Abstract
Approximately one third of the world's population is infected with Mycobacterium tuberculosis, the causative agent of tuberculosis. This bacterium has an unusual lipid-rich cell wall containing a vast repertoire of antigens, providing a hydrophobic impermeable barrier against chemical drugs, thus representing an attractive target for vaccine and drug development. Apart from the mycolyl–arabinogalactan–peptidoglycan complex, mycobacteria possess several immunomodulatory constituents, notably lipomannan and lipoarabinomannan. The availability of whole-genome sequences of M. tuberculosis and related bacilli over the past decade has led to the identification and functional characterization of various enzymes and the potential drug targets involved in the biosynthesis of these glycoconjugates. Both lipomannan and lipoarabinomannan possess highly variable chemical structures, which interact with different receptors of the immune system during host–pathogen interactions, such as Toll-like receptors-2 and C-type lectins. Recently, the availability of mutants defective in the synthesis of these glycoconjugates in mycobacteria and the closely related bacterium, Corynebacterium glutamicum, has paved the way for host–pathogen interaction studies, as well as, providing attenuated strains of mycobacteria for the development of new vaccine candidates. This review provides a comprehensive account of the structure, biosynthesis and immunomodulatory properties of these important glycoconjugates.
Keywords: bacterial, polysaccharides, cell wall, biosynthesis, glycosyltransferases, drug-targets
Introduction
Tuberculosis (TB) is a major cause of death worldwide, with approximately 9 million cases and 1.7 million deaths registered in 2008 (WHO, 2009). To compound this situation, 50 000 cases were reported as multidrug-resistant tuberculosis (MDR-TB) and 55 countries globally had reported at least one case of extensively drug-resistant tuberculosis (XDR-TB) (WHO, 2009). Mycobacterium tuberculosis is the causative agent of tuberculosis. It has an unusual lipid-rich cell wall that is unique to the order Actinomycetes, including the genera Mycobacterium, Rhodococcus, Corynebacterium and Nocardia (Brennan & Nikaido, 1995). The mycobacterial cell wall is composed of a mycolyl–arabinogalactan–peptidoglycan (mAGP) complex (Dafféet al., 1990; McNeil et al., 1990, 1991; Besra et al., 1995; Brennan, 2003; Dover et al., 2004), of which the mycolic acids and extractable lipids form the mycobacterial outer membrane (Hoffmann et al., 2008). The mycolic acid layer provides a hydrophobic mesh for intercalating additional complex lipids, resulting in a highly impermeable barrier for the penetration of antimicrobial drugs, such as penicillins (Amberson et al., 1931; Minnikin et al., 2002;). Other cell wall-associated lipids, such as phosphatidyl-myo-inositol mannosides (PIMs) and lipoglycans, termed lipomannan (LM) and lipoarabinomannan (LAM), are also found in the cell wall (Hill & Ballou, 1966; Brennan & Ballou, 1967, 1968a; Brennan & Nikaido, 1995; Besra et al., 1997; Morita et al., 2004). In addition to their physiological function, these complex glycoconjugates play a key role in modulating the host response during infection. PIMs, lipomannan and lipoarabinomannan all display several immunomodulatory properties by interaction with different receptors of the immune system. While lipomannan is mainly associated with Toll-like receptors (TLR) signaling, the higher-order PIMs and mannose-capped lipoarabinomannan (Man-LAM) are recognized by the C-type lectins, such as dendritic cell-specific intercellular adhesion molecule-3 (ICAM-3) grabbing nonintegrin (DC-SIGN) and the macrophage mannose receptor (MMR) (Schlesinger et al., 1994; Chatterjee & Khoo, 1998; Nigou et al., 2002; Geijtenbeek et al., 2003; Maeda et al., 2003;).
Because of the advent of MDR and XDR strains of M. tuberculosis (Sreevatsan et al., 1997; Telenti et al., 1997; Heymann et al., 1998; Chan & Iseman, 2008; Wright et al., 2009;), there is an urgent need to identify novel drug targets and the development of active compounds. In this respect, the biosynthetic machinery of the mycobacterial cell wall, which is the site of action of many front-line tuberculosis drugs, represents an attractive drug target (Bhatt et al., 2007; Bhowruth et al., 2007; Brennan & Crick, 2007; Dover et al., 2008;). Furthermore, a complete investigation of the roles of PIMs, lipomannan and lipoarabinomannan in mycobacterial pathogenicity requires mutants defective in their respective biosynthetic pathways. The availability of complete genome sequences of several mycobacteria and related actinomycetes and the development of novel tools for genetic manipulation have opened up the possibilities to achieve this (Cole et al., 1998).
Herein, we report recent advances in the biogenesis of lipoarabinomannan and related glycoconjugates, followed by a comprehensive analysis of their role in host–pathogen interactions. Furthermore, we review the localization and trafficking of these immunomodulatory lipoglycans and discuss recent findings concerning the role of CD1, TLR, DC-SIGN and MMR in M. tuberculosis infection.
Part I – Structure and biogenesis of PIMs, lipomannan and lipoarabinomannan
Structural features of PIMs, lipomannan and lipoarabinomannan
The majority of bacteria from suborder Corynebacterineae, including Corynebacterium diphtheriae, Corynebacterium glutamicum, pathogenic M. tuberculosis complex and nonpathogenic Mycobacterium smegmatis, possess the amphipathic lipoglycans, lipoarabinomannan and other related glycoconjugates, lipomannan and PIMs (Fig. 1). All Mycobacterium species possess two forms of acylated PIMs, tri- and tetra-acylated (Ac1- and Ac2-) phospho-myo-inositol-dimannoside (PIM2) and tri- and tetra-acylated phospho-myo-inositol-hexamannoside (Ac1/Ac2PIM6) (we have used Ac1/Ac2PIMx for two different acylated versions of PIMs throughout the text, and PIM as a synonym where the acylation state is not clear), and different acylated versions of lipomannan and lipoarabinomannan (Khoo et al., 1995a), which are believed to be noncovalently attached to the cell membrane via a lipid anchor (Fig. 2) (Hunter & Brennan, 1990).
Fig. 1.
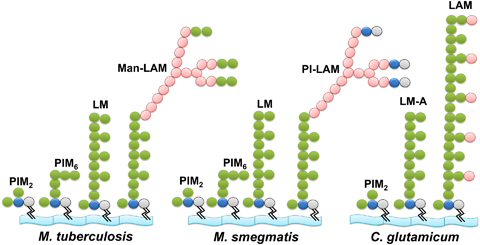
Lipoarabinomannan and related glycoconjugates found on the cell wall of Mycobacterium tuberculosis, Mycobacterium smegmatis and Corynebacterium glutamicum. Biochemical analysis of the mycobacterial cell wall suggests that different acylated variants of di- and hexa-mannosylated PIMs, Ac1/Ac2PIM2 and PIM6, and the higher glycosylated polymers lipomannan and lipoarabinomannan accumulate in the cell wall. However, in C. glutamicum, only PIM2, two types of lipomannan (LM-A and LM-B, Tatituri et al., 2007a; Mishra et al., 2008b;) and singular Araf capped lipoarabinomannan are present on the cell wall. For the purpose of simplicity, only diacylated forms of these glycoconjugates and LM-A, i.e. MPI anchored lipomannan, are shown. In these glycoconjugates, phosphatidyl-myo-inositol (phosphate in gray and inositol in blue) acts as an anchor to the plasma membrane and further glycosylated by Manp (green) and Araf (pink) sugars yielding different forms of PIMs, lipomannan and lipoarabinomannan that are species specific. In M. tuberculosis and other pathogenic mycobacteria, lipoarabinomannan is capped by mono, -di or -tri α(1→2)-Manp units, resulting in Man-LAM, while in nonpathogenic M. smegmatis, lipoarabinomannan is terminated by phospho inositol, yielding PI-LAM.
Fig. 2.
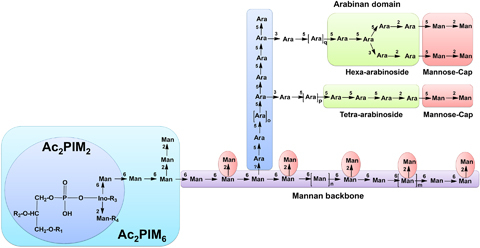
Schematic structures of lipoarabinomannan and related glycoconjugates. As described in the text, PI acts as an anchor around which PIMs, lipomannan and lipoarabinomannan are built. PI is glycosylated at the 2-OH and 6-OH positions of inositol by Manp residues, and acylated at position 3 of myo-inositol and position 6 of the Manp unit linked at O-2 of myo-inositol in Ac2PIM2 (See the inset in light blue color). Manp at the 6-OH position of inositol is linked to further three and two residues of α(1→6)-Manp and α(1→2)-Manp, respectively, in Ac2PIM6 (see the inset in light indigo color). In lipomannan and the mannan backbone of lipoarabinomannan, PIM2 is linked to another 17–19 residues of Manp in the α(1→6) direction and 7–9 singular branched α(1→2)-Manp units. Mature lipomannan is further linked via an unknown linkage to an arabinan domain made up of approximately 70 Araf residues. The majority of the arabinan domain consists of a linear α(1→5)-Araf polymer branched at certain positions, with α(3→5)-Araf residues towards its nonreducing end resulting in a linear tetra-arabinoside or/and branched hexa-arabinoside domain, which in turn is terminated by β(1→2)-Araf and capped by α(1→2)-Manp units. Here R1, R2, R3 and R4 show different acyl groups found at different locations in the MPI anchor, and n, m, o, p and q represent different degrees of species-specific glycosylation in lipomannan and lipoarabinomannan.
Structure of PIMs
PIMs are categorized as glycolipids composed of fatty acids attached to a glycerol unit, linked by a phosphodiester moiety to myo-inositol (Vilkas & Lederer, 1956; Ballou et al., 1963;) (see Fig. 2 for Ac2PIM2 and Ac2PIM6). This phosphatidyl-myo-inositol (PI) is based on an sn-glycero-3-phos-pho-(1-d-myo-inositol) unit and is further substituted at the O-2 and O-6 positions of myo-inositol with α-d-mannopyranosyl (Manp) units in case of PIM2, resulting in a mannosyl phosphate inositol (MPI) anchor, a derivative of the typical glycosyl phosphate inositol anchor, found in Eukaryotes (Lee & Ballou, 1964; Chatterjee et al., 1992a; Severn et al., 1998;).
