Abstract
OBJECTIVE
Long-term dietary intervention frequently induces a rapid weight decline followed by weight stabilization/regain. Here, we sought to identify adipokine biomarkers that may reflect continued beneficial effects of dieting despite partial weight regain.
RESEARCH DESIGN AND METHODS
We analyzed the dynamics of fasting serum levels of 12 traditional metabolic biomarkers and novel adipokines among 322 participants in the 2-year Dietary Intervention Randomized Controlled Trial (DIRECT) of low-fat, Mediterranean, or low-carbohydrate diets for weight loss.
RESULTS
We identified two distinct patterns: Pattern A includes biomarkers (insulin, triglycerides, leptin, chemerin, monocyte chemoattractant protein 1, and retinol-binding protein 4) whose dynamics tightly correspond to changes in body weight, with the trend during the weight loss phase (months 0–6) going in the opposite direction to that in the weight maintenance/regain phase (months 7–24) (P < 0.05 between phases, all biomarkers). Pattern B includes biomarkers (high molecular weight adiponectin, HDL cholesterol [HDL-C], high-sensitivity C-reactive protein [hsCRP], fetuin-A, progranulin, and vaspin) that displayed a continued, cumulative improvement (P < 0.05 compared with baseline, all biomarkers) throughout the intervention. These patterns were consistent across sex, diabetic groups, and diet groups, although the magnitude of change varied. Hierarchical analysis suggested similar clusters, revealing that the dynamic of leptin (pattern A) was most closely linked to weight change and that the dynamic of hsCRP best typified pattern B.
CONCLUSIONS
hsCRP, HDL-C, adiponectin, fetuin-A, progranulin, and vaspin levels display a continued long-term improvement despite partial weight regain. This may likely reflect either a delayed effect of the initial weight loss or a continuous beneficial response to switching to healthier dietary patterns.
Long-term dietary intervention typically induces a rapid weight decline that stabilizes by 6 months. This weight loss phase is followed by weight stabilization or partial to full weight regain despite continued dieting (1). Although it is clear that weight cycling as a result of repeated attempts to lose weight greatly diminishes the beneficial effects of healthier dietary habits (2), whether continued long-term dieting can indeed improve cardiovascular and metabolic risk even beyond weight loss and despite weight regain has remained unclear. Furthermore, it is not well established whether certain biomarkers primarily reflect weight changes or correspond to the continued dieting in long-term dietary intervention.
Here we sought to identify adipokines and other biomarkers that may reflect continued beneficial effects of dieting, despite partial weight regain, using new analyses from the Dietary Intervention Randomized Controlled Trial (DIRECT) (3). This 2-year weight loss trial was characterized by high retention rates (95% after 1 year and 85% after 2 years) and a high level of proven adherence (4) to the three distinct dietary strategies: low-fat, Mediterranean, and low-carbohydrate diets (3). Although the three interventions were different, in all three groups, the participants similarly increased the consumption of vegetables and decreased intake of snacks, sugared beverages, and processed foods, suggesting a common denominator of healthful dietary patterns across all groups compared with baseline (5). Diet intervention in the DIRECT study resulted in two segments: a rapid weight loss phase during the first 6 months and a partial regain/plateau phase during the subsequent 18 months of intervention (3,6).
To determine the correspondence between weight change dynamics and the change among biomarkers, we used both a nonbiased mathematical modeling approach and qualitative analysis, assessing traditional biomarkers (HDL cholesterol [HDL-C], triglycerides [TGs], insulin, high-sensitivity C-reactive protein [hsCRP], high molecular weight [HMW] adiponectin, and leptin) and more recently discovered adipokines, including chemerin (7), monocyte chemoattractant protein 1 (MCP-1) (8), progranulin (9), fetuin-A (10), retinol-binding protein 4 (RBP4) (11–13), and vaspin (14).
