Abstract
Mononuclear phagocytes are cells of the innate immunity that defend the host against harmful pathogens and heal tissues after injury. Contrary to expectations, in malignancies, tumour-associated macrophages (TAM) promote disease progression by supporting cancer cell survival, proliferation and invasion. TAM and related myeloid cells [Tie2+ monocytes and myeloid-derived suppressor cells (MDSC)] also promote tumour angiogenesis and suppress adaptive immune responses. These divergent biological activities are mediated by macrophages/myeloid cells with distinct functional polarization, which are ultimately dictated by microenvironmental cues. Clinical and experimental evidence has shown that cancer tissues with high infiltration of TAM are associated with poor patient prognosis and resistance to therapies. Targeting of macrophages in tumours is considered a promising therapeutic strategy: depletion of TAM or their ‘re-education’ as anti-tumour effectors is under clinical investigation and will hopefully contribute to the success of conventional anti-cancer treatments.
Keywords: angiogenesis, cancer, inflammation, myeloid cells, tumour-associated macrophages
Introduction
The complex relationship between tumours and the immune system has long been studied. Cancer cells express tumour-associated antigens able to trigger the host immune response [1–3]. Indeed, in recent decades there has been growing evidence that cells of the adaptive immunity are present at tumour sites and are usually associated with more favourable prognosis. The best evidence is in human colorectal cancer, where CD3+CD8RO+ lymphocytes are clearly associated with longer disease-free and -specific survival [4,5]. Also in other human tumours, such as ovary, melanoma and breast, the density of T cells is usually correlated with a more favourable outcome [6–9]. In marked contrast, in the majority of cancers, cells of the innate immunity, especially macrophages, most frequently favour tumour progression. Tumour-associated macrophages (TAM) are recruited early at tumour sites where they most frequently display pro-tumour functions, such as activation of the neo-angiogenic switch, the secretion of soluble factors that support tumour cell resistance to apoptotic stimuli and stimulate the proliferation and invasion of malignant cells. TAM have been also associated with the suppression of adaptive immunity [10–15].
Thus, in a simplified scheme, adaptive immunity is usually protective and limits tumour progression, while innate immunity favours disease development. However, biological mechanisms are more complex: recent evidence has been provided that components of adaptive immunity [e.g. interleukin (IL)-4-producing CD4 T cells and antibody-producing B cells] may activate innate immune cells in a pro-tumour manner [14,16]. Therefore, the dynamic interplay between innate and adaptive immunity is of paramount importance in the outcome of tumour progression or regression. A further level of complexity is dictated by the multi-faceted functional phenotypes of myeloid cells, especially macrophages, which allow them to have either anti-tumour or pro-tumour activities.
In this review we will summarize the current view on the roles of TAM in tumours and the available strategies to target or exploit them for anti-cancer therapies.
Macrophage heterogeneity
Macrophages are versatile cells that are capable of displaying different functional activities, some of which are antagonistic: they can be immunostimulatory or immune suppressive, and either promote or restrain inflammation [13,17–22]. This functional plasticity is regulated by local cues to which the macrophages respond. For instance, during bacterial infections macrophages first orchestrate the acute inflammatory response to eliminate the invading pathogens; at later times they transform into scavengers of tissue debris; further on they trigger the proliferative phase of healing by releasing a variety of growth factors and cytokines which recruit and activate fibroblasts and new vessels [20,23].
Macrophage heterogeneity has been simplified in the macrophage polarization concept where the two extreme phenotypes, the M1 and M2 macrophages, have distinct features [17,24–28], as depicted in Fig. 1. M1 or classically activated macrophages are stimulated by bacterial products and T helper type 1 (Th1) cytokines [e.g. interferon (IFN)-γ]; they are potent effectors that produce inflammatory and immunostimulating cytokines to elicit the adaptive immune response, secrete reactive oxygen species (ROS) and nitrogen intermediates and may have cytotoxic activity to transformed cells. M2 or alternatively activated macrophages differentiate in microenvironments rich in Th2 cytokines (e.g. IL-4, IL-13); they have high scavenging activity, produce several growth factors that activate the process of tissue repair and suppress adaptive immune responses [15,29,30].
Fig. 1.
