Abstract
Hepatic stellate cells (HSCs) undergo myofibroblastic trans-differentiation (activation) to participate in liver fibrosis, and identification of molecular targets for this cell fate regulation is essential for development of efficacious therapeutic modalities for the disease. Peroxisomal proliferator-activated receptor γ (PPARγ) is required for differentiation of HSCs and its epigenetic repression underlies HSC activation. The herbal prescription Yang-Gan-Wan (YGW) prevents liver fibrosis, but its active ingredients and molecular mechanisms are unknown. Here we demonstrate YGW prevents and reverses HSC activation via epigenetic de-repression of Pparγ involving reductions in MeCP2 expression and its recruitment to Pparγ promoter, suppressed expression of PRC2 methyltrasferase EZH2 and consequent reduction of H2K27di-methylation at the 3’ exon. HPLC/MS and NMR analyses identify polyphenolic rosmarinic acid (RA) and baicalin (BC) as active phytocompounds. RA and BC suppress the expression and signaling by canonical Wnts, which are implicated in the aforementioned Pparγ epigenetic repression. RA treatment in mice with existing cholestatic liver fibrosis inhibits HSC activation and progression of liver fibrosis. In conclusion, these results demonstrate a therapeutic potential of YGW and its active component RA and BC for liver fibrosis via Pparγ de-repression mediated by suppression of canonical Wnt signaling in HSCs.
Keywords: liver fibrosis, MeCP2, EZH2, Wnt, H3K27-methylation
INTRODUCTION
Excessive scarring of the liver results in cirrhosis, the end-stage liver disease of high mortality for which efficacious medical treatments are not currently available except for liver transplantation. Central to the pathogenesis of the disease is trans-differentiation or activation of hepatic stellate cells (HSCs), vitamin-A storing liver pericytes, into myofibroblastic cells with increased capacity for extracellular matrix (ECM) production and contractility. For better understanding of HSC trans-differentiation, primary efforts have been made on gene regulation and intracellular signaling for expression of activation-associated molecules such as collagens, cytokines (TGF-β, PDGF), chemokines (MCP-1), ECM degradation enzymes and inhibitors (MMPs, TIMPs), NADPH oxidase, renin-angiotensin system, and TLR4 ((1),(2) for review). Yet, fundamental questions concerning cell fate regulation of HSCs, remain largely underexplored. HSCs express many neuronal or glial cell markers, and their neuroectoderm origin was proposed with a subsequent failure to validate this notion using the Wnt1-Cre and ROSA26 reporter mice (3). This finding logically favored a hypothesis of mesoderm-derived multipotent mesenchymal progenitor cells (MMPC) as the origin of HSCs since MMPC also give rise to neural cells besides other mesenchymal lineages for smooth muscle cells, chondrocytes, osteoblasts, and adipocytes whose markers are also expressed by HSCs (4). In consistent with this notion, a recent study by Asahina, et al demonstrates the mesoderm origin of mouse fetal HSCs (5).
A fat-storing phenotype is a unique and distinct feature of quiescent HSCs, and our laboratory proposed a decade ago that there is a regulatory commonality between adipocytes and quiescent HSCs (6). Germane to this proposal is the expression and regulation by the master adipogenic transcription factor PPARγ, which is essential for both adipocyte differentiation (7) and HSC quiescence (8, 9). PPARγ promotes storage of intracellular fat including retinyl esters in HSCs (7) while suppressing α1(I) collagen promoter via inhibition of p300-facilitated NF-I binding (10). As shown for inhibition of adipogenesis, canonical Wnt signaling suppresses the expression and promoter activation of Pparγ in HSC trans-differentiation (11). Necdin, a member of the melanoma antigen family (MAGE) of proteins, inhibits differentiation of adipocytes (12) but promotes that of neurons (13), skeletal and smooth muscle cells (14, 15). Our recent study demonstrates Wnt10b, one of canonical Wnts expressed by activated HSCs, is a direct target of necdin and the necdin-Wnt pathway causes HSC trans-differentiation via epigenetic repression of Pparγ (16). This epigenetic regulation involves induction and recruitment of the methyl-CpG binding protein MeCP2 to the Pparγ promoter and concomitant H3K27 di- and tri-methylation in the 3’ exons of Pparγ, resulting in formation of a repressive chromatin structure as recently demonstrated by Mann, et al (17). Intriguingly, this study also demonstrates MeCP2-mediated induction of EZH2, a H3K27 methyltransferase of the polycomb repressive complex 2 (PRC2), responsible for H3K27 di- and tri-methylation (17). Most recently, this paradigm of the MeCP2-EZH2 regulatory relay has elegantly been characterized in neuronal differentiation where MeCP2-mediated epigenetic repression of miR137 is shown to result in EZH2 induction (18).
This epigenetic mechanism of Pparγ repression involving the MeCP2-EZH2 relay, identifies potential new therapeutic targets for liver fibrosis. To this end, the present study discovers that the herbal prescription Yang-Gan-Wan (YGW) which has been known for its protective effects on the liver (19), targets and abrogates the MeCP2–EZH2 relay of epigenetic Pparγ repression to reverse activated HSCs to their quiescent phenotype in culture. Our HPLC-MS and NMR analyses coupled with bioassays with primary HSCs, identify rosmarinic acid (RA) and baicalin (BC), the active component of Sho-Saiko-To, as the main active phytocompounds of YGW. RA and BC achieve the anti-fibrotic effect by supression of canonical Wnt signaling and epigenetic Pparγ de-repression.
MATERIALS AND METHODS
Animal Experiment
Male C57Bl/6 and collagen α1(I) promoter-GFP (Coll-GFP; kindly provided by Prof. David Brenner of UC San Diego) mice were subjected to ligation and scission of the common bile duct (BDL) to induce cholestatic liver fibrosis for HSC isolation or testing the therapeutic efficacy of RA. For the latter, after one week following BDL, RA was intraperitoneally injected daily at the dose of 0.1mg/25g body weight until the animals were sacrificed one week later for Sirius-red staining morphometry, immunohistochemistry, and qPCR analysis of the livers as described below.
Hepatic Stellate Cell Isolation and Culture
HSCs were isolated from normal male Wistar rats, C57Bl/6 and Coll-GFP mice by in situ digestion of the liver and arabinogalactan gradient ultracentrifugation by the Non-Parenchymal Liver Cell Core of the Southern California Research Center for ALPD and Cirrhosis as described previously (11, 16). The purity of the cells as determined by phase contrast microscopy and ultraviolet-excited fluorescence microscopy, exceeded 96%, and the viability as determined by trypan blue exclusion exceeded 94%. In vitro activation of HSC was achieved by culturing rat HSCs in Dulbecco’s modified Eagle’s medium (DMEM) with 1.0 g/liter glucose, 10% fetal bovine serum and 1% antibiotics on plastic dish for 3, 5 or 7 days. Culture-activated rat primary HSCs were treated with the YGW or starch (control) aqueous extract at 25% (v/v). To obtain the extract, the YGW or starch powder (provided by S.P. Pharmaceutics Inc.) was suspended in DMEM at the concentration of 35mg/ml, mixed thoroughly with a vortex for 5 min, and centrifuged at ×150g for 30 min to collect the supernatant. This supernatant was designated as 100% extract and used after filter-sterilization. RA and BC (Sigma Chemical Co) were dissolved in DMSO and tested at the concentration of 67.5~270 µM.
