE, del Aguila R, Gil A, Verastegui H, Gerna G, et al. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989;159:452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- 31.Santosham M, Letson GW, Wolff M, Reid R, Gahagan S, Adams R, et al. A field study of the safety and efficacy of two candidate rotavirus vaccines in a Native American population. J Infect Dis. 1991;163:483–487. doi: 10.1093/infdis/163.3.483. [DOI] [PubMed] [Google Scholar]
- 32.Lanata CF, Black RE, Flores J, Lazo F, Butron B, Linares A, et al. Immunogenicity, safety and protective efficacy of one dose of the rhesus rotavirus vaccine and serotype 1 and 2 human-rhesus rotavirus reassortants in children from Lima, Peru. Vaccine. 1996;14:237–243. doi: 10.1016/0264-410x(95)00132-k. [DOI] [PubMed] [Google Scholar]
- 33.Georges-Courbot MC, Monges J, Siopathis MR, Roungou JB, Gresenguet G, Bellec L, et al. Evaluation of the efficacy of a low-passage bovine rotavirus (strain WC3) vaccine in children in Central Africa. Res Virol. 1991;142:405–411. doi: 10.1016/0923-2516(91)90008-q. [DOI] [PubMed] [Google Scholar]
- 34.Midthun K, Kapikian AZ. Rotavirus vaccines: an overview. Clin Microbiol Rev. 1996;9:423–434. doi: 10.1128/cmr.9.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuker G, Oshiro LS, Schmidt NJ. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J Clin Microbiol. 1980;11:202–203. doi: 10.1128/jcm.11.2.202-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ukae S, Nakata S, Adachi N, Kogawa K, Chiba S. Efficacy of rhesus rotavirus vaccine MMU-18006 against gastroenteritis due to serotype 1 rotavirus. Vaccine. 1994;12:933–939. doi: 10.1016/0264-410x(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 37.Clark HF, Offit PA, Ellis RW, Eiden JJ, Krah D, Shaw AR, et al. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. J Infect Dis. 1996;174:73–80. doi: 10.1093/infdis/174.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein DI, Glass RI, Rodgers G, Davidson BL, Sack DA US Rotavirus Vaccine Efficacy Group, author. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. Jama. 1995;273:1191–1196. [PubMed] [Google Scholar]
- 39.Rennels MB, Glass RI, Dennehy PH, Bernstein DI, Pichichero ME, Zito ET, et al. United States Rotavirus Vaccine Efficacy Group, author. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines—report of the National Multicenter Trial. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 40.Joensuu J, Koskenniemi E, Pang XL, Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–1209. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 41.Linhares AC, Gabbay YB, Mascarenhas JD, de Freitas RB, Oliveira CS, Bellesi N, et al. Immunogenicity, safety and efficacy of tetravalent rhesus-human, reassortant rotavirus vaccine in Belem, Brazil. Bull World Health Organ. 1996;74:491–500. [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Schael I, Guntinas MJ, Perez M, Pagone V, Rojas AM, Gonzalez R, et al. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N Engl J Med. 1997;337:1181–1187. doi: 10.1056/NEJM199710233371701. [DOI] [PubMed] [Google Scholar]
- 43.Vesikari T, Karvonen A, Puustinen L, Zeng SQ, Szakal ED, Delem A, et al. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J. 2004;23:937–943. doi: 10.1097/01.inf.0000141722.10130.50. [DOI] [PubMed] [Google Scholar]
- 44.Salinas B, Perez Schael I, Linhares AC, Ruiz Palacios GM, Guerrero ML, Yarzabal JP, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J. 2005;24:807–816. doi: 10.1097/01.inf.0000178294.13954.a1. [DOI] [PubMed] [Google Scholar]
- 45.Araujo EC, Clemens SA, Oliveira CS, Justino MC, Rubio P, Gabbay YB, et al. Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy infants. Jornal de pediatria. 2007;83:217–224. doi: 10.2223/JPED.1600. [DOI] [PubMed] [Google Scholar]
- 46.Clark HF, Bernstein DI, Dennehy PH, Offit P, Pichichero M, Treanor J, et al. Safety, efficacy and immunogenicity of a live, quadrivalent human-bovine reassortant rotavirus vaccine in healthy infants. J Pediatr. 2004;144:184–190. doi: 10.1016/j.jpeds.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 47.Vesikari T, Itzler R, Matson DO, Santosham M, Christie CD, Coia M, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries) Int J Infect Dis. 2007;11:29–35. doi: 10.1016/S1201-9712(07)60019-8. [DOI] [PubMed] [Google Scholar]