The MPI anchor is heterogeneous, with variations occurring within the number, location and nature of the fatty acids. There are four potential sites of acylation within the MPI anchor, with different fatty acids at 1-OH and 2-OH of the glycerol unit in the anchor, 3-OH of myo-inositol and the 6-OH of the Manp residue linked at the O-2 position of myo-inositol (see R1, R2, R3 and R4 in Fig. 2) (Khoo et al., 1995a; Nigou et al., 2003;). Two different acylated forms of PIMs accumulate in the cell wall of mycobacteria, one with an acyl group at either the 3-OH of myo-inositol or the 6-OH of the Manp residue linked at the O-2 position of myo-inositol, Ac1PIMx, and secondly with an acyl group at both positions, Ac2PIMx. In mycobacteria, palmitic (C16) and tuberculostearic (10-methyl-octadecanoic, C19) acids are predominant, while myristic (C14) and octadecenoic acids (C18 : 1) are also found in significant amounts, with traces of stearic (C18), hexadecenoic (C16 : 1) and heptadecanoic acids (C17) (Ballou & Lee, 1964; Lee & Ballou, 1964; Gilleron et al., 2003; Nigou et al., 2003;). Furthermore, it was suggested that the 6-OH position of the O-2 mannose attached to the inositol of PIM2 is substituted by a C16 fatty acyl-substituent, which is also present in lipomannan and lipoarabinomannan from M. tuberculosis and Mycobacterium leprae (Khoo et al., 1995a).
Acylated forms of PIM2 serve as substrates for the synthesis of higher-order PIMs, such as Ac1/Ac2PIM6 (Figs 2 and 4). Studies with a crude cell extract of M. tuberculosis and Mycobacterium phlei identified PIM6, which is a pentamannoside attached to the position O-6 of the myo-inositol of PI of PIM1, Manp-α(1→2)-Manp-α(1→2)-Manp-α(1→6)-Manp-α(1→6)-Manp-α(1→.) (Lee & Ballou, 1965), which was later verified by others (Chatterjee et al., 1992a; Severn et al., 1998;) (Fig. 2). A biosynthetic relationship between PIM1 and PIM2 was also suggested, which involves a stepwise glycosylation of PI, first at the O-2 position and then at the O-6 position of the inositol ring (Ballou & Lee, 1964; Chatterjee et al., 1992a;). It was also suggested that this acylated version of PIM2 i.e. Ac1PIM2 is both a metabolic end-product and an intermediate in Ac1PIM6, lipomannan and lipoarabinomannan biosynthesis (Chatterjee et al., 1992a; Khoo et al., 1995a; Besra et al., 1997;).
Fig. 4.
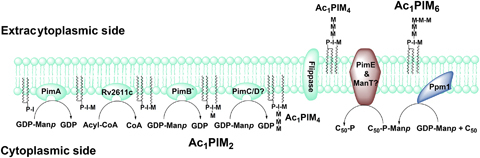
Overview of PIM biosynthesis in Mycobacterium tuberculosis. On the cytosolic side of the plasma membrane, PI is glycosylated by PimA, PimB' and an acyltransferase to form Ac1PIM2, which is further mannosylated by PimC and/or PimD? to form Ac1PIM4, an intermediate in Ac1PIM6 and lipomannan biosynthesis. Ac1PIM4 is probably transported across the plasma membrane by unidentified flippases and further mannosylated by α(1→2) mannopyranosyltransferases, PimE and/or another unidentified enzyme to form Ac1PIM6. For simplicity, only triacylated versions of PIMs are shown.
More recently, the presence of a glucuronic acid diacyl-glycerol-based glycolipid, Manp-α(1→4)-d-glucopyranosyluronic acid-diacyl glycerol, ManGlcAGroAc2, and a novel lipomannan variant (Cg-LM-B) were reported in C. glutamicum, in addition to the MPI anchored-LM (Cg-LM-A) and lipoarabinomannan, and to date, these glycoconjugate variants have not been identified in mycobacteria (Tatituri et al., 2007a; Lea-Smith et al., 2008; Mishra et al., 2008b, 2009). However, Rv0557 [MgtA] of M. tuberculosis was shown to have the ability to synthesize these novel lipids and lipoglycans in vitro and in vivo (Tatituri et al., 2007a; Mishra et al., 2009;), and the majority of the genus Mycobacterium possesses an ortholog of MgtA. Therefore, theoretically, the possibility remains for the identification of glucuronic acid-anchor-based glycolipids in mycobacteria in addition to MPI anchor-based ones.
Structure of lipomannan and lipoarabinomannan
In 1930, Masucci and colleagues isolated a polysaccharide containing d-arabinofuranose (Araf) and Manp with high serological activity from mycobacteria (Masucci et al., 1930), which was also identified in Mycobacterium bovis bacilli Calmette-Guérin (BCG) (Chargaff & Schaefer, 1935), and separated using electrophoresis (Seibert & Watson, 1941). Later, one of the polysaccharides was identified as arabinogalactan (AG), the basic constituent of the mAGP complex (Misaki & Yukawa, 1966), and the other as a pool of immunologically active lipoarabinomannan and inactive lipomannan (Misaki et al., 1977), both sharing a similar mannan core (Hunter et al., 1986; Hunter & Brennan, 1990; Chatterjee et al., 1992a;) (Fig. 2). Further studies identified Ac1/Ac2PIM2 as the attachment point (Hunter & Brennan, 1990) for the synthesis of the α(1→6)-mannan backbone of lipomannan and lipoarabinomannan, which is composed of around 21–34 residues of α(1→6)-Manp and decorated by singular 5–10 units of α(1→2)-Manp, resulting in the formation of lipomannan (Chatterjee et al., 1992a; Kaur et al., 2008;). The mannan core is further elaborated by the addition of an arabinan domain consisting of approximately 55–70 Araf residues in a linear α(1→5)-d-Araf fashion with 3,5-α-d-Araf branches (Kaur et al., 2008; Birch et al., 2010;). The arabinan domain is highly branched and conserved with two types of chain arrangements. Firstly, linear tetra-arabinoside (Ara-4) of the structure β-d-Araf(1→2)-α-d-Araf(1→5)-α-d-Araf(1→5)-α-d-Araf, and secondly, branched hexa-arabinoside (Ara-6) motifs with the structure [β-d-Araf(1→2)-α-d-Araf]2-3,5-α-d-Araf(1→5)-α-d-Araf (Chatterjee et al., 1991; Chatterjee et al., 1993;). In both cases, the nonreducing end is characterized by the disaccharide unit, Araf-β(1→2)-Araf-α(1→.) (Fig. 2) (Chatterjee et al., 1991, 1993; McNeil et al., 1994).
The arabinan termini of lipoarabinomannan from the Erdman strain of M. tuberculosis were shown to be capped with Manp residues and it was established that the tetra-/hexa-arabinofuranoside unit was further extended by mono, di- and tri-α(1→2)-d-Manp saccharide units (Fig. 2) (Chatterjee et al., 1992b, 1993; Venisse et al., 1993). The number of mannose caps is species specific, with M. tuberculosis H37Rv and M. bovis BCG Man-LAM equally capped with around seven residues per molecule of lipoarabinomannan (Khoo et al., 1995b; Nigou et al., 2003;). Surprisingly, lipoarabinomannan from a fast-growing Mycobacterium species is devoid of any mannose cap (Chatterjee et al., 1992b) and, in turn, a novel phosphoinositol capping motif was identified from M. smegmatis strains ATCC 14468 and mc2155 (PI-LAM) (Fig. 1) (Khoo et al., 1995b) and the absence of a capping motif in lipoarabinomannan from Mycobacterium chelonae, AraLAM (Guérardel et al., 2002).
Further chemical modifications of lipoarabinomannan
In its physiological form, Man-LAM is found in two different fractions: parietal and cellular (Vercellone et al., 1998; Gilleron et al., 2000; Hoffmann et al., 2008;). These fractions differ in terms of the percentage of mannose caps and acylation groups of the MPI anchor. Parietal Man-LAM possess a novel fatty acid assigned as 12-O-(methoxypropanoyl)-12-hydroxystearic acid, esterified at C-1 of the glycerol residue of PI, while cellular Man-LAMs are largely heterogeneous with palmitic and tuberculostearic acid (Nigou et al., 1997). More likely, cellular lipoarabinomannan is more strongly attached to the cell wall due to higher acylation as compared with parietal lipoarabinomannan (Pitarque et al., 2008). Furthermore, in different M. bovis BCG strains (Pasteur, Glaxo, Copenhagen and Japanese strains), the presence of succinyl groups on O-2 of the 3,5-di-α-d-Araf residue of Man-LAM was also reported (Delmas et al., 1997). Recently, Treumann et al. (2002) identified a 5-methylthiopentose substituent on the terminal Manp in the cap structure of Man-LAM in several strains of M. tuberculosis, which was later characterized as 5-deoxy-5-methylthio-xylofuranose (Turnbull et al., 2004) with a d-configuration (Joe et al., 2006) and linked by an α(1→4) linkage to a Manp residue in the mannan portion of the glycan (Guérardel et al., 2003).
Biogenesis of PIMs, lipomannan and lipoarabinomannan
Biosynthesis of substrates
GDP-Manp biosynthesis
Besides being part of glycolipids and lipoglycans, mannose is also involved in the synthesis of a number of glycosylated proteins (VanderVen et al., 2005) and a few other key components, such as polymethylated polysaccharides in mycobacteria (Jackson & Brennan, 2009). These molecules are synthesized by both pathogenic and nonpathogenic species, raising the possibility of as yet undefined ‘housekeeping’ functions in these organisms. The mannose metabolism is essential for growth in M. smegmatis and it was suggested that apart from glycolipid and lipoglycan biosynthesis, mannose-containing molecules may also play a role in regulating septation and cell division (Patterson et al., 2003).
In mycobacteria, mannose is probably obtained by two distinct pathways: firstly, by transport of extracellular mannose from the medium or the extracellular environment with the activity of a hexokinase (Kowalska et al., 1980). The phosphorylated mannose, mannose-1-phosphate, is then converted into GDP-Manp by GDP-mannose pyrophosphorylase, ManC [Rv3264c] (Ning & Elbein, 1999; Ma et al., 2001;) (Fig. 3). Secondly, in the absence of extracellular mannose, it can be derived from glucose and other sugars via the glycolytic pathway, where fructose-6-phosphate is converted to mannose-6-phosphate by an essential enzyme, phosphomannose isomerase, encoded by manA [Rv3255c] (Patterson et al., 2003). Mannose-6-phosphate is then converted to mannose-1-phosphate by a phosphomannomutase, ManB [Rv3257c] (McCarthy et al., 2005), followed by conversion into GDP-Manp by ManC (Ning & Elbein, 1999; Ma et al., 2001;) (Fig. 3).
