Abstract
A vaginal gel containing 1% tenofovir (TFV) was found to be safe and effective in reducing HIV infection in women when used pericoitally. Because of the long intracellular half-life of TFV and high drug exposure in vaginal tissues, we hypothesized that a vaginal gel containing TFV may provide long-lasting protection. Here, we performed delayed-challenge experiments and showed that vaginal 1% TFV gel protected 4/6 macaques against vaginal simian-human immunodeficiency virus (SHIV) exposures occurring 3 days after gel application, demonstrating long-lasting protection. Despite continued gel dosing postinfection, neither breakthrough infection had evidence of drug resistance by ultrasensitive testing of SHIV in plasma and vaginal lavage. Analysis of the active intracellular tenofovir diphosphate (TFV-DP) in vaginal lymphocytes collected 4 h to 3 days after gel dosing persistently showed high TFV-DP levels (median, 1,810 fmol/106 cells) between 4 and 24 h that exceed the 95% inhibitory concentration (IC95), reflecting rapid accumulation and long persistence. In contrast to those in peripheral blood mononuclear cells (PBMCs) following oral dosing, TFV-DP levels in vaginal lymphocytes decreased approximately 7-fold by 3 days, exhibiting a much higher rate of decay. We observed a strong correlation between intracellular TFV-DP in vaginal lymphocytes, in vitro antiviral activity, and in vivo protection, suggesting that TFV-DP above the in vitro IC95 in vaginal lymphocytes is a good predictor of high efficacy. Data from this model reveal an extended window of protection by TFV gel that supports coitus-independent use. The identification of protective TFV-DP concentrations in vaginal lymphocytes may facilitate the evaluation of improved delivery methods of topical TFV and inform clinical studies.
INTRODUCTION
Human immunodeficiency virus (HIV) continues to spread primarily through heterosexual routes, with women being disproportionately infected in many parts of the world (30, 35). While condoms have been shown to be one of the most reliable methods for preventing HIV transmission, such interventions are often limited by adherence and the ability of women to negotiate their use (24, 37). In the absence of an effective HIV vaccine, increasing efforts have been made toward developing vaginal gels formulated with antiretroviral (ARV) drugs, as a female-controlled option for women to protect themselves against HIV acquisition (10, 21, 33, 37).
Topical gels containing tenofovir (TFV), a nucleotide analogue reverse transcriptase inhibitor, have recently shown great promise against vaginal HIV transmission. Results of the CAPRISA 004 trial demonstrated for the first time that women who used a vaginal gel containing 1% TFV were 39% less likely overall to contract HIV than those who used a placebo gel (1). The trial evaluated a coitus-dependent before-and-after (BAT) modality, with one gel application administered up to 12 h before sex followed by a second gel application up to 12 h after sex. Importantly, effectiveness was found to be dependent on reported adherence. Tenofovir gel showed 54% protection in women who self-reported >80% adherence, compared to only 38 and 28% in women who reported 50 to 80% and <50% adherence, respectively. Moreover, the study found that TFV gel reduced the risk of herpes simplex virus 2 (HSV-2) infection in women by 52%. This added benefit was unique to TFV, since all other ARV-containing gels have no reported activity against HSV-2, and thus further enhances the importance of the use of TFV gel as a prevention strategy for both HIV and HSV-2 (1).
The observed efficacy of the TFV gel in CAPRISA 004 is consistent with previous data in our vaginal repeat-challenge macaque model that showed protection from simian-human immunodeficiency virus (SHIV) infection when 1% TFV gel was applied at 30 min before each vaginal challenge (1, 21). In this previous study, six female pigtail macaques were challenged twice weekly for a total of 20 exposures, and all remained protected from SHIV infection. The reasons for the higher protection in the macaques than in the women in CAPRISA 004 are not clear, but it may be explained by lower adherence than currently estimated by self-reports or possibly be related to other model-related parameters that are not fully optimized. However, the protection seen in both CAPRISA 004 and the nonhuman primate model highlights the importance of using the macaque model to investigate important questions and modalities that are otherwise difficult to assess in human studies, such as defining the prophylactic window of protection with respect to pharmacokinetic (PK) parameters. An improved understanding of the window of protection can help define clinical dosing and timing parameters. For instance, a short window of protection would support a coitus-dependent use of the gel. In contrast, a long window of protection would support a coitus-independent use, thereby obviating gel application with each coital activity, which may be more desirable to women and help improve adherence. To this end, the macaque model was used as a tool to provide critical information about tissue drug levels and defining protective PK and pharmacodynamic (PD) parameters, all of which will be important for selecting dosing strategies and informing clinical evaluations.