Fluorescence-Activated Cell Sorting (FACS)
Two weeks after BDL or sham operation, nonparenchymal cells (NPCs) were isolated from the Coll-GFP mice and subjected to FACS using FACS AriaII sorter (BD Bioscience) at the USC-CSCRM/NCCC Flow Cytometry Core. GFP expression was analyzed by an argon laser at 488 nm and a 530 nm filter. Vitamin A autofluorescence was analyzed by a solid-state laser at 350 nm and a 450 nm filter. As a negative control for vitamin A autofluorescence, we used the spontaneously immortalized rat HSC line (BSC) established from cholestatic liver fibrosis in rats (20).
Immunohistochemistry, TUNEL and Lipid Staining
After 3 days of the extract treatment, the cells were washed with cold phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PF). To stain α-smooth muscle actin (SMA), a fluorescein isothiocyanate (FITC) conjugated antibody (1:200, Sigma, Saint Louis, MO) was added as a primary antibody at 4°C for overnight. After washing and blocking with 5% nonfat milk, fluorescence images were viewed by a Nikon microscope as described above. For intracellular lipid staining, HSCs treated with the extract for 3 days, were cultured with retinol (5µM) and palmitic acid (100µM) (Sigma, Saint Louis, MO) for 48 hr, and fixed with 10% formalin in PBS. Oil Red O (0.5%w/v in isopropanol) was diluted with 67% volume of water, filtered, and added to the fixed HSCs. Apoptosis was detected in cultured HSCs and liver sections from BDL mice using a Cell Death Detection kit from Roche. For liver section immunostaining, liver tissues were fixed with 4% PF and embedded in freezing medium. Cryosections (7 µm) were washed with PBS, digested with 20 µg/ml proteinase K (Invitrogen, Carlsbad, CA), and blocked with 5% goat serum and 0.2% bovine serum albumin. The sections were then incubated with mouse anti-SMA antibody conjugated with FITC (Sigma, 1:400) and rabbit anti-desmin antibody (Thermo Scientific, Rockford, IL, 1:400). After washing, the sections were incubated with goat anti-rabbit antibody conjugated with AlexaFluor 568 (Invitrogen, 1:400) and mouse anti-FITC antibody conjugated with DyLight 488 (Jackson ImmunoResearch, West Grove, PA, 1:400). The sections were mounted with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) and fluorescence images were visualized under a microscope. To quantify the percentage and density of HSCs in the liver after BDL with or without treatment of RA, 6 images were randomly captured using a 10× objective lens in 3 different sections and SMA+ and desmin+ HSCs in the parenchyma were counted.
Real Time Quantitative PCR
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen) or RNeasy Mini kit (Qiagen). One microgram of RNA was reverse transcribed to cDNA by using SuperScript III First-Strand Synthesis System (Invitrogen) and amplified by 40 cycles using primers listed below and the SYBR Green PCR Master mix reagent (AB Applied Biosystem). Each threshold cycle (Ct) value was first normalized to the 36B4 Ct value of a sample and subsequently compared between the treatment and control samples. Primer sequences used are shown in Supplemental Information : Pparγ, CCT GAA GCT CCA AGA ATA CCA AA; and 5’-AGA GTT GGG TTT TTT CAG AAT AAT AAGG; α1(I)Coll, 5’-TCG ATT CAC CTA CAG CAC GC and 5’- GAC TGT CTT GCC CCA AGT TCC; 36B4, 5’- TTC CCA CTG GCT GAA AAG GT and 5’- CGC AGC CGC AAA TGC; Ezh2, 5’-AGT GGA GTG GTG CTG AAG and 5’-GCC GTC CTT TTT CAG TTG; Tgfβ1, 5’-AGA AGT CAC CCG CGT GCTA and 5’-TGT GTG ATG TCT TTG GTT TTG TCA; Suz12, 5’-GTG AAG AAG CCG AAA ATG and 5’-AAT GTT TTC CTT TTG ATG; Eed, 5’-ATC CTA TAA CAA TGC AGT and 5’-TTC ATC TCT GTG CCC TTC; α-Sma, 5’-TGT GCT GGA CTC TGG AGA TG and 5’-GAT CAC CTG CCC ATC AGG; Wnt10b, 5’-CGA GAA TGC GGA TCC ACAA and 5’-CCG CTT CAG GTT TTC CGTTA; Wnt3a, 5’-CAT CGC CAG TCA CAT GCA CCT and 5’-CGT CTA TGC CAT GCG AGC TCA; Desmin, 5’-CAG GAC CTG CTC AAT GTG and 5’-GTA GCC TCG CTG CTG ACA ACC TC; Gapdh, 5’-CTG CCC GTA GAC AAA ATG GT and 5’-GAA TTT GCC GTG AGT GGA GT; Sma, 5’-CTG AGC GTG GCT ATT CCT TC and 5’-CCT CTG CAT CCT GTC AGC AA; Timp1,5’-CAG TAA GGC CTG TAG CTG TGC and 5’-CTC GTT GAT TTC GG GGA AC.
Transfection and reporter assay and IKK assay
TCF promoter-luciferase construct TOPFLASH (a gift from Dr. Randall Moon, Univ. of Washington, Seattle, WA) or a κB-luciferase construct was used for transient transfection in the rat primary HSCs by electroporation using the Neon™ Transfection System (Invitrogen). The Renilla pRL-TK construct was used for standardization for transfection efficiency. Cell lysates were analyzed by the dual luciferase assay (Promega) on a luminometer. To assess the activity of IKK, IKK was immunoprecipitated by IKKα antibody and protein G-Sepharose, and the assay was performed at 30°C for 1hr in buffer containing 20 mM Tris HCl, pH 7.5, 20 mM MgCl2, 2mM dithiothreitol, 20 µM ATP, 2 µg GST-IκBα, and [γ-32P]ATP. The reaction was stopped by addition of Laemmi buffer and was resolved by 10% SDS-PAGE followed by a transfer onto a membrane for imaging.
Immunoblot Analysis
Whole cell extracts were prepared as previously described (8). Equal amount of the extract (20µg) was separated by 8–15% SDS-PAGE and the proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). MeCP2, type I collagen, and β-actin were detected by incubating with rabbit polyclonal anti-MeCP2 (1:1000) (Abcam), anti-type I collagen (1:4000), and anti-β-actin (1:5000) primary antibodies (Santa Cruz Biotechnology) in TBS (100 mM Tris-HCl, 1.5 M NaCl, pH 7.4) with 5% non fat milk overnight at 4°C followed by incubation with horseradish peroxidase conjugated goat anti-rabbit secondary antibodies (1:4000) (Sigma) at room temperature for 2 hr. The antigen-antibody complexes’ chemiluminescence was detected by using the ECL detection kit (Pierce).
Chromatin immunoprecipitation (ChIP)
For assessing Pparγ epigenetic regulation, carrier ChIP was performed using Raji cells as the source of carrier chromatin. For native ChIP, 20µg of HSC chromatin was mixed with 80µg of Raji cell chromatin. For cross-link ChIP, Raji cells (1.4 × 107 cells) were mixed to HSCs (0.2 × 106 cells) and fixed with 1% formaldehyde on the rotating platform for 5–10 minutes at room temperature followed by addition of glycine to a final concentration of 0.125M. After lysis of the cells with SDS buffer (1% SDS, 10mM EDTA, 50mM Tris- HCl pH8.1) with protease inhibitors, the lysates were sonicated and snap frozen in aliquots. For chromatin IP, diluted samples were first pre-cleared using protein G-agarose beads and then incubated with antibody against Ser2P RNApolyII, MeCP2, H3K27me2, H3K4me2 and H3Kacetylated (Abcam) at 1 µg/µl at 4°C for overnight followed by precipitation with protein G-agarose beads. After elution of immunoprecipitated complex, crosslinking was reversed with 5N NaCl and proteins digested with protease K. Extracted chromatin was subjected to real-time PCR using the primers flanking a segment within Pparγ promoter or exon as previously described (17). Ct values of the samples with non-immune IgG were subtracted and compared to their respective input Ct values.