Fig. 3.
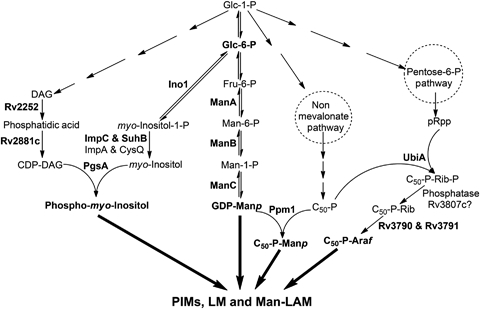
Biosynthetic pathways of important nucleotide and lipid-linked sugar donors involved in the synthesis of PIMs, lipomannan and Man-LAM. Most of the sugars utilized by mycobacteria are derived from glycolytic intermediates or glucose as the major carbon source. The experimentally characterized enzymes have been indicated in bold. Apart from the glycolytic pathway, GDP-Manp, C50-P-Manp, C50-P-Araf and PI are also derived from exogenous sources, which have not been shown here to retain simplicity.
Synthesis of β-d-mannosyl-1-monophosphoryldecaprenol
GDP-Manp serves as an intracellular nucleotide-derived mannose donor for the synthesis of several glycolipids and mannosylated proteins by the GT-A/B superfamily of glycosyltransferases (Liu & Mushegian, 2003). However, for periplasmic biosynthetic events, a polyprenyl-phosphate-based mannose donor is required, which acts as a mannose donor for the GT-C superfamily of glycosyltransferases for the synthesis of higher PIMs, lipomannan and lipoarabinomannan. Takayama & Goldman (1970) were the first to report the presence of a C50-polyprenol-based mannolipid, C50-decaprenol-phospho-mannose (C50-P-Manp, PPM), in M. tuberculosis (Takayama & Goldman, 1970). Later on, another alkali-stable, C35-octahydroheptaprenyl-phospho-mannose, C35-P-Manp, was identified in M. smegmatis (Wolucka & Hoffmann, 1998). Based on similarities to the known eukaryotic dolichol monophosphomannose synthases, Rv2051c [Ppm1] from M. tuberculosis was identified as a polyprenol monophosphomannose synthase, PPM synthase (Gurcha et al., 2002) (Figs 3 and 4). Surprisingly, Ppm1 possesses an unusual two-domain architecture in M. tuberculosis, of which the second domain, Mt-Ppm1/D2, is sufficient for PPM synthesis (Gurcha et al., 2002; Gibson et al., 2003;). However, M. smegmatis, Mycobacterium avium and M. leprae produce two distinct proteins, one for each of the two domains found in Mt-Ppm1, with Ms-Ppm2 and Ma-Ppm2 having a catalytic activity similar to that of domain 2 of Mt-Ppm1. Recently, a transmembrane glycosyltransferase, Rv3779, was identified and suggested to be involved in the synthesis of C35/50-P-Manp as a second PPM synthase (Scherman et al., 2009). However, Skovierova et al. (2010) recently described the function of Rv3779 as the glycosyltransferase involved in transferring galactosamine from a polyprenyl-phospho-N-acetylgalactosamine to arabinogalactan in M. tuberculosis.
Origin and synthesis of decaprenyl-phospho-arabinose
Generally, in nature, d-arabinose exists in two cyclic forms: a rare pyranose-ring (Arap) and the furanose-ring (Araf) (Wolucka, 2008). Araf forms a key component of both arabinogalactan and lipoarabinomannan in mycobacteria and the only known Araf sugar donor is a lipid-linked decaprenyl-phospho-arabinose (DPA and also termed C50-P-Araf) (Wolucka et al., 1994). However, a putative role of a nucleotide-based Araf donor was also suggested in the addition of the single terminal d-Araf residues of lipoarabinomannan in C. glutamicum (Tatituri et al., 2007b). The majority of DPA synthesized in mycobacteria comes from the pentose phosphate pathway (Marks, 1956). A transketolase, Rv1449, links the glycolytic and pentose phosphate pathway to produce ribose-5-phosphate (Wolucka, 2008). Alternatively, ribose-5-phosphate isomerase [Rv2465] isomerizes d-ribulose-5-phosphate into ribose-5-phosphate (Roos et al., 2004; Roos et al., 2005;). Furthermore, Rv1017c, a ribose-5-phosphate diphosphokinase (PrsA), converts ribose 5-phosphate into 5-phosphoribosyl-α-1-pyrophosphate (pRpp) (Alderwick et al., 2010), which is dephosphorylated by a phosphatase as the first committed step in decapolyprenol-phosphoribose (DPR and also termed C50-P-Rib) and DPA biosynthesis (Mikusováet al., 2005) (Fig. 3). In the genome of M. tuberculosis, an unknown poly-(A)-polymerase2 (PAP2)-superfamily phospholipid phosphatase [Rv3807c] exists that is present in the arabinogalactan biosynthetic cluster (Rv3779-Rv3809c) and next to Rv3806c, UbiA (Huang et al., 2005), which may be responsible for pRpp phosphatase activity. Furthermore, the deletion of the Rv3807c homolog in C. glutamicum remains unsuccessful, suggesting it as a prime candidate (L. Eggeling & G.S. Besra, unpublished data).
The synthesis of DPA and DPR from pRpp was shown experimentally and it was concluded that DPA is formed from pRpp via a two-step pathway, with an additional epimerization step that converts DPR to DPA (Scherman et al., 1996; Mikusováet al., 2005;) (Fig. 3). Recently, a 5-phospho-α-d-ribose-1-diphosphate:decaprenyl-phosphate 5-phospho-ribosyltransferase, UbiA [Rv3806c], was identified in M. tuberculosis (Huang et al., 2005). The deletion of ubiA in C. glutamicum produced a mutant that possessed a galactan core consisting of alternating β(1→5)-galactofuranose (Galf) and β(1→6)-Galf residues and completely devoid of arabinan and cell-wall-bound corynomycolic acids, confirming its role in the synthesis of DPR and DPA biosynthesis in Corynebacterineae (Alderwick et al., 2005; Alderwick et al., 2006a;). More recently, Mikusováet al. (2005) identified an epimerase, which is involved in the epimerization of DPR to DPA. It was established that the 2-OH of ribose is oxidized to decaprenylphosphoryl-2-keto-β-d-erythro-pentofuranose, which is then reduced to form DPA. These activities are encoded by Rv3790 and Rv3791, respectively, and the simultaneous expression of both is required for complete activity of the epimerase reaction (Mikusováet al., 2005) (Fig. 3). Interestingly, Rv3790 has been shown to be a target of benzothiazinones, potential tuberculosis drugs (Christophe et al., 2009; Makarov et al., 2009;).
Synthesis of phosphatidyl-myo-inositol
Inositol is an essential metabolite in Mycobacterium (Kataoka & Nojima, 1967), Corynebacterium (Brennan & Lehane, 1971), Nocardia (Yano et al., 1969), Micromonospora (Tabaud et al., 1971) and Propionibacterium (Brennan & Ballou, 1968b). In mycobacteria, inositol is essential for growth and derived directly via glycolysis (Jackson et al., 2000). Glucose-6-phosphate is cyclized by an inositol-1-phosphate synthase, Ino1 [Rv0046c] (Bachhawat & Mande, 1999; Movahedzadeh et al., 2004;), into myo-inositol-1-phosphate, followed by its dephosphorylation utilizing an inositol monophosphatase (IMP) (Fig. 3). On the basis of homology, the M. tuberculosis genome shows four ORFs encoding putative proteins with an IMP domain: Rv1604 (ImpA), Rv2701c (SuhB), Rv2131c (CysQ) and Rv3137 (ImpC). Out of these, impC was found to be essential for mycobacterial growth, and it was suggested that impA, suhB and cysQ may make a minor contribution towards inositol biosynthesis (Movahedzadeh et al. 2010) as suggested by the in vitro IMP activity of SuhB (Fig. 3) (Parish et al., 1997; Nigou & Besra, 2002a; Brown et al., 2007;).
The first step in the production of many phospholipids, including PI, is the phosphorylation of diacylglycerol (DAG) by a diacylglycerol kinase [Rv2252] to form phosphatidic acid (Owens et al., 2006). Phosphatidic acid is then activated by CTP to form CDP-DAG by a CDP-DAG synthase [Rv2881c], a homolog of which has been characterized in M. smegmatis (Nigou & Besra, 2002b). Furthermore, it was shown that a cell wall fraction (Percoll-60, P60) from M. smegmatis is able to synthesize P-[3H]-I in the presence of the exogenous substrate, CDP-dipalmitoyl-DAG, concluding that myo-inositol reacts with CDP-DAG and forms PI (Salman et al., 1999). Recently, the gene encoding the PgsA [Rv2612c] has been identified and shown to be essential in M. tuberculosis (Jackson et al., 2000) (Fig. 3).
Overview of PIM biosynthesis
The current model of mycobacterial PIM biosynthesis supported by biochemical and genetic studies follows a linear pathway from PI→PIM2→PIM4→PIM6 (Fig. 4) (Chatterjee et al., 1992a; Besra & Brennan, 1997; Morita et al., 2004, 2006). Glycosylation of PI by different α-mannopyranosyltransferases, PimA, PimB', PimC, unidentified PimD?, PimE, unidentified PimF? and acylation by acyltransferase(s), results in the synthesis of Ac1/Ac2PIMs (Kordulakova et al., 2002, 2003; Kremer et al., 2002; Morita et al., 2006; Lea-Smith et al., 2008; Guerin et al., 2009; Mishra et al., 2009), out of which Ac1/Ac2PIM2 and Ac1/Ac2PIM6 accumulate onto the mycobacterial cell wall (Figs 1 and 4).