The long intracellular half-life (∼60 to 175 h) of the active TFV diphosphate (TFV-DP) in peripheral blood mononuclear cells (PBMCs) after oral dosing in both humans and macaques is unique among ARVs (5, 8). Both the long intracellular half-life and the high dose of TFV (30 to 40 mg) delivered in a single gel application suggest that TFV gel can provide long-lasting antiviral activity in vaginal tissues. Here, we performed delayed-challenge experiments to evaluate the efficacy of 1% TFV gel administered 3 days prior to virus challenge. We used a repeated-low-dose vaginal challenge macaque model. This model has several advantages, including the use of female pigtail macaques, which have a menstrual cycle and vaginal anatomy similar to those of women, an inoculum dose with viral RNA levels in the range similar to those detected in seminal fluid, twice-weekly challenges to mimic high-risk human exposure, and a SHIV-162p3 inoculum which constitutes an R5-tropic envelope similar to that of most transmitted HIV (14, 22). We performed detailed PK analysis of intracellular TFV-DP drug levels in vaginal lymphocytes over time to better understand the relationship between tissue drug levels and protective efficacy. We show that 1% TFV gel administered 3 days prior to exposure provided effective protection, and we relate this protection indirectly to persistent intracellular TFV-DP levels in vaginal lymphocytes at time of exposure. While topical dosing resulted in high TFV-DP levels in vaginal lymphocytes that were long lasting, we also show that the half-life of TFV-DP was significantly shorter than that previously predicted from oral dosing.
MATERIALS AND METHODS
Gel formulation.
Tenofovir [(R)-9-(2-phosphonylmethoxypropyl)adenine] (TFV) was kindly provided by Gilead Sciences; 1% (wt/wt) TFV was formulated in 2% hydroxyethyl cellulose (HEC) gel as previously described, with minor modifications (16). Gels were formulated at pH 6.5 to mimic the average vaginal pH of pigtailed macaques (12). A placebo 2% HEC gel was formulated similarly and used as a control. Products were stored at room temperature, and antiviral activity was confirmed throughout the course of the study using a TZM-bl reporter assay to measure drug activity (23).
Virus stock.
SHIVSF162P3 (simian immunodeficiency virus SIVmac239 backbone with an HIV-1 subtype B, CCR5-tropic envelope) (6, 7) was obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program and was propagated in PBMCs from pigtailed macaques as previously described (20, 29). The virus stock titer was calculated in pigtailed macaque PBMCs, and virus was diluted to the challenge dose of 10 50% tissue culture infective doses (TCID50) (1.5× 106 RNA copies) and stored separately in 1-ml aliquots in liquid nitrogen. Individual vials were thawed on ice prior to challenge.
Efficacy study using the repeat-exposure macaque model.
We previously reported the efficacy of 1% TFV gel administered 30 min before vaginal SHIV exposures in female pigtailed macaques (21). Macaques were challenged twice weekly for 10 weeks (20 challenges over ∼2 menstrual cycles), and all remained protected. Here we assessed the window of protection conferred by 1% TFV gel using a similar study design. Macaques received a single vaginal application of placebo (n = 1) or 1% TFV (n = 6) gel but were exposed to two SHIVSF162P3 challenges at 30 min and 3 days after the gel application (Fig. 1A). The once-weekly gel application followed by two virus exposures was repeated for 10 weeks or up to 20 challenges, occurring over two menstrual cycles. All animals were anesthetized and remained recumbent for 30 min after each gel application and 15 min after virus inoculation. Blood was collected after each challenge throughout the study period and twice per week, for 10 weeks, after the last virus challenge. Virus exposures were stopped when a macaque became SHIV RNA positive in plasma. Infected macaques continued to receive weekly gel dosing for a median of 13 weeks (range, 12 to 16 weeks) after infection. All experiments were done under highly controlled conditions by the same personnel using the same virus stock, inoculum dose, and procedures as described in previous studies (21). These studies adhered to the Guide for the Care and Use of Laboratory Animals (18a); all procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of both the Centers for Disease Control and Prevention (CDC) and the Yerkes National Primate Research Center (Emory University).
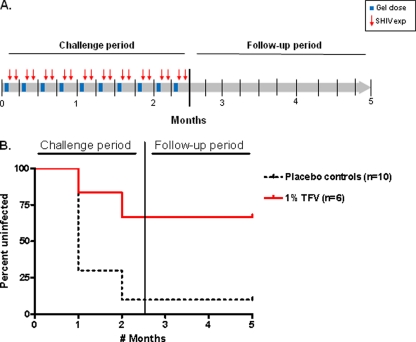
Fig 1.
TFV gel provides durable protection against vaginal SHIV162P3 infection. (A) Study design. Macaques received one weekly vaginal gel application (solid blue bars) and were exposed to SHIV162P3 at 30 min and again at 72 h after the gel dose (red arrows). (B) Survival curves representing the cumulative percentage of uninfected macaques as a function of the number of months in the study period (8 challenges per month). After 2.5 months (20 SHIV162p3 exposures), challenges were stopped and animals were monitored for 10 additional weeks for infection in the follow-up period. Controls include 1 real-time control and 9 historic controls (dotted black line).
Menstrual cycle monitoring.