RESEARCH DESIGN AND METHODS
The 2-year DIRECT
The DIRECT, previously described in detail (3), was conducted in one phase between July 2005 and June 2007 in a research center workplace in Dimona, Israel, among 322 participants. In brief, the trial compared the effect of low-fat, restricted-calorie diet; Mediterranean, restricted-calorie diet; or low-carbohydrate, non–restricted calorie diet on long-term weight loss and various health parameters. The participants were randomized by strata of sex, age (below or above the median), BMI (below or above the median), history of coronary heart disease (yes/no), type 2 diabetes mellitus (yes/no), and current use of statins (none, <1 year, or ≥1 year). Eligible participants were aged 40–65 years with BMI ≥27 kg/m2. In addition, individuals with type 2 diabetes mellitus or coronary heart disease were eligible regardless of age or BMI. Pregnant or lactating women and participants with a serum creatinine ≥2 mg/dL (≥176 μmol/L), liver dysfunction (greater than or equal to twofold higher than the upper limit of normal in alanine aminotransferase or aspartate aminotransferase), intestinal problems that would prevent adherence to any of the test diets, or active cancer were excluded. The participants received no financial compensation or gifts for participating. The study was approved and monitored by the human subjects committee of Soroka Medical Center and Ben-Gurion University. Each participant provided written informed consent.
Assessment of dietary adherence
The interventions were reported in detail previously (3). After analyzing the recipes using the Israeli nutritional database, we color coded the labels of all food dishes, for each diet type, that were served daily in the central workplace cafeteria during the 2-year span of the trial to promote dietary adherence (3).
Adherence to the diets was evaluated by a validated food-frequency questionnaire (FFQ) (15) that included 127 food items and three portion-size pictures for 17 items (16). A subgroup of participants completed two repeated 24-h dietary recalls to verify absolute intake. At baseline and at 6, 12, and 24 months of follow-up, the questionnaires were self-administered electronically through the workplace intranet. The electronic questionnaire helped to ensure completeness of the data by prompting the participant when a question was not answered, and it permitted rapid automated reporting by the group dietitians. The 15% of participants who requested aid in completing the questionnaires were assisted by the study nurse.
The FFQs (3) revealed that the Mediterranean diet group consumed the highest dietary fiber and monounsaturated-to-saturated fat ratio (P < 0.05) and that the low-carbohydrate diet group consumed the least carbohydrates and the most fat, protein, and cholesterol and had a higher percentage of positive urinary ketone determinations (P < 0.05). Caloric deficit was similar among groups. The food diaries obtained from a subset of participants during the weight loss phase (4) further showed distinct differences between low-carbohydrate and low-fat diet, respectively, in fat intake (41 vs. 26%), carbohydrate intake (28 vs. 48%), and dietary cholesterol intake (358 vs. 174 mg/day).
Measurement of outcome parameters
Body weight was measured every month without shoes to the nearest 0.1 kg. Height was measured to the nearest millimeter with the use of a wall-mounted stadiometer at baseline for BMI determination. A blood sample was drawn by venipuncture at 8 a.m., after a 12-h fast, at baseline and at 6 and 24 months and stored at −80°C. Blood biomarkers were analyzed in Leipzig University Laboratories, Leipzig, Germany. Plasma insulin, serum hsCRP, total cholesterol, HDL-C, LDL cholesterol, TGs, leptin, and HMW total adiponectin were measured as described previously (3). RBP4 was measured using an ELISA (AdipoGen, Seoul, Korea). Serum MCP-1 concentrations were measured by immunoassay system (Quantikine Human MCP-1 Immunoassay; R&D Systems Inc., Minneapolis, MN). Serum vaspin and progranulin were measured as previously described (14). Serum chemerin and fetuin-A were measured by ELISAs (Biovendor, Heidelberg, Germany).
Statistical analyses
For intention-to-treat analyses, we included all 322 participants and used the most recent values for weight and blood pressure. We characterized the study population across quartiles of BMI and stratified by sex for leptin and adiponectin because their levels significantly differed between sexes. The pattern analysis, however, was similar in men and women. We tested for statistically significant differences between different time points within dietary intervention groups by t test and for given time points between dietary groups by ANOVA. We used SPSS software, version 18 (SPSS Inc., Chicago, IL), and Stata software, version 9 (StataCorp LP, College Station, TX), for the statistical analysis.
Methods of the nonbiased approaches: trend analysis and clustering.
