SUMMARY
Androgen deprivation is still the standard systemic therapy for metastatic prostate cancer (PCa), but patients invariably relapse with a more aggressive form of PCa termed hormone refractory, androgen independent, or castration resistant PCa (CRPC). Significantly, the androgen receptor (AR) is expressed at high levels in most cases of CRPC, and these tumors resume their expression of multiple AR-regulated genes, indicating that AR transcriptional activity becomes reactivated at this stage of the disease. The molecular basis for this AR reactivation remains unclear, but possible mechanisms include increased AR expression, AR mutations that enhance activation by weak androgens and AR antagonists, increased expression of transcriptional coactivator proteins, and activation of signal transduction pathways that can enhance AR responses to low levels of androgens. Recent data indicate that CRPC cells may also carry out intracellular synthesis of testosterone and DHT from weak adrenal androgens and may be able to synthesize androgens from cholesterol. These mechanisms that appear to contribute to AR reactivation after castration are further outlined in this review.
Keywords: androgen receptor, prostate cancer, testosterone, androgen, androgen deprivation therapy, AR antagonist
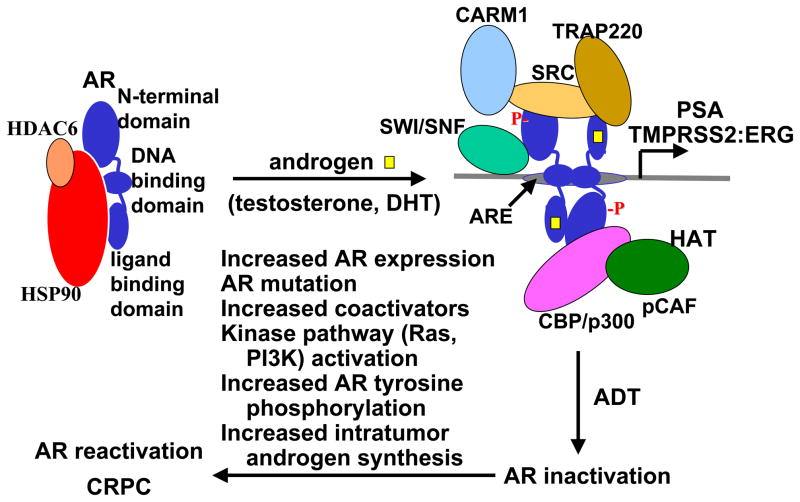
The androgen receptor (AR) is a steroid receptor member of the larger nuclear receptor superfamily, and plays a central role in normal prostate development and in prostate cancer (PCa) initiation and progression. In the absence of androgens (testosterone or dihydrotestosterone, DHT) the AR is inactive and associates with an Hsp90 chaperone complex. Androgen binding results in conformational changes that lead to dimerization, binding to androgen responsive elements (AREs) in androgen regulated genes, and increased transcription of these AR target genes (Fig. 1). The major role of AR in normal prostate is to drive differentiation of luminal epithelial cells and regulate the transcription of protein products that are required for prostate function, such prostate specific antigen (PSA). The critical function or functions of AR in PCa have been less clear, but are presumably to stimulate the expression of a series of genes that regulate cell cycle and are required for PCa survival or growth [1,2]. A recent major breakthrough was the discovery of gene fusions between Ets transcription factors (primarily ERG) and the strongly androgen regulated TMPRSS2 gene in a large fraction of primary PCa and in associated PIN lesions [3–5]. This gene fusion is presumed to result in AR stimulated overexpression of Ets transcription factors, which may then mediate or strongly contribute to PCa development (Fig. 1).
Figure 1. AR activation in normal prostate/hormone naïve PCa and reactivation after androgen deprivation therapy.
Androgen binding causes a conformational change in AR that results in dissociation from Hsp90, nuclear translocation, dimerization on androgen responsive elements (AREs) in androgen regulated genes, recruitment of steroid receptor coactivator proteins (SRC1–3), and recruitment of multiple other transcriptional coactivator proteins that acetylate histone (histone acetyltransferases, HATs) (CBP/p300, pCAF), methylate histones (CARM1), unwind DNA (SWI/SNF), or recruit the RNA polymerase II complex (TRAP220). AR activity is initially suppressed by androgen deprivation therapy (ADT), but the tumor cells eventually adapt by one or more mechanisms to reactivate AR activity, resulting in (or contributing to) the emergence of castration resistant prostate cancer (CRPC).