Menstrual cycles in macaques were monitored by plasma progesterone levels. Plasma samples collected twice weekly were shipped to the University of Wisconsin National Primate Research Center and analyzed for progesterone levels using an enzyme immunoassay (EIA) (25).
Infection monitoring by PCR and serology.
SHIV infection was monitored twice weekly by the detection of SHIV RNA in plasma using a reverse transcriptase PCR (RT-PCR) method with a sensitivity of 50 RNA copies per milliliter as previously described (4, 20, 21). Proviral DNA was monitored in peripheral blood mononuclear cells (PBMCs) using primers and probes specific for SIVmac239 pol (20, 21). Serologic testing was done using a synthetic-peptide EIA (Genetic Systems HIV-1/HIV-2 plus O; Bio-Rad). The estimated time of infection was defined as 7 days prior to the first positive RNA RT-PCR in plasma to account for the eclipse period between virus inoculation and detection of SHIV RNA in infected macaques (13). Animals were considered protected if they tested negative for SHIV plasma RNA and SHIV DNA in PBMCs and remained seronegative during the course of the study and the following 10 weeks of washout in the absence of challenge and gel application.
Drug resistance testing.
Drug resistance emergence was monitored in both plasma and cervicovaginal lavage (CVL) fluid from infected animals using standard sequence analysis of the RT-coding region (551 bp; amino acids 52 to 234) and by sensitive allele-specific real-time PCR methods for the K65R mutation as previously described (9, 29). CVL specimens were collected by instilling and recollecting approximately 5 ml of sterile phosphate-buffered saline in the vaginal cavity. CVL fluids were centrifuged for 15 min at 400 × g to pellet cells and debris. Virion-associated RNA was extracted from CVL supernatant (1 ml) using a Qiagen viral RNA kit.
Measurement of drug concentrations in plasma, tissue lymphocytes, and PBMCs.
TFV concentrations in plasma were measured in macaques at 30 min after vaginal administration of TFV gel, resulting in the analysis of at least 10 samples from each macaque. Briefly, TFV was extracted from 100 μl of plasma by protein precipitation with 350 μl of methanol containing 200 ng of 13C-labeled TFV as an internal standard. Supernatant containing the drug from precipitation was evaporated to near dryness under vacuum and then resuspended in high-pressure liquid chromatography (HPLC) buffer containing 9.9 mM acetic acid, 5.9 mM ammonium hydroxide, and 9.4 mM formic acid (pH ∼3). Drug levels were analyzed by using liquid chromatography-mass spectrometry (LC-MS) (21, 36). The assay had a lower limit of quantification (LLOQ) of 5 ng/ml and standard curve r2 values of greater than 0.99.
For tissue lymphocyte drug levels, SHIV-infected female macaques (n = 10) were sacrificed at 4 h (n = 3), 24 h (n = 2), 2 days (n = 2), and 3 days (n = 3) after receiving a single dose of 1% TFV gel (30 mg). All animals were within the luteal (progesterone-dominant) phase of their menstrual cycle at the time of dosing. Vaginal tissue collected at time of necropsy were dissociated using an enzyme cocktail containing collagenase type II (62.5 U/ml), elastase (0.07 U/ml), hyaluronidase (0.8 U/ml), and DNase I (0.083 U/ml) following procedures kindly provided by Craig Hendrix with minor modifications. Crude cell preparations isolated from tissues were separated using lymphocyte separation medium (LSM) (MP Biomedicals, Aurora, OH) to enrich for mononuclear cells. Total cell populations were gated and counted for lymphocytes using a Guava cell counter with CytoSoft data acquisition and analysis software version 6.0.2 (Millipore, Billerica, MA).
To compare TFV-DP concentrations observed in vivo with those achieved in vitro, macaque PBMCs were incubated with various concentrations of TFV. Intracellular TFV-DP and extracellular TFV concentrations were then plotted in a standard curve. Briefly, PBMCs (5 × 106) were incubated for 24 h in RPMI medium containing TFV concentrations within approximately the range of the 100% inhibitory concentration (IC100) to the IC0 (300, 60, 12, 2.4, 0.48, and 0.096 μM) (28, 31). Additional PBMCs were incubated with 32.8 mM TFV, which corresponds to the TFV concentration in the gel. Cells were washed twice with saline buffer solution and counted using a Guava cell counter.
In addition, we measured intracellular TFV-DP levels in PBMCs following oral administration of tenofovir disoproxil-fumarate (TDF) as previously described (4). Briefly, six macaques each received a single human equivalent oral TDF dose (22 mg/kg), and TFV-DP levels were measured in PBMCs from blood collected at 2, 5, 24, 48, 120, and 168 h.
