Abstract
Large cytomegalovirus (CMV)-specific CD8 T-cell responses are observed in both young and, somewhat more often, old people. Frequent CMV reactivation is thought to exhaust these cells and render them dysfunctional so that larger numbers of them are needed to control CMV. Expansions of CMV-specific CD4 T cells are also seen but are less well studied. In this study, we examined the T-cell response to the dominant CMV pp65 and IE-1 antigens in healthy CMV-infected people across a wide age range (20 to 84 years) by using multicolor flow cytometry. CMV-specific T cells were characterized by the activation markers CD40 ligand (CD40L), interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) and the memory markers CD27 and CD45RA. The proportions of effector memory T cells increased in large responses, as did the proportions of polyfunctional CD8 (IFN-γ+ IL-2+/− TNF-α+) and CD4 (CD40L+/− IFN-γ+ IL-2+ TNF-α+) T-cell subsets, while the proportion of naïve T cells decreased. The bigger the CD4 or CD8 T-cell response to pp65, the larger was the proportion of T cells with an advanced memory phenotype in the entire (including non-CMV-specific) T-cell compartment. In addition, the number of activation markers per cell correlated with the degree of T-cell receptor downregulation, suggesting increased antigen sensitivity in polyfunctional cells. In summary, our findings show that polyfunctional CMV-specific T cells were not superseded by dysfunctional cells, even in very large responses. At the same time, however, the memory subset composition of the entire T-cell compartment correlated with the size of the T-cell response to CMV pp65, confirming a strong effect of CMV infection on the immune systems of some, but not all, infected people.
INTRODUCTION
Large cytomegalovirus (CMV)-specific T-cell responses are quite common in both young and old CMV-infected people. This is the result of memory T-cell inflation, which is best described for the murine cytomegalovirus (MCMV) model and consists of a persistence and steady increase of the number of certain T cells selected during (primary) infection (18, 43). These enlarged T-cell responses, in particular CD8 responses, have been described as “degenerated” because they appear to accumulate T cells with a limited cytokine profile and reduced proliferative potential that have an advanced to late differentiation phenotype (14, 30, 31). It is important that dysfunctional T cells may occur in young people and that this may be related to the duration of the infection rather than the age of the donor, but at an older age, the chances of having been infected with CMV for a longer period are obviously greater (16). Unfortunately, there is usually no way of knowing the time of primary CMV infection, except when it occurs as a result of transplantation or transfusion of an organ or cells, respectively, from a CMV-infected donor to a CMV-negative recipient.
CMV-induced changes, along with other changes of the immune system, are thought to result in increased susceptibility to infection and decreased vaccine efficiency, in particular in older life (24, 32). Unfortunately, in terms of immunological parameters, the decline of anti-infectious immunity is difficult to quantify because no clear and measurable correlates of protective immunity are known to date. The most commonly used parameter to investigate CMV-specific T-cell responses is the frequency of either CD4 or CD8 T cells producing gamma interferon (IFN-γ) after short-term stimulation (4, 6, 13, 21, 22, 37, 40, 41, 44). Although IFN-γ is thought to play an important role in the control of various infections, the frequency of IFN-γ-producing T cells alone does not seem to be a good correlate of protection (11, 12). However, a recent and widely accepted concept suggests that polyfunctional T cells, in particular those simultaneously producing interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), and IFN-γ upon antigen challenge, are a crucial factor in controlling infection (1, 3, 9, 11, 27). Khan et al. previously reported that the proportion of IFN-γ-producing effector cells among CMV tetramer-binding CD8 T cells is reduced in old age (22). However, the number of tetramer-binding CD8 T cells was increased so strongly in older people that there was an actual net increase in the overall number of IFN-γ-producing CD8 T cells. This observation was confirmed in principle by Vescovini et al., who recently reported that CMV-specific IFN-γ- and TNF-α-producing T cells (functions measured separately) are increased in old age, in both absolute (cells per ml of blood) and relative (% of respective T-cell subset) terms (49).
We were intrigued by the idea that in CMV-infected individuals—contrary to the prevailing opinion—the numbers of fully functioning polyfunctional CMV-specific T cells might actually be stable or even increase in very large responses. Our study therefore examined pp65-specific CD4 and CD8 T cells (CD4/pp65 and CD8/pp65 responses) and IE-1-specific CD8 T cells (CD8/IE-1 response) with regard to memory subset distribution and polyfunctionality in people between 20 and 84 years of age. The results showed an unexpected accumulation of polyfunctional CMV-specific T cells in individuals with large CMV-specific responses, in both relative (% of respective subset [CD4 or CD8]) and absolute (cells per ml of blood) terms. Moreover, we found that the size of the pp65-specific T-cell response (rather than just its presence) positively correlated with an advancing degree of terminal T-cell differentiation in general, including that of non-CMV-specific T cells.
MATERIALS AND METHODS
Volunteer sample collection and isolation.