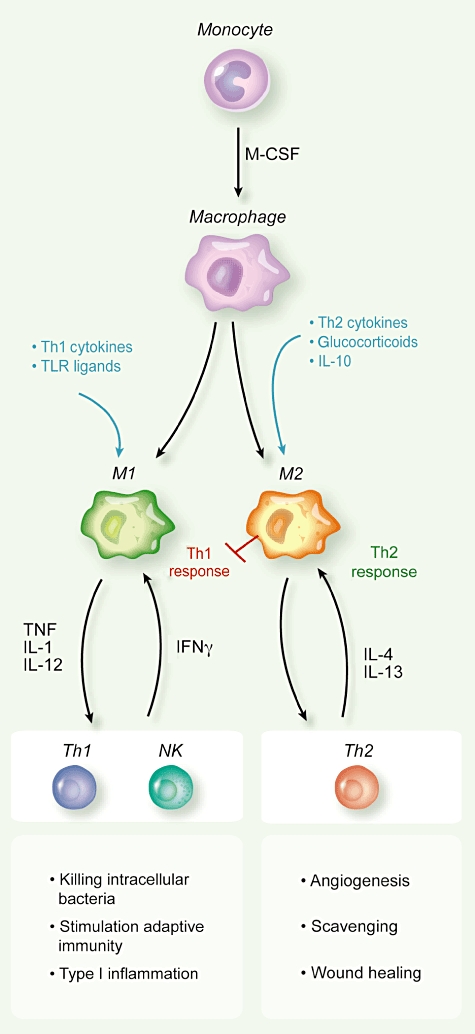
Macrophage polarization is modulated by microenvironmental signals. T helper type 1 (Th1) cytokines [e.g. interferon (IFN-γ)] and Toll-like receptor (TLR) ligands [e.g. lipopolysaccharide (LPS)] promote M1 macrophages which elicit Th1 immune responses and fight intracellular pathogens. Th1 cells produce IFN-γ, which further sustains M1 polarization. Th2 cytokines [e.g. interleukin (IL)-4, IL-13], IL-10 and glucocorticoids promote M2 macrophages which block Th1 immune responses and promote wound healing, scavenging of damaged tissues and angiogenesis. Th2 cells produce IL-4, which further sustains M2 polarization.
Macrophages infiltrate neoplastic lesions from the early stages of tumorigenesis and usually precede other leucocytes (e.g. lymphocytes) having potential anti-tumour function [31].
Tumour and stromal cells secrete a number of diverse chemoattractants that recruit blood circulating monocytes. For instance, the chemokine CCL2 was discovered as a tumour-derived factor inducing chemotaxis in monocytes [32,33]. Once in tumours, monocytes differentiate to macrophages primarily because of the presence of macrophage colony-stimulating factor (M-CSF), produced by tumour cells. M-CSF production in human tumours correlates with poor prognosis in ovarian, breast and endometrial cancer [34,35]. Conditioned by the tumour milieu, monocytes differentiate as tumour-educated macrophages and acquire properties of immune-suppressive and pro-tumoural effectors.
In established tumours, TAM resemble M2-like macrophages [36–40]. While M2-related activities are of extreme importance during wound healing to return to the homeostatic state, in the context of a growing tumour they may favour disease progression [11,12,36,39,41–43].
In molecular profiling studies, murine TAM display hallmarks of M2 macrophages: arginase-I, YM1, FIZZ1, MGL2, vascular endothelial growth factor (VEGF), osteopontin and matrix metalloproteinases (MMPs), as well as an immunosuppressive phenotype: high IL-10, transforming growth factor (TGF-β) and low IL-12, reactive nitrogen intermediates (RNI) and major histocompatibility complex (MHC) II, which correlate functionally to reduced cytotoxicity and antigen-presenting capacity [44–46].
Similar findings were found in human TAM from ovarian cancer patients [47]. We compared the expression of up-regulated genes in human TAM with the profiling of in-vitro-polarized M1 and M2 macrophages. Some up-regulated genes (e.g. osteopontin, fibronectin, scavenger and mannose receptors) were up-regulated similarly in TAM and in M2 macrophages. Indeed, using principal component analysis, the global profiling of TAM fell much closer to that of M2-polarized macrophages [40].
In fact, TAM heterogeneity is starting to emerge, probably depending on the tumour type and microenvironmental cues [39,48]. Notably, murine TAM from fibrosarcoma also showed the expression of typical M1 factors such as IFN-inducible chemokines (CCL5, CXCL9, CXCL10, CXCL16) [44,49].
TAM as promoters of cancer-related inflammation
Conditions of persistent inflammation in tissues predispose to carcinogenesis and, in established malignancies, accelerate tumour development [42,50–54]. Cancer-related inflammation is now recognized as a hallmark of cancer [55,56]. Macrophages are key initiators of the subtle chronic inflammation present in the tumour microenvironment, as they are major producers of inflammatory mediators. Several experimental studies in inflammation-induced murine tumour models have demonstrated the requirement of nuclear factor (NF)-κB activation in TAM for tumour promotion [46,54,57–60]. Within the tumour context the transcription factor NF-κB can be triggered in macrophages by factors released by necrotic tissues [e.g. high-mobility group protein 1 (HMGB1) degraded matrix proteins] and by inflammatory cytokines [e.g. tumour necrosis factor (TNF)] produced by neoplastic cells [61]. Activated TAM, in turn, produce cytokines (IL-6, TNF) and chemokines, which perpetuate and amplify the inflammatory cascade [42].