Androgen deprivation therapy (ADT) is still the standard systemic treatment used for locally advanced or metastatic PCa. Approximately 80% of patients treated with androgen deprivation therapies, which suppress testicular androgen production (surgical castration or administration of LHRH superagonists) or block AR by treatment with AR antagonists (flutamide or bicalutamide), show clinical and biochemical (decrease in serum PSA) evidence of improvement. Unfortunately, patients invariably relapse with a more aggressive form of PCa that has been termed hormone refractory, androgen independent, or castration resistant PCa (CRPC). Significantly, multiple immunohistochemical studies have shown that AR protein is expressed at high levels (comparable to levels in untreated tumors) in most cases of CRPC, although the expression may be heterogeneous with a fraction of cells in some tumors being AR low or negative [6–8]. Consistent with the immunohistochemical results, AR mRNA is also highly expressed in CRPC, with levels being several fold higher than in primary untreated tumors [9–11]. At least one mechanism for the increased AR mRNA expression is AR gene amplification, which occurs in about one-third of CRPC cases [12,13].
In addition to expressing AR, these CRPC resume their expression of multiple AR regulated genes (such as PSA and including TMPRSS2:ERG fusion genes), indicating that AR transcriptional activity becomes reactivated at this stage of the disease [8,10,11,14,15]. Studies using PCa cell lines and xenografts similarly show that progression to CRPC is associated with high levels of AR and resumed expression of androgen regulated genes [16–22]. Moreover, AR downregulation in cell lines at this CRPC stage (by siRNA or other methods) can suppress tumor growth, indicating that AR continues to provide critical functions [17,22–24].
Although these data indicate that AR transcriptional activity is reactivated in CRPC, the molecular basis for this reactivation remains unclear and multiple mechanisms are likely to be involved (Fig. 1). Increased AR expression may enhance any weak residual AR activity that remains after castration, and can enhance growth of a PCa cell line in castrated mice [17]. Several groups have identified AR mutations in CRPC that can enhance AR activation by weak adrenal androgens and other steroid hormones such as progesterone, estradiol, and cortisol. These mutations may also alter AR responses to certain antagonists such as hydroxyflutamide (the active metabolite of flutamide) and bicalutamide, so that these drugs act as potent agonists [9,25–29].
However, the overall frequency of AR mutations in patients treated initially with surgical castration or LHRH agonists (androgen deprivation monotherapy) is quite low, and this is unlikely to be a major mechanism for progression to CRPC [30]. Nonetheless, mutant ARs that are stimulated by the AR antagonist flutamide are much more frequent in patients treated long term with this drug in combination with castration as their initial hormonal therapy (combined androgen blockade). Moreover, these patients also have increased responses to another AR antagonist (bicalutamide) that can still block the mutant ARs [26,31,32]. Finally, the AR mutation in codon 741 that allows bicalutamide to function as an agonist has been found in patients treated with bicalutamide, and in LNCaP cells after long term culture with bicalutamide [30,33,34]. Taken together, these findings demonstrate that AR antagonists can generate strong selective pressure for mutations that enhance AR activity, but that alternative mechanisms are responsible for AR reactivation in most patients.