Active compounds isolation and identification
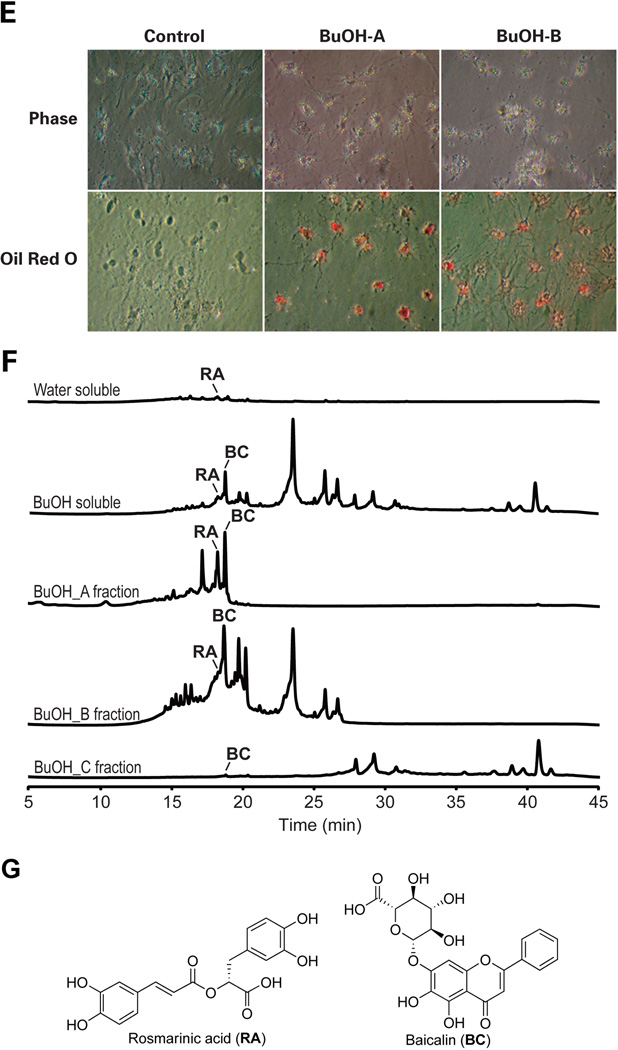
The aqueous YGW extract (350mg/ml in PBS) was applied to size exclusion chromatography using Super Prep Grade gel in XK 16/70 column (Amersham Pharmacia Biotech, Piscataway, NJ) and PBS as a mobile phase solvent. The fractions were tested for their bioactivity toward activated HSCs as determined by the morphological reversal of the cells to their quiescence under microscope, and the fractions with the molecular size range of 200–750 Da, were shown to contain the most of the activity. To improve the extraction efficiency of both water soluble and lipophilic phytocompounds and to allow their structural elucidation, n-butanol (BuOH) was added to the YGW water suspension. Briefly, 10 grams of YGW powder suspended in 200 ml of ddH2O were partitioned with 200 ml of BuOH. After centrifugation and phase separation, BuOH and ddH2O were evaporated in vacuo and lyophilized to yield water (3.59 g) and BuOH (0.54 g) soluble part. Based on the bioactivity-guided fractionation, BuOH soluble phytocompounds (500 mg) were fractionated by column chromatography on RP-18 gel (COSMOSIL 75C18-OPN, 20 by 70 mm, Nacalai, USA) eluting with MeCN-H2O mixtures of decreasing polarity. Fraction A (250 ml of 10% MeCN-H2O, 192.3 mg), B (250 ml of 40% MeCN-H2O, 196.6 mg), and C (250 ml of 100% MeCN, 64.2 mg) were then subjected to bioassay and high performance liquid chromatography-photodiode array detection-mass spectrometry (HPLC-DAD-MS) analysis after removing the solvent by using the rotavapor and lyophilizer. HPLC-DAD-MS analysis was carried out on a ThermoFinnigan LCQ Advantage ion trap mass spectrometer with a RP C18 column (Alltech Prevail C18 3 µm 2.1 × 100 mm) at a flow rate of 125 µl/min with a 10 µl injection. The solvent gradient system and the conditions for MS analysis were as described (21). For quantification of RA and BC in each fraction, linear curves of each compound were generated by using extract ion chromatograms (EIC) in negative mode at the molecular weight of each corresponding parent ion. For identification of the major phytocompounds in fraction A, fraction A (135.0 mg) was purified by reverse phase HPLC [Phenomenex Luna 5µm C18 (2), 250 × 10 mm] with a flow rate of 5.0 ml/min and measured by a UV detector at 254 nm. The gradient system was MeCN (solvent B) in 5% MeCN/H2O (solvent A) both containing 0.05% TFA: 10% B from 0 to 5 min, 10 to 30% B from 5 to 25 min, 30 to 100% B from 25 to 27 min, 100% B from 27 to 30 min, 100 to 10% B from 30 to 32 min, and re-equilibration with 20% B from 32 to 35 min. RA (4.3 mg) and BC (8.7 mg) were eluted at 22.1 and 23.6 min, respectively. NMR spectral data were collected on a Varian Mercury Plus-400 spectrometer. The structures were elucidated by their mass, 1H-, 13C-, and 2D-NMR data and also confirmed by comparing their spectroscopic data with those of literatures (22, 23) and commercial authentic samples from Sigma.
Data Analysis
Data were presented as the means - S.E. Student’s t test was performed to assess the statistical significance between the two sets of data, and p values less than 0.05 were considered significant.