In the trend analysis, we define the trends correspondence measure (TCM) to represent the correspondence between the trend in the first (0–6 months) and second (7–24 months) parts of the trial for each biomarker. This analysis represents the fraction of the change in the second part of the intervention that continues the trend observed in the first part. Formally, it is defined as follows: Let 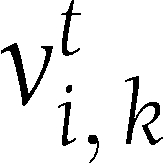 represent the change in variable i in individual
represent the change in variable i in individual 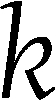 from time 0 to time t, in percentages of its value at time 0:
from time 0 to time t, in percentages of its value at time 0:
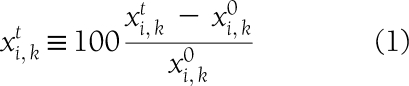 |
where 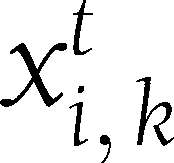 represents the value of that variable in that individual at time t. Let
represents the value of that variable in that individual at time t. Let 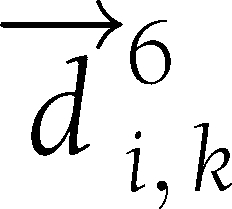 be the vector connecting the points (0, 0) and (6,
be the vector connecting the points (0, 0) and (6, 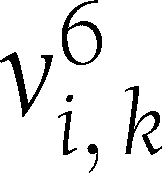 ). Let
). Let 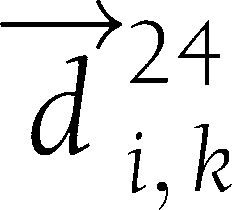 be the vector connecting the points (6,
be the vector connecting the points (6, 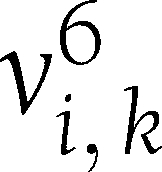 ) and (6,
) and (6, 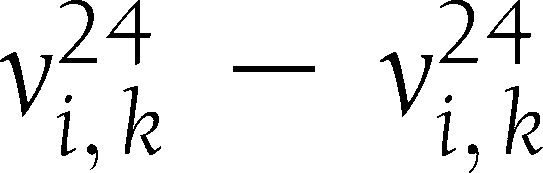 ). TCM is defined as the cosine of the angle between
). TCM is defined as the cosine of the angle between 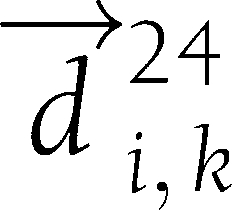 and the line containing
and the line containing 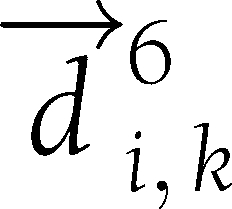 (Supplementary Fig. 1). Hierarchical clustering variables were used. The h-clust function in R was used with default parameters. A distance matrix was derived from the TCM values of every two variables using the following:
(Supplementary Fig. 1). Hierarchical clustering variables were used. The h-clust function in R was used with default parameters. A distance matrix was derived from the TCM values of every two variables using the following:
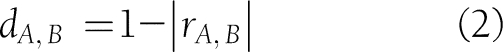 |
RESULTS
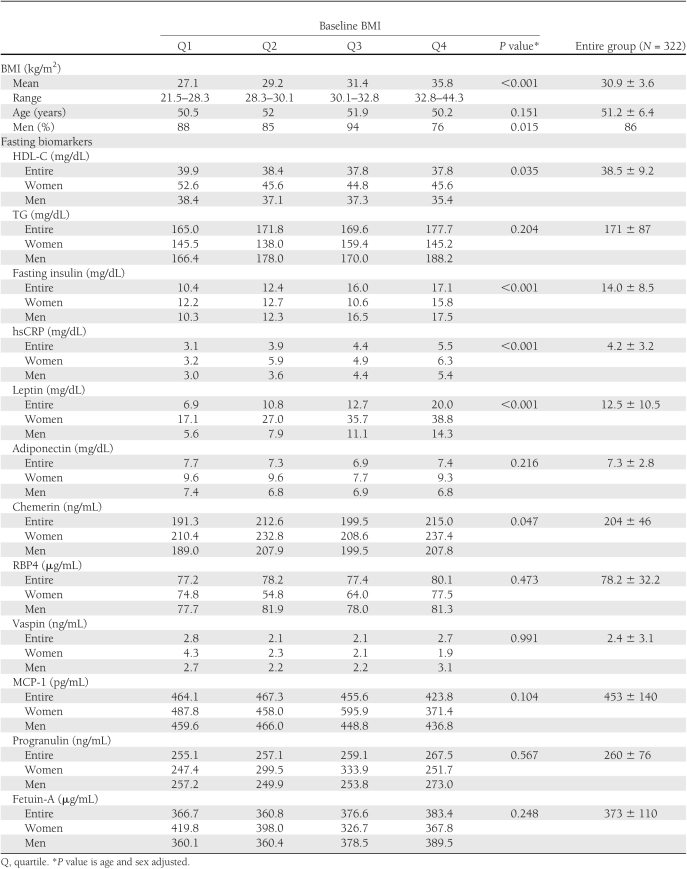
The mean age of the study population was 52 ± 7 years with mean BMI of 30.9 ± 3.6 kg/m2. Most of the participants (86%) were men. Higher baseline BMI was significantly associated with increased proportion of women and higher baseline fasting insulin, hsCRP, leptin, and chemerin serum concentrations and lower levels of HDL-C (Table 1). A significant sex difference was found for leptin and adiponectin. The three diet interventions resulted in two segments: a rapid weight loss phase during the first 6 months followed by partial or complete reversal at the 7–24 months interval (weight maintenance/regain phase) (Fig. 1A).