While castration reduces the levels of circulating testosterone and DHT, there are still substantial levels of these hormones in serum and in tissues (see below), and there are multiple mechanisms by which tumors with wild-type AR may adapt and respond to these decreased androgen levels. One general mechanism is by increased expression of transcriptional coactivator proteins such as SRC-1 and SRC-2 (TIF2), which can enhance AR transcriptional responses to low levels of androgen [35]. A second general mechanism is by activation of certain kinases or kinase signal transduction pathways. These include protein kinase A, Cdk1, PI3 kinase/Akt, and the Ras-Raf-MAP kinase pathway that may be activated by upstream receptor tyrosine kinases. Studies from many groups have shown that activation of these kinase pathways in PCa cell lines or in AR transfected cells can enhance AR activation in response to low levels of androgen, with the dose-response curve to androgens shifting by one hundred to one thousand fold lower androgen concentrations in some cases [20,35–46]. Some studies also suggest that the AR may be activated in the total absence of ligand, but it is not clear that very low levels of residual ligands can be excluded. Importantly, activation of the PI3 kinase/Akt and Ras-Raf-MAP kinase pathway, and increased expression of receptor tyrosine kinases such as HER2/Neu/ErbB2 are observed in more aggressive primary PCa and in CRPC [20,22,40,47]. Therefore, it appears likely that these pathways do contribute to the generation of an AR that is “hypersensitive” to low levels of androgen, and that this is an important mechanism for PCa progression after castration.
While these kinase pathways can enhance AR activity, the effects in general appear to be indirect and mediated through phosphorylation of coactivator proteins, rather than through direct AR phosphorylation [43]. Previous studies have established that the AR is phosphorylated at multiple serine/threonine sites, but the specific kinases mediating phosphorylation at each site and the functional importance of these sites are not clear [48–51,51]. In the case of Cdk1, which enhances AR activity and can directly phosphorylate AR, the effects of Cdk1 are not abrogated by mutation of individual Cdk1 target sites (indicating that either multiple sites are involved or that Cdk1 is modulating AR indirectly through phosphorylation of other proteins) [45]. However, an exciting recent advance has been the finding that AR can be phosphorylated, at least transiently, at several tyrosine residues. This tyrosine phosphorylation can be mediated by at least two tyrosine kinases, Src and Ack1, and enhances AR responses to low levels of androgen [52–54].
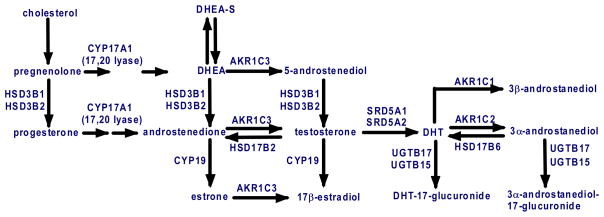
A further mechanism that may contribute to AR activation after castration is increased androgen synthesis by the tumor cells (Fig. 2). Previous studies have established that stromal cells in normal prostate express the enzyme that can reduce androstenedione to testosterone, aldo-keto reductase family 1, member C3 (AKR1C3, also referred to as 17β-hydroxysteroid dehydrogenase type 5, 17βHSD5; or 3α-hydroxysteroid dehydrogenase type 2) [55–59]. Interestingly, testosterone synthesis in Leydig cells in the testes is mediated by a distinct enzyme, type 3 17β-HSD [60]. Prostate epithelium also expresses the type 1 and type 2 5α-reductases that reduce testosterone to the higher affinity ligand DHT, with the type 2 5α-reductase being the predominant isoform in normal prostate. The function of these enzymes is presumably to buffer the prostate against fluctuations in serum androgen levels and insure that the AR remains constitutively liganded. Consistent with this intraprostatic synthesis of testosterone and DHT, healthy men treated with a GnRH antagonist had a 94% decline in serum testosterone, but only a 70–80% decline in prostate tissue levels of testosterone and DHT [61]. Other studies in healthy men or patients undergoing neoadjuvant hormone deprivation therapy prior to radical prostatectomy have similarly shown that intraprostatic androgen levels do not decline as markedly as systemic levels [62–65].
Figure 2. Androgen synthesis and metabolism in normal prostate and prostate cancer cells.
The enzymes mediating testosterone and DHT synthesis from DHEA-S, DHEA, and androstenedione from precursor steroids are expressed in normal prostate, and many are increased in CRPC (HSD3B, AKR1C3, and SRD5A1), while SRD5A2 (the type 2 5α-reductase expressed at highest levels in normal prostate) is reduced. Enzymes mediating DHT catabolism are similarly increased, and CRPC cells may also express CYP17A1 (the enzyme inhibited by ketoconazole and abiraterone) and thereby synthesize androgens de novo from cholesterol.