Heparinized peripheral blood was collected from over 100 healthy donors. CMV serostatus was tested in all individuals. T-cell responses to the CMV proteins pp65 and IE-1 were tested in all seropositive individuals and all individuals for whom serology was not available at the time of testing, which included some seronegative individuals. A total of 41 CMV-seropositive donors with T-cell responses to pp65 or IE-1 above 1/10,000 of the reference subset (CD4 or CD8 T cells) were included in this study. The age range was 20 to 84 years; 27 donors were female (mean age ± standard deviation [SD], 53 ± 21 years), and 14 were male (57 ± 20 years). Young donors were recruited from healthy university staff and students. Older volunteers were recruited from the general public through the Clinical Investigation and Research Unit (Brighton and Sussex University Hospital Trust) and through general practitioner (GP) practices, with assistance from the NIHR Primary Care Research Network; all older subjects were mobile, were not suffering from acute or chronic illness, and were not on medication known to affect the immune system. The study was in agreement with the Declaration of Helsinki and was approved by the relevant University and National Health Service (NHS) ethics committees. All donors gave written informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation.
Antibodies.
Fluorochrome-conjugated antibodies were obtained from the following companies: anti-TNF-α–Alexa 700, anti-IL-2–allophycocyanin (APC), anti-CD8–APC–H7, anti-CD8–peridinin chlorophyll protein (PerCP), anti-CD4–PerCP, anti-CD40 ligand (CD40L)–phycoerythrin (PE), anti-IFN-γ–PE–Cy7, anti-CD27–PE, anti-CD16–fluorescein isothiocyanate (FITC), anti-CD56–FITC, and anti-CD45–PerCP were from BD Biosciences (San Jose, CA); anti-CD45RA–Texas Red–PE and anti-CD4–Texas Red–PE were from Beckmann Coulter (Fullerton, CA); anti-CD3–QDot605 and aqua blue live-dead stain were from Invitrogen (Paisley, United Kingdom); and anti-CD3–Pacific blue was from Cambridge Biosciences (Cambridge, United Kingdom).
CMV serology.
Plasmas or sera from all donors were tested for CMV-specific IgG and IgM by an automated CMV enzyme-linked immunosorbent assay (ELISA) (Department of Microbiology, Royal Sussex County Hospital, Brighton, United Kingdom).
Whole-blood counting and staining.
Leukocytes in fresh whole blood were counted for all but two donors. One hundred microliters of whole blood was stained with pretitrated surface antibodies (to CD45, CD3, CD4, and CD8) for 30 min at 4°C, lysed for 10 min at room temperature, and washed, and flow cytometry data were acquired immediately.
Cell stimulation and staining.
The method of cell stimulation was described previously in more detail (4). In brief, 400 μl of PBMC suspension (2 × 106 cells/ml) was stimulated with pp65 or IE-1 spanning peptide pools (1 μg/ml per peptide; JPT Peptide Technologies, Berlin, Germany) dissolved in dimethyl sulfoxide (DMSO; Pierce, Germany) for 2 h at 37°C. Peptide pools were composed of 15 amino acid peptides with 11 overlaps, beginning at the first amino acid of the published sequence for pp65 (Swiss-Prot accession no. P06725; 138 peptides) or IE-1 (Swiss-Prot accession no. P13202; 121 peptides). Stimulation with purified protein derivative (PPD) (10 μg/ml; Statens Serum Institute, Copenhagen, Denmark) was performed for a subgroup of individuals. OKT3 (0.5 μg/ml) was used as a positive control, and DMSO (equivalent to the amount added with peptide pools) was added to unstimulated samples (negative control). After the addition of brefeldin A (10 μg/ml; Sigma), samples were incubated for another 14 h and then washed (in phosphate-buffered saline [PBS] containing 0.5% bovine serum albumin and 0.1% sodium azide) and stained with pretitrated surface antibodies for 30 min at 4°C. After washing, lysis, and permeabilization (Perm 2 and Lysis reagents [BD Biosciences], used according to the manufacturer's instructions), cells were stained intracellularly (30 min, 4°C). Following staining, cells were washed, fixed in PBS containing 0.5% paraformaldehyde, and acquired on a BD LSRII flow cytometer using FACSdiva 6.1 software (both from BD Biosciences).
Data analysis.
Data analysis was performed using FlowJo software (Treestar, OR). A lymphocyte gate, singlet gate (FSC-A versus FSC-H), live/dead gate, CD3 T-cell gate, CD4 and CD8 T-cell gates, memory T-cell subset gates, and activated cell gates (activation marker positive) were applied sequentially according to a set gating strategy. For the purpose of this study, cells displaying at least one of the four analyzed activation markers upon antigen stimulation were called antigen specific.
Statistical analysis.