Table 1.
Baseline characteristics of the fasting blood biomarkers across quartiles of BMI stratified for sex in the DIRECT population
Figure 1.
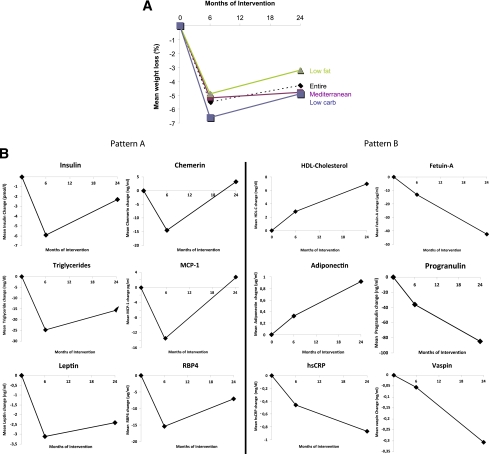
Two major patterns of biomarkers during the course of 2 years of dietary weight loss intervention. A: Weight loss percentages across the diet groups in the entire DIRECT population. B: Patterns of biomarkers in the entire DIRECT population. The y-axes represent the 6- and 24-month change (Δ) of the biomarkers, as compared with baseline by the specific biomarker units, as indicated in Table 1. Pattern A included biomarkers whose dynamics closely reflect changes in body weight, which exhibited a rapid decline (0–6 months, weight loss phase) followed by weight stabilization or regain (7–24 months, weight maintenance/regain phase). All the biomarkers showed a significant difference (P < 0.05 for all) between baseline and 6 months and between 6 and 24 months (in the opposite direction), with chemerin and MCP-1 exhibiting a full return to baseline levels at 24 months and leptin, RBP4, vaspin, progranulin, and fetuin-A remaining significantly different between baseline and 24 months (P < 0.05). Pattern B consisted of biomarkers that displayed a continuing, cumulative increase or decrease throughout the 24 months of dietary intervention, despite the partial regain in mean body weight. All biomarkers were significantly (P < 0.05 for all) improved after 24 months as compared with baseline, and except for HMW adiponectin, all exhibited a significant change between time 6 and 24 months (in the same direction, P < 0.05), reflecting additional improvement during the weight stabilization/regain phase. Patterns A and B were similar among patients with type 2 diabetes and nondiabetics.
We identified two distinct major patterns in the dynamics of biomarker serum concentrations measured at baseline and at 6 and 24 months of intervention (Fig. 1B). The first, pattern A, included biomarkers whose dynamics closely corresponded to changes in body weight, exhibiting a rapid change between 0 to 6 months, during the weight loss phase, followed by partial or complete reversal at the 7–24 months interval, during the weight maintenance/regain phase. The following biomarkers exhibited this pattern: fasting plasma insulin, TGs, leptin, chemerin, MCP-1, and RBP4 serum concentrations (Fig. 1B). All biomarkers showed a significant difference (P < 0.05 for all) between baseline and 6 months and between 6 and 24 months, with chemerin and MCP-1 exhibiting a full return at 24 months to the baseline levels and leptin, RBP4, vaspin, progranulin, and fetuin-A remaining significantly different between baseline and 24 months (P < 0.05).
In sharp contrast, a second pattern of dynamics, pattern B, could be observed for HDL-C, hsCRP, HMW adiponectin, fetuin-A, progranulin, and vaspin during the 24 months of dietary intervention (Fig. 1B). Each of these parameters displayed a continuing, cumulative change in the same direction throughout the 24 months of dietary intervention, irrespective of weight stabilization or partial weight regain. Consistently, all of these biomarkers were significantly (P < 0.05 for all) improved after 24 months as compared with baseline, and except for HMW adiponectin, all exhibited a significant change between 6 and 24 months (P < 0.05), reflecting additional improvement during the weight stabilization/regain phase. It is interesting that we found significant intercorrelations at baseline between several parameters of pattern A, whereas for parameters of pattern B, the only significant correlations were found between adiponectin and CRP, adiponectin and HDL-C, and CRP and HDL-C (Supplementary Table 1).
