Abstract
Mutations in the lamin A/C gene (LMNA) encoding A-type nuclear lamins cause dilated cardiomyopathy. We have uncovered a novel connection between these mutations and hyper-activation of the extracellular signal-regulated kinase 1/2 and c-jun N-terminal kinase branches of the mitogen-activated protein kinase signaling pathway in a mouse model of the disease. This discovery has identified targets that can be inhibited by drugs thatimprove heart function and prevent fibrosis.
LMNA Cardiomyopathy
Dilated cardiomyopathy is characterized by increased myocardial mass and volume with thinning and stretching of the ventricular walls; this compromises cardiac contractility, ultimately resulting in poor left ventricular function (Luk et al. 2008). Genetic disorders are responsible for at least 20% of dilated cardiomyopathies (Michels et al., 1992). Causative mutations occur in genes that encode components of a wide variety of cellular structures, including the contractile apparatus, the force transduction apparatus and the nuclear envelope (Morita et al., 2005). One such gene is LMNA encoding Atype lamins of the nuclear envelope (Bonne et al. 1999, Fatkin et al. 1999).
LMNA may be the most prevalent dilated cardiomyopathy gene, as mutations appear to be responsible for approximately 8% of inherited cases (Taylor et al. 2003, Millat et al. 2011). Affected individuals sometimes have associated skeletal muscular dystrophy, most frequently the Emery-Deifuss phenotype (Bonne et al. 1999). The onset of symptoms in LMNA cardiomyopathy is variable, ranging from the first to sixth decade of life and occurring most frequently in the third decade (Ben Yaou et al. 2006). LMNA cardiomyopathy has a more aggressive course than most other inherited dilated cardiomyopathies (Taylor et al. 2003, van Berlo et al. 2005, Pasotti et al. 2008). In addition to left ventricular dilatation, affected individuals have early atrioventricular conduction block followed by ventricular arrhythmias. Arrhythmias gradually become more frequent with age, potentially leading to sudden death (Sanna et al. 2003). While sudden death from arrhythmias may be prevented by implantation of a pacemaker and defibrillator, progressive heart failure eventually becomes resistant to treatment (van Berlo et al. 2005, Meune et al., 2006, Golzio et al. 2007). No therapies are curative and heart transplantation is often necessary.
A-type Nuclear Lamins
LMNA is located on human chromosome 1q21.2–21.3 and encodes the A-type nuclear lamins, of which lamin A and lamin C are the major isoforms expressed in somatic cells (Lin and Worman 1993, Wydner et al. 1996). Lamins are intermediate filament proteins that polymerize to form the nuclear lamina, a fibrous meshwork underlining the inner nuclear membrane of most metazoan cells (Aebi et al. 1986, Fisher et al. 1986, McKeon et al. 1986). The nuclear lamina is attached to the inner nuclear membrane via interactions with integral proteins. The lamina also interacts with the cytoskeleton through a multi-protein complex called the linker of nucleoskeleton and cytoskeleton complex (Stewart et al. 2007b). A-type lamins appear to be essential for maintaining normal nuclear and cytoskeletal mechanics and stress-induced activation of transcription (Broers et al. 2004, Lammerding et al. 2004). These biomechanical functions may be particularly significant in contractile cells such as cardiomyocytes. Lamins are also believed to be involved in several cellular processes such as chromatin organization, gene regulation, DNA replication and RNA splicing (Dechat et al. 2008). The pleiotropic functions of A-type lamins are perhaps best appreciated by the fact that LMNA mutations different from (and rarer than) those causing cardiomyopathy cause phenotypically diverse diseases including partial lipodystrophy, peripheral neuropathy and Hutchinson-Gilford progeria syndrome (Worman et al. 2009).
Mitogen-activated Protein (MAP) Kinases in LMNA Cardiomyopathy
A-type lamins are expressed in most differentiated somatic cells in virtually all tissues, making it difficult to readily explain the tissue-selective defects that result from LMNA mutations. However, clues about the in vivo functions of A-type lamins have been gained from studies of mouse models in which their gene has been targeted by homologous recombination to generate either knockout or knock-in mutations (Stewart et al. 2007a). One of these mouse models has provided data that partially explain the pathogenesis of LMNA cardiomyopathy and give clues about potential therapies.