Intracellular TFV-DP concentrations in lymphocytes were measured with an automated online weak anion-exchange solid-phase extraction method coupled with ion-pair chromatography-MS/MS (4, 11). Briefly, TFV-DP was extracted from lymphocytes using 80% methanol. Lymphocytes were washed with 0.9% NaCl buffer and then resuspended in 1 ml of ice-cold 80% methanol containing 13C-labeled TFV-DP as an internal standard. Samples were extracted overnight at −80°C, centrifuged, and dried to completion. Samples were reconstituted in 100 μl of 50 mM ammonium acetate buffer (pH 7.0) and centrifuged at 17,000 × g to remove insoluble particulates. Calibration curves were generated from standards of tenofovir-DP by serial dilutions in blank human PBMC extract over the range from 0.25 to 10 nM. The lower limit of quantification for tenofovir-DP was 10 ng/ml. All calibration curves had r2 values of greater than 0.99.
Statistical methods.
Fisher's exact test was used to compare the number of infections per number of months at risk for new infection by study group. Risk for infection per month, or approximately per menstrual cycle, with biweekly challenge doses throughout each month, considers that cycling pigtailed macaques are more susceptible to infection during the luteal phase of the menstrual cycle, as we previously demonstrated in this model (34). Intervention efficacy was calculated as 1-(p1/p0), where p1 and p0 denote the proportions of months with incident infection for intervention and control animals, respectively (15).
Linear mixed-effects regression models (17) were used to estimate the TFV-DP half-life and 95% confidence limits (CL) based upon observations at and after peak drug absorption and to test for differences in rate of drug elimination between vaginal lymphocytes and systemic PBMCs following topical TFV gel and oral TDF dosing, respectively. A delta approximation method was used to calculate variance for drug half-life (19). The use of this methodology to estimate drug half-life assumes first-order drug PKs. SAS version 9.2.1 was used for all statistical analyses (17).
RESULTS
TFV gel maintains protection at 3 days against challenge with SHIV-162P3.
Figure 1B shows the infection survival rates in animals administered TFV gel at 3 days before SHIV exposure. The infection outcome was compared to that seen in 10 untreated controls receiving a placebo 2% HEC gel application (1 real-time control and 9 historic controls). The historic controls were from a study completed 2 months prior to the start of this work in which control animals received the same placebo gel and were challenged with the same virus stock under the same experimental conditions (21). Figure 1B shows that 9 of 10 controls became infected after 10 weeks, 7 animals (including the real-time control) became infected during the first month of challenges, and 2 additional animals became infected in the following weeks. In contrast, 4 of the 6 macaques receiving 1% TFV gel remained uninfected after 10 weeks of twice-weekly SHIV exposures; 2 treated animals became infected, 1 during the first month and 1 in the following weeks. All infected macaques had detectable proviral DNA and seroconverted at 1 to 3 weeks after first detectable plasma SHIV RNA was observed (data not shown). Risk for infection differed by study group (P = 0.02 by Fisher's exact test). TFV gel efficacy was estimated at 74%, based upon 9/13 infections per number of months at risk among controls and 2/11 among treated animals.
Intracellular half-life of TFV-DP in vaginal tissue lymphocytes.
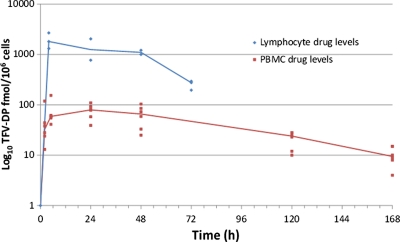
To better understand the relationship between protection and tissue drug levels, we assessed the decay of intracellular TFV-DP over time in vaginal lymphocytes by administration of a single vaginal gel application to 10 macaques followed by necropsy at specified time points and collection of vaginal tissues. Gel was applied at 4 h (n = 3), 1 day (n = 2), 2 days (n = 2), or 3 days (n = 3) prior to tissue collection. Figure 2 shows TFV-DP concentrations detected in tissue lymphocytes from all 10 macaques. The TFV-DP levels detected in vaginal tissues were highest at 4 h (median, 1,810 fmol/106 cells; range, 1,312 to 2,684) and remained above 1,000 fmol/106 cells within 1 to 2 days (median, 1,107.5 fmol/106 cells; range, 771 to 2,043), demonstrating rapid accumulation and persistence of TFV-DP. However, the median TFV-DP concentrations at 3 days precipitously dropped to 252 fmol/106 cells (range, 196 to 295). We compared the half-life of TFV-DP in vaginal lymphocytes after vaginal gel dosing with that in circulating PBMCs in six pigtail macaques after oral TDF dosing. In lymphocytes extracted from vaginal tissues, the estimated half-life was 25 h (95% CL, 16 to 34). In contrast, TFV-DP persisted much longer in PBMCs (P = 0.02) following oral dosing, with a half-life of 49 h (95% CL, 39 to 60).
Fig 2.
Intracellular TFV-DP concentrations after vaginal gel or oral dosing. TFV-DP concentrations in mononuclear cells isolated from vaginal tissues collected 4, 24, 48, and 72 h after a single vaginal TFV gel dose (solid blue line) are shown. For oral dosing, six macaques each received a single human equivalent oral TDF dose (22 mg/kg) and, TFV-DP levels were measured in PBMCs from blood collected at 2, 5, 24, 48, 120, and 168 h (red line).