We next used a nonbiased hierarchical clustering approach to analyze the dynamics of the various parameters tested at baseline and at 6 and 24 months and to further understand the extent of similarity of trends within the clusters (i.e., to quantify the distance from the weight change pattern). A hierarchical clustering dendrogram depicts the results of this analysis (Supplementary Fig. 2), revealing that leptin is the parameter whose dynamic most closely corresponded during the 24-month period of dietary intervention to that of body weight, followed by insulin, chemerin, and MCP-1 (Supplementary Fig. 2). Of interest, several parameters showed a distinct pattern that differed from the pattern of weight change, including hsCRP, HDL-C, RBP4, and the adipokines vaspin and adiponectin, whose dynamic patterns closely corresponded to each other, suggesting a cumulative improvement in the same direction (pattern B). However, our statistical approach does not allow defining whether the distance of a specific biomarker pattern from the weight change pattern is statistically significant.
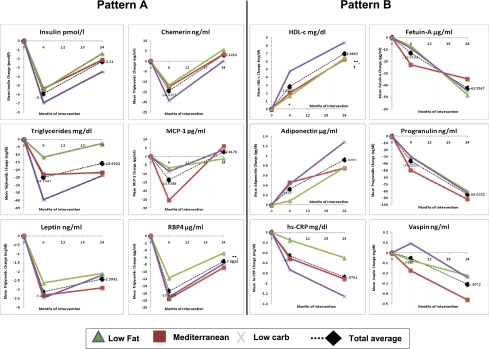
The DIRECT included low-fat, Mediterranean, and low-carbohydrate dietary intervention arms in which unique dietary compositions with respect to carbohydrate, total fat, cholesterol, and monounsaturated-to-saturated fat intake were documented. We next assessed whether the dynamics in the aforementioned parameters depended on the dietary strategy (Fig. 2). Although there were (for some biomarkers) significant differences in the extent of the effects of the dietary strategies on the changes of specific biomarkers (3), the three diets similarly exhibited the distinct two patterns of dynamics. Both patterns were also similar across sex and type 2 diabetic groups (data not shown).
Figure 2.
Dynamics of biomarkers in the three different dietary interventions. The two distinct patterns (A and B) of biomarkers were confirmed with differences in the extent across low-fat, Mediterranean, and low-carbohydrate groups during 2 years of dietary weight loss intervention. At baseline, for all 12 displayed biomarkers, no significant differences were found between the diet groups. At 6 months, significant differences (P < 0.05) were found between all diet groups for TGs, between the Mediterranean diet and the other diets for MCP-1 and Fetuin-A, between the low-fat diet and the other diets for adiponectin and RBP4, and between the low-carbohydrate diet and the other diets for HDL-C and vaspin. At 24 months, significant differences were found between all diet groups for CRP, between the Mediterranean diet and the other diets for vaspin, between the low-fat diet and the other diets for TGs, and between the low-carbohydrate diet and the other diets for HDL-C. The following statistically significant differences (P < 0.05 for all) were found between time points within the diet groups: 1) Low-fat group, 0–6 months: CRP, leptin, TG, HDL-C, insulin, adiponectin, MCP-1, vaspin, fetuin-A, and chemerin; 6–24 months: CRP, leptin, TG, HDL-C, insulin, adiponectin, MCP-1, vaspin, fetuin-A, and chemerin; 0–24 months: CRP, leptin, HDL-C, insulin, adiponectin, progranulin, and fetuin-A; 2) Mediterranean group, 0–6 months: CRP, leptin TG, HDL-C, insulin, adiponectin, MCP-1, vaspin, fetuin-A, chemerin, RBP4, and progranulin; 6–24 months: CRP, leptin, TG, HDL-C, insulin, adiponectin, MCP-1, vaspin, fetuin-A, chemerin, and progranulin; 0–24 months: CRP, leptin, TG, HDL-C, insulin, adiponectin, vaspin, fetuin-A, RBP4, and progranulin; and 3) Low-carbohydrate group, 0–6 months: CRP, leptin, TG, HDL-C, insulin, adiponectin, MCP-1, vaspin, chemerin, RBP4, and progranulin; 6–24 months: CRP, leptin, TG, HDL-C, insulin, adiponectin, MCP-1, vaspin, fetuin-A, chemerin, and progranulin; 0–24 months: CRP, leptin, TG, HDL-C, insulin, adiponectin, vaspin, fetuin-A, RBP4, and progranulin.