Conversion of PI into Ac1PIM1
The enzymes involved in the synthesis of early PIMs are encoded by a conserved cluster of six ORFs in an operon, which is found in all members of Corynebacterineae (Cole & Barrell, 1998; Cole et al., 1998;). The first ORF of this cluster, Rv2614c, encodes a protein with an aminoacyl-tRNA synthase class-II motif and is similar to Escherichia coli threonyl-t-RNA synthases. The second ORF, Rv2613c, has similarity to the proteins involved in nucleotide biosynthesis, while the third ORF, Rv2612c, encodes for PgsA and the fourth ORF, Rv2611c, encodes an acyltransferase. An M. smegmatis Rv2611c mutant exhibited severe growth defects and accumulated nonacylated PIM1 and PIM2, suggesting its role in acylation of PIMs. Further biochemical analysis suggested that Rv2611c acylates the 6-position of Manp residue linked to the 2-OH position of myo-inositol (Kordulakova et al., 2003). Very recently, the identification of an α-d-mannose-α(1→6)-phosphatidyl-myo-inositol-mannopyranosyltransferase, PimB', involved in the biosynthesis of Ac1/Ac2PIM2, shed further light on the acylation step in PIM biosynthesis. The deletion of pimB' in C. glutamicum resulted in the abrogation of Ac1/Ac2PIM2 and the accumulation of Ac1PIM1 (Lea-Smith et al., 2008; Mishra et al., 2008b;), suggesting that the first acylation step, i.e. acylation of PIM1 (Kordulakova et al., 2003), precedes the second mannosylation step, resulting in the formation of Ac1PIM2 (Schaeffer et al., 1999).
PimA [Rv2610c] is the fifth ORF of the operon and is essential in M. smegmatis (Kordulakova et al., 2002). In cell-free assays with partially purified Rv2610c and/or membranes from M. smegmatis overexpressing PimA and GDP-[14C]-Manp, Kordulakova et al. (2002) identified the incorporation of radioactivity into PIM1 and Ac1PIM1. They deduced that Rv2610c encodes for an α-mannopyranosyltransferase and that PimA is responsible for the formation of PIM1 from PI and GDP-Manp (Kordulakova et al., 2002). The crystal structure of PimA in complex with GDP-Manp from M. smegmatis shows a two-domain organization with the catalytic machinery typical of GT-B glycosyltransferases (Guerin et al., 2007). The sixth ORF, Rv2609c, encodes for a putative GDP-Manp hydrolase containing a mutT domain (see below for further discussion).
Synthesis of Ac1PIM2, an important step in higher PIMs, lipomannan and lipoarabinomannan biosynthesis
Recently, Rv2188c and its homologs in C. glutamicum (Lea-Smith et al., 2008; Mishra et al., 2008b;) and M. smegmatis (Guerin et al., 2009) were identified as PimB'. This identification augmented the confusion in the field, as another gene, Rv0557, had already been assigned the function of PimB as an α-d-mannose-α(1→6)-phosphatidyl-myo-inositol-mannopyranosyltransferase (Schaeffer et al., 1999). This study was based on the utilization of cell-free assays using GDP-[14C]-Manp, Ac1PIM1, M. smegmatis membranes and/or partially purified recombinant Rv0557 (Schaeffer et al., 1999). Furthermore, the disruption of Rv0557 in M. tuberculosis did not affect the biosynthesis of Ac1PIM2 (Torrelles et al., 2009), suggesting either gene duplication or that Rv0557 performed another function in M. tuberculosis. Recently, Rv0557 was shown to be involved in the biosynthesis of ManGlcAGroAc2 and a Cg-LM-B (also see Structure of PIMs) in C. glutamicum, and has been suggested to have an α-mannosyl-glucopyranosyluronic acid transferase, MgtA, activity (Tatituri et al., 2007a).
To solve this puzzle, involving PimB, PimB' and MgtA, and to assign the correct function to each ORF, a double mutant deficient in orthologs of Rv0557 and Rv2188c was generated in C. glutamicum, and subsequently, Rv0557 and Rv2188c were overexpressed in the double mutant. Consequently, the in vivo complementation of α-d-mannose-α(1→6)-phosphatidyl-myo-inositol-mannopyranosyltransferase activity was restored using plasmid-borne copies of Rv2188c resulting in the synthesis of Ac1PIM2 and the related lipoglycan in the C. glutamicum double mutant, while overexpression of Rv0557 resulted in the synthesis of ManGlcAGroAc2, suggesting that Rv0557 has an α-mannosyl-glucopyranosyluronic acid transferase activity, and therefore, Rv2188c was suggested to be Mt-PimB, while Rv0557 was renamed as Mt-MgtA (Mishra et al., 2009). For consistency with the recent literature, we retain the designation PimB' for Rv2188c (and its orthologs in M. smegmatis and C. glutamicum) (Lea-Smith et al., 2008; Mishra et al., 2008b, 2009; Guerin et al., 2009). The crystal structure of C. glutamicum PimB' in complex with GDP vs. GDP-Manp shows the selectivity of PimB' for 6-OH of the inositol moiety of PI (Batt et al., 2010). Rv0557 possesses relaxed substrate specificity towards Ac1PIM1 (Schaeffer et al., 1999; Mishra et al., 2009;) and its deletion from M. tuberculosis resulted in a viable mutant with a subtle decrease in the lipomannan and lipoarabinomannan contents (Torrelles et al., 2009), indicating a superficial role of Rv0557 in the biosynthesis of PIMs, lipomannan and lipoarabinomannan. In contrast, Rv2188c is essential in M. smegmatis (Guerin et al., 2009), illustrating an example of a high-duplication event that lead to extensive functional redundancy in mycobacteria (Cole et al., 1998; Tekaia et al., 1999;).
More recently, the role of glycosyl hydrolases has been suggested in the regulation of glycolipid flux inside and outside the cell membrane and it was suggested that these glycosyl hydrolases work in close coordination with glycosyltransferases (Crespo et al., 2010). The presence of GDP-Manp hydrolases in the vicinity of pimA and pimB', suggests the metabolic role of these glycosyl hydrolases in the regulation of the sugar donors and glycolipids, such as GDP-Manp, PPM and PIMs. In addition, the presence of putative transporters Rv2190c in M. tuberculosis and NCgl2107 and NCgl2108 in C. glutamicum in the vicinity of pimB' region suggests their role in PIM or PPM transport in Corynebacterineae. However, the deletion of the homolog of Rv2190c in C. glutamicum resulted in a viable mutant with no phenotype, suggesting gene redundancy, which is not surprising as these putative transporters are present in multicopies elsewhere in the genome (L. Eggeling & G.S. Besra, unpublished data). Future studies targeting the role of these glycosyl hydrolases and transporters may shed further light on the regulation of PIMs, lipomannan and lipoarabinomannan in mycobacteria.
Synthesis of higher-order PIMs
Bioinformatical analysis of the genome of M. tuberculosis CDC1551 has led to the identification of RvD2-ORF1 from M. tuberculosis CDC1551 as an Ac1PIM2:α-d-mannose-α(1→6)-phosphatidyl-myo-inositol-mannopyranosyltransferase, PimC, involved in the addition of Manp from GDP-Manp to the 6-OH of mannose at the nonreducing end of Ac1/Ac2PIM2 (Kremer et al., 2002). The use of a cell-free assay containing GDP-Manp, amphomycin (an antibiotic that inhibits the synthesis of PPMs by inhibiting the PPM synthase) and membranes from M. smegmatis-overexpressing PimC led to the synthesis of Ac1/Ac2PIM3. However, the inactivation of pimC in M. bovis BCG did not affect the production of higher PIMs, lipomannan and lipoarabinomannan, and the fact that genes orthologous to pimC were found in only 22% of clinical isolates suggests the existence of redundant gene(s) or an alternate pathway that may compensate for PimC deficiency (Kremer et al., 2002).
Ac1/Ac2PIM3 is further α(1→6) mannosylated at the nonreducing termini by an unidentified α(1→6)-mannopyranosyltransferase [PimD] or PimC itself, resulting in the formation of Ac1/Ac2PIM4. This step in the biosynthesis of higher PIMs, lipomannan and lipoarabinomannan, has been suggested as a key branch point towards the synthesis of Ac1/Ac2PIM6, lipomannan and lipoarabinomannan (Morita et al., 2004; Morita et al., 2006; Mishra et al., 2008a;). It has been proposed that a transition occurs from glycosyltransferases, utilizing nucleotide-derived sugar substrates, characterized by the GT-A/B superfamily, to the glycosyltransferases utilizing polyprenyl-phosphate sugars, the GT-C superfamily (Liu & Mushegian, 2003), for the elongation and branching of lipomannan and lipoarabinomannan (Morita et al., 2006). Rv1159 [PimE] has been identified as an α(1→2)-mannopyranosyltransferase that utilizes PPM as a substrate and adds an α(1→2)-Manp to Ac1/Ac2PIM4, resulting in the synthesis of Ac1/Ac2PIM5 (Fig. 4) (Morita et al., 2006). However, it is not clear whether PimE is solely responsible for the synthesis of both Ac1/Ac2PIM5 and Ac1/Ac2PIM6. So far, most of the putative glycosyltransferases belonging to the GT-C family (Liu & Mushegian, 2003) in M. tuberculosis have been functionally characterized. That leaves us with fewer possibilities, in which either PimE or one of the other uncharacterized GT-Cs (Rv0051 and Rv0541c) (Liu & Mushegian, 2003; Berg et al., 2007;) adds the second Manp residue onto Ac1/Ac2PIM5. However, an Rv0051 deletion mutant showed no phenotypic change in the cell wall glycolipid of M. tuberculosis (A.K. Mishra & G.S. Besra, unpublished data), leaving Rv0541c as a promising candidate.
Morita et al. (2005) suggested that enzymes involved in the biosynthesis of early PIM intermediates (PIM1 and Ac1PIM1) are localized to a membrane subdomain termed PMf in the plasma membrane, while the majority of Ac1/Ac2PIM2 (and biosynthetic enzymes) involved in higher-order PIM (Ac1/Ac2PIM4 and Ac1/Ac2PIM6) biosynthesis are localized to a denser fraction that contains both plasma membrane and cell wall markers (PM-CW) (Morita et al., 2005). On the basis of various cell-free assays, they concluded that higher PIM biosynthesis occurs in the plasma membrane rather than the PM-CW fraction, followed by their subsequent transport to the cell wall (Morita et al., 2005). The relative amount of higher PIMs and lipoglycans was suggested to be regulated by a recently identified lipoprotein [LpqW] in M. smegmatis (Kovacevic et al., 2006; Marland et al., 2006;). However, the exact mechanism of PIM flux and its segregation for Ac1/Ac2PIM6 or lipomannan biosynthesis is unknown. Furthermore, Ac1/Ac2PIM4 was suggested to be a key regulatory product involved in the biosynthesis of Ac1/Ac2PIM6 and/or lipomannan biosynthesis (Morita et al., 2004, 2006). PimE directs Ac1/Ac2PIM4 towards Ac1/Ac2PIM6 synthesis, while LpqW channels Ac1/Ac2PIM4 for lipomannan synthesis (Crellin et al., 2008). It is speculated that Ac1/Ac2PIM4 is transported by a flippase or a sugar transporter across the plasma membrane, where subsequent mannosylation occurs by distinct mannopyranosyltransferases belonging to the GT-C family (Liu & Mushegian, 2003; Mishra et al., 2008a;).