Intracellular TFV-DP in PBMCs following in vitro TFV dosing and comparison with in vivo concentrations.
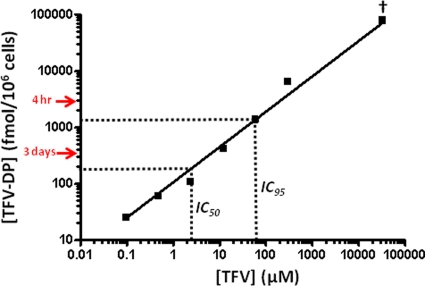
We compared the TFV-DP concentrations observed in vivo with those achieved at 24 h after in vitro dosing. Figure 3 shows a standard curve relating extracellular TFV to intracellular TFV-DP concentrations. TFV-DP levels increased proportionally with extracellular TFV concentrations. Exposure of PBMCs to 2.4 and 60 μM TFV, the in vitro IC50 and IC95 (28, 31), resulted in intracellular TFV-DP concentrations of 109 and 1,373 fmol/106 cells, respectively. Figure 3 also denotes the TFV-DP concentrations found in vaginal lymphocytes collected 4 h (median, 1,810 fmol/106 cells) and 3 days (median, 252 fmol/106 cells) after gel dosing in macaques, which were found to exceed the IC95 and IC50, respectively. Interestingly, the TFV-DP concentrations in PBMCs (79,244 fmol/106 cells) after in vitro dosing at 32.8 mM, the concentration of TFV in 1% TFV gel, were 72-fold higher than those achieved in vivo in vaginal lymphocytes at 24 h after TFV gel dosing (1,107 fmol/106 cells). These data demonstrate a lower in vivo permeability of TFV across the vaginal mucosa.
Fig 3.
Correlation between extracellular TFV and intracellular TFV-DP. Intracellular TFV-DP levels measured in PBMCs incubated for 24 h in RPMI medium containing various concentrations of TFV are shown. Dotted lines indicate extracellular TFV concentrations in the range of the previously reported in vitro IC50 and IC95 (28, 31). Red arrows denote intracellular TFV-DP levels in vaginal lymphocytes at 4 h and 3 days after in vivo dosing with 1% TFV gel. †, TFV concentration in gel (32.8 mM).
No evidence of blunted viremia in breakthrough infections.
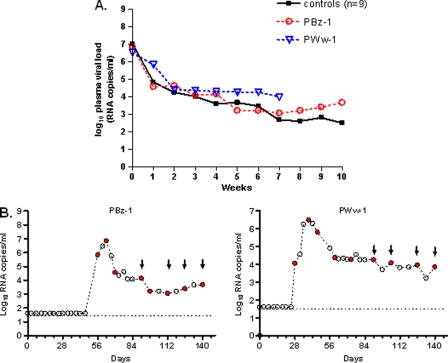
We next explored the impact of continuous gel dosing on systemic virus replication by maintaining the once-weekly TFV gel applications in the two breakthrough animals. Animals identified as PBz-1 and PWw-1 received an additional 12 and 16 TFV gel applications postinfection, respectively. Figure 4A shows the kinetics of plasma viral replication compared to that in 9 untreated macaques. The peak viremia in the two treated macaques, 6.8 log10 RNA copies/ml and 6.5 log10 RNA copies/ml, was similar to the mean peak viremia of the 9 untreated controls (7.0 ± 0.96 log10 RNA copies/ml), showing no difference in systemic virus levels during acute viremia.
Fig 4.
Breakthrough infections show no evidence of blunted viremia or K65R emergence (A) Individual virus load kinetics in breakthrough infections under continued once-weekly gel dosing (n = 2, red and blue dotted lines) and median virus load kinetics of placebo-infected macaques (n = 9, black line). Time zero indicates the peak plasma virus load. (B) Undetectable K65R mutation in plasma or cervicovaginal (CVL) viral RNA in breakthrough infections. Red circles and black arrows indicate samples tested for plasma and CVL fluid, respectively. The broken line denotes the limit of detection of the virus load assay (50 copies/ml).
No drug resistance emergence despite continued gel dosing.
Both breakthrough infections were initiated with wild-type viruses. As shown in Fig. 4B, sequence analysis of longitudinal specimens revealed no evidence of K65R emergence in viruses from plasma at the time of infection, peak viremia, or the following 8 to 12 weeks of the washout period. Likewise, despite weekly gel dosing, K65R was not detected by sequencing of SHIV RNA from CVL samples collected periodically up to 14 weeks from the time of initial infection. Additional sensitive testing by allele-specific PCR was also negative for all samples sequenced, thus indicating the absence of low-frequency K65R mutants in both plasma and CVL samples.
Systemic drug absorption following vaginal dosing with 1% TFV gel.