Statistical analysis was performed using SPSS 18. Both relative and absolute counts for CD4 T cells, CD8 T cells, and the pp65- and IE-1-specific responses fitted a lognormal distribution. For visualization of data, cell counts were represented as log-transformed data except where non-log-transformed data were better suited to show the effects of small changes in response size. The relationship between the analyzed parameters was generally assumed to be nonlinear, and Spearman's correlation coefficient (RS) was used for all correlations. When a normal distribution could not be shown (by the Kolmogorov-Smirnoff test and Q-Q plots), statistical tests for nonparametric data were used. P values of <0.05 were considered significant. Mean fluorescence intensity (MFI) values were standardized using Z scores in order to allow comparisons between different subjects.
RESULTS
CMV-specific T-cell response size correlates only loosely with age.
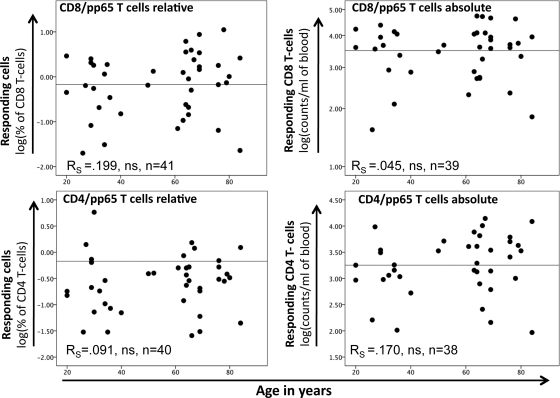
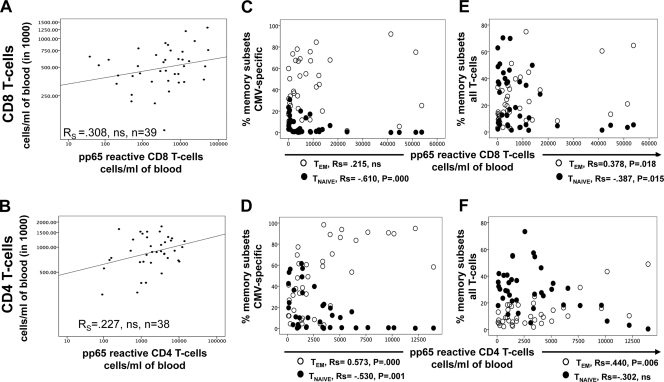
T cells expressing one or more of the activation markers CD40L, IFN-γ, IL-2, and TNF-α were considered activated and antigen specific. Response size was measured either as a percentage of the reference subset (CD8 or CD4 T cells) or as the number of cells per ml of blood (absolute counts). Figure 1 shows CD8/pp65 and CD4/pp65 responses as percentages and absolute counts versus donor age. Up to approximately 11% of CD8 T cells and 6% of CD4 T cells responded to pp65 in this cohort, which is in line with previously published data (19, 20, 45). As expected for a human population, large variations in response size were visible across all ages. A (nonsignificant) trend toward an increase of response size with age was visible for both CD8/pp65 and CD8/IE-1 responses (the latter not shown) and also for CD4/pp65 responses. CD4/IE-1 responses were inconsistent and small and therefore were not considered further. Very large CD8/pp65 and CD4/pp65 responses seemed to occur preferentially in older people. Statistical analysis revealed that both relative and absolute counts of these responses fitted a lognormal distribution. Notably, there was no significant correlation between the absolute size of the entire CD4 or CD8 T-cell compartment and the size of the CMV-specific CD8 or CD4 T-cell response (in cells/ml of blood) (Fig. 2A and B).
Fig 1.
Frequencies and absolute numbers of pp65-specific T cells are not closely correlated with age. Responding T cells were defined as those displaying at least one of the activation markers CD40L, IL-2, IFN-γ, and TNF-α. Frequencies (left) and absolute numbers (right) of responding CD8/pp65 (top) and CD4/pp65 (bottom) T cells are displayed. Absolute and relative values followed a lognormal distribution and were log transformed to better visualize changes with age. There was no detectable correlation of T-cell responsiveness to pp65 with age.
Fig 2.
Large pp65-specific T-cell responses show a more advanced memory T-cell subset composition. (A and B) The absolute counts of CMV pp65-specific CD8 and CD4 T cells do not correlate significantly with the absolute numbers of CD8 and CD4 T cells in peripheral blood. (C and D) There is an overproportioned (nonlinear) relative increase in pp65-specific TEM cells and decrease in TNAIVE cells within these responses. (E and F) The proportions of the entire TNAIVE and TEM CD8 and CD4 T-cell compartments (C and D) are changed in the presence of large pp65-specific responses. ns, not significant.
The increase of the effector memory T-cell compartment is overproportioned in large responses.