The primary inflammatory cytokine TNF, produced by immune cells but also by malignant and stromal cells, is an activating mediator and at low concentrations sustains the growth of tumour cells and blood vessels. TNF is also associated with increased release of chemokines (CCL2, CXCL8, CXCL12) and activation of matrix degrading enzymes [61].
IL-6 is a key growth-regulating and anti-apoptotic cytokine, having tumour-inducing activities on both malignant and stromal cells. In recent years, the involvement of IL-6 in cancer has been closely investigated, highlighting NF-kB as a link. In mouse models of colitis, IL-6 is produced mainly by macrophages in response to intestinal injury and in an NF-kB-dependent manner. Inhibition of NF-kB in these cells resulted in reduced tumour growth, which was attributed to decreased IL-6 production by TAM [57,62,63]. In murine models of hepatocellular carcinoma, NF-kB inhibition in liver macrophages (Küpffer cells) resulted in marked delay of tumour onset [64,65].
Further, in tumour areas of low oxygen tension, where TAM usually accumulate, hypoxia induces a hypoxia-induced factor (HIF)-1alpha-mediated transcriptional programme, which includes also the activation of NF-kB in macrophages [66,67]. Collectively, these studies clearly emphasize the essential requirement of NF-kB activation in TAM for maintaining the inflammatory circuit(s) that promote tumour growth.
IL-6 activates the signal transducer and activator of transcription 3 (STAT3) pathway, another crucial mediator of the cancer-related inflammation. In tumour cells STAT3 induces the expression of genes important for cell cycle progression [such as cyclin D and proliferating cell nuclear antigen (PCNA)] and suppression of apoptosis (Bcl-XL, Bcl-2 and Mcl-1) [53,68,69]. In a mouse genetic model of pancreatic cancer, STAT3 activation was contributed mainly by macrophage-released IL-6; ablation of IL-6 production or STAT3 activation resulted in decreased carcinogenesis and inflammatory cell infiltration [70,71].
Chemokines and their receptors are key players in cancer-related inflammation, and TAM are a rich source of different inflammatory chemokines. The CXC chemokines bearing the ELR motif (e.g. CXCL1, CXCL2 and the most popular CXCL8) have potent angiogenic function and activate the neo-angiogenic switch [72,73]. CCL2 is highly produced by TAM and amplifies the recruitment of myeloid cells within tumours. Further, it may play an important role in the regulation of angiogenesis [72,74].
During the last decade there has been recognition that degraded/proteolytic fragments of extracellular matrix (ECM) molecules, or their aberrant expression, can sustain the activation of inflammatory cells and contribute to fuel inflammation at tumour sites. A cryptic peptide of laminin-10, a prominent component of basement membranes, is chemotactic for neutrophils and macrophages and induces the up-regulation of TNF, chemokines and MMP-9 [75]; versican activates Toll-like receptor (TLR)-2 and TLR-6 on TAM and stimulates the expression of inflammatory genes [76], while hyaluronan fragments trigger through TLR-4, TLR-2 and the CD44 receptor [77].
Pro-tumour functions of TAM
TAM influence fundamental aspects of tumour biology, as shown in Fig. 2. Among the well-documented pro-tumour functions of TAM is the production of trophic and activating factors for tumour and stromal cells [e.g. endothelial growth factor (EGF), fibroblast growth factor (FGF), VEGF, platelet-derived growth factor (PDGF), TGF-β]. These growth factors directly promote the proliferation of tumour cells and increase resistance to apoptotic stimuli [42,78–80]. TAM are also a major source of proteolytic enzymes that degrade the ECM, thus favouring the release of matrix-bound growth factors [42,81]. As mentioned above, IL-6 released by TAM plays a key role in sustaining the survival and proliferation of malignant cells in tumours of epithelial and haematopoietic origin [63,70,71,82,83].
Fig. 2.