As previously reported, the median detectable TFV levels in plasma at 30 min after vaginal application were low (15.5 ng/ml) relative to median peak levels following oral dosing with TDF (80 ng/ml) (5, 21). Overall, the proportion of measurements with TFV detected in plasma of the 6 treated macaques was 60%, i.e., 36 of a total of 60 measurements (range, 20 to 90%), likely reflecting temporal changes in drug absorption associated with the menstrual cycle (Table 1). Both the infrequent (once-weekly) gel dosing and low systemic drug exposure may likely explain the lack of virus blunting and drug resistance emergence in the breakthrough infections.
Table 1.
Systemic drug absorption at 30 min following vaginal dosing with 1% TFV gela
| Subject | Infected | Median detectable plasma TFV (ng/ml)b | % of measurements with detectable TFV | Challenges received | TFV (ng/ml) 3 days prior to infection |
|---|---|---|---|---|---|
| PEd-1 | − | 8.6 | 90 | 20 | NAd |
| PPy-1 | − | <5.0 | 20 | 20 | NA |
| PVd-2 | − | 12.0 | 70 | 20 | NA |
| PWu-1 | − | <5.0 | 40 | 20 | NA |
| PBz-1 | + | 10.8 | 70 | 14c | 8.6 |
| PWw-1 | + | 18.5 | 70 | 6c | 14.9 |
n = 10 measurements per subject.
Lower limit of detection = 5 ng/ml.
Estimated infectious challenge.
NA, not applicable.
DISCUSSION
In this study, we used normal cycling female pigtailed macaques in a repeat-challenge model to evaluate the window of protection conferred by a vaginal 1% TFV gel. We predicted that both the high concentration of TFV in the gel and the long intracellular half-life of TFV-DP would result in durable protection. Our data show that 1% TFV gel maintained significant efficacy (74%) at 3 days after application, thus providing a wide window of protection to macaques against vaginal SHIV transmission. To better understand the tissue drug levels needed for protection, we examined TFV-DP concentrations in vaginal lymphocytes collected between 4 h and 3 days after administering a single gel dose and related these concentrations to the TFV-DP levels associated with in vitro inhibition. Intracellular drug analysis revealed persistently high TFV-DP concentrations detected as early as 4 h and up to 2 days, reflecting rapid accumulation and long persistence in vaginal lymphocytes. Furthermore, these TFV-DP concentrations exceeded the intracellular TFV-DP IC95, which likely explains the complete protection previously observed in macaques challenged 30 min after gel application (21). The persistence of these TFV-DP levels in vaginal lymphocytes for up to 2 days further implies that this level of high efficacy would likely extend to 2 days. In contrast, our data show an unexpected 7-fold decrease in TFV-DP concentrations in vaginal lymphocytes at 3 days and, thus, a shorter half-life (25 h) than that in PBMCs after oral dosing (49 h). The reduced TFV-DP levels in the vaginal lymphocytes are in the range of the in vitro IC75 and likely explain the reduction in efficacy from 100 to 74% when TFV gel was applied at 3 days as opposed to 30 min before virus exposure. While our findings could be strengthened by larger numbers of macaques and more than two efficacy measurements, the strong correlation we observed between TFV-DP concentrations, their associated in vitro activity, and in vivo protection suggests that TFV-DP in vaginal lymphocytes is a good predictor of efficacy. Thus, data from this model provide the first information on the protective TFV-DP levels and point to TFV-DP concentrations in vaginal lymphocytes that are above the in vitro IC95 as predictors of high protection by TFV gels.
Although we have not measured the TFV-DP concentrations in vaginal lymphocytes after oral TDF dosing in macaques, recent data for humans are consistent with our macaque data and have demonstrated much higher TFV-DP exposures in vaginal tissues after TFV gel compared to oral TDF dosing, implying that oral TDF may not confer the same level of protection as topical TFV gel against vaginal infection (3, 27, 39). An interim analysis of two human clinical trials has recently shown conflicting efficacy results. In the VOICE trial, oral TDF was found to be ineffective, while the Partners PREP study showed a reduction in HIV-1 infection by 68% among women who have HIV-1-infected partners (J. Baeten et al., presented at the 6th IAS Conference on HIV Pathogenesis Treatment and Prevention, 17 to 20 July 2011, Rome, Italy, and MTN-003). The reasons for the discrepancy between the two study results are not clear at this time, as further analyses of adherence and other parameters are needed. If oral TDF is found to be highly protective in women despite the lower vaginal TFV exposures, this would suggest a mechanism of dual protection whereby systemic and local antiviral activities both contribute to prevent infection.
We also found that vaginal permeability reduces intracellular TFV-DP concentrations in vaginal lymphocytes by about two orders of magnitude relative to in vitro dosing, which underscores the need for highly concentrated (mM) TFV gels to achieve in vivo protection. The scale-up in drug concentration needed for in vivo protection with topical gels has been previously observed with other drugs and likely reflects the permeability of drug through the vaginal mucosa, as well as the need to protect a large vaginal surface area for a significant period of time (32, 33). The TFV-DP concentrations in vaginal lymphocytes associated with high efficacy that were identified in this study may provide reference values to guide the development of devices for vaginal TFV delivery by different gel formulations, vaginal rings, or films.