By convention, T-cell memory compartments are referred to as naïve (TNAIVE; CD45RA+ CD27+), central memory (TCM; CD45RA− CD27+), effector memory (TEM; CD45RA− CD27−), and revertant (TEMRA; CD45RA+ CD27−) cells (2, 15). Please note that with respect to the naïve compartment, this terminology is not entirely accurate, since naïve cells are not expected to respond to antigen stimulation by producing effector cytokines or to occur in large frequencies. The size of the pp65-specific CD8 T-cell response correlated significantly with a relative loss of TNAIVE cells within the pp65-specific CD8 T-cell compartment but not with an increase in TEM cells (Fig. 2C). However, the proportions of TNAIVE and TEM cells in the entire CD8 T-cell compartment, which usually includes a very large majority of non-CMV-specific T cells (45), both correlated significantly with the size of the pp65-specific T-cell response (Fig. 2E). The decline of the proportion of all naïve CD8 T cells also correlated significantly with the size of the CD8/IE-1 response (−0.521; P = 0.001; n = 36) (data not shown). Changes in the proportions of T-cell memory subsets within and outside the pp65-specific CD4 T-cell compartment correlated in a very similar way with the size of the CD4/pp65 response (Fig. 2D and F). Not all memory compartments were affected in the same clear way by response size (see Fig. S1 and S2 in the supplemental material). As a result, the degree of advanced T-cell memory differentiation within the CMV-specific T-cell compartment (Fig. 2C and D), but also outside it (Fig. 2E and F), seemed to be related to the size of the CMV pp65-specific T-cell response.
Polyfunctional components of CMV-specific T-cell responses grow with increasing response size.
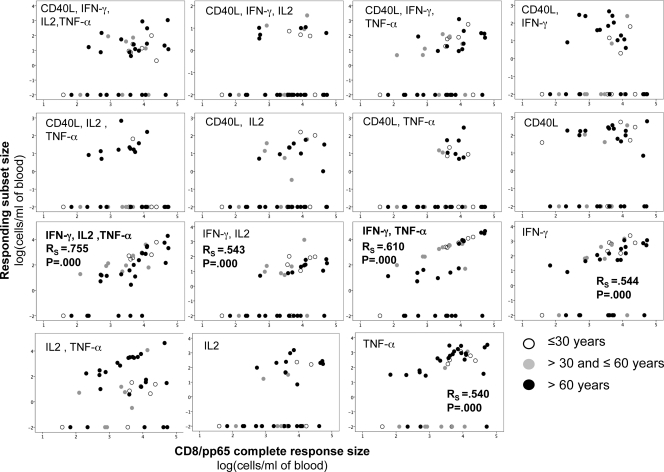
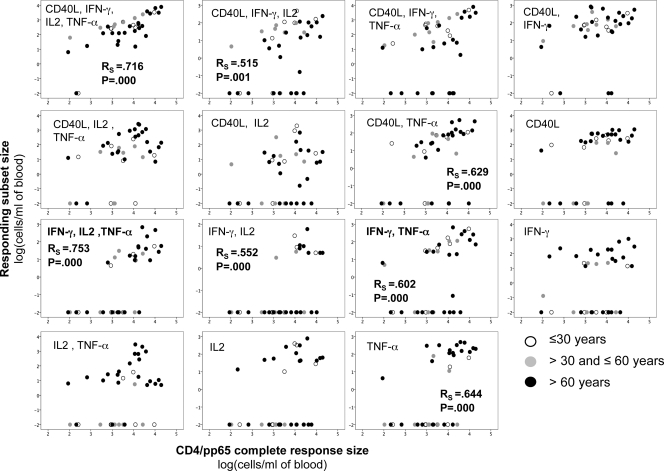
Two functional subsets correlated particularly well with the sizes of CD8/pp65 responses (Fig. 3). These included CD8 T cells that were either IFN-γ+ IL-2+ TNF-α+ or IFN-γ+ TNF-α+. These subsets were found primarily in TEM cells and, to some extent, in the TCM and TEMRA compartments. Both subsets also correlated with CD8/IE-1 cell expansion (RS = 0.609, P = 0.000, and n = 36 and RS = 0.475, P = 0.003, and n = 36, respectively). The subsets whose size correlated most strongly with the size of the CD4/pp65 response were CD40L+ IFN-γ+ IL-2+ TNF-α+ and IFN-γ+ IL-2+ TNF-α+ cells; these corresponded to the subset that was most strongly associated with the CD8/pp65 response, with or without additional CD40L upregulation (Fig. 4). For each subset, there was a number of donors not expressing this particular marker combination, but these donors varied from subset to subset. Note that an increase of pp65-specific T cells having none of the functions we measured is not ruled out by our findings. However, because the frequencies of polyfunctional T cells were measured as percentages of all CD4 or all CD8 T cells or in absolute counts per ml of blood, it can be excluded fully that nonfunctional T cells replaced polyfunctional T cells. Figure 5 shows representative raw data for an older individual.
Fig 3.