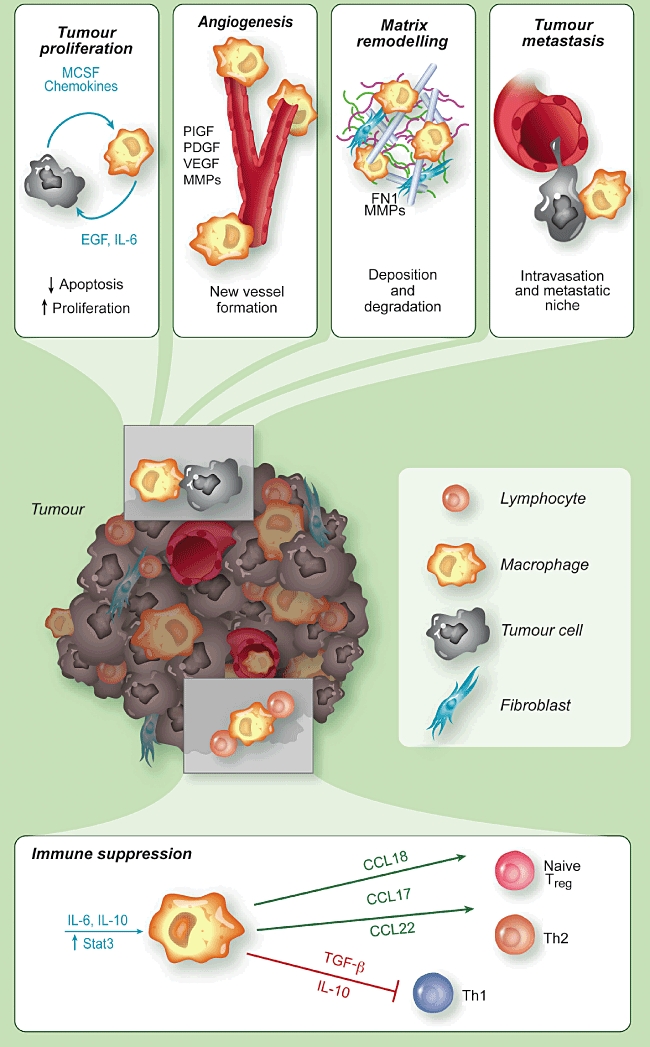
Pro-tumour functions of tumour-associated macrophages (TAM). TAM promote the survival of neoplastic cells from apoptotic stimuli and their proliferation, by producing several growth factors and cytokines [e.g. epithelial growth factor (EGF), interleukin (IL)-6], and the tumour angiogenesis, via vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs) and other angiogenic factors. TAM have an intense proteolityic activity and degrade the extracellular matrix, but also produce matrix proteins, such as fibronectin (FN1). They favour tumour cell intravasation and dissemination to distant sites. TAM have immune suppressive functions by producing IL-10 and transforming growth factor (TGF-β) which suppress T helper type 1 (Th1) lymphocytes, and by secreting chemokines (e.g. CCL17, CCL18, CCL22) which recruit lymphoid cells devoid of cytotoxic activity (Th2, naive lymphocytes) or having suppressive functions [regulatory T cells (Treg)].
TAM are key effectors of the ‘angiogenic switch’ where the balance between pro- and anti-angiogenic factors, commonly present in tissues, tilts towards a pro-angiogenic outcome [84–87]. In hypoxic conditions the transcription factor HIF-1alpha induces in TAM the production of VEGF and the angiogenic chemokine CXCL8 [88].
In addition, a unique subset of monocytes has been identified recently in tumours by the expression of the angiopoietin (Ang) receptor Tie-2, named Tie-2 expressing monocytes (TEMs) [89] Thus, TAM and related myeloid cells directly produce angiogenic factors and are also a major source of proteolytic enzymes which mobilize VEGF from extracellular matrix stores, indirectly sustaining tumour angiogenesis [90].
It is emerging that the expression pattern of genes that regulate iron homeostasis is distinct in polarized macrophages. For instance, M1 macrophages showed ferroportin repression and H ferritin induction, thus favouring iron sequestration, whereas M2 macrophages had an inverse expression profile (ferroportin up-regulation and down-regulation of H ferritin and haem oxygenase) and enhanced iron release [91]. Therefore, M1 cells mediate iron retention to control pathogen expansion during the acute phase of inflammation, while M2 cells donate iron that is important to tissue repair in the resolution phase. Interestingly, haem oxygenase 1 is highly expressed in TAM; it is tempting to speculate that the increased iron availability in the tumour microenvironment might represent a previously unknown mechanism that underlies the tumour-promoting activity of TAM [92].