The persistently high levels of TFV-DP detected in vaginal lymphocytes for up to 2 days, which were previously shown to be associated with complete protection, suggest that the same level of protection could be maintained within 2 days after dosing. These data and the 74% efficacy observed at 3 days all point to a long window of protection that supports coitus-independent gel use, which may be more desirable to women and help improve adherence. The window of protection for TFV gel is longer than that recently reported for macaques with a gel containing the CCR5 entry inhibitor maraviroc (33). Maraviroc gel (6 mM) fully protected macaques from vaginal SIV infection when administered 30 min before exposure, but the protection was short-lived, as complete loss of protection was observed by 12 h (33). The reasons for the short window of protection by the maraviroc gel are not known but could be due to rapid CCR5 receptor recycling and lymphocyte turnover. Lymphocyte turnover in vaginal tissues could also explain the precipitous drop in TFV-DP levels at 3 days following TFV gel dosing. It will be important to compare the half-lives of TFV-DP in vaginal lymphocytes from macaques with those in women following TFV gel dosing.
Drug resistance emergence when antiretroviral prophylaxis fails is a concern, particularly if the intervention includes drugs that are widely used for treatment, as is the case with TFV. Consistent with human data from CAPRISA 004 showing only wild-type virus detected in women who became infected while using TFV gel, we demonstrated that two macaques who failed TFV gel treatment had no evidence of the TFV-associated K65R mutation in plasma by conventional sequencing and ultrasensitive testing, despite continued gel dosing postinfection (1). We also found only wild-type SHIV populations in the vaginal secretions of both macaques, which was reassuring because it indicated that the higher drug selection in vaginal tissues, as a result of vaginal dosing, had no impact on K65R emergence.
We further showed that TFV was detected in 60% of plasma samples collected 30 min after gel dosing, confirming very rapid absorption through vaginal tissues. Plasma TFV was detected at higher levels in macaques than in women who applied vaginal TFV gel containing 40 mg (16, 27). This difference may be explained by the approximately 12-fold-smaller blood volume in macaques, which may concentrate TFV, thus making it more easily detected in macaques than in women, especially if vaginal absorption is similar in both species (26). We also found no reduction in the acute viremia in breakthrough infections compared to controls, reflecting insignificant antiviral activity from any systemic TFV exposure by the once-weekly gel dosing modality.
Our study has several limitations. First, the virus inoculum was clonal, and all challenges were done in the absence of semen or semen-derived factors shown to enhance HIV infection in vitro (18). Second, exposures were to an intact vaginal mucosa without trauma associated with coitus, concurrent genital ulcers, or bacterial vaginosis, all of which can increase the risk of HIV acquisition (2, 38). Third, the gel volume was decreased to 3 ml (30 mg TFV), compared to 4 ml (40 mg TFV) in women, to accommodate the smaller vaginal cavity in macaques, which may not fully mimic the drug exposure in women. Although the drug exposure may be greater based on the smaller vaginal surface area in the macaques than in humans, it is unclear whether the difference in scale is critical because the drug may not be limiting in either humans or macaques given the high local drug dose and the small amount (∼0.02%) that is absorbed (21). Importantly, data from humans dosed with 4 ml of 1% TFV gel show intracellular TFV-DP concentrations in cells collected from vaginal tissue at 24 h (∼1,700 fmol/106 cells) that are similar to the levels in macaques at 24 h (∼1,100 fmol/106 cells), suggesting little impact of the difference in scale (27).
In conclusion, we demonstrate that topical application of a gel containing 1% TFV administered 3 days before virus exposure provided durable protection in macaques. Data from this model suggest that gel application immediately before sex may not be required to provide high protection. We also show that gel dosing results in very high TFV-DP levels in vaginal lymphocytes but demonstrate a faster decay of TFV-DP than previously predicted from oral dosing. Our data are the first to identify protective intracellular drug levels in vaginal lymphocytes, which should facilitate the development of improved methods for vaginal delivery of TFV and inform next-generation efficacy and PK studies in humans.
ACKNOWLEDGMENTS
This work was partially supported by Interagency Agreement Y1-AI-0681-02 between CDC and NIH.
We thank Stephanie Ehnert, Christopher Souder, Elizabeth Strobert, and the animal care staff at the Yerkes National Primate Center (Emory University) as well as James Mitchell, Elizabeth Sweeney, and Shanon Bachman at the CDC for monitoring, maintaining, and performing animal procedures using our macaque cohort. We thank Jim Rooney at Gilead Sciences for providing tenofovir. We thank T. E. Ziegler and Dan Wittwer at the University of Wisconsin National Primate Research Center for providing progesterone measurement services. Reagents SHIVSF162P3 and TZM-bl were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH): SHIVSF162P3 from Janet Harouse, Cecilia Cheng-Mayer, Ranajit Pal, DAIDS, and NIAID and TZM-bl cells from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Centers for Disease Control and Prevention or the Department of Health and Human Services.