In a study of gene expression in CRPC bone metasteses versus laser capture microdissected primary “androgen dependent” PCa, we found that AKR1C3 expression was increased ~5-fold in the CRPC samples [11]. This study also found increased expression of the type 1 5α-reductase and reduced expression of the type 2 5α-reductase, which has been confirmed in other studies [66]. Expression of the AKR1C3 related genes, AKR1C2 and AKR1C1, was also increased in the CRPC tumors. AKR1C2 reduces DHT to the less active 5α-androstane-3α,17β-diol (3α-diol), which is subsequently glucuronidated to 5α-androstane-3α,17β-diol glucuronide (3α-diolG) [58,67,68]. AKR1C1 reduces DHT to 5α-androstane-3β,17β-diol (3β-diol), a possible endogenous ligand for the estrogen receptor β [69,70]. Finally, there was increased expression of UDP glycosyltransferase 2, B15 (UGT2B15), which in conjunction with UGT2B17 mediates the glucuronidation of DHT metabolites. This increase in genes mediating DHT catabolism (AKR1C2, AKR1C1, and UGT2B15) may be in response to relatively high intracellular testosterone synthesis in cells overexpressing AKR1C3. Consistent with the increased synthesis of testosterone and increased DHT metabolism, a study of intraprostatic testosterone and DHT in castrated men with CRPC (obtained from transurethral resections performed to relieve obstruction) showed that levels of testosterone were not significantly reduced relative to controls with normal serum androgen levels, while DHT levels were lower than in the controls [8].
Taken together, these data indicate that testosterone synthesis from weak adrenal androgens, which occurs physiologically in the stroma and basal epithelium of normal prostate, is upregulated in CRPC cells and may provide a substantial source of ligand for AR. Consistent with this hypothesis, early studies found that about one-third of patients with CRPC had objective responses (tumor shrinkage) to adrenalectomy or hypophysectomy, and that the majority of patients improved symptomatically [71]. This surgical approach was later replaced by treatment with aminoglutethimide or ketoconazole, which both suppress adrenal androgen synthesis. However, responses are generally partial and transient, and it appears clear that even total elimination of adrenal androgen production has limited efficacy. This may reflect other alternative ligands or additional sources of androgen precursors, including the possible synthesis of androgens from cholesterol by CRPC cells [10,72], but may also reflect the eventual emergence of tumor cells that are no longer dependent on AR transcriptional activity.
A final poorly understood feature of CRPC is that it does not generally respond to treatment with AR antagonists, including high-dose (150–200 mg) bicalutamide, which can effectively block the AR when used as single agents for initial hormone therapy [31,32,73]. One explanation may be that bicalutamide (as a relatively weak competitive antagonist) can no longer effectively compete for AR binding due to increased intracellular testosterone and/or molecular changes that have markedly increased the AR affinity for agonist ligands (“hypersensitive” AR). An alternative general hypothesis is that bicalutamide can compete for AR binding in CRPC cells, but no longer functions as an antagonist. Recent studies have shown that AR can recruit corepressor proteins (NCoR and SMRT), which may contribute to the antagonist activity of bicalutamide, and that AR overexpression or removal of NCoR from the nucleus may convert bicalutamide to an agonist [17,74–77]. However, other data show that NCoR and SMRT downregulation by siRNA does not reveal bicalutamide agonist activity, and indicate that bicalutamide functions as an antagonist because it fails to mediate coactivator recruitment [78,79]. In any case, a better understanding of the molecular basis for AR antagonist resistance in CRPC is clearly important for the generation of more effective antagonists.