RESULTS
YGW reverses activated HSCs to quiescent cells
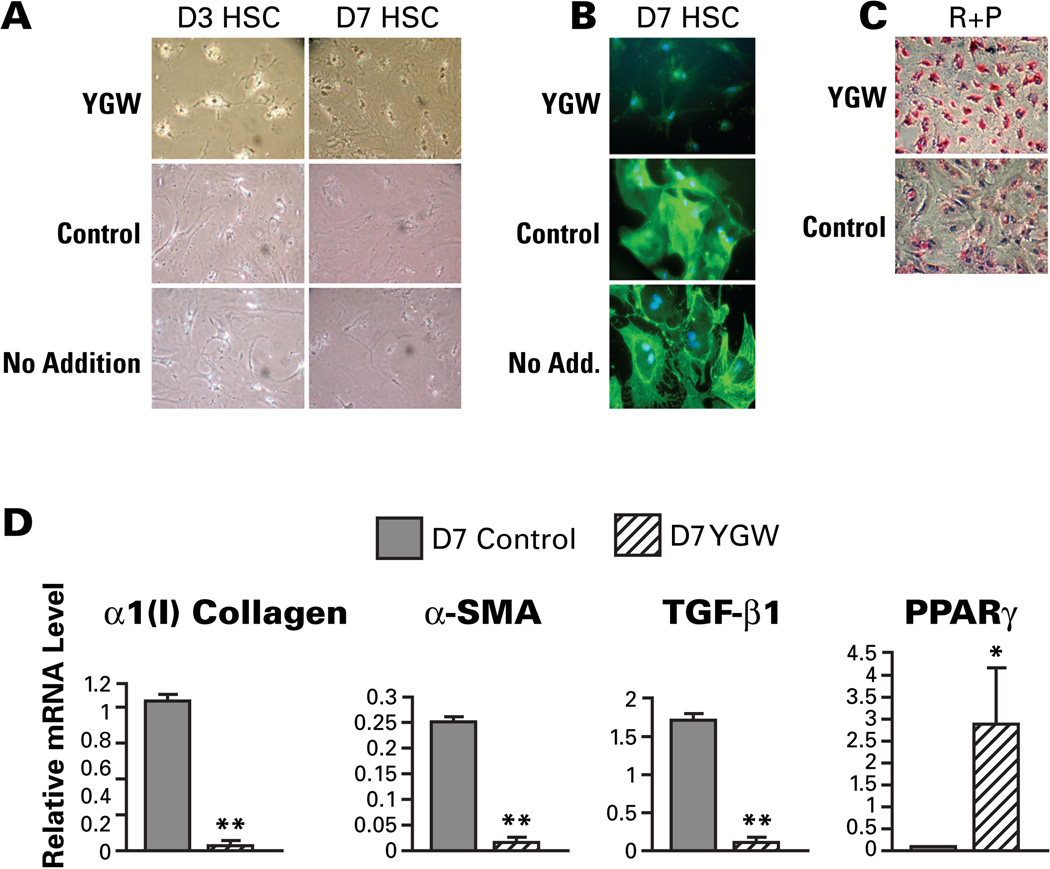
We previously demonstrated attenuation of liver fibrosis in two etiologically distinct animal models (porcine serum-induced liver fibrosis in rats and CCl4-induced liver fibrosis in mice) by administration of the YGW aqueous extract (19). In order to understand the mechanisms of the demonstrated anti-fibrotic effect of YGW at the cellular level, primary cultures of rat HSCs were treated with the YGW extract or the solvent as a control. Rat HSCs cultured on plastic dish spontaneously undergo myofibroblastic transdifferentiation (“activation”) from day 2~3 and become fully activated by day 5~7. Upon treatment of day 3 activating or day 7 fully activated HSCs with the YGW extract for 2 days, activation of HSC is morphologically attenuated as compared to the cells treated with the solvent control or no treatment (Fig. 1A). The YGW decreases the expression of SMA, the bona fide marker for the HSC activation as detected by immunohistochemistry (Fig. 1B) and increases oil red O staining upon addition of retinol and palmitic acid, the parameter for vitamin A storage and the unique feature of quiescent HSCs (Fig. 1C). In addition, the YGW treatment markedly suppresses mRNA expression of markers for HSC activation such as α1(I) procollagen, SMA, and TGF-β1 while upregulating the HSC quiescence marker PPARγ (Fig. 1D). As restored expression of PPARγ reverses activated HSCs to quiescent cells (8, 9), the observed YGW’s effect to prevent or reverse culture-activation of HSCs, is most likely mediated via PPARγ induction.
Fig. 1.
YGW prevents and reverses hepatic stellate cell (HSC) activation in culture. A. Phase contrast microscopy of activating day 3 or fully-activated day 7 rat HSCs cultured for the last 48 hr with YGW extract, vehicle control, or no addition. Note a morphologic reversal of activated HSCs to quiescent cells. B. Immunostaining for SMA. Note a marked reduction in SMA with YGW extract. C. Oil red O staining after retinol and palmitate addition is increased in YGW-treated 7 day HSCs. D. The mRNA levels for activation marker genes, α1(I)collagen, αSMA, TGF-β1 are conspicuously suppressed in day 7 HSC by the 48-hr treatment with YGW extract while PPARγ mRNA is induced. *p<0.05, **p<0.01 compared to the vehicle control treatment.
YGW epigenetically de-represses Pparγ
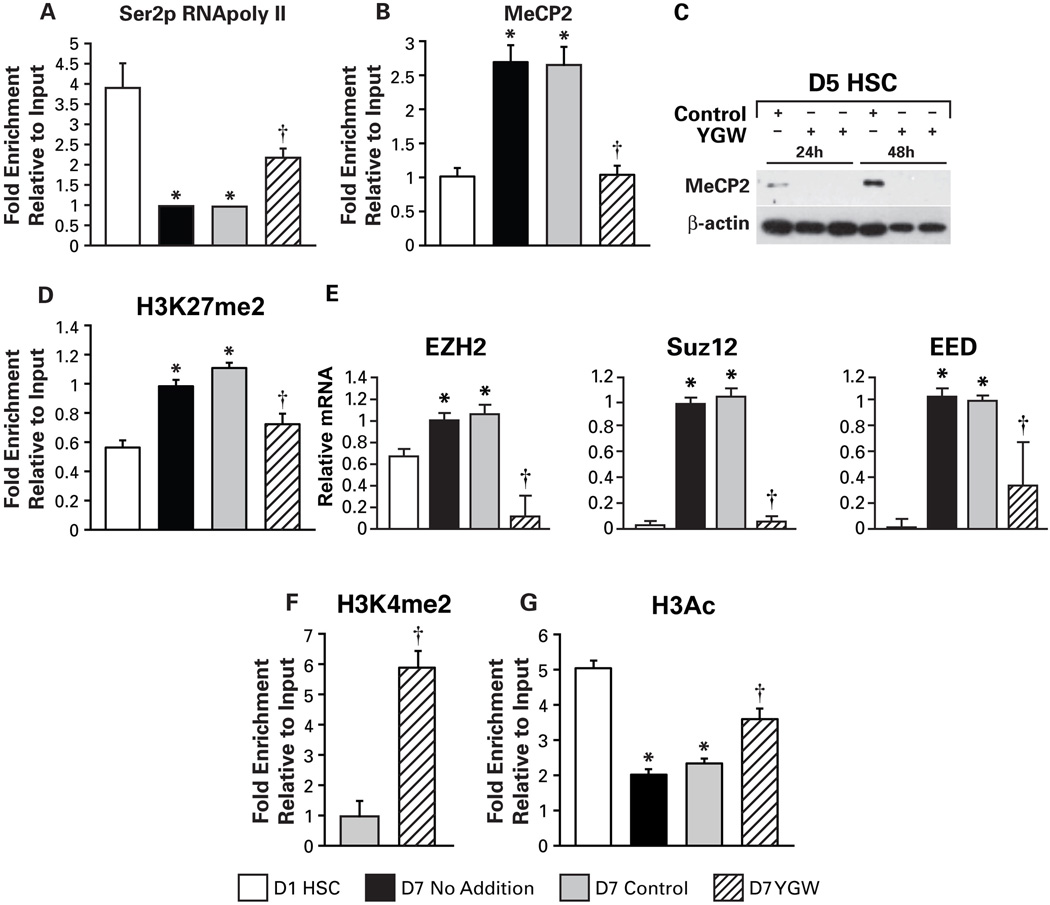
Our recent study revealed the epigenetic mechanisms of Pparγ repression in HSC activation involving upregulation and recruitment of the DNA methyl-CpG binding protein MeCP2 to the Pparγ promoter resulting in the recruitment of the HP-1α corepressor (17). This study also demonstrated MeCP2-dependent upregulation of EZH2, the histone H3 lysine 27 (H3K27) methyltransferase of polychrome repressor complex 2 (PRC2), increasing H3K27 di- and tri-methylation in the Pparγ exons with consequent formation of a repressive chromatic structure (17). Thus, we tested whether YGW’s inductive effect on Pparγ is associated with epigenetic effects on this gene. First, we examined the recruitment of elongating RNA polymerase II (Ser2-p RNAPoly II) to the Pparγ gene. As previously shown, culture-activated HSCs at day 7 have a markedly reduced recruitment of the Ser2-p RNAPoly II as compared to day 1 quiescent HSCs, and this suppression is attenuated by the YGW treatment (Fig. 2A). MeCP2 enrichment to the Pparγ promoter is increased in Day 7 culture-activated HSCs but reduced by the YGW treatment to the level seen in Day 1 HSCs (Fig. 2B). This reduction is associated with abrogation of MeCP2 protein induction seen in day 5 HSCs subsequently incubated with the YGW extract for 24 or 48 hr (Fig. 2C). Increased H3K27 di-methylation (H3K27me2) noted at the exon 2 of Pparγ in culture-activated HSCs (17) with or without the solvent, is also normalized by the YGW extract (Fig. 2D), most likely attributable to suppressed expression of PRC2 components, EZH2, Suz12, and EED (Fig. 2E). H3K4 di-methylation (H3K4me2) and H3 acetylation (H3Ac), the histone modifications for active transcription, are both increased at the Pparγ promoter locus by the YGW treatment (Fig. 2F and G). These data collectively demonstrate that epigenetic repression of the Pparγ gene in culture-activated HSCs is lifted by the YGW extract treatment, and this effect must be responsible for restored PPARγ expression and HSC quiescence.