- 48.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J. Med;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 49.Santosham M, Moulton LH, Reid R, Croll J, Weatherholt R, Ward R, et al. Efficacy and safety of high-dose rhesus-human reassortant rotavirus vaccine in Native American populations. J Pediatr. 1997;131:632–638. doi: 10.1016/s0022-3476(97)70076-3. [DOI] [PubMed] [Google Scholar]
- 50.Lanata CF, Midthun K, Black RE, Butron B, Huapaya A, Penny ME, et al. Safety, immunogenicity and protective efficacy of one and three doses of the tetravalent rhesus rotavirus vaccine in infants in Lima, Peru. J Infect Dis. 1996;174:268–275. doi: 10.1093/infdis/174.2.268. [DOI] [PubMed] [Google Scholar]
- 51.Linhares AC, Lanata CF, Hausdorff WP, Gabbay YB, Black RE. Reappraisal of the Peruvian and Brazilian lower titer tetravalent rhesus-human reassortant rotavirus vaccine efficacy trials: analysis by severity of diarrhea. Pediatr Infect Dis J. 1999;18:1001–1006. doi: 10.1097/00006454-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 53.Patel MM, Haber P, Baggs J, Zuber P, Bines JE, Parashar UD. Intussusception and rotavirus vaccination: a review of the available evidence. Expert review of vaccines. 2009;8:1555–1564. doi: 10.1586/erv.09.106. [DOI] [PubMed] [Google Scholar]
- 54.WHO, author. Meeting of the Strategic Advisory Group of Experts on immunization, October 2009—conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:518–518. [PubMed] [Google Scholar]
- 55.Macartney KK, Burgess MA. Rapid impact of rotavirus vaccination in the United States: implications for Australia. Med J Aust. 2009;191:131–132. doi: 10.5694/j.1326-5377.2009.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 56.Orozco M, Vasquez J, Pedreira C, De Oliveira LH, Amador JJ, Malespin O, et al. Uptake of rotavirus vaccine and national trends of acute gastroenteritis among children in Nicaragua. J Infect Dis. 2009;200:125–130. doi: 10.1086/605053. [DOI] [PubMed] [Google Scholar]
- 57.Tate JE, Panozzo CA, Payne DC, Patel MM, Cortese MM, Fowlkes AL, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124:465–471. doi: 10.1542/peds.2008-3528. [DOI] [PubMed] [Google Scholar]
- 58.Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein DI, Ward RL. Rotarix: development of a live attenuated monovalent human rotavirus vaccine. Pediatr Ann. 2006;35:38–43. doi: 10.3928/0090-4481-20060101-12. [DOI] [PubMed] [Google Scholar]
- 60.Cunliffe NA, Kirsten M, Madhi S, et al. Efficacy of human rotavirus vaccine RIx4414 in Africa during the first year of life; Presented at the 26th meeting of the European Society of Pediatric Infectious Diseases; June 9–13, 2009; Brussels, Belgium. 2009. [Google Scholar]
- 61.de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert review of vaccines. 2008;7:345–353. doi: 10.1586/14760584.7.3.345. [DOI] [PubMed] [Google Scholar]
- 62.Nakagomi T, Patel M, Nakagomi O, Cunliffe NA, Parashar U, et al. Effectiveness of a monovalent G1P[8] human rotavirus vaccine against severe diarrhea caused by heterotypci G2P[4] rotavirus strains in Recife, Brazil; Presented at the 26th meeting of the European Society of Pediatric Infectious Diseases; June 9–13, 2009; Brussels, Belgium. 2009. [Google Scholar]
- 63.Snelling TL, Andrews RM, Kirkwood CD, Carapetis JR. Evaluation of the Monovalent Human Rotavirus Vaccine RIX4414 following a G2P[4] Outbreak; Presented at the 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); San Francisco CA, USA. 2009. [Google Scholar]
- 64.Snelling TL, Schultz R, Graham J, Roseby R, Barnes GL, Andrews RM, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis. 2009;49:428–431. doi: 10.1086/600395. [DOI] [PubMed] [Google Scholar]
- 65.WHO, author. Generic protocol for monitoring impact of rotavirus vaccination on rotavirus disease burden and viral strains. Document WHO/IVB/0816 Geneva. 2009:1–73. [Google Scholar]
- 66.Vesikari T, Karvonen A, Korhonen T, Espo M, Lebacq E, Forster J, et al. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine. 2004;22:2836–2842. doi: 10.1016/j.vaccine.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 67.Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Human rotavirus vaccine RIX4414 is highly efficacious in Asian infants during the third year of life; Presented at the 26th meeting of the European Society of Pediatric Infectious Diseases, P608; June 9–13, 2009; Brussels, Belgium. 2009. [Google Scholar]