Recently, the role of a putative acyl transferase, Rv1565c, was suggested in the acylation of higher-order PIMs, lipomannan and lipoarabinomannan. An Rv1565c deletion mutant in Mycobacterium marinum showed a reduced incorporation of 1,2-[14C]-acetate into the PIMs, lipomannan and lipoarabinomannan as compared with the wild type. Furthermore, lipoarabinomannan from the mutant lacks mannose caps and showed a higher degree of branching of both the arabinan domain and the mannan core, suggesting some important and unidentified role of Rv1565c in mycobacteria (Driessen et al., 2010).
Overview of lipomannan and lipoarabinomannan biosynthesis
Synthesis of the mannan core
Using mutant constructs in C. glutamicum, and cell-free assays, two α(1→6)-mannopyranosyltransferase activities were reported from C. glutamicum, of which one enzyme (M. tuberculosis homolog, Rv2174) was characterized as MptA and shown to be involved in the synthesis of the distal end of the α(1→6) mannan backbone of lipomannan (Kaur et al., 2007; Mishra et al., 2007;), while a second α(1→6)-mannopyranosyltransferase, Rv1459c (MptB), was shown to be involved in the synthesis of the proximal end of the mannan backbone and speculated to extend an Ac1/Ac2PIM4 acceptor (Fig. 5) (Mishra et al., 2008a). The deletion of the MptB ortholog in C. glutamicum resulted in the absence of lipomannan and lipoarabinomannan and a reduction in α(1→6)-mannopyranosyltransferase activity. Furthermore, cell-free assays involving C50-P-Manp and heterologously expressed Rv1459c and/or its M. smegmatis homolog MSMEG_3120 in C. glutamicum showed that these enzymes possessed α(1→6)-mannopyranosyltransferase activity. On this basis, it was suggested that after the transport of Ac1PIM4 outside the plasma membrane by an unidentified flippase, Mt-MptB catalyzes the addition of further 12–15 Manp units (Mishra et al., 2008a). MptB is part of an operon that consists of four ORFs encoding ATP-binding cassette (ABC) transporters (Wang et al., 2006). This enhances a strong possibility for a functional coupling of the glycosyltransferase MptB with ABC transporters, Rv1458c, Rv1457c and Rv1456c. However, a C. glutamicum mutant deficient in these ABC transporters showed no difference in their PIM, lipomannan and lipoarabinomannan profiles (L. Eggeling & G.S. Besra, unpublished data), suggestive of gene redundancy, which is not surprising as these ABC transporters are found at multiple locations in the genome of Corynebacterineae.
Fig. 5.
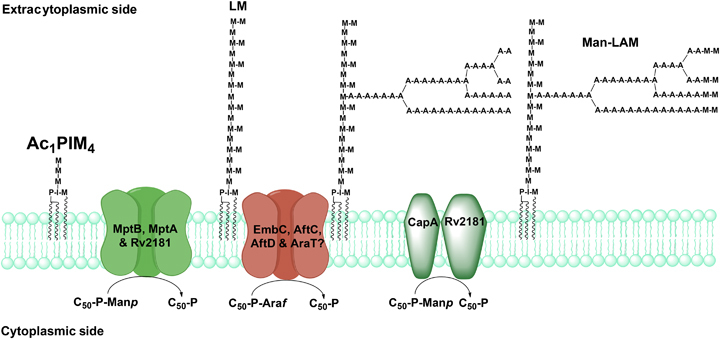
Biogenesis of Man-LAM from Mycobacterium tuberculosis. Ac1/Ac2PIM4 plausibly serves as an acceptor and extended by MptB in the α(1→6) direction, followed by MptA and further decorated by singular α(1→2)-Manp units by Rv2181 (MptC), resulting in lipomannan. Mature lipomannan is subsequently primed by a singular d-Araf at an unknown position, which is extended by EmbC and/or unidentified α(1→5) arabinofuranosyltransferases. The linear α(1→5)-d-Araf chain is further primed by AftC, which is subsequently extended by AftD and unknown arabinofuranosyltransferases and terminated by the action of AftB to form linear Ara-4 or branched Ara-6. The penultimate Araf of the arabinan domain is further capped by Manp residues by CapA and Rv2181 (MptC) to form Man-LAM. For simplicity, only triacylated versions of different lipoglycans are shown.
Interestingly, α(1→6) mannan extension is more complex in mycobacteria, based on the evidence that Mt-MptB and Ms-MptB fail to complement the C. glutamicum▵mptB mutant, suggesting a slightly different substrate specificity of the MptB orthologs of M. tuberculosis and M. smegmatis as compared with Cg-MptB (Mishra et al., 2008a). Furthermore, the redundancy of Ms-MptB in M. smegmatis▵mptB indicates that either another as yet unidentified mannopyranosyltransferase is substituting for MptB in the mutant or the distal α(1→6)-mannopyranosyltransferase, MptA, is substituting for the deficiency of Ms-MptB. A mycobacterial strain devoid of MptA and MptB may shed further light on this aspect.
In order to identify the α(1→6)-mannopyranosyltransferase involved in the synthesis of the distal α(1→6) mannan backbone, the homologs of putative glycosyltransferase, Rv2174, were deleted from C. glutamicum (Mishra et al., 2007) and M. smegmatis (Kaur et al., 2007). The cell wall phenotype of the mutants suggested the accumulation of a truncated lipoglycan (t-LM), deficient in α(1→6)-Manp units. A cell-free assay involving C50-P-Manp and a synthetic disaccharide acceptor, Man-α(1→6)-Man-C8, established that the mutant lacked α(1→6)-mannopyranosyltransferase activity and was termed MptA (Mishra et al., 2007). Pfam analysis (Bateman et al., 2004) of the ORF upstream of mptA revealed that Rv2173 (putative geranylgeranyl pyrophosphate synthetase, idsA2) bears structural similarities to polyprenyl synthetases, which could be functionally related to MptA, and both genes may form a transcriptional unit. Interestingly, mycobacterial MptA contains 13 transmembrane helixes (TMH), of which TMH 3 and 4 are conserved and contain the catalytic DXD motif typified by glycosyltransferases (Liu & Mushegian, 2003), while the C-terminus extracellular loop is nonexistent, unlike other GT-C glycosyltransferases (Zhang et al., 2003; Alderwick et al., 2011;), suggesting the existence of a different model for chain extension as reported already in the case of M. tuberculosis EmbC (Shi et al., 2006). Furthermore, the recent genome analysis of Actinobacterium and Micrococcus luteus identified the presence of homologs of MptA and MptB in a cluster with another gene encoding for a GT-C glycosyltransferase, which are cotranscribed and probably translationally coupled (Young et al., 2010). In contrast, MptA and MptB are dispersed in corynebacteria and mycobacteria.
The α(1→6) mannan core in lipomannan and lipoarabinomannan is further decorated by single α(1→2)-Manp branches (Hunter & Brennan, 1990; Chatterjee et al., 1992a;). On the basis of known polyprenol-dependent glycosyltransferases, Rv2181 (MptC), was identified from an 18-kb conserved region and suggested to be involved in the synthesis of α(1→2)-Manp side chains of lipomannan (Fig. 5) (Kaur et al., 2006; Kaur et al., 2008; Sena et al., 2010; Mishra et al., 2011;). More recently, it was suggested that MptA and MptC may act in close coordination to synthesize mature lipomannan and lipoarabinomannan, and the length of the mannan core may be regulated by a branching-dependent chain termination mechanism (Sena et al., 2010).
Arabinan domain assembly of lipoarabinomannan
Ac1/Ac2-PIM2 is extended by MptB, MptA and MptC to yield a mature lipomannan that probably serves as an acceptor for an uncharacterized arabinofuranosyltransferase to initiate lipoarabinomannan synthesis (Besra et al., 1997). However, the number of arabinofuranosyltransferases required for arabinan domain biosynthesis will depend on the types of arabinan linkage present in mycobacterial lipoarabinomannan (Fig. 5). It is quite likely that mature lipomannan is primed by a few Araf units in a similar fashion as AftA primes the galactan of arabinogalactan in mycobacteria (Alderwick et al., 2006c). However, the enzyme responsible for this activity is not known. The primed Araf-LM is then further extended by EmbC (Rv3793) (Zhang et al., 2003; Shi et al., 2006; Alderwick et al., 2011;) for 12–16 α(1→5)-Araf residues (Birch et al., 2010). Recently, AftC (Rv2673) was shown to introduce the α(1→3)-Araf branch points in both arabinogalactan (Birch et al., 2008) and lipoarabinomannan (Birch et al., 2010). This α(1→3)-Araf branched product, [Araf]12–16-LM, is then further extended by an unidentified α(1→5)-arabinofuranosyltransferase.
More recently, Skovierova et al. (2009) proposed a second branching α(1→3)-arabinofuranosyltransferase, AftD (Rv0236c). However, unlike the role of AftC as an α(1→3)-arabinofuranosyltransferase, which was experimentally validated by creating a knockout in M. smegmatis defective in α(1→3)-arabinofuranosyltransferase activity (Birch et al., 2008), the role of AftD is debatable as Skovierova et al. (2009) were unable to create a viable mutant displaying a clear phenotype, and the study was solely based on the usage of artificial chemically defined acceptors using crude M. smegmatis and C. glutamicum membranes (Skovierova et al., 2009). It is also interesting to note that the same authors also discussed the possibility of AftD as an α(1→5)-arabinofuranosyltransferase involved in α(1→5)-Araf extension of the nonreducing termini of the arabinan domain of lipoarabinomannan and arabinogalactan (Skovierova et al., 2009) (Fig. 5).