In summary, current data indicate that PCa cells adapt to androgen deprivation therapy by multiple mechanisms, which include increasing AR gene expression and androgen biosynthesis, and activation of multiple pathways that can directly or indirectly enhance AR activation by low levels of androgen. Secondary hormonal therapies with available inhibitors of androgen synthesis (ketoconazole, finasteride, dutasteride) or AR antagonists (bicalutamide, flutamide, nilutamide) have modest efficacy, but responses may be improved with more potent agents that are in clinical trials. An alternative approach is to target AR folding and stability by the Hsp90 chaperone complex, which can be suppressed by direct Hsp90 inhibitors or indirectly by HDAC inhibitors that prevent HDAC6 mediated deacetylation of Hsp90. Finally, more data are needed to understand precisely how AR becomes sensitized to low androgen levels, and to identify agents that target these mechanisms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–20222. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 3.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 4.Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–888. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 5.Cerveira N, Ribeiro FR, Peixoto A, Costa V, Henrique R, Jeronimo C, et al. TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia. 2006;8:826–832. doi: 10.1593/neo.06427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 7.Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, Verleun-Mooijman MC, Trapman J, Brinkmann AO, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994;144:735–746. [PMC free article] [PubMed] [Google Scholar]
- 8.Mohler JL, Gregory CW, Ford OH, III, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 9.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen- independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 10.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 12.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 13.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 14.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 15.Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–138. doi: 10.1016/s0090-4295(02)01593-5. [DOI] [PubMed] [Google Scholar]
- 16.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, et al. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58:5718–5724. [PubMed] [Google Scholar]
- 17.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 18.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 19.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 20.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase [see comments] Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 21.Abreu-Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan X, Li T, Wang H, Zhang T, Barua M, Borgesi RA, et al. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol. 2006;169:682–696. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–1013. [PubMed] [Google Scholar]
- 24.Wright ME, Tsai MJ, Aebersold R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol. 2003;17:1726–1737. doi: 10.1210/me.2003-0031. [DOI] [PubMed] [Google Scholar]
- 25.Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7:1541–1550. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 26.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–2515. [PubMed] [Google Scholar]
- 27.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 28.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 29.Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11:450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 30.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- 31.Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol. 1997;15:2928–2938. doi: 10.1200/JCO.1997.15.8.2928. [DOI] [PubMed] [Google Scholar]
- 32.Joyce R, Fenton MA, Rode P, Constantine M, Gaynes L, Kolvenbag G, et al. High dose bicalutamide for androgen independent prostate cancer: effect of prior hormonal therapy. J Urol. 1998;159:149–153. doi: 10.1016/s0022-5347(01)64039-4. [DOI] [PubMed] [Google Scholar]
- 33.Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–153. [PubMed] [Google Scholar]
- 34.Yoshida T, Kinoshita H, Segawa T, Nakamura E, Inoue T, Shimizu Y, et al. Antiandrogen bicalutamide promotes tumor growth in a novel androgen-dependent prostate cancer xenograft model derived from a bicalutamide-treated patient. Cancer Res. 2005;65:9611–9616. doi: 10.1158/0008-5472.CAN-05-0817. [DOI] [PubMed] [Google Scholar]
- 35.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 36.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, et al. Androgen receptor activation in prostatic tumor cell lines by insulin- like growth factor-I, keratinocyte growth factor and epidermal growth factor. Eur Urol. 1995;27 (Suppl 2):45–47. doi: 10.1159/000475232. [DOI] [PubMed] [Google Scholar]
- 37.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 38.Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 39.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–284. [PubMed] [Google Scholar]
- 41.Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63:1981–1989. [PubMed] [Google Scholar]
- 42.Bakin RE, Gioeli D, Bissonette EA, Weber MJ. Attenuation of Ras signaling restores androgen sensitivity to hormone-refractory C4-2 prostate cancer cells. Cancer Res. 2003;63:1975–1980. [PubMed] [Google Scholar]