Male LmnaH222P/H222P mice develop left ventricular dilatation and decreased ejection fraction at approximately 10 weeks of age that progressively worsen with subsequent cardiac fibrosis followed by death at a median age of approximately 26 weeks (Arimura et al. 2005). This mimics, albeit on a different timescale, what occurs in human subjects with LMNA cardiomyopathy. We therefore examined the transcriptome in hearts of LmnaH222P/H222P mice using Affymetrix GeneChips (Muchir et al. 2007). We found a prominent increase in the expression of genes involved in fibrosis and in the “fetal gene program” that is induced in stressed and failing hearts (Schwartz et al. 1993). In addition, genes encoding proteins in the MAP kinase signaling pathway demonstrated significantly altered expression compared to controls. Further analysis revealed hyper-activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) branches of the MAP kinase pathway and increased expression of genes stimulated by these enzymes. Activation of the same signaling cascades occurs in dilated hearts of human subjects with late-stage cardiac failure of several different causes (Haq et al. 2001). However, in hearts of LmnaH222P/H222P mice, there is increased ERK1/2 and JNK signaling as early as four weeks of age, before the onset of clinically detectable signs of cardiac dysfunction (Muchir et al. 2007).
MAP kinase signaling is activated by mitogens, growth factors and cytokines that bind to cell surface receptors as well as by mechanical and chemical stresses independent of receptors (Seger and Krebs 1995, Chang and Karin 2001). These stimuli induce the phosphorylation (activation) of enzymes that through a cascade of sequential reactions leads to phosphorylation a MAP kinase. For example, Raf phosphorylates MAP kinase/ERK kinase (MEK) 1/2, which directly phosphorylates ERK1/2. Similarly, MAP kinase kinase kinase 1 phosphorylates MEK4 and MEK7, which phosphorylate JNK. Activated ERK1/2 and JNK in turn phosphorylate a wide array of substrates including transcription factors, other protein kinases and cytoskeletal components. This affects gene expression, crosstalk with other signal transduction pathways and the control of processes such as cell proliferation, cell death and acute stress responses. Members of the dual-specificity phosphatase family dephosphorylate MAP kinases, leading to deactivation of signaling (Theodosiou and Ashworth 2002).
The simultaneous activation of multiple signaling pathways in dilated failing hearts has made deciphering the specific pathogenic roles of the different branches of the MAP kinase pathway difficult. The ERK1/2 branch has been classically associated with the hypertrophic response, during which cardiomyocytes increase in size without cell division (McKinsey and Olsen 1999, Molkentin 2004). While this may initially be compensatory in nature, a prolonged hypertrophic response can become detrimental, leading to dilated cardiomyopathy. JNK branch hyper-activation is thought to influence contractile function, extracellular matrix remodeling, intercellular communication and metabolic regulation (Petrich and Wang 2004). Examination of transcriptomes in hearts of transgenic mice with constitutive hyper-activation of ERK1/2 or JNK signaling has revealed distinct gene expression patterns with several clearly overlapping features that change over time (Mitchell et al. 2006). So while the specific contributions of increased ERK1/2 and JNK signaling to the development of dilated cardiomyopathy remain to be elucidated, there is little doubt that it plays an important pathogenic role. The fact that ERK1/2 and JNK are hyper-activated in a mouse model of LMNA cardiomyopathy prior to the onset of clinical disease lead us to hypothesize that inhibiting their activities to restore a more physiological balance would be beneficial.
Inhibition of MAP kinases as Treatment for LMNA Cardiomyopathy
To test our hypothesis, we treated male LmnaH222P/H222P mice with PD098059, which inhibits MEK1/2 and blocks ERK1/2 phosphorylation. We also treated them with SP600125, which inhibits JNK activity. We first treated the mice starting at 8 weeks of age, prior to the onset of clinically detectable cardiac abnormalities, and analyzed them at 16 weeks (Muchir et al. 2009, Wu et al. 2010). Systemic administration of either of these compounds inhibited their respective targets in heart by approximately 50%. At 16 weeks of age, placebo-treated LmnaH222P/H222P mice had left ventricular dilatation and decreased ejection fraction whereas these parameters were normal in mice treated with PD098058 or SP600125. Treatment with these inhibitors also blocked increased cardiac expression of RNAs encoding natriuretic peptide precursors and the induction of elements of the “fetal gene program” that occurred in placebo-treated mice.