CONCLUSIONS
In this study, we analyzed the circulating levels of traditional biomarkers and common and more recently discovered adipokines and their dynamics during 2 years of dietary intervention, using both a nonbiased mathematical modeling approach and qualitative analysis. We found that the adipokines leptin, chemerin, and MCP-1 correspond to body weight dynamics, similar to plasma insulin and serum TG levels. In contrast, HMW adiponectin, fetuin-A, progranulin, and vaspin exhibited cumulative change despite partial weight regain, similar to the changes in hsCRP and HDL-C.
Pattern B dynamics may reflect either a delayed effect of the initial weight loss or a continuous beneficial response to switching to healthier dietary patterns. The two distinct biomarker patterns suggest different underlying biological mechanisms through which these biomarkers cluster together during different phases of weight loss and maintenance. Even the classical adipokines leptin (pattern A) and adiponectin (pattern B) seem to reflect different biological processes associated with weight loss and weight regain: Changes in circulating leptin are a function of changes in fat mass, whereas adiponectin serum concentrations may reflect changes in adipose tissue function beyond the effects on fat mass. We therefore hypothesize that biomarkers cluster in pattern A because they directly reflect body (fat) mass dynamic. This hypothesis is further supported by several intercorrelations among parameters of pattern A. In contrast, parameters in pattern B may reflect alterations in other or in addition to fat mass–related biological mechanisms, including improved chronic subclinical inflammation (as reflected by intercorrelations with CRP), adipose tissue function (as reflected by intercorrelations with adiponectin), or lipid metabolism (as reflected by intercorrelations with HDL-C) upon diet interventions.
Regardless of the yet-to-be-discovered mechanisms linking healthful diet and beneficial health effects independent of body weight dynamics, we demonstrate that 1) sustained moderate weight loss is sufficient to improve insulin and TG levels, and 2) adhering to long-term dietary intervention, even when experiencing body weight plateau or partial regain, results in clinically relevant cumulatively improved HDL-C, adiponectin, and hsCRP levels.
Our study has several limitations, including the fact that only a few female participants were studied. The statistical approach does not allow defining whether the distance of a specific biomarker pattern from the weight change pattern is statistically significant or not. We relied on self-reported dietary intake, but we validated the dietary assessment in two different dietary-assessment tools (FFQs and food diaries) and one subjective questionnaire, asking the participants about the extent of their adherence to the assigned diet, and used electronic questionnaires to minimize missing data. The unique nature of the workplace in this study, which permitted a closely monitored dietary intervention for 2 years, makes it difficult to generalize the results to other free-living conditions. However, we believe that similar strategies to maintain adherence could be applied elsewhere and should be used. Finally, in these analyses, the end points constituted changes in biomarkers, which are only surrogate markers for cardiometabolic disease risk. Yet some of these biomarkers (hsCRP, adiponectin, and HDL-C) are well-documented biomarkers of disease risk, whereas data of the clinical relevance of other markers is still accumulating. The strengths of the study include the one-phase design in which all participants started simultaneously, the long duration of the study, the relatively large study-group size, the high rate of adherence, and the comprehensive list of traditional biomarkers and novel adipokines.
Our data suggest that reduction in circulating TGs and insulin could be achieved only if weight loss, even if moderate, is maintained. TGs, as a sensitive biomarker of lifestyle, were recently reviewed in a new scientific statement of the American Heart Association (17), which concluded that lifestyle interventions (diet and exercise) are the key factors for treating hypertriglyceridemia. Weight loss is an established treatment option to improve insulin sensitivity in patients with type 2 diabetes (18). It is noteworthy that fasting plasma insulin concentration has been shown to be the best predictor of higher baseline carotid vessel wall volume in the DIRECT study population (19).
Our finding that increases in circulating HDL-C and HMW adiponectin and decreases in hsCRP levels continued in a cumulative manner throughout the entire study period may be clinically important. Although it is clear that adiponectin and CRP are strong markers for the development of chronic disease, the best strategy to favorably alter the inflammatory response is uncertain (20). We previously reported that increased HDL-C and apoA1 are associated with a significant regression in carotid vessel wall volume achieved within 2 years of the DIRECT (19).