The final enzyme involved in arabinan domain biosynthesis is AftB (Rv3805c), which results in a terminal tetra- and hexa-arabinofuranoside structure (Figs 2 and 5). The role of AftB has been experimentally shown to be a β(1→2)-arabinofuranosyltransferase in the synthesis of arabinogalactan (Seidel et al., 2007). However, its role in the synthesis of similar Araf residues in lipoarabinomannan is highly likely after the discovery of a dual role of AftC in arabinogalactan (Birch et al., 2008) and lipoarabinomannan (Birch et al., 2010) biosynthesis.
Mannan priming and Man-LAM synthesis
All pathogenic species of the genus Mycobacterium are known to possess Man-LAM, which is responsible for some of the immunomodulatory properties of these strains (Briken et al., 2004). A close inspection of the M. tuberculosis genome in comparison with M. smegmatis that possesses lipoarabinomannan without mannose caps provided the first indication of the role of Rv1635c in Man-LAM biosynthesis. On this basis, the homolog of Rv1635c in M. tuberculosis CDC1551 was identified as a glycosyltransferase that could be involved in Man-LAM capping (Dinadayala et al., 2006). Simultaneously, mutants of Rv1635c homologs in M. marinum and M. bovis BCG showed that the gene encoded for an α(1→5)-mannopyranosyltransferase, CapA, was involved in the addition of the first Manp residue on the nonreducing arabinan termini of lipoarabinomannan (Appelmelk et al., 2008). More recently, it was also shown that MptC (Rv2181), which adds α(1→2)-Manp residues onto the α(1→6) mannan backbone of lipomannan and lipoarabinomannan, also adds α(1→2)-Manp caps at the nonreducing end of lipoarabinomannan in combination with CapA (Kaur et al., 2008) (Fig. 5). Our recent studies with an M. bovis BCG mutant defective in pimE also suggested its tentative role in α(1→2)-Manp capping of Man-LAM (G.S. Besra & B.J. Appelmelk, unpublished data). However, more studies are needed to establish the exact interplay of these mannopyranosyltransferases involved in the mannan caps of Man-LAM.
Almost the entire repertoire of enzymes and genes involved in the biogenesis of lipoarabinomannan and related glycoconjugates has been identified (Table 1), and some of these genes are essential for the survival of M. tuberculosis, therefore representing excellent drug targets. However, the roles of lipoarabinomannan and related glycoconjugates in mycobacterial pathogenicity require the availability of mycobacterial mutants defective in their respective biosynthetic pathways, as most of the studies have been carried out using purified molecules that do not represent the true in vivo condition during infection. The availability of complete genome sequences of several mycobacteria and related actinomycetes and the development of novel tools for genetic manipulation have enhanced these possibilities.
Table 1.
Experimentally characterized genes involved in the biosynthesis of LAM and related glycoconjugates
| ORF | Function | Role | References |
|---|---|---|---|
| PgsA (Rv2612c) | PI synthase | Synthesis of Phosphatidyl-myo-inositol | Jackson et al. (2000) |
| PimA (Rv2610c) | α(1→2)-Mannopyranosyltransferase | Synthesis of PIM1 | Kordulakova et al. (2002) |
| Rv2611c | Acyltransferase | Synthesis of Ac1/Ac2-PIM1 | Kordulakova et al. (2003) |
| PimB' (Rv2188c) | α(1→6)-Mannopyranosyltransferase | Synthesis of Ac1/Ac2-PIM2 | Lea-Smith et al. (2008); Mishra et al. (2008b, 2009) |
| MgtA/PimB (Rv0557) | α(1→6)-Mannopyranosyltransferase | Synthesis of ManGlcGroAc2 and Ac1/Ac2-PIM2 | Tatituri et al. (2007a,b); Mishra et al. (2008b); |
| PimC (RvD2-ORF1) | α(1→6)-Mannopyranosyltransferase | Synthesis of Ac1/Ac2-PIM3 | Kremer et al. (2002) |
| PimE (Rv1159) | α(1→2)-Mannopyranosyltransferase | Synthesis of Ac1/Ac2-PIM5 | Morita et al. (2006) |
| MptB (Rv1459c) | α(1→6)-Mannopyranosyltransferase | Synthesis of proximal mannan backbone i.e. Ac1/Ac2-PIM12–17 | Mishra et al. (2008a) |
| MptA (Rv2174) | α(1→6)-Mannopyranosyltransferase | Synthesis of distal mannan backbone i.e. Ac1/Ac2-PIM22–25 | Mishra et al. (2007) |
| MptC (Rv2181) | α(1→2)-Mannopyranosyltransferase | Adds α(1→2)-Manp units on the mannan backbone, and also adds a second mannose cap on ManLAM | Kaur et al. (2008, 2010); Mishra et al. (2011) |
| EmbC (Rv3793) | α(1→5)-Arabinofuranosyltransferase | Involved in the synthesis of the α(1→5)-arabinan backbone | Zhang et al. (2003); Alderwick et al. (2011); |
| AftC (Rv2673) | α(1→3)-Arabinofuranosyltransferase | Adds Araf on α(1→5)-arabinan backbone in the α(3→5)-direction | Birch et al. (2010) |
| AftD (Rv0236c) | α(1→3) or α(1→5)-Arabinofuranosyltransferase | Either adds α(1→3)-Araf units to the non-reducing end of the α(1→5)-arabinan branch or synthesize α(1→5) itself | Skovierova et al. (2009) |
| CapA (Rv1635c) | α(1→5)-Mannopyranosyltransferase | Adds first mannose cap on ManLAM | Appelmelk et al. (2008) |
Part II – Interactions with host immune system
Accessibility of lipoglycans to the immune system: localization and trafficking
Lipoarabinomannan and related lipoglycans are not only essential for mycobacterial growth and cell viability (Haites et al., 2005; Kovacevic et al., 2006;), but are also thought to be important in the interactions between the mycobacteria and their host. The nature of these host–pathogen interactions is determined by the accessibility of the lipoglycans to the immune system, i.e. can cell wall-bound lipoglycans be recognized by the pattern-recognition receptors (PRRs) of the immune system and how do the lipoglycans traffic, once released from the mycobacterial cell wall?
The localization of PIMs and lipoarabinomannan in the mycobacterial cell envelope has been assessed in multiple ways, including biotin tagging of lipoarabinomannan and extraction of lipids from the cell wall with detergents or by mechanical treatment with glass beads. Because of the strong conditions needed to extract lipoarabinomannan from the cell wall, and the possibility to detect lipoarabinomannan with lipoarabinomannan-recognizing antibodies on whole cells, it was hypothesized that lipoarabinomannan is firmly attached via its MPI anchor to the surface of the cell (Chatterjee & Khoo, 1998). Biotin labeling, assumed to be restricted to the cell surface, showed two fractions of lipoarabinomannan: one anchored to the cytosolic membrane and one in the mycobacterial outer membrane (mycomembrane) (Hoffmann et al., 2008; Pitarque et al., 2008;). However, biotin is only a small molecule and may have easier access to lipoarabinomannan more buried in the cell wall as compared with the large PRRs, which may reduce the potential of lipoarabinomannan to be recognized by the immune system. Furthermore, native mycobacterial cells are surrounded by a capsule, which could cover lipoarabinomannan. The mycobacterial capsule mainly consists of polysaccharides and proteins (Daffé & Etienne, 1999). Electron microscopy (EM) with immunogold-labeled cells using ConA and anti-arabinan antibodies, combined with nuclear magnetic resonance studies, showed the presence of mannose-capped arabinomannan (Man-AM; i.e. without the lipid anchor present in Man-LAM) in the capsule (Ortalo-Magnéet al., 1995). The capsule has been reported to have only a very low lipid content, among which are small amounts of PIMs and virtually no lipoarabinomannan (Ortalo-Magnéet al., 1996a,b;). A recent study used immunogold-EM with monoclonal antibodies against PIM6 (F183-24), and against the mannose cap (55.92.1A1) and the arabinan domain (F30-5) of Man-AM and Man-LAM to detect surface localization of these lipoglycans. Unperturbed mycobacterial cells bearing an intact capsule display good labeling with these antibodies (Sani et al., 2010), which confirms the presence of PIM6 and Man-AM in the capsule. In contrast, mycobacteria without a surrounding capsule due to growth under perturbing conditions (in the presence of 0.05% Tween-80 and mechanical agitation) hardly become labeled with these antibodies. As lipoarabinomannan is localized in the cell wall and not in the capsule, this suggests limited surface exposure of lipoarabinomannan and related glycans buried in the mycomembrane, even if the capsule is not covering the cell wall. However, the amount of lipoarabinomannan or its accessibility in these cells grown under perturbing conditions has not been assessed further.
Although culture filtrate has been reported to contain only trace amounts of lipids (Lemassu & Daffé, 1994), studies with infected macrophages (Mϕ) showed intracellular trafficking of PIMs and lipoarabinomannan, suggesting that these glycolipids are substantially released from mycobacteria. Even release into noninfected bystander cells (Xu et al., 1994; Beatty et al., 2000; Rhoades et al., 2003;) and subsequent presentation through CD1 glycoproteins (Schaible et al., 2000) has been observed. Furthermore, isolated PIMs and lipoarabinomannan can be incorporated into the endomembranes and plasma membranes of different cell types, a process requiring the MPI anchor and the mannan core (Ilangumaran et al., 1995; Shabaana et al., 2005; Welin et al., 2008;). It can be hypothesized that the lipoglycans released are able to modulate the immune response, for example by interfering with phagosome maturation.
Phagosome maturation arrest
At least two strategies used by M. tuberculosis to survive in Mϕ have been described. One is delay of the phagosome maturation, i.e. prevention of fusion of the phagosome with late endosomal and lysosomal organelles, which normally leads to killing and digestion of a pathogen in an acid environment (Armstrong & Hart, 1971; Russell, 2001; Nguyen & Pieters, 2005;). The other strategy is based on escape from the phagosome to the cytosol (van der Wel et al., 2007). In the phagosome maturation arrest, a role for both Man-LAM and PIMs has been implicated (Vergne et al., 2003b; Welin et al., 2008;).