Fig. 2.
PPARγ epigenetic repression is lifted with YGW extract. A. Recruitment of Ser2-p RNA polymerase II to the Pparγ gene is significantly reduced in day 7 culture-activated HSCs with no addition or with the vehicle control treatment, and this reduction is attenuated by the YGW extract treatment. B. Increased MeCP2 recruitment to Pparγ promoter in day 7 culture-activated HSCs is normalized with the YGW extract. C. MeCP2 protein detected by immunoblotting in day 5 HSCs cultured for 24 and 48 hr with the vehicle control becomes undetectable by the YGW treatment. D. Increased H3K27me2 at the Pparγ exon 2 locus in day 7 HSCs is reduced with the YGW extract. E. Increased mRNA levels of the PRC2 component EZH2, Suz12, and EED in day 7 HSCs are reduced by the YGW treatment. F. H3K4me2 at the Pparγ promoter locus is increased by the YGW extract treatment in day 7 HSCs compared to HSCs treated with the vehicle. G. Reduced H3 acetylation (H3Ac) in 7 day HSCs is attenuated with the YGW extract. *p<0.05 compared to day 1 HSCs, †p<0.05 compared to the vehicle control.
YGW suppresses IKK and NF-κB activity in HSC
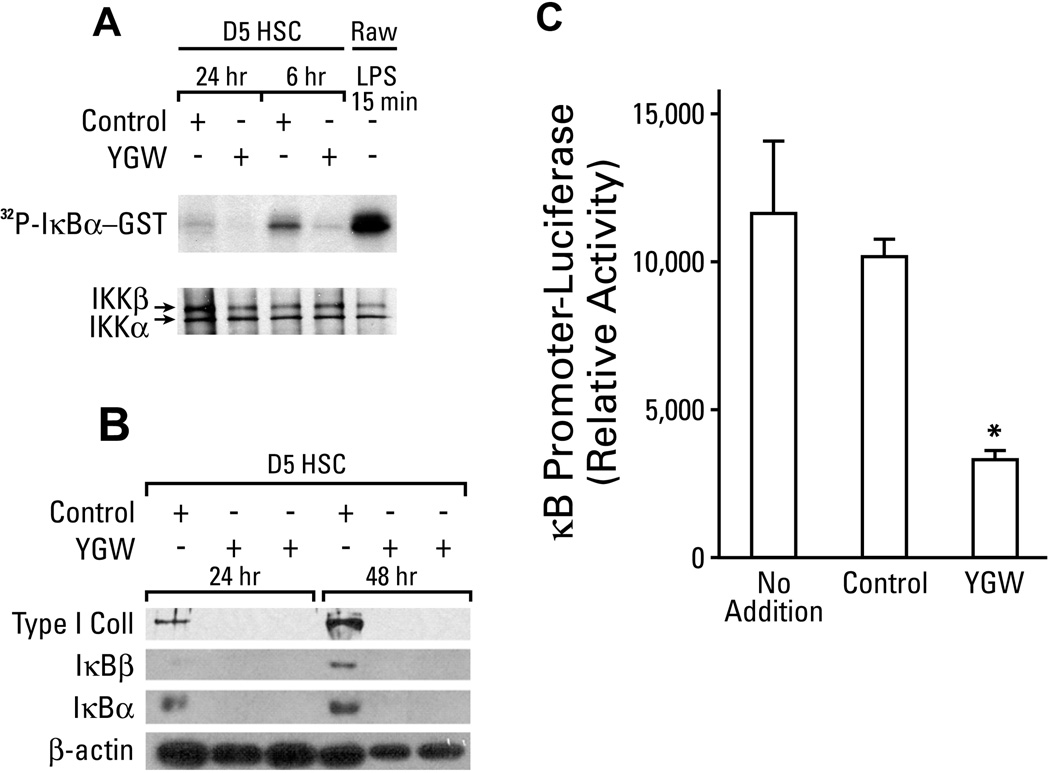
Another important biochemical feature of activated HSCs is increased activity of NF-κB (24). We tested how the YGW extract affects this parameter. The treatment with the YGW extract markedly inhibits the activity of IκB kinase (IKK) as assessed by phosphorylation of IκBα-GST fusion protein (Fig. 3A), the expression of IκBα and β, both targets of NF-κB (Fig. 3B) in day-5 HSCs, and NF-κB promoter activity in the rat HSC line (BSC) (Fig. 3C). The demonstrated suppressive effects of YGW on IKK and NF-κB suggest that it may promote apoptotic death of HSCs. Only after a prolonged extract treatment exceeding 4–5 days with replenishment of the medium containing the extract every 2 days, apoptosis of cultured HSCs begins to appear and becomes apparent after 8 days as assessed by TUNEL staining (Suppl Fig. 1A).
Fig. 3.
Suppression of IKK and NF-κB with YGW. A. Day 5 HSCs cultured with the YGW extract vs. the vehicle control for 6 or 24 hr in serum-free medium, show reduced IKK activity as assessed by phosphorylation of IκBα-GST fusion protein. A positive control for IKK activation is shown with LPS-stimulated RAW macrophage cell line (last lane). B. Day 5 HSCs cultured with the YGW extract for 24 or 48 hr, show marked reductions in the levels of IκBα and IκBβ proteins, as well as in type I collagen protein. C. The activity of κB promoter is significantly reduced by the YGW extract in the rat HSC line (BSC) as assessed by a transient transfection-reporter analysis. *p<0.05.