Large CMV-specific CD8 T-cell responses have large polyfunctional components. Data are presented as dots correlating the absolute numbers of CD8/pp65 T cells displaying the indicated combinations of activation markers to the absolute number of all responding CD8/pp65 T cells (displaying at least one of the activation markers) (n = 39). The Spearman rank correlation coefficient for nonparametric data (RS) is indicated for significant correlations (RS of >0.50). Certain donors did not express certain functional subsets, resulting in what appears to be two groups with respect to each subset, but the nonresponding donors varied. However, the IFN-γ+ IL-2+ TNF-α+ subset was expressed by most donors. Cell counts were log transformed, with zeroes assigned the value 0.01 prior to transformation. The size of responding subsets is given as log(cells/ml of blood).
Fig 4.
Large CMV-specific CD4 T-cell responses have large polyfunctional components. Data are presented as dots correlating the absolute numbers of CD4/pp65 T cells displaying the indicated combinations of activation markers to the absolute number of all responding CD4/pp65 T cells (displaying at least one of the activation markers) (n = 38). The Spearman rank correlation coefficient for nonparametric data (RS) is indicated for significant correlations (RS of >0.50). Certain donors did not express certain functional subsets, resulting in what appears to be two groups with respect to each subset, but the nonresponding donors varied. However, the CD40L+ IFN-γ+ IL-2+ TNF-α+ subset was expressed by most donors. Cell counts were log transformed, with zeroes assigned the value 0.01 prior to transformation. The size of responding subsets is given as log(cells/ml of blood).
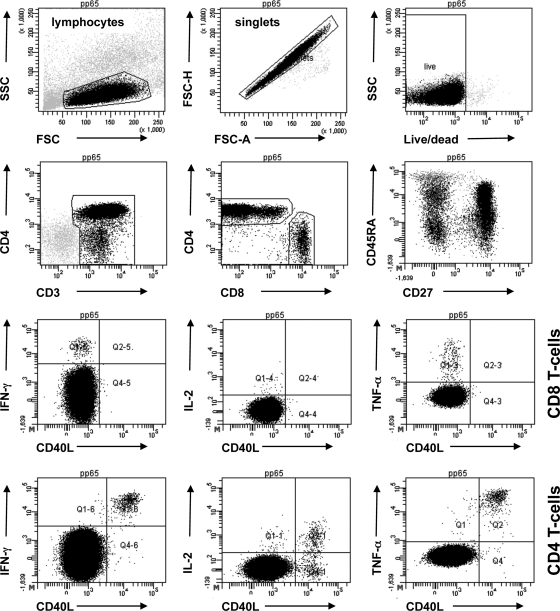
Fig 5.
Example of original data for an older individual. Representative dot plots show different views of the same data. CD4 and CD8 T cells were gated from single lymphocytes (FCS-H/FSC-A). Activated cells were gated individually for each T-cell subset and activation marker, and then Boolean gating combinations were computed. For the purpose of illustration only, all cytokines were plotted against CD40L. Plots show log fluorescence intensities for all markers. Side scatter (SSC) and forward scatter (FSC) are shown on a linear scale. More than 400,000 lymphocytes were gated in the example.
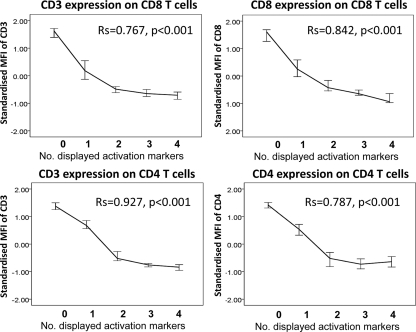
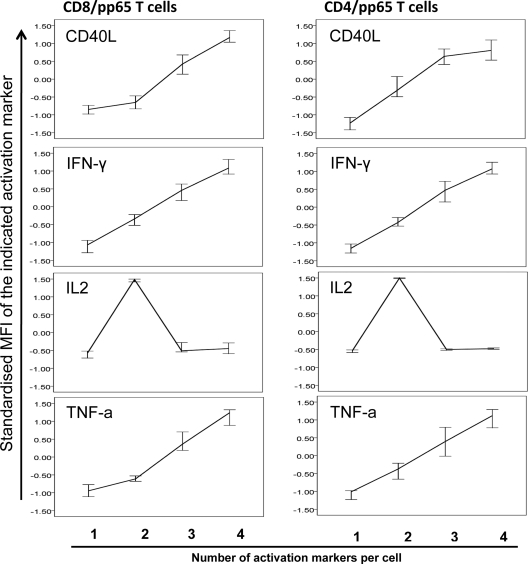
The degree of polyfunctionality of CMV-specific T cells correlates with the degree of TCR downregulation and the amounts of cytokines per cell.