The phagosome–lysosome fusion process starts after cytosolic Ca2+ increase. The Ca2+/calmodulin-dependent PI3-kinase hVPS34 and its modulatory subunit p150 will then generate the membrane-trafficking lipid phosphatidylinositol 3-phosphate (PI3P) on the phagosomal membrane (Vergne et al., 2004a). Both PI3P and early endocytic small GTPase Rab5 mediate in the subsequent recruitment of membrane tethering protein early endosome autoantigen 1 (EEA1) to the phagosome (Vergne et al., 2003a) (Fig. 6). EEA1 plays an essential role in phagosome maturation by interacting directly with syntaxin-6, a soluble NSF attachment protein receptor (SNARE) protein involved in the delivery of cathepsins (lysosomal hydrolases) and VoH+-ATPase from the trans-Golgi network to the phagosome (Simonsen et al., 1999). The normal cytosolic Ca2+ increase upon an infection is absent during mycobacterial uptake. This has been hypothesized to lead to a reduced activity of the PI3-kinase hVPS34 and an altered production of PI3P in the case of phagocytosis of mycobacteria (Malik et al., 2001; Chua & Deretic, 2004;) (Fig. 6).
Fig. 6.
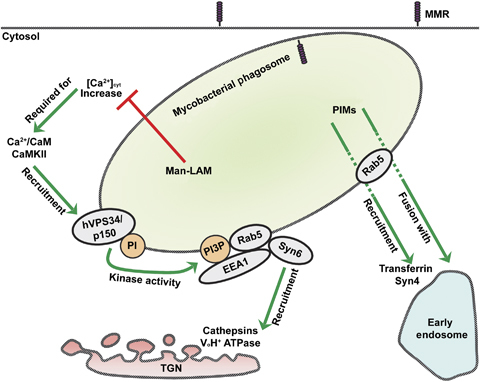
The role of PIMs and Man-LAM in phagosome maturation arrest by mycobacteria. While Man-LAM prevents lysosomal fusion and acidification, PIMs induce fusion with early endosomes to obtain nutrients required for phagosomal residence of mycobacteria. Man-LAM appears to inhibit cytosolic-Ca2+ increase and thereby blocks the successive steps of hVPS34 kinase activity at the phagosomal membrane, the recruitment of Rab5, EEA1 and Syn6 to the phagosome, and the delivery of cathepsins and VoH+ ATPase. The activity of PIMs in phagosome maturation is dependent on Rab5, but the exact mechanism is not known yet. MMR, macrophage mannose receptor; TGN, trans-Golgi network; CaM, calmodulin; PI, phosphatidylinositol; PI3P, phosphatidylinositol 3-phosphate; Syn, syntaxin; EEA1, early endosome autoantigen 1.
This immune evasion strategy of blocking phagosome maturation can be mimicked by Man-LAM, which is also able to inhibit cytosolic Ca2+ increase (Fratti et al., 2003b; Vergne et al., 2003b;) (Fig. 6). In Mϕ infected with M. bovis BCG or Man-LAM-coated beads, EEA1 is excluded from the early endosome, thereby inhibiting phagosome maturation at the stage of recruitment of late endosomal and lysosomal constituents and, hence, preventing acidification (Fratti et al., 2001). The exact mechanism of [Ca2+]cyt-modulation by Man-LAM is not known. For M. tuberculosis to infect Mϕ without the induction of a cytosolic Ca2+ increase, phagocytosis via the complement receptor is required (Malik et al., 2000), but the inhibition of phagosome maturation by Man-LAM appears to involve binding to the MMR instead (Kang et al., 2005). Furthermore, a role for macrophage phosphatase SHP-1 has been suggested, which is activated by Man-LAM and impairs Ca2+ signaling (Ono et al., 1997; Knutson et al., 1998; Vergne et al., 2004a;).
Another possibility of interference by Man-LAM in phagosome maturation, distinct from blocking the rise in [Ca2+]cyt, considers a role for the activation of p38 mitogen-activated protein kinase (p38 MAPK). p38 MAPK activity may indirectly maintain Rab5 in an inactive GDP-bound form (Cavalli et al., 2001; Vergne et al., 2004a;). This is consistent with the report that the induction of p38 MAPK reduces the recruitment of Rab5-effector protein EEA1 to the early endosome (Fratti et al., 2003a). Moreover, the level of p38 MAPK activation is significantly increased upon infection with M. bovis BCG (Fratti et al., 2003a) and Man-LAM was hypothesized to be a triggering component (Vergne et al., 2004a). However, recently, it has been shown experimentally that p38 MAPK activation is neither induced nor influenced by isolated Man-LAM, and thus must be linked to other mycobacterial components (Welin et al., 2008).
Noteworthy, lipoarabinomannan is incorporated into membrane lipid rafts, a process that is also required for the phagosome maturation arrest (Welin et al., 2008). Lipid rafts are highly dynamic lipid domains, enriched in cholesterol and glycosphingolipids, and that have been associated with cell signaling (Simons & Toomre, 2000; Pike, 2009;). It has been suggested that the presence of lipoarabinomannan in the endomembrane causes drastic reorganization of the lipid domains and thereby fusion of the lipid vesicles (Hayakawa et al., 2007). However, PI-LAM from avirulent M. smegmatis is also incorporated into endomembranes, although to a lesser extent, but it cannot prevent phagosome–lysosome fusion nor inhibit cytosolic Ca2+ increase (Vergne et al., 2003b; Kang et al., 2005; Welin et al., 2008;). Given that the MMR only recognizes the mannose-capped Man-LAM and not Ara-LAM or PI-LAM (Schlesinger et al., 1994), this is further evidence that ligation of the Man-LAM to the MMR is required for the phagosome maturation block, which appears to be restricted to the more virulent Mycobacterium spp. A role for the MMR has been confirmed recently by showing that glycopeptidolipids from M. avium delay phagosome–lysosome fusion by interaction with the MMR and by an MMR siRNA knockdown in human monocyte-derived Mϕ, resulting in increased phagosome–lysosome fusion upon M. avium infection (Sweet et al., 2009).
As mammalian phosphoinositide PI3P plays an important role in phagosome maturation, next to lipoarabinomannan, other mycobacterial PI-analogs, PIMs and lipomannan, were investigated as well for their possible interference in phagosome maturation. While for lipomannan no role in phagosome maturation arrest could be detected (Kang et al., 2005), PIMs do have an effect, although in a way distinct from Man-LAM (Vergne et al., 2004b). Similar to lipoarabinomannan, PIMs can be incorporated into lipid rafts and, moreover, the addition of PIMs competitively inhibits lipoarabinomannan insertion (Ilangumaran et al., 1995; Welin et al., 2008;). Although PIMs seem to reverse the effect of lipoarabinomannan of preventing endosomal fusions (Welin et al., 2008), however, indications exist of a different role of PIMs in the phagosome maturation arrest. PIMs do prevent phagosome acidification, but not by reducing the recruitment of syntaxin-6 (Fratti et al., 2003b; Vergne et al., 2004b;). Instead, PIMs induce the acquisition of endosomal SNARE protein syntaxin-4 and the transferrin receptor (Fratti et al., 2003b; Vergne et al., 2004a,b;) (Fig. 6). Transferrin and its receptor are recycling endosomal markers involved in iron delivery (Clemens & Horwitz, 1996; Sturgill-Koszycki et al., 1996;). While Man-LAM arrests phagosome maturation by blocking the recruitment of late endosomal and lysosomal markers, PIMs appear to stimulate fusion with early endosomes and thereby retrieve nutrients necessary for mycobacteria residing in the phagosomal compartments (Kelley & Schorey, 2003; Vergne et al., 2004b;). This process is also Rab5-dependent (Gorvel et al., 1991), in particular when Rab5 activity is rate limiting, but whether PIMs affects Rab5 directly or indirectly is not yet known (Vergne et al., 2004b).
Interestingly, also in the effect that PIMs exert on the phagosome maturation, the MMR seems to play a role. While the lower-order PIMs (PIM2) are not recognized by the MMR, the MMR has a high affinity for higher-order PIMs (PIM5 and PIM6) (Torrelles et al., 2006). This is consistent with the report that only in Mϕ stimulated with higher-order PIMs was a significant increase in phagosome-lysosome fusion seen upon MMR blockade (Torrelles et al., 2006). Thus, although PIMs and Man-LAM influence the phagosome maturation by distinct mechanisms, both may involve recognition by the MMR. This indicates a balance between Man-LAM preventing maturation into the phagolysosome on the one hand and PIMs stimulating early endosomal fusion to retrieve nutrients on the other (Vergne et al., 2004b).
Inhibition of phagosome maturation by pathogenic Mycobacterium spp. may be a critical first step for their intracellular survival. Mycobacteria probably display several mechanisms to prevent lysosomal transfer, of which one is interference of Man-LAM in the phagosome maturation process. A M. marinum mutant that only produces lipoarabinomannan devoid of mannose caps showed a significant increase in colocalization with phagolysosomes in murine Mϕ as compared with its parent strain. However, the absolute numbers remained low in this assay (7.4%, 12.0% and 5.0% for the wild-type, mutant and complemented strain, respectively), and importantly, no significant differences in bacterial survival were observed (Appelmelk et al., 2008). Other mechanisms of phagosome maturation blockade, independent of Man-LAM, have been reported, for example the secretion of SapM by M. tuberculosis, a lipid phosphatase that hydrolyzes the PI3P on the endomembranes (Vergne et al., 2005), and the secretion of a eukaryotic-like serine/threonine protein kinase G (PknG) (Walburger et al., 2004).
Clusters of differentiation (CD)1
CD-1 glycoproteins have been identified as important antigen-presenting molecules of the immune system, next to major histocompatibility complex (MHC) class I and II molecules. While MHC class I and II present peptide antigens, CD1 molecules present glycolipids, thereby covering the presentation of a large variety of both self as well as microbial antigens (Young & Moody, 2006; de Libero & Mori, 2009;). In mycobacterial infection, CD1 ensures the presentation of the glycolipids unique to the mycobacterial cell wall to activate CD1-restricted T cells and is thereby involved in shaping the immune response (Porcelli et al., 1998; Sieling et al., 1999; Barral & Brenner, 2007;).