Identification of YGW’s active ingredients
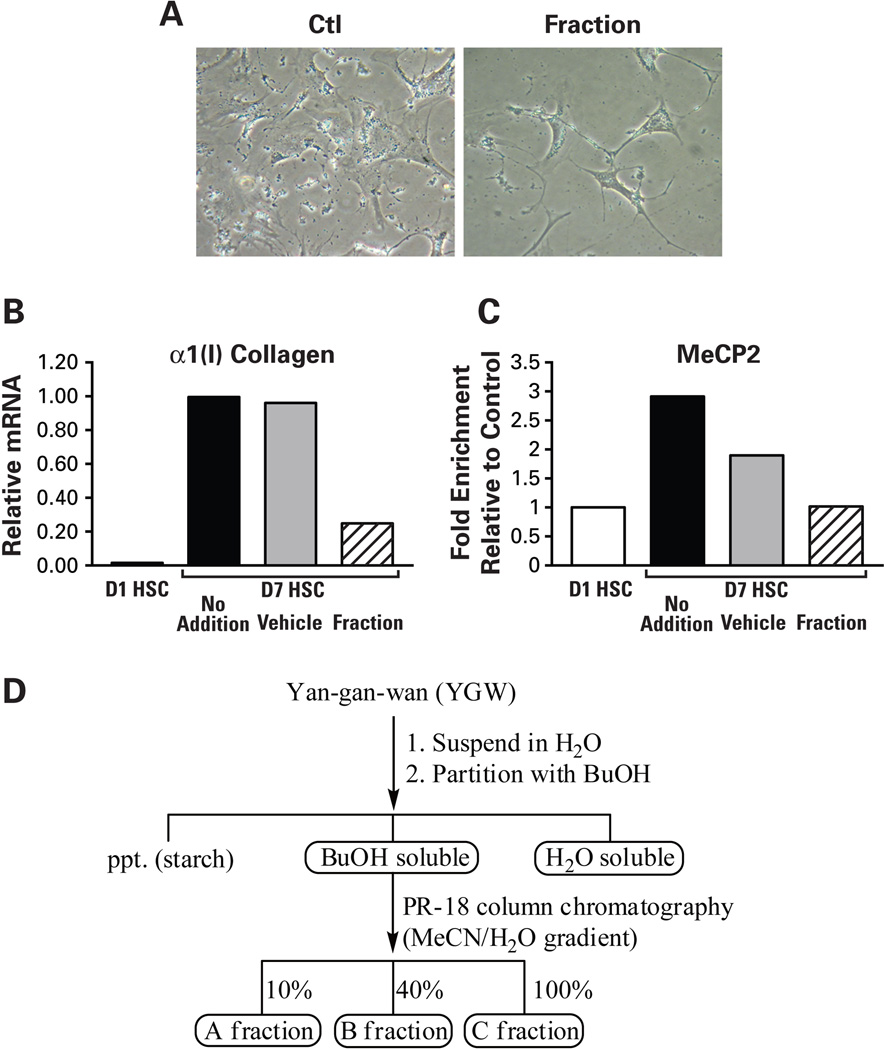
As the first step in identifying active ingredients of YGW rendering the above reversal effects on activated HSCs, we first tested different fractions of gel filtration of the YGW water extract in culture-activated HSCs. This analysis reveals a fraction with a molecular mass range of 200~750Da, reproduces the YGW effects including the morphological reversal (Fig. 4A), down-regulation of α1(I)procollagen mRNA (Fig. 4B), and decreased MeCP2 enrichment at the Pparγ promoter (Fig. 4C). This gel filtration fraction was next applied to LC/MS for identification of active ingredients. This analysis identifies small peaks with the retention time of 14~15 min (boxed in the UV254 tracing of Fig. 4D). Due to low amounts of these molecules detected in the water extract to allow their purification and identification, we next analyzed YGW ingredients extracted with butanol (BuOH). This method ensures that most hydrophilic and lipophilic organic compounds are extracted into the butanol layer while most of the sugar and ionic inorganic components remain in the water layer. After lyophilization, the water-soluble portion of YGW shows reduced activity of the HSC morphologic reversal when compared with the YGW water extract before the butanol partitioning. In contrast, the butanol-soluble portion of YGW shows clear bioactivity toward HSCs (data not shown), suggesting that the bioactive phytocompounds are enriched in the butanol soluble portion. We further fractionated the butanol soluble portion by reverse phase chromatography eluted with 10% (A fraction), 40% (B fraction), and 100% (C fraction) acetonitrile-water mixtures (Fig. 4D). The butanol A fraction shows a reproducible effect on HSC morphologic reversal (Fig. 4E) while the C fraction causes immediate cytotoxicity evident by detachment of the cells (data not shown). The B fraction shows a moderate reversal effect (Fig. 4E). The HPLC profiles clearly show the metabolites distribution of each fraction and suggest that the bioactive compound(s) may be eluted from 15 min to 20 min in fraction A (Fig. 4F). In order to identify the bioactive phytocompounds in the A fraction, total of eight subfractions were further purified by semi-preparative HPLC (data not shown). Two major compounds were then isolated and identified to be the bioactive principles. They are rosmarinic acid (RA) and baicalin (BC) (Fig. 4G) by analyzing their mass, 1H-, 13C-, and 2D-NMR data as well as by comparing their 1H-, 13C-NMR data with those of commercial authentic samples (data not shown).
Fig. 4.
Identification of active components. A. Treatment of day 7 HSCs with a gel filtration fraction with a molecular mass range of 200~750Da, causes a morphologic reversal of HSCs as compared to the cells treated with the elution buffer control (phase contract microscopy). B and C. Addition of the fraction to 7 day HSC culture reduces increased α1(I)collagen mRNA and MeCP2 enrichment to the Pparγ promoter as shown with the YGW extract. D. A summary of chromatographic methods for separation of YGW’s active ingredients. E. Butanol (BuOH) fraction A and B eluted with 10% acetonitrile-90% water and 40% acetonitrile-60% water, respectively, produce reproducible effects of HSC morphologic reversal as shown by phase contrast microscopy and oil red O staining. F. LC/MS tracing of butanol fractions identifies 5 peaks of which two are identified to be RA and BC. G. Molecular structures of rosmarinic acid and baicalin.
In vitro effects of RA and BC on HSCs
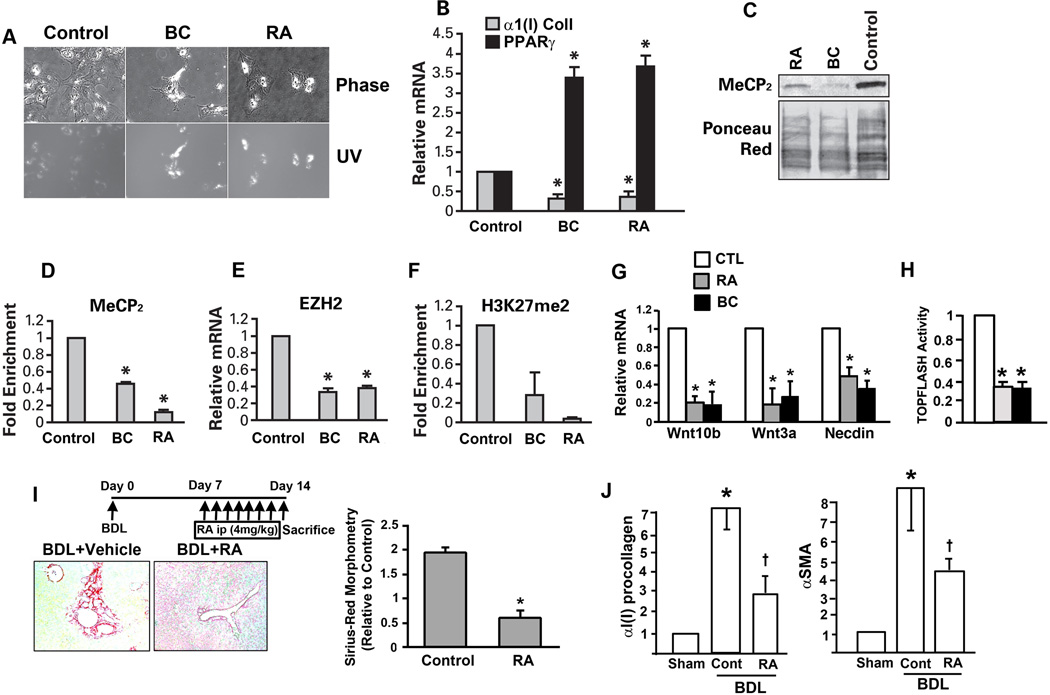
We tested next whether authentic RA and BC reproduce the effects observed with the YGW extract by testing a wide range of concentrations for HSC morphologic reversal. Indeed, both RA and BC morphologically reverse activated HSCs to quiescent cells with increased UV-excited autofluorescence at the concentration of 135 and 270 µM (Fig. 5A). Using the concentration of 270 µM, RA and BC are shown to downregulate α1(I) procollagen 2~3 fold and to induce PPARγ 3~4 fold (Fig. 5B). Both RA and BC reduce MeCP2 protein level (Fig. 5C) and its enrichment in the Pparγ promoter (Fig. 5D). RA and BC also reduce EZH2 expression and H3K27me2 at the Pparγ exon (Fig. 5E and F). Collectively, these results support that RA and BC are indeed active phytocompounds that render the YGW’s effect to inhibit or reverse HSC activation via epigenetic de-repression of Pparγ.