T-cell receptor (TCR) downregulation was examined because it is a marker of T-cell sensitivity and correlates with the degree of T-cell activation. It was measured as the MFI of CD3, CD4, or CD8 expression. To allow for comparisons between individuals in different experimental runs, MFIs were normalized. The higher the number of activation markers displayed simultaneously per T cell, the stronger was not only CD3 but also CD8 and CD4 downregulation (Fig. 6). This was observed for CD8/pp65 T cells, CD8/IE-1 T cells, and CD4/pp65 T cells, as well as in OKT3-stimulated CD8 and CD4 T cells and PPD-stimulated CD4 T cells. OKT3 and PPD were tested for comparison (data not shown). Furthermore, and in agreement with other reports, the degree of polyfunctionality (i.e., the number of different activation markers displayed at the same time) correlated with the amount of each activation marker expressed per cell (normalized MFI), except for IL-2, which was expressed more highly in cells displaying just two functions (Fig. 7) (11, 17, 38, 39). This is further illustrated for IFN-α and TNF-α in Fig. S3 in the supplemental material.
Fig 6.
Downregulation of CD3, CD8, and CD4 on T cells after pp65-specific stimulation. Line curves (medians ± 95% confidence intervals [95% CI]) represent CD3 and CD8 expression on CD8/pp65 T cells (top panels), as well as CD3 and CD4 expression on CD4/pp65 T cells (bottom panels), as a function of the number of activation markers displayed simultaneously following overnight stimulation with a pp65 peptide pool. Lineage marker expression is represented by the standardized MFIs of CD3, CD8, and CD4 within the respective populations. There was a nearly linear correlation between TCR downregulation and the number of displayed activation markers (RS is indicated; n = 20 for all diagrams).
Fig 7.
Polyfunctional T cells display larger quantities of each activation marker except for IL-2. Line curves (medians ± 95% CI) show standardized MFIs of IFN-γ, IL-2, TNF-α, and CD40L. MFIs for T cells expressing one, two, three, or four activation markers are shown (n = 20). Note that although IL-2 was displayed by the major polyfunctional CD8/pp65 and CD4/pp65 subsets, its levels in responses including more than 2 markers were lower, which probably reflects its role in early antigen-dependent T-cell differentiation/proliferation.
DISCUSSION
In the literature, large CMV-specific T-cell responses are frequently described in the context of old age; these responses are usually reported as percentages of IFN-γ-producing or tetramer-binding CD8 T cells (22, 29, 31–34, 49). However, large CMV-specific responses also occur in young people (19, 45). Studies carried out on CMV-negative transplant recipients of CMV-positive grafts suggested that the size of the T-cell response to CMV correlates with the duration of CMV infection (36), and it is likely that this holds true for healthy CMV-infected individuals, too. However, we believe that in addition to the length of infection, host factors are crucial for the development of very large responses. This was suggested by long-term prospective studies of ageing cohorts, the OCTO and NONA studies, where an immune risk phenotype (IRP) thought to result from CMV infection was identified only in a subset of CMV-infected participants. It included CMV seropositivity and the expansion (inflation) of CD8 T cells with an advanced memory phenotype. This is probably the result of advanced memory differentiation of CMV-specific and non-CMV-specific T cells, where the latter may be driven in a still unknown way by an inflated CMV-specific T-cell response. Why this inflation occurs in some but not all CMV-infected individuals is unclear; to date, CMV-related memory T-cell inflation is best studied in MCMV models but is still not fully understood (18). It is one attractive hypothesis that the selection of “inflating” T-cell specificities is related to the composition of the naïve TCR repertoire (35). Other factors could also be involved in this process, including viral factors. Unfortunately, no study of humans or mice has yet reported a clear effect of viral variations or gene expression patterns on memory T-cell inflation. Also, viral load testing is usually negative for long-term virus-infected humans, and there is no method to retrospectively determine the CMV load during primary infection, so these factors cannot be included in the analysis. With regard to viral gene expression, it appears that even interference with antigen presentation has little effect on TCR selection and memory inflation patterns in the MCMV model (25). A recent study of married couples where one of the partners, but not the other, was the offspring of very-long-lived parents found that the hallmarks of CMV infection (immune system changes pertaining to the IRP) were absent in those with long-lived parents, pointing again to an important role of host genetic factors (10).
It was previously reported by several authors that a higher degree of T-cell differentiation (i.e., the presence of more cells of a more advanced memory type) in general is found in CMV-infected individuals than in noninfected individuals (7, 33, 46), but no correlation with any quantitative measure of CMV-specific immunity was established. Our results now clarify that the degree of T-cell differentiation in general correlates with the actual size of CD4/pp65 and CD8/pp65 responses. This is a novel and potentially very important finding, because it suggests that it may be the way in which the immune system deals with CMV that determines if the entire T-lymphocyte compartment (including a majority of non-CMV-specific cells) shows moderate (if the pp65-specific response is small) or advanced (if the pp65-specific response is large) differentiation.