As suggested by the hierarchical clustering analysis, leptin has the closest dynamic to weight changes, followed by insulin. Adipokines may play a role as signals from adipose tissue to the brain, directly contributing to weight regain, or at least as predictors of body weight dynamics. Although metabolic, behavioral, neuroendocrine, and autonomic responses all contribute to the maintenance of body energy stores at a defined state, part of the opposition to sustained weight loss can be attributed to leptin (21). Circulating leptin levels and leptin mRNA expression highly correlate with adipose tissue mass and, thus, can be used as surrogate parameters for changes in the amount of adipose tissue (22). We recently reported that plasma leptin reduction, combined with the degree of initial weight loss and with genetic variations in the leptin gene, constitutes a significant predictor of subsequent long-term weight regain (6). Additional adipokines signal the functional status of adipose tissue to other tissues and frequently reflect weight changes.
In our study, chemerin, MCP-1, and RBP4 serum concentrations corresponded to body weight dynamics. Our data of chemerin dynamic are in contrast to a recent study demonstrating that a decrease in circulating chemerin concentrations occurs despite weight maintenance after bariatric surgery (23). Chemerin, a proinflammatory protein, is highly expressed in the liver and adipose tissue and is associated with impaired insulin sensitivity, altered glucose, and lipid metabolism, with a dual role in inflammation and metabolism (7). MCP-1, primarily secreted by monocytes and macrophages, is a potent proinflammatory chemokine. It is synthesized by adipose tissue, elevated in obesity, and has a role in monocyte recruitment and, apparently, in the development of type 2 diabetes (8). RBP4, secreted by adipocytes and hepatocytes, correlates with the degree of obesity, insulin resistance, visceral fat accumulation, and fasting glucose (11–13). In our study, chemerin, MCP-1, and RBP4 serum concentration patterns closely followed the body weight pattern, suggesting that these adipokines reflect rather than cause changes in body weight.
In contrast, the adipokines fetuin-A, progranulin, and vaspin exhibited cumulative improvement despite partial weight regain throughout the study. Fetuin-A, a glycoprotein synthesized by hepatocytes, is associated with insulin resistance and fat accumulation in the liver in humans (10). Progranulin levels are associated with macrophage infiltration into omental adipose tissue and with markers of inflammation, hyperglycemia, and predominantly visceral fat accumulation (9). Vaspin, suggested as a visceral adipose tissue–derived factor, is associated with obesity and impaired insulin sensitivity (14). Our data on the dynamics of fetuin-A, progranulin, and vaspin during the long-term diet intervention suggest that these adipokines are directly linked to reduced food intake and/or diet intervention effects, which are independent of body weight dynamics. Because insulin sensitivity and carotid vessel wall volume (19) are improved during the entire DIRECT, fetuin-A, progranulin, and vaspin serum concentrations may provide a mechanistic link between healthful diet and improvements in these obesity-related parameters.
The two patterns of dynamics described herein were similarly observed among the three intervention arms that were proven to be distinct in dietary composition. Although our study may be underpowered to detect minor differences between the three arms, it is also plausible that factors common to the three diets are major drivers of the change in the various parameters. One obvious common factor is the degree of caloric and total food weight deficit achieved (5), which was similar between the three dietary groups despite the fact that the low-carbohydrate group was not instructed to restrict caloric intake. Yet additional dietary changes were common to the three arms, including decrease in trans fatty acids and sweetened beverages and increase in vegetables and fiber. Although we were unable to link a specific dietary component to a particular parameter, this is an important area for future research, because specific dietary factors could then be implemented in larger public health measures to combat obesity-associated morbidity, regardless of whether they are part of a more comprehensive dietary change.
In conclusion, we demonstrate for the first time that two major classes of dynamics of biomarkers can be described during long-term diet interventions. One pattern closely reflects weight change, and the other is suggestive of cumulative beneficial effects, alternatively as a delayed response to the initial weight loss or perhaps to continued healthful dieting. Thus, weight reduction is not the sole indicator of the beneficial effects of healthful dieting, which may be discernable by measuring a specific group of biomarkers despite partial weight maintenance/regain.
Supplementary Material
Acknowledgments
This work was supported by grants from the Israeli Ministry of Health, Chief Scientist Office (project 300000-4850), the Deutsche Forschungsgemeinschaft of the Clinical Research Group Atherobesity KFO 152-2 (project BL 833/1-1) (to M.B.), the Dr. Robert C. and Veronica Atkins Research Foundation, and the German-Israeli Science Foundation (project 995-41.2/2008). The German-Israeli Science Foundation was not involved in any stage of the design, conduct, or analysis of the study and had no access to the study results before publication.