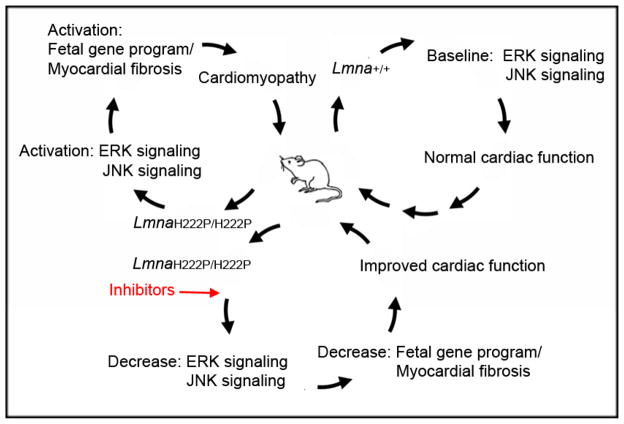
As treatment for cardiomyopathy in human subjects may more likely be administered after the onset of symptoms or measurable cardiac abnormalities, we next treated male LmnaH222P/H222P mice with MEK1/2 or JNK inhibitors starting at 16 weeks of age (Wu et al. 2011). At this time, male LmnaH222P/H222P mice have left ventricular dilatation and an ejection fraction approximately 70% that of wild type mice. We then analyzed the mice at 20 weeks. Treatment with PD98059 or SP600125 prevented further left ventricular end-systolic dilatation, increased ejection fraction, reduced cardiac expression of RNAs encoding natriuretic peptide precursors and reversed elements of the “fetal gene program” compared to placebo-treated mice. LmnaH222P/2PH222P mice receiving either of these inhibitors also had significantly less cardiac fibrosis than those receiving placebo, indicating that blocking ERK1/2 or JNK signaling could prevent an irreversible late-stage feature of LMNA cardiomyopathy. Overall, this research showed that inhibiting ERK1/2 or JNK signaling has beneficial effects on heart function and fibrosis in a mouse model of LMNA cardiomyopathy (Figure 1).
Figure 1.
Diagram of molecular and cellular events linking an Lmna point mutation in mice to MAP kinase activation and the development of cardiomyopathy. In Lmna+/+ mice (upper right), baseline ERK1/2 (ERK) and JNK signaling maintain normal cardiac function. In LmnaH222P/H222P mice (upper left), there is hyper-activation of ERK1/2 and JNK signaling, leading to induction of a “fetal gene program” and myocardial fibrosis that over time results in cardiomyopathy. Treatment of LmnaH222P/H222P mice with inhibitors of ERK1/2 and JNK signaling (bottom) reverse induced elements of the “fetal gene program” and prevent myocardial fibrosis, leading to improved cardiac function.
Remaining Questions
An intriguing riddle is how certain alterations in A-type lamins, which are expressed in virtually all differentiated somatic cells, activate MAP kinase signaling pathways specifically in striated muscle (Muchir et al. 2007). Research suggests that LMNA mutations can be divided into two categories - those leading to functionally hypoactive A-type lamins and those leading to expression of “toxic” variants, with mutations causing cardiomyopathy falling into the former category (Davies et al. 2011). One study has shown that ERK1/2 interacts with A-type lamins at the nuclear periphery (Gonzales et al. 2008), suggesting that phosphorylated ERK1/2 may translocate to the nucleus where binding to A-type lamins inhibits accessibility to other nuclear substrates. This can lead to the hypothesis that functionally hypoactive A-type lamins reduce a nuclear envelope-mediated buffering of ERK1/2 activity on gene expression. Given their role in maintaining normal cellular mechanics, another hypothesis is that functionally hypoactive A-type lamins make contractile cells more susceptible to stress-induced damage, which activates ERK1/2 and JNK signaling. The crosstalk between these MAP kinases and other signal transduction pathways, as well as the deactivation of ERK1/2 and JNK, also require further study in LMNA cardiomyopathy.
A key remaining question is whether discoveries showing that inhibitors of MAP kinase signaling are beneficial in a mouse model of LMNA cardiomyopathy can be translated to humans. Drug development programs have produced highly specific, orally bioavailable inhibitors of MEK1/2 that are being studied in clinical trials for cancer (Adjei et al. 2008, Bekaii-Saab et al. 2011, O’Neil et al. 2011). Whether these drugs can be safely administered at appropriate doses over prolonged periods to effectively treat human subjects with LMNA cardiomyopathy needs to be determined. Nonetheless, the availability of such drugs makes clinical trials for this debilitating cardiovascular disease an imminent and exciting possibility.
Acknowledgments
Supported by grants from the National Institutes of Health (AR048997, NS059352), the Muscular Dystrophy Association (MDA172222) and l’Association Française contre les Myopathies and by a BioAccelerate NYC Prize from the New York City Partnership, Inc.