TAM are probably the most active contributors to the incessant matrix remodelling present within tumours, as they produce several MMPs and other proteolytic enzymes [93]. Tumour cells exploit the ECM degradation mediated by TAM to invade locally, penetrate into vessels and disseminate to give distant metastasis [94]. TAM aiding cancer cell invasion have been visualized directly in experimental tumours in vivo by multi-photon microscopy: by using fluorescently labelled cells, Wyckoff and colleagues showed that tumour cell intravasation occurs next to perivascular macrophages in mammary tumours [94,95]. Further, it has been shown recently that cathepsin protease activity, by IL-4-stimulated TAM, promotes tumour invasion [96]. IL-4 is produced by tumour-infiltrating CD4 T cells and there is mounting evidence of its relevance in the polarization of macrophages with pro-tumour functions [14,16]. The chemokine CCL18 produced by TAM has been shown recently to play a critical role in promoting breast cancer invasiveness by activating tumour cell adherence to ECM [97].
We found recently that human TAM and in-vitro tumour-conditioned macrophages express high levels of the migration stimulation factor (MSF) [40], a truncated isoform of fibronectin [98]. Macrophage-secreted MSF displays potent chemotactic activity to tumour cells in vitro[40], confirming that the proinvasive phenotype of cancer cells is modulated by macrophage products released in the tumour microenvironment.
Further support to the concept of a reciprocal interaction between tumour cells and TAM was provided by a recent paper where SNAIL-expressing keratinocytes became locally invasive after macrophage recruitment elicited by M-CSF [99].
Tumour macrophages have the ability to suppress the adaptive immune response, thus contributing directly to the phenomenon of immune evasion of cancer [1]. TAM are poor antigen-presenting cells, have defective IL-12 secretion [100], produce IL-10 and TGF-β and inhibit T cell proliferation [27,36,101]. At least some of these immune-suppressive activities of TAM are mediated by over-activation of the transcription factor STAT3. In immune cells STAT3 enables suppression of tumour immunity by opposing STAT1-regulated Th1 anti-tumour immune responses and promoting the differentiation of immature myeloid cells with suppressive activity [102].
Myeloid-derived suppressor cells (MDSC), identified in tissues and lymphoid organs of tumour-bearing hosts, contribute to tumour-induced immune suppression [101,103–105]. These cells share properties and gene expression profiles with M2-polarized TAM, yet also display distinct features [106]. MDSC use two enzymes involved in the arginine metabolism to control T cell response: inducible nitric oxide synthase (NOS2) and arginase (Arg1), which deplete the milieu of arginine, causing peroxinitrite generation and T cell apoptosis [107].
The immune-suppressive activity of TAM is also exerted indirectly by their release of chemokines (e.g. CCL17 and CCL22) that preferentially attract Th1, Th2 lymphocytes and regulatory T cells (Treg), devoid of cytotoxic functions [72]. The chemokine CCL18, produced abundantly by TAM from human ovarian carcinoma [108], recruits naive T cells, which eventually turn into anergic cells within a microenvironment dominated by M2 macrophages and immature DC [109,110].
In line with the above experimental evidence, in the majority of human tumours high numbers of infiltrating TAM have been associated significantly with advanced tumours and poor patient prognosis [11,15,42,111]. There are, however, notable exceptions to this pro-tumour phenotype, probably dictated by TAM functional polarization. One such exception is human colorectal cancer, where some studies have reported that TAM density is associated with better prognosis [112–114]. The localization of TAM within colorectal cancers appears to be of primary importance: the number of peritumoural macrophages with high expression of co-stimulatory molecules (CD80 and CD86), but not of those within the cancer stroma, was associated with improved disease-free survival [115,116].
Specific TAM subsets identified by surface markers may have predictive values: in lung adenocarcinoma, the number of TAM CD204+ (scavenger receptor) showed a strong association with poor outcome, while the total CD68+ population did not [117].
Macrophage-related gene signatures have been identified in human tumours such as ovarian and breast cancer, soft tissue sarcoma and follicular B lymphoma [118–121]; in classic Hodgkin's lymphoma, tumours with increased number of CD68+TAM were associated significantly with shortened progression-free survival [122].
In recent years there has been increasing evidence that TAM and related myeloid cells with pro-angiogenic (Tie-2+ monocytes) and/or immune suppressive functions (MDSC) [101,103,104,123] are implicated strongly in the failure of anti-tumour therapies [124,125]. Accumulation of myeloid CD11b+Gr1+ cells (including TAM, MDSC and immature cells) in tumours renders them refractory to angiogenic blockade by VEGF antibodies [126]. This effect was traced to a VEGF-independent pathway driven by the granulocyte colony-stimulating factor (G-CSF)-induced protein Bv8 [127]. Further, depletion or pharmacological inhibition of TEMs in tumour-bearing mice markedly increased the efficacy of therapeutic treatment with a vascular-disrupting agent. Overall, these data indicate that myeloid cells, including TAM, considerably limit the clinical efficacy of anti-angiogenic therapies [124].