No potential conflicts of interest relevant to this article were reported.
M.B. researched data and wrote the manuscript. A.R. wrote the manuscript. N.K., Y.H., E.R., and Y.G. researched data. R.G. and D.S. performed statistical analyses. M.J.S. conducted the original clinical trial, contributed to discussion, and edited the manuscript. M.F. researched data and edited the manuscript. J.T. contributed to discussion. M.S. reviewed and edited the manuscript. I.S. conducted the original clinical trial, contributed to discussion, and wrote the manuscript.
M.B. is the guarantor and takes responsibility for the contents of the article.
Footnotes
Clinical trial reg. no. NCT00160108, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1267/-/DC1.
References
- 1.Aronne LJ, Wadden T, Isoldi KK, Woodworth KA. When prevention fails: obesity treatment strategies. Am J Med 2009;122(Suppl. 1):S24–S32 [DOI] [PubMed] [Google Scholar]
- 2.Muls E, Kempen K, Vansant G, Saris W. Is weight cycling detrimental to health? A review of the literature in humans. Int J Obes Relat Metab Disord 1995;19(Suppl. 3):S46–S50 [PubMed] [Google Scholar]
- 3.Shai I, Schwarzfuchs D, Henkin Y, et al. ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–241 [DOI] [PubMed] [Google Scholar]
- 4.Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I; DIRECT Group Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). J Am Coll Nutr 2009;28:159–168 [DOI] [PubMed] [Google Scholar]
- 5.Canfi A, Gepner Y, Schwarzfuchs D, et al. Effect of changes in the intake of weight of specific food groups on successful body weight loss during a multi dietary strategy intervention trial. J Am Coll Nutr. In press [DOI] [PubMed] [Google Scholar]
- 6.Erez G, Tirosh A, Rudich A, et al. Phenotypic and genetic variation in leptin as determinants of weight regain. Int J Obes (Lond) 2011;35:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab 2010;21:660–667 [DOI] [PubMed] [Google Scholar]
- 8.Sell H, Eckel J. Chemotactic cytokines, obesity and type 2 diabetes: in vivo and in vitro evidence for a possible causal correlation? Proc Nutr Soc 2009;68:378–384 [DOI] [PubMed] [Google Scholar]
- 9.Youn BS, Bang SI, Klöting N, et al. Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes 2009;58:627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefan N, Hennige AM, Staiger H, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006;29:853–857 [DOI] [PubMed] [Google Scholar]
- 11.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552–2563 [DOI] [PubMed] [Google Scholar]
- 12.Klöting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab 2007;6:79–87 [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–362 [DOI] [PubMed] [Google Scholar]
- 14.Youn BS, Klöting N, Kratzsch J, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008;57:372–377 [DOI] [PubMed] [Google Scholar]
- 15.Shai I, Rosner BA, Shahar DR, et al. ; DEARR study Dietary evaluation and attenuation of relative risk: multiple comparisons between blood and urinary biomarkers, food frequency, and 24-hour recall questionnaires: the DEARR study. J Nutr 2005;135:573–579 [DOI] [PubMed] [Google Scholar]
- 16.Shai I, Shahar DR, Vardi H, Fraser D. Selection of food items for inclusion in a newly developed food-frequency questionnaire. Public Health Nutr 2004;7:745–749 [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Stone NJ, Ballantyne C, et al. ; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–2333 [DOI] [PubMed] [Google Scholar]
- 18.Brown A, Desai M, Taneja D, Tannock LR. Managing highly insulin-resistant diabetes mellitus: weight loss approaches and medical management. Postgrad Med 2010;122:163–171 [DOI] [PubMed] [Google Scholar]
- 19.Shai I, Spence JD, Schwarzfuchs D, et al. ; DIRECT Group Dietary intervention to reverse carotid atherosclerosis. Circulation 2010;121:1200–1208 [DOI] [PubMed] [Google Scholar]
- 20.Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J Nutr 2008;138:2293–2296 [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl. 1):S47–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birsoy K, Soukas A, Torrens J, et al. Cellular program controlling the recovery of adipose tissue mass: An in vivo imaging approach. Proc Natl Acad Sci U S A 2008;105:12985–12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sell H, Divoux A, Poitou C, et al. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 2010;95:2892–2896 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.