- 68.Vesikari T, Karvonen A, Allen S, Lawrence J, Ciarlet M. Serotype-specific efficacy of the pentavalent rotavirus vaccine against hospitalizations and emergency department visits up to three years [abstract]; Presented at: the 48th ICAAC/46th IDSA Annual Meeting; October 25–28, 2008; Washington DC. [Google Scholar]
- 69.Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–332. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 70.Glass RI, Bresee JS, Turcios R, Fischer TK, Parashar UD, Steele AD. Rotavirus vaccines: targeting the developing world. J Infect Dis. 2005;192:160–166. doi: 10.1086/431504. [DOI] [PubMed] [Google Scholar]
- 71.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200:39–48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danchin MH, Bines JE. Defeating rotavirus? The global recommendation for rotavirus vaccination. N Engl J Med. 2009;361:1919–1921. doi: 10.1056/NEJMp0905091. [DOI] [PubMed] [Google Scholar]
- 73.Gotuzzo E, Butron B, Seas C, Penny M, Ruiz R, Losonsky G, et al. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD 103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun. 1993;61:3994–3997. doi: 10.1128/iai.61.9.3994-3997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hallander HO, Paniagua M, Espinoza F, Askelof P, Corrales E, Ringman M, et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine. 2002;21:138–145. doi: 10.1016/s0264-410x(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 75.Lagos R, Fasano A, Wasserman SS, Prado V, San Martin O, Abrego P, et al. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J Infect Dis. 1999;180:1709–1712. doi: 10.1086/315051. [DOI] [PubMed] [Google Scholar]
- 76.Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, Manna B, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med. 2009;361:335–344. doi: 10.1056/NEJMoa0807521. [DOI] [PubMed] [Google Scholar]
- 77.Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, et al. Mucosal Immunity after Vaccination with Monovalent and Trivalent Oral Poliovirus Vaccine in India. J Infect Dis. 2009 doi: 10.1086/605330. [DOI] [PubMed] [Google Scholar]
- 78.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 79.Jiang B, Gentsch JR, Glass RI. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine. 2008;26:6754–6758. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Kapikian AZ, Simonsen L, Vesikari T, Hoshino Y, Morens DM, Chanock RM, et al. A hexavalent human rotavirus-bovine rotavirus (UK) reassortant vaccine designed for use in developing countries and delivered in a schedule with the potential to eliminate the risk of intussusception. J Infect Dis. 2005;192:22–29. doi: 10.1086/431510. [DOI] [PubMed] [Google Scholar]
- 81.Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282–287. doi: 10.1093/infdis/168.2.282. [DOI] [PubMed] [Google Scholar]
- 82.Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983;309:72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- 83.Chiba S, Yokoyama T, Nakata S, Morita Y, Urasawa T, Taniguchi K, et al. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986;2:417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- 84.Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 85.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 86. [2010]. Available at http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0,,contentMDK: 20420458∼menuPK:64133156∼pagePK:64133150∼piPK:64133175∼theSitePK:239419,00.html.
- 87.Boom J, Tate JE, Sahni L, Rench M, Patel M, Baker C, et al. Effectiveness of Pentavalent Rotavirus Vaccine in a Large Urban Population in the United States. Pediatrics. In press January 2009. [DOI] [PubMed]
- 88.Payne DC, Staat MA, Szilagyi PG, Edwards K, Gentsch J, Weinberg GA, et al. Decline in rotavirus hospitalizations in 3 U.S. counties after introduction of rotavirus vaccine; Paper presented at: Pediatric Academic Societies Conference; May 2–5, 2009; Baltimore, MD. 2009. [Google Scholar]
- 89.WHO, author. Report of the meeting on future direction for rotavirus vaccine research in developing countries. Geneva: World Health Organization. Document WHO/V & B/00.23. 2000:1–56. [Google Scholar]