Fig. 5.
Rosmarinic acid (RA) and baicalin (BC) are the YGW’s active components to render epigenetic de-repression of Pparγ. A. Note both rosmarinic acid (RA) and baicalin (BC) reverse activated HSC to quiescent cells as shown by phase contrast and UV-excited autofluorescence microscopy. B. RA and BC (270 µM) reduce mRNA expression for α1(I)collagen and increase that for PPARγ. C. RA and BC reduce MeCP2 protein level in day 7 HSCs. D. MeCP2 enrichment to the Pparγ promoter is reduced with RA and BC. E. EZH2 mRNA level is reduced equally by RA and BC. F. H3K27me2 at the Pparγ exon 2 is reduced by RA and BC. * p<0.05 compared to the solvent control. G. RA and BC (270 µM) reduce the expression of Wnt10b, Wnt3a, and Necdin in day 7 HSCs compared to the vehicle control as determined by qPCR analysis. H. RA (shaded bar) and BC (black bar) reduces the TOPFLASH promoter activity in day 7 primary HSCs ad determined by a transient transfection using an electroporation method. I. RA treatment (ip injection daily at 0.1mg/25g body weight) given during the last one week of the 2-week cholestasis caused by the bile duct ligation, attenuates liver fibrosis in mice as assessed by digital morphometric analysis of Sirius red staining. *p<0.05 compared to the vehicle control. J. Hepatic expression of α1(I)procollagen and SMA are also significantly reduced by the RA treatment. *p<0.05 compared to Sham. +p<0.05 compared to vehicle-treated (Cont) mice.
We have previously shown that activation of canonical Wnt signaling underlies HSC activation (11) via epigenetic repression of Pparγ involving MeCP2 and H3K27me2 (16). Thus we thought epigenetic de-repression of Pparγ achieved by RA and BC is due to their ability to inhibit canonical Wnt signaling. Indeed, both RA and BC suppress the expression of Wnt10b and Wnt3a (Fig. 5G), the canonical Wnts upregulated in HSC activation (11) and TOPFLASH activity (Fig. 5H). Expression of Necdin which transcriptionally upregulates Wnt10b (16), is also reduced by RA and BC (Fig. 5G), suggesting that these phytocompounds target the Necdin-Wnt-MeCP2 pathway for reversal of HSC activation.
RA inhibits HSC activation and progression of biliary liver fibrosis in mice
BC is the active ingredient of Sho-Saiko-To, a Japanese herbal medicine which has been tested for its anti-fibrotic effects in experimental models (25) and patients (26). In contrast, studies on the effects of RA on liver fibrosis are limited to a few recent reports (27, 28). In one of these studies, RA was shown to prevent the development of CCl4-induced liver fibrosis in rats (27). As RA is an anti-oxidant, this effect on CCl4-induced oxidative liver damage and consequent liver fibrosis are rather expected. To extend this observation in a different etiological model, we considered testing the efficacy of RA for inhibiting progression of pre-existing cholestatic liver fibrosis induced by BDL in mice. As portal myofibroblasts (MFs) rather than HSCs are thought to be the primary source of the fibrotic response in the BDL model (29), we first examined whether HSCs are activated in the model by analyzing HSCs isolated by FACS from α1(I) collagen promoter-GFP (Coll-GFP) mice subjected to 2-wk BDL. As shown in Suppl. Fig. 2A, the percentage of GFPlow cells (minimal collagen promoter activity) in UV+ (vitamin A containing) HSCs is reduced from 9.5% to 2.1% while the percentage of GFPhigh/UV+ HSCs increases in BDL mice as compared to sham-operated animals, indicating activation of HSCs in the model. Further, qPCR analysis of all UV+ HSCs from BDL vs. sham mice, reveals induction of HSC activation markers such as α1(I)procollagen (Col1a1), Sma, and Timp1 in BDL HSCs but not Desmin (Suppl Fig. 2B). Having confirmed that HSCs are indeed activated in the model, we tested the effects of daily intraperitoneal administration of RA vs. vehicle during the second week of BDL. The liver to body weight percentage is not different between RA or vehicle-treated mice (6.8+0.7 vs. 6.3+0.3), nor are the plasma ALT levels (157+71 vs. 283+95, p=0.29). However, the digital morphometric analysis of Sirius red-stained collagen fibers shows a significant attenuation of liver fibrosis by RA treatment (Fig. 5I). To examine whether this anti-fibrotic effect of RA is associated with suppressed activation of HSCs in vivo, immunohistochemistry for SMA and Desmin were performed (Suppl. Fig. 2C). In the sham-operated liver, expression of SMA is primarily seen in the hepatic artery and a very few cells around the bile duct, but not in HSCs in the sinusoid (Suppl. Fig. 2C, upper and lower left panel). In the vehicle-treated BDL liver, expression of SMA increases in Desmin+ portal MFs and HSCs (Suppl. Fig. 2C, upper and lower middle panel). RA treatment reduces the percentage of SMA+ MFs by 40% and that of SMA+ HSCs by 75% (Suppl. Fig. 2C and 2D). The density of Desmin+ HSCs increases by BDL, but RA treatment has no effect on this change (Suppl. Fig. 2D). No TUNEL+ HSCs or hepatocytes are detected in the liver parenchyma of either RA- or vehicle-treated BDL livers. These data indicate that RA suppresses activation of both portal MFs and HSCs in BDL-induced liver injury. Hepatic mRNA levels of α1(I)procollagen and SMA are also significantly reduced by RA treatment (Fig. 5J), further supporting anti-fibrotic effects of RA in this model. Taken together, these data indicate that RA suppresses activation of HSCs and liver fibrosis in BDL-induced liver injury.
DISCUSSION
The present study demonstrates that the MeCP2 -EZH2 relay of Pparγ epigenetic repression is an important target for the anti-fibrotic effect of the herbal prescription YGW. Polyphenolic RA and flavonoid BC are identified as active phytochemicals in YGW that reverse epigenetic Pparγ repression and activated phenotype of HSCs. Both RA and BC inhibit MeCP2 induction and its recruitment to the Pparγ promoter while suppressing the expression of PRC2 components including the H3K27 methyltrasferase EZH2 resulting in reduced H3K27me2 at the Pparγ exon locus. These epigenetic effects which result in the formation of eurochromatin at the Pparγ locus, increase a recruitment of RNA polymerase to Pparγ and its transcription, and restore expression of the gene which is essential for HSC differentiation (8, 9). In essence, these results provide the molecular basis of the anti-fibrotic effects of YGW and its ingredients, RA and BC at the epigenetic level.
Due likely to the ability to suppress NF-κB, the prolonged treatment of cultured HSCs with the YGW extract for 8 days, causes apoptosis in cultured HSCs (Suppl Fig. 1). However, no apoptosis is evident during the first 2 days of the treatment when the epigenetic Pparγ de-repression and phenotypic reversal of HSCs are achieved. RA treatment of BDL mice attenuates liver fibrosis, and this effect is accompanied by suppressed activation of HSCs as demonstrated by a marked reduction in SMA+ HSCs. In these livers, apoptosis of HSCs is not evident and the number of HSCs is not reduced (Suppl Fig. 2C and 2D). Thus, these results suggest that suppressed activation rather than apoptosis of HSCs is responsible at least in part for RA’s anti-fibrotic effect in the BDL model. Portal MFs which are considered as a major source of a fibrogenic response in the BDL model (29), are indeed increased in number after BDL (Suppl Fig. 2D), and this change is attenuated by RA treatment. At present, we do not know the molecular basis of this suppression of MFs by YGW and its active ingredients RA and BC, and a future study will need to address this question.