It has become increasingly clear in recent years that response size is not directly related to protection. Recent studies suggested a critical role of T-cell response quality in the prediction of disease outcome, particularly assigning a protective role to subsets displaying several functions/activation markers simultaneously (1, 3, 8, 9, 11, 27, 28, 42). In particular, subsets producing IL-2, IFN-γ, and TNF-α at the same time have been associated with protection from infection after vaccination (9). We found that, surprisingly, polyfunctional CD4 and CD8 T cells were markedly increased in large pp65-specific responses. These polyfunctional subsets were located predominantly in the TEM compartment but also, to some extent, in the TCM and TEMRA compartments, in agreement with the expansion of TEM and TEMRA compartments in large responses. Several published studies reported larger numbers of CMV-specific T cells, with a relative loss of IFN-γ- or TNF-α-producing cells, in older CMV-positive donors (14, 22, 29, 32). Our results clarify that irrespective of response size or age, a robust population of polyfunctional CMV-specific T cells is present.
The degree of polyfunctionality of a T-cell response is likely to depend on the type of antigen encountered, antigen concentration, effectiveness of antigen presentation, and stage of antigen-dependent differentiation of the responding T cell (1, 5, 26, 47). In T cells with high antigen sensitivity, antigen contact results in a stronger signal than in cells with lower antigen sensitivity, and a stronger signal then translates into a more polyfunctional profile (1). TCR downregulation directly follows T-cell activation, and the degree of TCR downregulation is known to correlate with antigen sensitivity (23, 48, 50). As a result, one would expect TCR downregulation to be linked directly to polyfunctionality, and our results confirm exactly this prediction. Our results also confirm that CMV-specific T cells producing multiple cytokines express more of each cytokine per cell than single producers, which supports the idea that they are more efficient effector cells (11, 17, 38, 39). This was observed for CD4 and CD8 T cells after stimulation with CMV antigens but also after stimulation with PPD and OKT3, indicating that it is a general feature of T cells and is independent of the antigen recognized. IL-2 appeared to be an exception to this rule, which might be explained by its role early in an immune response where sustained T-cell proliferation is required.
In conclusion, our results show that large expansions of nonfunctional CMV-specific T cells do not occur at the expense of functional cells. This observation should help to reconcile some of the divergent views in the literature on the role of CMV-specific T-cell expansions. At the same time, our findings clarify that the magnitude of the effect that CMV has on general memory T-cell subset distribution (CMV-specific and non-CMV-specific cells) depends on the size of the pp65-specific T-cell response. This is a significant extension of previous observations, as it links this effect to the characteristics of the CMV-specific immune response itself rather than the presence of CMV infection alone.
Supplementary Material
ACKNOWLEDGMENTS
We thank Becky Dilley (NIHR Primary Care Research Network South-East) and all participating GP practices for help with recruiting suitable donors. We acknowledge Elaine Noon for handling the informed consent procedure, donor assessment, and sample acquisition. We are grateful to all blood donors for their contributions.
This work was supported in part by the Brighton and Sussex Medical School and by grant R107/0209 from the Dunhill Medical Trust (United Kingdom).
We declare no commercial affiliations or competing financial interests.
Footnotes
Published ahead of print 9 November 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Almeida JR, et al. 2009. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 113:6351–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appay V, van Lier RA, Sallusto F, Roederer M. 2008. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 73:975–983 [DOI] [PubMed] [Google Scholar]
- 3. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bunde T, et al. 2005. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 201:1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casazza JP, et al. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen SF, et al. 2004. Antiviral CD8 T cells in the control of primary human cytomegalovirus infection in early childhood. J. Infect. Dis. 189:1619–1627 [DOI] [PubMed] [Google Scholar]
- 7. Chidrawar S, et al. 2009. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 155:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciuffreda D, et al. 2008. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur. J. Immunol. 38:2665–2677 [DOI] [PubMed] [Google Scholar]
- 9. Darrah PA, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850 [DOI] [PubMed] [Google Scholar]
- 10. Derhovanessian E, et al. 2010. Hallmark features of immunosenescence are absent in familial longevity. J. Immunol. 185:4618–4624 [DOI] [PubMed] [Google Scholar]
- 11. Duvall MG, et al. 2008. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur. J. Immunol. 38:350–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elias D, Akuffo H, Britton S. 2005. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 99:363–368 [DOI] [PubMed] [Google Scholar]
- 13. Engstrand M, et al. 2003. Cellular responses to cytomegalovirus in immunosuppressed patients: circulating CD8+ T cells recognizing CMVpp65 are present but display functional impairment. Clin. Exp. Immunol. 132:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hadrup SR, et al. 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 176:2645–2653 [DOI] [PubMed] [Google Scholar]