Targeting of TAM in tumours
The pro-tumour functions of TAM make these cells attractive targets of biological anti-cancer therapies. Macrophage depletion in experimental settings has been successful to limit tumour growth and metastatic spread [25,128,129], and to achieve better responses to conventional chemotherapy and anti-angiogenic therapy [101,103,123–125].
A number of studies have shown that the bisphosphonate clodronate encapsulated in liposomes is an efficient reagent for the depletion of macrophages in vivo. Clodronate-depletion of TAM in tumour-bearing mice resulted in reduced angiogenesis and decreased tumour growth and metastatization [130,131]. Moreover, the combination of clodronate with sorafenib, an available inhibitor of tyrosine protein kinases [e.g. VEGFR and platelet-derived growth factor receptor (PDGFR)], increased significantly the efficacy of sorafenib alone in a xenograft model of hepatocellular carcinoma. In clinical practice, bisphosphonates are employed to treat osteoporosis; current applications in cancer therapy include their use to treat skeletal metastases in multiple myeloma, prostate and breast cancer. Treatment with zoledronic acid was associated with a significant reduction of skeletal-related events and, possibly, direct apoptotic effects in tumour cells [132–134].
Our group reported that the anti-tumour agent of marine origin, trabectedin (Yondelis), was found unexpectedly to be highly cytotoxic to mononuclear phagocytes, including TAM. This cytotoxic effect is remarkably selective, as neutrophils and lymphocytes are not affected [135–137]. Trabectedin has now been registered in 2007 in Europe for the treatment of soft tissue sarcoma and in 2009 for ovarian cancer [136,138–140].
Another approach is to inhibit the recruitment of circulating monocytes in tumour tissues.
The M-CSF receptor (M-CSFR) is expressed exclusively by monocytes–macrophages. In patients with advanced tumours, clinical studies are under way to check the feasibility and possibly clinical efficacy of inhibitors to the CSF-1R. Among the many chemokines expressed in the tumour microenvironment, CCL2 (or monocyte chemotactic protein-1) occupies a prominent role and has been selected for therapeutic purposes. Preclinical studies have shown that anti-CCL2 antibodies or antagonists to its receptor CCR2, given in combination with chemotherapy, were able to induce tumour regression and yielded to improved survival in mouse models of prostate cancer or colitis-associated carcinogenesis [141–143].
A third and more recent approach is to ‘re-educate’ TAM to exert anti-tumour responses protective for the host, ideally by using factors able to revert TAM into M1-macrophages, with potential anti-tumour activity. It is becoming accepted that macrophages are flexible and able to switch from one polarization state to the other [144]. This was achieved in experimental mouse tumours, by injecting the TLR-9 agonist cytosine–guanine dinucleotide-oligodeoxynucleotide (CpG-ODN), coupled with anti-IL-10 receptor [145] or the chemokine CCL16 [146]. CpG-ODN also synergized with an agonist anti-CD40 mononuclear antibody (mAb) to revert TAM displaying anti-tumour activity [147].
A remarkable anti-tumour effect of redirected macrophages has been reported recently in human pancreatic cancer with the use of agonist anti-CD40 mAb [148]. Still in the same direction, a recent report showed that the plasma protein histidine-rich glycoprotein (HRG), known for its inhibitory effects on angiogenesis [149,150], is able to skew TAM polarization into M1-like phenotype by down-regulation of the placental growth factor (PlGF), a member of the VEGF family. In mice, HRG promoted anti-tumour immune responses and normalization of the vessel network [151].
The use of IL-12 in cancer patients is now under clinical investigation. This cytokine is pivotal for the simulation of Th1 circuits of adaptive immunity, leading to the production of IFN-γ. In experimental mouse tumour models, IL-12 injection reduced the tumour-supportive activities of TAM, suggestive of an M1 polarization [152]. Along the same line, therapies inhibiting IL-6, a main product of TAM, with specific monoclonal antibodies, may result in reduction of their M2-skewed phenotype.
Conclusion
The last decade witnessed a growing understanding of the promoting role of chronic inflammation in cancer initiation and progression [42,50,51,56,153]. TAM are present in large numbers in tumour tissues and are key promoters of cancer-related inflammation [10,11,13–15]. They produce a host of growth factors and inflammatory cytokines that contribute to tumour cell survival, development of full-blown angiogenesis and resistance to therapies. In addition, immunosuppressive mediators released by TAM and related myeloid cells extinguish host-mediated anti-tumour responses and ease tumour progression. Therefore, TAM appear to be attractive candidates of future therapeutic strategies. Depletion of the disloyal TAM in tumours, or their ‘re-education’ to potential anti-tumour effectors, may contribute to increase the efficacy of current anti-tumour therapies.