BC is an active ingredient of Sho-saiko-to, a Japanese herbal medicine known for its anti-fibrotic effects, and its mechanism of action has primarily been ascribed to its antioxidant property and its ability to reduce lipid peroxidation (25). RA is also a polyphenolic antioxidant which may also render the similar protective effects against oxidant liver damage and fibrosis. Suppression of IKK and NF-κB activities by YGW shown in HSCs is also consistent with its ability to suppress oxidant stress which is a well known signal for activation of IKK. Oxidant stress generated by NADPH oxidase is recognized as a key signaling event in activation of HSC induced by a wide array of agonists such as angiotensin II (30), PDGF (31), and leptin (32). Accordingly, antioxidants which scavenge NADPH oxidase-derived ROS are expected to suppress activation of HSCs. However, the present study demonstrates that BC and RA inhibit the canonical Wnt signaling which we have recently shown to mediate epigenetic repression of Pparγ involving MeCP2 and EZH2 (16). Further, Necdin which transcriptionally activates Wnt10b via its binding to a GN box in its proximal promoter (16), is also reduced by both RA and BC. Taken together, these results suggest that both phytocompounds target the Necdin-Wnt-MeCP2-EZH2 pathway for their epigenetic effects. Whether and how this novel effect is related to antioxidant activity of the phytocompounds, are yet to be determined.
Supplementary Material
Acknowledgments
The present study was supported by NIH grants: U01AA018663 (HT and DM), P50AA11199 (HT), R24AA12885 (HT), and R01AA020753 (KA) and by Medical Research Service of Department of Veterans Affairs (HT); Medical Research Council, Welcome Trust, and British Liver Trust; and the Newcastle Health Care Charity and Newcastle upon Tyne Hospitals NHS Charity (JM and DM); and a grant from S.P. Pharmaceutics, Inc. (HT).
REFERENCES
- 1.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008 Jan;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 3.Cassiman D, Barlow A, Vander BS, Libbrecht L, Pachnis V. Hepatic stellate cells do not derive from the neural crest. J Hepatol. 2006 Jun;44(6):1098–1104. doi: 10.1016/j.jhep.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001 Aug;21(3):311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 5.Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Jr., Sucov HM, et al. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2008 Nov 5;49(3):998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Jr., Motomura K, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000 Nov 17;275(46):35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 7.Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996 Nov 1;87(3):377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 8.Hazra S, Xiong S, Wang J, Rippe RA, Krishna V, Chatterjee K, et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004 Mar 19;279(12):11392–11401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 9.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005 Feb 11;280(6):4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 10.Yavrom S, Chen L, Xiong S, Wang J, Rippe RA, Tsukamoto H. Peroxisome proliferator-activated receptor gamma suppresses proximal alpha1(I) collagen promoter via inhibition of p300-facilitated NF-I binding to DNA in hepatic stellate cells. J Biol Chem. 2005 Dec 9;280(49):40650–40659. doi: 10.1074/jbc.M510094200. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007 Nov 15; doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- 12.Tseng YH, Butte AJ, Kokkotou E, Yechoor VK, Taniguchi CM, Kriauciunas KM, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005 Jun;7(6):601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 13.Kuwajima T, Nishimura I, Yoshikawa K. Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J Neurosci. 2006 May 17;26(20):5383–5392. doi: 10.1523/JNEUROSCI.1262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwajima T, Taniura H, Nishimura I, Yoshikawa K. Necdin interacts with the Msx2 homeodomain protein via MAGE-D1 to promote myogenic differentiation of C2C12 cells. J Biol Chem. 2004 Sep 24;279(39):40484–40493. doi: 10.1074/jbc.M404143200. [DOI] [PubMed] [Google Scholar]
- 15.Brunelli S, Tagliafico E, De Angelis FG, Tonlorenzi R, Baesso S, Ferrari S, et al. Msx2 and necdin combined activities are required for smooth muscle differentiation in mesoangioblast stem cells. Circ Res. 2004 Jun 25;94(12):1571–1578. doi: 10.1161/01.RES.0000132747.12860.10. [DOI] [PubMed] [Google Scholar]
- 16.Zhu NL, Wang J, Tsukamoto H. The Necdin-Wnt pathway causes epigenetic peroxisome proliferator-activated receptor gamma repression in hepatic stellate cells. J Biol Chem. 2010 Oct 1;285(40):30463–30471. doi: 10.1074/jbc.M110.156703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010 Feb;138(2):705–714. doi: 10.1053/j.gastro.2009.10.002. 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010 Apr 5;189(1):127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang MD, Deng QG, Chen S, Xiong S, Koop D, Tsukamoto H. Hepatoprotective mechanisms of Yan-gan-wan. Hepatol Res. 2005 Aug;32(4):202–212. doi: 10.1016/j.hepres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Sung CK, She H, Xiong S, Tsukamoto H. Tumor necrosis factor-alpha inhibits peroxisome proliferator-activated receptor gamma activity at a posttranslational level in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004 May;286(5):G722–G729. doi: 10.1152/ajpgi.00411.2003. [DOI] [PubMed] [Google Scholar]
- 21.Bok JW, Chiang YM, Szewczyk E, Reyes-Dominguez Y, Davidson AD, Sanchez JF, et al. Chromatin-level regulation of biosynthetic gene clusters. Nat Chem Biol. 2009 Jul;5(7):462–464. doi: 10.1038/nchembio.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin YL, Wang WY, Kuo YH, Chen CF. Nonsteroidal constituents from Solanum incanum L. [Abstract] Journal of the Chinese Chemical Society. 2000 Feb 1;(47):247–251. [Google Scholar]
- 23.Tezuka Y, Kasimu R, Li JX, Basnet P, Tanaka K, Namba T, et al. Constituents of roots of Salvia deserta Schang. (Xinjiang-Danshen). [Abstract] Chemical and Pharmaceutical Bulletin. 1998;(46):107–112. [Google Scholar]
- 24.Oakley F, Mann J, Ruddell RG, Pickford J, Weinmaster G, Mann DA. Basal expression of IkappaBalpha is controlled by the mammalian transcriptional repressor RBP-J (CBF1) and its activator Notch1. J Biol Chem. 2003 Jul 4;278(27):24359–24370. doi: 10.1074/jbc.M211051200. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu I, Ma YR, Mizobuchi Y, Liu F, Miura T, Nakai Y, et al. Effects of Sho-saiko-to, a Japanese herbal medicine, on hepatic fibrosis in rats. Hepatology. 1999 Jan;29(1):149–160. doi: 10.1002/hep.510290108. [DOI] [PubMed] [Google Scholar]
- 26.Stickel F, Brinkhaus B, Krahmer N, Seitz HK, Hahn EG, Schuppan D. Antifibrotic properties of botanicals in chronic liver disease. Hepatogastroenterology. 2002 Jul;49(46):1102–1108. [PubMed] [Google Scholar]
- 27.Li GS, Jiang WL, Tian JW, Qu GW, Zhu HB, Fu FH. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomedicine. 2010 Mar;17(3–4):282–288. doi: 10.1016/j.phymed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JJ, Wang YL, Feng XB, Song XD, Liu WB. Rosmarinic acid inhibits proliferation and induces apoptosis of hepatic stellate cells. Biol Pharm Bull. 2011 Mar;34(3):343–348. doi: 10.1248/bpb.34.343. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007 Nov;46(5):1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003 Nov;112(9):1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, et al. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005 Jun;41(6):1272–1281. doi: 10.1002/hep.20719. [DOI] [PubMed] [Google Scholar]
- 32.De MS, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology. 2008 Dec;48(6):2016–2026. doi: 10.1002/hep.22560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.