- 15. Hamann D, et al. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hecker M, Qiu D, Marquardt K, Bein G, Hackstein H. 2004. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 86:41–44 [DOI] [PubMed] [Google Scholar]
- 17. Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. 2007. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J. Virol. 81:8468–8476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karrer U, et al. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022–2029 [DOI] [PubMed] [Google Scholar]
- 19. Kern F, et al. 2002. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J. Infect. Dis. 185:1709–1716 [DOI] [PubMed] [Google Scholar]
- 20. Kern F, et al. 2000. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur. J. Immunol. 30:1676–1682 [DOI] [PubMed] [Google Scholar]
- 21. Khan N, et al. 2007. T cell recognition patterns of immunodominant cytomegalovirus antigens in primary and persistent infection. J. Immunol. 178:4455–4465 [DOI] [PubMed] [Google Scholar]
- 22. Khan N, et al. 2004. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 173:7481–7489 [DOI] [PubMed] [Google Scholar]
- 23. Kohrt HE, et al. 2005. Rapid assessment of recognition efficiency and functional capacity of antigen-specific T-cell responses. J. Immunother. 28:297–305 [DOI] [PubMed] [Google Scholar]
- 24. McElhaney JE, Effros RB. 2009. Immunosenescence: what does it mean to health outcomes in older adults? Curr. Opin. Immunol. 21:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munks MW, Pinto AK, Doom CM, Hill AB. 2007. Viral interference with antigen presentation does not alter acute or chronic CD8 T cell immunodominance in murine cytomegalovirus infection. J. Immunol. 178:7235–7241 [DOI] [PubMed] [Google Scholar]
- 26. Ndhlovu ZM, Oelke M, Schneck JP, Griffin DE. 2010. Dynamic regulation of functionally distinct virus-specific T cells. Proc. Natl. Acad. Sci. U. S. A. 107:3669–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nebbia G, et al. 2008. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am. J. Transplant. 8:2590–2599 [DOI] [PubMed] [Google Scholar]
- 28. Nesbit L, Johnson SM, Pappagianis D, Ampel NM. 2010. Polyfunctional T lymphocytes are in the peripheral blood of donors naturally immune to coccidioidomycosis and are not induced by dendritic cells. Infect. Immun. 78:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ouyang Q, et al. 2003. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1). Exp. Gerontol. 38:911–920 [DOI] [PubMed] [Google Scholar]
- 30. Ouyang Q, Wagner WM, Wikby A, Remarque E, Pawelec G. 2002. Compromised interferon gamma (IFN-gamma) production in the elderly to both acute and latent viral antigen stimulation: contribution to the immune risk phenotype? Eur. Cytokine Netw. 13:392–394 [PubMed] [Google Scholar]
- 31. Ouyang Q, et al. 2003. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J. Clin. Immunol. 23:247–257 [DOI] [PubMed] [Google Scholar]
- 32. Ouyang Q, et al. 2004. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp. Gerontol. 39:607–613 [DOI] [PubMed] [Google Scholar]
- 33. Pita-Lopez ML, et al. 2009. Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun. Ageing 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pourgheysari B, et al. 2007. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J. Virol. 81:7759–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quigley MF, et al. 2010. Convergent recombination shapes the clonotypic landscape of the naive T-cell repertoire. Proc. Natl. Acad. Sci. U. S. A. 107:19414–19419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rentenaar RJ, et al. 2000. Development of virus-specific CD4(+) T cells during primary cytomegalovirus infection. J. Clin. Invest. 105:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwanninger A, et al. 2008. Age-related appearance of a CMV-specific high-avidity CD8+ T cell clonotype which does not occur in young adults. Immun. Ageing 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seder RA, Ahmed R. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835–842 [DOI] [PubMed] [Google Scholar]
- 39. Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258 [DOI] [PubMed] [Google Scholar]
- 40. Sester M, et al. 2002. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J. Virol. 76:3748–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sester U, et al. 2005. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am. J. Transplant. 5:1483–1489 [DOI] [PubMed] [Google Scholar]
- 42. Sester U, et al. 2008. PD-1 expression and IL-2 loss of cytomegalovirus-specific T cells correlates with viremia and reversible functional anergy. Am. J. Transplant. 8:1486–1497 [DOI] [PubMed] [Google Scholar]
- 43. Snyder CM, et al. 2009. CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J. Immunol. 183:3932–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sund F, et al. 2010. CMV-specific T-cell immunity, viral load, and clinical outcome in seropositive renal transplant recipients: a pilot study. Clin. Transplant. 24:401–409 [DOI] [PubMed] [Google Scholar]
- 45. Sylwester AW, et al. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turner JE, et al. 2010. Latent cytomegalovirus infection amplifies CD8 T-lymphocyte mobilisation and egress in response to exercise. Brain Behav. Immun. 24:1362–1370 [DOI] [PubMed] [Google Scholar]
- 47. Valitutti S, Muller S, Dessing M, Lanzavecchia A. 1996. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 183:1917–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Valitutti S, Muller S, Salio M, Lanzavecchia A. 1997. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J. Exp. Med. 185:1859–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vescovini R, et al. 2007. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J. Immunol. 179:4283–4291 [DOI] [PubMed] [Google Scholar]
- 50. Weyand CM, Goronzy J, Fathman CG. 1987. Modulation of CD4 by antigenic activation. J. Immunol. 138:1351–1354 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.