Acknowledgments
The Authors are supported by grants from the Italian Association for Cancer Research (AIRC) and from Regione Lombardia ‘NEPENTE’ under Institutional Agreement n. 14501A.
Disclosure
The authors have no financial conflict of interest.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–83. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 5.Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–84. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 7.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–7. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 12.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 14.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HW, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: priming for protumoral functions. Cell Cycle. 2010;9:4824–35. doi: 10.4161/cc.9.24.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 20.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 21.Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol. 2009;86:1105–9. doi: 10.1189/jlb.0209073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89:557–63. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 24.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–70. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 26.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 27.Allavena P, Sica A, Garlanda C, Mantovani A. The yin–yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 28.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 32.Bottazzi B, Polentarutti N, Acero R, et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–12. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 33.Zachariae CO, Anderson AO, Thompson HL, et al. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–82. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kacinski BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995;27:79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- 35.Smith HO, Anderson PS, Kuo DY, et al. The role of colony-stimulating factor 1 and its receptor in the etiopathogenesis of endometrial adenocarcinoma. Clin Cancer Res. 1995;1:313–25. [PubMed] [Google Scholar]
- 36.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 37.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, et al. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 40.Solinas G, Schiarea S, Liguori M, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–52. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–22. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 43.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 44.Biswas SK, Gangi L, Paul S, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–22. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 45.Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702–12. doi: 10.4049/jimmunol.0902360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–46. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allavena P, Chieppa M, Bianchi G, et al. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010;2010:547179. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 49.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 53.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–31. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 55.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 56.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 58.Mancino A, Lawrence T. Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res. 2010;16:784–9. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas SK, Lewis CE. NF-kappaB as a central regulator of macrophage function in tumors. J Leukoc Biol. 2010;88:877–84. doi: 10.1189/jlb.0310153. [DOI] [PubMed] [Google Scholar]
- 60.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 61.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 62.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 66.Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang HY, Hughes R, Murdoch C, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–59. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamb J, Ramaswamy S, Ford HL, et al. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–34. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Li PK, Li C, Lin J. Inhibition of STAT3 signaling blocks the anti-apoptotic activity of IL-6 in human liver cancer cells. J Biol Chem. 2010;285:27429–39. doi: 10.1074/jbc.M110.142752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukuda A, Wang SC, Morris JPt, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–44. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 75.Adair-Kirk TL, Atkinson JJ, Kelley DG, Arch RH, Miner JH, Senior RM. A chemotactic peptide from laminin alpha 5 functions as a regulator of inflammatory immune responses via TNF alpha-mediated signaling. J Immunol. 2005;174:1621–9. doi: 10.4049/jimmunol.174.3.1621. [DOI] [PubMed] [Google Scholar]
- 76.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 78.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 79.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–9. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 80.Moussai D, Mitsui H, Pettersen JS, et al. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J Invest Dermatol. 2011;131:229–36. doi: 10.1038/jid.2010.266. [DOI] [PubMed] [Google Scholar]
- 81.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Ribatti D, Vacca A. The role of monocytes–macrophages in vasculogenesis in multiple myeloma. Leukemia. 2009;23:1535–6. doi: 10.1038/leu.2009.55. [DOI] [PubMed] [Google Scholar]
- 84.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 85.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19:329–37. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS ONE. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–8. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 89.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Recalcati S, Locati M, Marini A, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40:824–35. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]
- 92.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32:241–7. doi: 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 93.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–37. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wyckoff JB, Wang Y, Lin EY, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 95.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–30. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gocheva V, Wang HW, Gadea BB, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J, Yao Y, Gong C, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schor SL, Ellis IR, Jones SJ, et al. Migration-stimulating factor: a genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res. 2003;63:8827–36. [PubMed] [Google Scholar]