- 43.Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, et al. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem. 2004;279:7119–7130. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- 44.Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 45.Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci U S A. 2006;103:15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 47.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 48.Kuiper GG, Brinkmann AO. Phosphotryptic peptide analysis of the human androgen receptor: detection of a hormone-induced phosphopeptide. Biochemistry. 1995;34:1851–1857. doi: 10.1021/bi00006a005. [DOI] [PubMed] [Google Scholar]
- 49.Zhou ZX, Kemppainen JA, Wilson EM. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9:605–615. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]
- 50.Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, et al. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277:29304–29314. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- 51.Wong HY, Burghoorn JA, Van Leeuwen M, de Ruiter PE, Schippers E, Blok LJ, et al. Phosphorylation of androgen receptor isoforms. Biochem J. 2004;383:267–276. doi: 10.1042/BJ20040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66:11047–11054. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- 55.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3 alpha-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3 alpha/17 beta-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 56.El Alfy M, Luu-The V, Huang XF, Berger L, Labrie F, Pelletier G. Localization of type 5 17beta-hydroxysteroid dehydrogenase, 3beta-hydroxysteroid dehydrogenase, and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinology. 1999;140:1481–1491. doi: 10.1210/endo.140.3.6585. [DOI] [PubMed] [Google Scholar]
- 57.Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:568–574. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- 58.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Andersson S, Geissler WM, Patel S, Wu L. The molecular biology of androgenic 17 beta-hydroxysteroid dehydrogenases. J Steroid Biochem Mol Biol. 1995;53:37–39. doi: 10.1016/0960-0760(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 61.Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 62.Geller J, Albert J. Effects of castration compared with total androgen blockade on tissue dihydrotestosterone (DHT) concentration in benign prostatic hyperplasia (BPH) Urol Res. 1987;15:151–153. doi: 10.1007/BF00254427. [DOI] [PubMed] [Google Scholar]
- 63.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 64.Mizokami A, Koh E, Fujita H, Maeda Y, Egawa M, Koshida K, et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res. 2004;64:765–771. doi: 10.1158/0008-5472.can-03-0130. [DOI] [PubMed] [Google Scholar]
- 65.Belanger B, Belanger A, Labrie F, Dupont A, Cusan L, Monfette G. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extratesticular androgens in men. J Steroid Biochem. 1989;32:695–698. doi: 10.1016/0022-4731(89)90514-1. [DOI] [PubMed] [Google Scholar]
- 66.Thomas LN, Douglas RC, Lazier CB, Too CK, Rittmaster RS, Tindall DJ. Type 1 and Type 2 5alpha-Reductase Expression in the Development and Progression of Prostate Cancer. Eur Urol. 2007 doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 67.Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM. Human type 3 3alpha-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells. Endocrinology. 2003;144:2922–2932. doi: 10.1210/en.2002-0032. [DOI] [PubMed] [Google Scholar]
- 68.Ji Q, Chang L, VanDenBerg D, Stanczyk FZ, Stolz A. Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate. 2003;54:275–289. doi: 10.1002/pros.10192. [DOI] [PubMed] [Google Scholar]
- 69.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 70.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, et al. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahoney EM, HARRISON JH. Bilateral adrenalectomy for palliative treatment of prostatic cancer. J Urol. 1972;108:936–938. doi: 10.1016/s0022-5347(17)60911-x. [DOI] [PubMed] [Google Scholar]
- 72.Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, Gleave ME, et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64:2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 73.Fowler JE, Jr, Pandey P, Seaver LE, Feliz TP. Prostate specific antigen after gonadal androgen withdrawal and deferred flutamide treatment. J Urol. 1995;154:448–453. doi: 10.1097/00005392-199508000-00030. [DOI] [PubMed] [Google Scholar]
- 74.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 75.Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, et al. Macrophage/Cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 76.Baek SH, Ohgi KA, Nelson CA, Welsbie D, Chen C, Sawyers CL, et al. Ligand-specific allosteric regulation of coactivator functions of androgen receptor in prostate cancer cells. Proc Natl Acad Sci U S A. 2006;103:3100–3105. doi: 10.1073/pnas.0510842103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng S, Brzostek S, Lee SR, Hollenberg AN, Balk SP. Inhibition of the dihydrotestosterone-activated androgen receptor by nuclear receptor corepressor. Mol Endocrinol. 2002;16:1492–1501. doi: 10.1210/mend.16.7.0870. [DOI] [PubMed] [Google Scholar]
- 78.Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002;277:26321–26326. doi: 10.1074/jbc.M203310200. [DOI] [PubMed] [Google Scholar]
- 79.Hodgson MC, Astapova I, Hollenberg AN, Balk SP. Activity of Androgen Receptor Antagonist Bicalutamide in Prostate Cancer Cells Is Independent of NCoR and SMRT Corepressors. Cancer Res. 2007;67:8388–8395. doi: 10.1158/0008-5472.CAN-07-0617. [DOI] [PubMed] [Google Scholar]