Footnotes
Published ahead of print 9 November 2011
REFERENCES
- 1. Abdool Karim Q, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. 2008. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dumond JB, et al. 2007. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 21:1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García-Lerma JG, et al. 2008. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia-Lerma JG, et al. 2010. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14ra4 [DOI] [PubMed] [Google Scholar]
- 6. Harouse JM, et al. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J. Virol. 75:1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816–819 [DOI] [PubMed] [Google Scholar]
- 8. Hawkins T, et al. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406–411 [DOI] [PubMed] [Google Scholar]
- 9. Johnson JA, Rompay KK, Delwart E, Heneine W. 2006. A rapid and sensitive real-time PCR assay for the K65R drug resistance mutation in SIV reverse transcriptase. AIDS Res. Hum. Retroviruses 22:912–916 [DOI] [PubMed] [Google Scholar]
- 10. Klasse PJ, Shattock R, Moore JP. 2008. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 59:455–471 [DOI] [PubMed] [Google Scholar]
- 11. Kuklenyik Z, et al. 2009. Effect of mobile phase pH and organic content on liquid chromatography mass spectrometry analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J. Chromatogr. Sci. 47:365–372 [DOI] [PubMed] [Google Scholar]
- 12. Lichtenwalner AB, Patton DL, Klebanoff SJ, Headley CM, Hillier SL. 2000. Vaginal myeloperoxidase and flora in the pig-tailed macaque. J. Med. Primatol. 29:36–41 [DOI] [PubMed] [Google Scholar]
- 13. Liu J, et al. 2010. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J. Virol. 84:10406–10412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Livingston L, et al. 2011. Hormonal synchronization of the menstrual cycles of pigtail macaques to facilitate biomedical research including modeling HIV susceptibility. J. Med. Primatol. 40:164–170 [DOI] [PubMed] [Google Scholar]
- 15. Longini IM, Jr, Datta S, Halloran ME. 1996. Measuring vaccine efficacy for both susceptibility to infection and reduction in infectiousness for prophylactic HIV-1 vaccines. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:440–447 [DOI] [PubMed] [Google Scholar]
- 16. Mayer KH, et al. 2006. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS 20:543–551 [DOI] [PubMed] [Google Scholar]
- 17. Molenberghs G, Verbeke G. 1997. Linear mixed models for longitudinal data. Springer, Berlin, Germany [Google Scholar]
- 18. Münch J, et al. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059–1071 [DOI] [PubMed] [Google Scholar]
- 18a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 19. Oehlert GW. 1992. A note on the delta method. Am. Stat. 46:27–29 [Google Scholar]
- 20. Otten RA, et al. 2005. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J. Infect. Dis. 19:164–173 [DOI] [PubMed] [Google Scholar]
- 21. Parikh UM, et al. 2009. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pilcher CD, et al. 2004. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 189:1785–1792 [DOI] [PubMed] [Google Scholar]
- 23. Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. 2009. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J. Virol. 83:8289–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Potts M. 1994. The urgent need for a vaginal microbicide in the prevention of HIV transmission. Am. J. Public Health 84:890–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. 1994. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol. Behav. 56:801–810 [DOI] [PubMed] [Google Scholar]
- 26. Salvato MS, Rater M, Pauza CD. 1996. Tracking of dye-labeled lymphocytes in rhesus macaques. J. Med. Primatol. 25:112–121 [DOI] [PubMed] [Google Scholar]
- 27. Schwartz JL, et al. 2011. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One 6:e25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Srinivas RV, Fridland A. 1998. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob. Agents Chemother. 42:1484–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Subbarao S, et al. 2006. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J. Infect. Dis. 194:904–911 [DOI] [PubMed] [Google Scholar]
- 30. UNAIDS/WHO 2008. Report on the global HIV/AIDS epidemic 2008. UNAIDS, Geneva, Switzerland [Google Scholar]
- 31. van Rompay KK, et al. 1996. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veazey RS, et al. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438:99–102 [DOI] [PubMed] [Google Scholar]
- 33. Veazey RS, et al. 2010. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J. Infect. Dis. 202:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vishwanathan SA, et al. 2011. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J. Acquir. Immune Defic. Syndr. 57:261–264 [DOI] [PubMed] [Google Scholar]
- 35. Voelker R. 2005. Women shoulder growing HIV/AIDS burden. JAMA 293:281–282 [DOI] [PubMed] [Google Scholar]
- 36. von Ehrenstein OS, et al. 2009. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod. Toxicol. 27:239–245 [DOI] [PubMed] [Google Scholar]
- 37. Weber J, Desai K, Darbyshire J. 2005. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Med. 2:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weiler AM, et al. 2008. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J. Virol. 82:4154–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeh RF, et al. 2009. Genital tract, cord blood, and amniotic fluid exposures of seven antiretroviral drugs during and after pregnancy in human immunodeficiency virus type 1-infected women. Antimicrob. Agents Chemother. 53:2367–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]