- 99.Du F, Nakamura Y, Tan TL, et al. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010;70:10080–9. doi: 10.1158/0008-5472.CAN-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sica A, Saccani A, Bottazzi B, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762–7. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 101.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 102.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bianchi G, Borgonovo G, Pistoia V, Raffaghello L. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histol Histopathol. 2011;26:941–51. doi: 10.14670/HH-26.941. [DOI] [PubMed] [Google Scholar]
- 105.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–6. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 106.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 108.Schutyser E, Struyf S, Proost P, et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem. 2002;277:24584–93. doi: 10.1074/jbc.M112275200. [DOI] [PubMed] [Google Scholar]
- 109.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 110.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 111.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 112.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–9. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 113.Ohno S, Inagawa H, Dhar DK, et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res. 2003;23:5015–22. [PubMed] [Google Scholar]
- 114.Sconocchia G, Zlobec I, Lugli A, et al. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128:2663–72. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible antitumor immunity? Lab Invest. 1997;77:231–41. [PubMed] [Google Scholar]
- 116.Sugita J, Ohtani H, Mizoi T, et al. Close association between Fas ligand (FasL; CD95L)-positive tumor-associated macrophages and apoptotic cancer cells along invasive margin of colorectal carcinoma: a proposal on tumor-host interactions. Jpn J Cancer Res. 2002;93:320–8. doi: 10.1111/j.1349-7006.2002.tb02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohtaki Y, Ishii G, Nagai K, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5:1507–15. doi: 10.1097/JTO.0b013e3181eba692. [DOI] [PubMed] [Google Scholar]
- 118.Beck AH, Espinosa I, Edris B, et al. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res. 2009;15:778–87. doi: 10.1158/1078-0432.CCR-08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–27. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 120.Ghassabeh GH, De Baetselier P, Brys L, et al. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108:575–83. doi: 10.1182/blood-2005-04-1485. [DOI] [PubMed] [Google Scholar]
- 121.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–24. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 124.Welford AF, Biziato D, Coffelt SB, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–73. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferrara N. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr Opin Hematol. 2010;17:219–24. doi: 10.1097/MOH.0b013e3283386660. [DOI] [PubMed] [Google Scholar]
- 126.Shojaei F, Ferrara N. Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res. 2008;68:5501–4. doi: 10.1158/0008-5472.CAN-08-0925. [DOI] [PubMed] [Google Scholar]
- 127.Shojaei F, Wu X, Zhong C, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 128.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aharinejad S, Sioud M, Lucas T, Abraham D. Targeting stromal-cancer cell interactions with siRNAs. Methods Mol Biol. 2009;487:243–66. doi: 10.1007/978-1-60327-547-7_12. [DOI] [PubMed] [Google Scholar]
- 130.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brown HK, Holen I. Anti-tumour effects of bisphosphonates – what have we learned from in vivo models? Curr Cancer Drug Targets. 2009;9:807–23. doi: 10.2174/156800909789760339. [DOI] [PubMed] [Google Scholar]
- 132.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–99. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–30. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 134.Martin CK, Werbeck JL, Thudi NK, et al. Zoledronic acid reduces bone loss and tumor growth in an orthotopic xenograft model of osteolytic oral squamous cell carcinoma. Cancer Res. 2010;70:8607–16. doi: 10.1158/0008-5472.CAN-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Allavena P, Signorelli M, Chieppa M, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–71. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 136.D'Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther. 2010;9:2157–63. doi: 10.1158/1535-7163.MCT-10-0263. [DOI] [PubMed] [Google Scholar]
- 137.Germano G, Frapolli R, Simone M, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70:2235–44. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 138.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 139.Carter NJ, Keam SJ. Trabectedin: a review of its use in soft tissue sarcoma and ovarian cancer. Drugs. 2010;70:355–76. doi: 10.2165/11202860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 140.Monk BJ, Herzog TJ, Kaye SB, et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28:3107–14. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]
- 141.Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–24. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 142.Li X, Loberg R, Liao J, et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–92. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Popivanova BK, Kostadinova FI, Furuichi K, et al. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–92. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 144.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–27. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–46. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 146.Cappello P, Caorsi C, Bosticardo M, et al. CCL16/LEC powerfully triggers effector and antigen-presenting functions of macrophages and enhances T cell cytotoxicity. J Leukoc Biol. 2004;75:135–42. doi: 10.1189/jlb.0403146. [DOI] [PubMed] [Google Scholar]
- 147.Buhtoiarov IN, Sondel PM, Wigginton JM, et al. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology. 2011;132:226–39. doi: 10.1111/j.1365-2567.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–16. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Juarez JC, Guan X, Shipulina NV, et al. Histidine-proline-rich glycoprotein has potent antiangiogenic activity mediated through the histidine-proline-rich domain. Cancer Res. 2002;62:5344–50. [PubMed] [Google Scholar]
- 150.Olsson AK, Larsson H, Dixelius J, et al. A fragment of histidine-rich glycoprotein is a potent inhibitor of tumor vascularization. Cancer Res. 2004;64:599–605. doi: 10.1158/0008-5472.can-03-1941. [DOI] [PubMed] [Google Scholar]
- 151.Rolny C, Mazzone M, Tugues S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 152.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–62. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 153.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]