Footnotes
Potential Conflict of Interest
Drs. Worman and Muchir are inventors on a pending PCT patent application on methods for treating and/or preventing cardiomyopathies by ERK and JNK inhibition filed by the trustees of Columbia University in the City of New York.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetics and pharmacodynamics study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Arimura T, Helbling-Leclerc A, Massart C, et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancer. J Clin Oncol. 2011;29:2357–2363. doi: 10.1200/JCO.2010.33.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Yaou R, Gueneau L, Demay L, et al. Heart involvement in lamin A/C related diseases. Arch Mal Coeur Vaiss. 2006;99:848–855. [PubMed] [Google Scholar]
- Bonne G, Di Barletta MR, Varnous S, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- Broers JL, Peeters EA, Kuijpers HJ, et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- Chang Y, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Davies BSJ, Coffinier C, Yang SH, et al. Investigating the purpose of prelamin A processing. Nucleus. 2011;2:4–9. doi: 10.4161/nucl.2.1.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatkin D, MacRae C, Sasaki T, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci USA. 1986;83:6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio PG, Chiribiri A, Gaita F. Unexpected sudden death avoided by implantable cardioverter-defibrillator in Emery-Dreifuss patient. Europace. 2007;9:1158–1160. doi: 10.1093/europace/eum236. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Navarro-Puche A, Casar B, Crespo P, Andres V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J Cell Biol. 2008;183:653–666. doi: 10.1083/jcb.200805049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq S, Choukroun G, Lim H, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, et al. Lamin A/C deficiency causes defective nuclear mechanics and machanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- Luk A, Ahn E, Soor GS, Butany J. Dilated cardiomyopathy: a review. J Clin Pathol. 2009;62:219–225. doi: 10.1136/jcp.2008.060731. [DOI] [PubMed] [Google Scholar]
- McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Olsen EN. Cardiac hypertrophy: sorting out the circuitry. Curr Opin Genet Dev. 1999;9:267–274. doi: 10.1016/s0959-437x(99)80040-9. [DOI] [PubMed] [Google Scholar]
- Meune C, Van Berlo JH, Anselme F, et al. Primary prevention of sudden death in patients with lamin A/C gene mutations. N Engl J Med. 2006;354:209–210. doi: 10.1056/NEJMc052632. [DOI] [PubMed] [Google Scholar]
- Michels VV, Moll P, Miller FA, et al. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992;326:77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- Millat G, Bouvagnet P, Chevalier P, et al. Clinical and mutational spectrum in a cohort of 105 unrelated patients with dilated cardiomyopathy. Eur J Med Genet. 2011;54:e570–575. doi: 10.1016/j.ejmg.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Ota A, Foster W, et al. Distinct gene expression profiles in adult mouse heart following targeted MAP kinase activation. Physiol Genomics. 2006;25:50–59. doi: 10.1152/physiolgenomics.00224.2005. [DOI] [PubMed] [Google Scholar]
- Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Morita H, Seidman J, Seidman CE. Genetic causes of human heart failure. J Clin Invest. 2005;115:518–526. doi: 10.1172/JCI200524351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Pavlidis P, Decostre V, et al. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchir A, Shan J, Bonne G, et al. Inhibition of extracellular signal-regulate kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet. 2009;18:241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil BH, Goff LW, Kauh JS, et al. Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcionoma. J Clin Oncol. 2011;29:2350–2356. doi: 10.1200/JCO.2010.33.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasotti M, Klersy C, Pilotto A, et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52:1250–1260. doi: 10.1016/j.jacc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- Petrich BG, Wang Y. Stress-activated MAP kinases in cardiac remodeling and heart failure; new insights from transgenic studies. Trends Cardiovasc Med. 2004;14:50–55. doi: 10.1016/j.tcm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Sanna T, Dello Russo A, Toniolo D, et al. Cardiac features of Emery-Dreifuss muscular dystrophy caused by lamin A/C gene mutations. Eur Heart J. 2003;24:2227–2236. doi: 10.1016/j.ehj.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Schwartz K, Chassagne C, Boheler KR. The molecular biology of heart failure. J Am Coll Cardiol. 1993;22:30A–33A. doi: 10.1016/0735-1097(93)90459-e. [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp Cell Res. 2007a;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007b;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- Taylor MRG, Fain PR, Sinagra G, et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol. 2003;41:771–780. doi: 10.1016/s0735-1097(02)02954-6. [DOI] [PubMed] [Google Scholar]
- Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3:REVIEWS3009. doi: 10.1186/gb-2002-3-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, de Voogt WG, van der Kooi AJ, et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med. 2005;83:79–83. doi: 10.1007/s00109-004-0589-1. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Muchir A, Shan J, et al. Mitogen activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by lamin A/C gene mutation. Circulation. 2011;123:53–61. doi: 10.1161/CIRCULATIONAHA.110.970673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Shan J, Bonne G, et al. Pharmacological inhibition of c-Jun N-terminal kinase signaling prevents cardiomyopathy caused by mutation in LMNA gene. Biochim Biophys Acta. 2010;1802:632–638. doi: 10.1016/j.bbadis.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydner KL, McNeil JA, Lin F, et al. Chromosomal assignment of human nuclear envelope protein genes LMNA, LMNB1, and LBR by fluorescence in situ hybridization. Genomics. 1996;32:474–478. doi: 10.1006/geno.1996.0146. [DOI] [PubMed] [Google Scholar]