Human CD1 molecules are expressed by a variety of antigen-presenting cells (APC) and can be divided into three groups: CD1a, CD1b and CD1c together form group 1, and CD1d and CD1e form group 2 and group 3, respectively. Murine homologs for group 1 CD1 molecules have not been identified, but mice do express CD1d. Group 1 CD1 molecules present lipids to a clonally diverse T-cell population in which the precursors have unique specificity for a single antigen (Barral & Brenner, 2007). The expression of group 1 CD1 on isolated human myeloid APC is hardly detectable, but it is upregulated to high levels within a couple of days after infection with M. tuberculosis or activation by mycobacterial lipids (Roura-Mir et al., 2005) (Felio et al., 2009). This demonstrates an apparent role of antigen presentation by the group 1 CD1 in the clonal expansion of T cells and, hence, the adaptive immune response against mycobacterial infection (Roura-Mir et al., 2005; Barral & Brenner, 2007;). CD1d presents lipids to CD1d-restricted natural killers T (NKT) cells including the subset of clonally less diverse invariant NKT cells that display a rapid innate-like response (Barral & Brenner, 2007). In contrast to group 1 CD1, CD1d molecules are constitutively expressed and are reported to be downregulated during mycobacterial infection, confirming their association with the innate immune response (Roura-Mir et al., 2005; Moody, 2006;). CD1e is only restricted to myeloid dendritic cells (DCs) and is not expressed at the cell surface and thus does not present antigens to TCRs. In this review, we focus on CD1b and CD1d, because these CD1 molecules bind and present PIMs and related lipoglycans. Group 2 CD1d has been reported to only bind lower-order PIMs, PIM2 and PIM4, but not lipomannan or Man-LAM (Fischer et al., 2004; Zajonc et al., 2006;). In contrast, group 1 CD1b binds several mycobacterial lipid including PIM2 and Man-LAM (Sieling et al., 1995; Prigozy et al., 1997; Ernst et al., 1998;).
The structure of CD1 molecules has similarities to the MHC class I molecules, but shows some important differences in its binding groove, which is deeper and facilitates the binding of two acyl chains as present in the MPI anchor of PIMs and Man-LAM (Zeng et al., 1997; Porcelli et al., 1998; Fischer et al., 2004;). While the lipid anchoring in the hydrophobic CD1 groove is relatively nonspecific, the TCR recognizes the hydrophilic carbohydrate head group of the antigens with high specificity (Moody et al., 1997). As compared with CD1b, additional interactions between the center of the binding groove of CD1d and the polar head group of the PIM2 are of additive importance for the formation of a stable glycolipid complex and subsequent T cell recognition (Zajonc et al., 2006). In the presentation of PIM4 by CD1d, the two additional α(1→6)-linked Manp residues are probably orientated away from the binding groove (Zajonc et al., 2006). Considering the low abundance of PIM4 in the mycobacterial cell wall in contrast to (diacylated) PIM2 (Gilleron et al., 2001), presentation of PIM4 by CD1d may not be of high biological significance. For group 1 CD1b, mycobacterial antigens with head groups much larger than present in PIM2 have been described, which raises questions regarding how these large carbohydrates fit in the narrow space between the TCR and CD1 (Young & Moody, 2006). Higher-order PIM6 needs processing into the smaller PIM2 before being able to stimulate CD1b-restricted T cells. A role in this antigen processing has been implicated for CD1e, because the presence of CD1e is required for the activation of CD1b-restricted T cells by PIM6, and not by PIM2 (de la Salle et al., 2005). As mentioned above, CD1e does not present lipid antigens at the cell surface, but probably aids in endosomal/lysosomal α-mannosidase activity to produce PIM2 by binding PIM6 similar to the other antigen-presenting CD1 molecules (de la Salle et al., 2005). Secondly, CD1e may facilitate the loading of other CD1 molecules (de Libero & Mori, 2009). How Man-LAM is presented in the interaction between the TCR and the CD1b-Man-LAM complex has not yet been resolved. Man-LAM may be partly digested similar to PIM6 (Ernst et al., 1998), which is most likely, as already PIM6 with its short carbohydrate head group requires processing. Of note, CD1b and Man-LAM do colocalize in the cell (Prigozy et al., 1997) and CD1b is able to bind Man-LAM (Ernst et al., 1998). Two other options have been suggested by Young & Moody (2006). One possibility is flattening of the glycan part of Man-LAM between the TCR and CD1, so that only one or two carbohydrate units are positioned directly between the TCR and CD1. Multiple TCR-docking orientations may play a role in this as well. In the second option, Man-LAM is not presented by CD1b, but stimulates the process of CD1-dependent T cell activation indirectly via the mechanisms discussed below (Young & Moody, 2006).
CD1b is the predominant group 1 CD1 molecule present in the late endosomes/lysosomes and MHC class II compartments (Prigozy et al., 1997; Ernst et al., 1998; Schaible et al., 2000;) and shares with MHC class II molecules the requirement for acidification in order to function (Benaroch et al., 1995; Sugita et al., 1999;). PIMs and Man-LAM have been observed to be released in the phagosomes of infected cells and transported into the same intracellular compartments (Xu et al., 1994; Prigozy et al., 1997; Schaible et al., 2000;). The low pH in these compartments causes conformational changes in the structure of CD1b including relaxation of certain parts of its binding groove to facilitate subsequent antigen loading (Ernst et al., 1998; Sugita et al., 1999; Kronenberg & Sullivan, 2008; Relloso et al., 2008; de Libero & Mori, 2009;). As described above, PIMs and Man-LAM interfere in phagosome maturation and in particular Man-LAM has been shown to prevent endosomal acidification (Fratti et al., 2001). Hence, PIMs and Man-LAM likely impede their own presentation by CD1b. On the other hand, mycobacterial lipids have been shown to induce the transcription and expression of group 1 CD1 glycoproteins at the surface of the APC by signaling through TLR-2 (Roura-Mir et al., 2005; Moody, 2006;). Possible lipids involved were reported to be PIM2 and Ara-LAM extracted from the mycobacterial cell wall (Roura-Mir et al., 2005). However, PIM2 and Ara-LAM are poor TLR2 ligands as discussed in the next section (Nigou et al., 2008). Copurified lipopeptides, which are more potent inducers of TLR2 signaling, may also have induced CD1 expression in this assay (Nigou et al., 2008; Zahringer et al., 2008;).
Mycobacteria are able to interfere with the immune response against mycobacterial infection including the modulation of peptide antigen presentation by MHC class I and II molecules (Kaufmann & Schaible, 2005). Therefore, the lipid antigen presentation via four different CD1 glycoproteins forms an important alternative mechanism to induce an effective immune response (Sugita et al., 1999). Although the function and expression of CD1 molecules can be impaired by mycobacteria or mycobacterial components such as capsular α-glucan (Gagliardi et al., 2007, 2009; Balboa et al., 2010), the many distinct pathways for antigen sampling from various intracellular localizations and their subsequent presentation circumvents the immune evasion strategies exploited by mycobacteria (Sugita et al., 1999; Kaufmann & Schaible, 2005; de Libero & Mori, 2009;). Furthermore, both group 1 and group 2 CD1 presentation of lipid antigensseem to play a potential role in the protection against tuberculosis by vaccination with BCG (Watanabe et al., 2006; Venkataswamy et al., 2009;).
TLRs
Three TLRs have been implicated to play a role in the mycobacterial infection: TLR2, TLR4 and TLR9 (Ozinsky et al., 2000; Quesniaux et al., 2004a; Jo, 2008;). PIMs, lipomannan and lipoarabinomannan have all been examined for signaling via TLR2 and via TLR4, of which an overview is given here.
Lipoproteins are the major ligands for TLR2 (Brightbill et al., 1999), but MPI-anchored mannosylated lipoglycans can signal via TLR2 as well, depending on their degree of acylation and mannosylation (Gilleron et al., 2006; Doz et al., 2007; Nigou et al., 2008;). TLR2 dimerizes with either TLR1 or TLR6 in order to function (Ozinsky et al., 2000). TLR1/TLR2 heterodimers mainly recognize triacylated lipoproteins, while the diacylated forms bind TLR2/TLR6 (Takeuchi et al., 2002; Akira & Takeda, 2004;). Lipoglycan-induced signaling occurs via the TLR1/TLR2 complex (Elass et al., 2005; Gilleron et al., 2006; Nigou et al., 2008;). A positive relation exists between the length of the mannan chain and the ability of the lipoglycan to activate TLR2 (Nigou et al., 2008). The lipoglycan bearing the largest accessible mannan chain (i.e. not substituted with an arabinan domain) – lipomannan – showed to be a potent inducer of TLR2-signaling (Quesniaux et al., 2004b), although this activity is restricted to the tri- and tetra-acylated forms (Ac1/Ac2LM) (Gilleron et al., 2006; Doz et al., 2007;). Next to the induction of cytokines, Mϕ stimulated with lipomannan displayed increased production of matrix metalloproteinase (MMP)-9 (Elass et al., 2005). This was due to the downregulation of the transcription of the MMP-9 inhibitor, tissue inhibitor of metalloproteinases-1, and dependent on TLR2. This implies a role for lipomannan in tissue destruction by MMP-9 during mycobacterial infection via interaction with TLR2 (Elass et al., 2005). Furthermore, lipomannan induces granuloma macrophage fusion in an in vitro granuloma model in a TLR2-dependent way (Puissegur et al., 2007).
In the group of PIMs, both Ac1/Ac2PIM2 and Ac1/Ac2PIM6 have been reported to signal via TLR2, irrespective of their acylation pattern (Jones et al., 2001; Gilleron et al., 2003;). Further, in two studies, cellular activation via TLR2 by non-mannose-capped lipoarabinomannan (PI-LAM/Ara-LAM) from rapidly growing species has been observed (Means et al., 1999; Underhill et al., 1999;), but not for M. tuberculosis or BCG-derived Man-LAM. In addition, an inflammatory response induced by PI-LAM from M. smegmatis in mice appeared to be TLR2 dependent (Wieland et al., 2004). However, in a comparative study of all lipoglycans, Ac1/Ac2PIM2, PI-LAM and Ara-LAM were shown to be poor inducers of TLR2 signaling as compared with lipomannan and Ac1/Ac2PIM6 (Nigou et al., 2008). This is consistent with an earlier study showing that in contrast to lipomannan, neither Ara-LAM from M. chelonae nor Man-LAM and Ac1/Ac2PIM2 from Mycobacterium kansasii mediate TLR2-dependent activation (Vignal et al., 2003). Moreover, chemical degradation of the arabinan domain of Man-LAM from M. kansasii restored its ability to induce cytokine secretion via TLR2, which suggests that the arabinan domain prevents the proper interaction of Man-LAM with TLR2 (Vignal et al., 2003). This was confirmed by a recent study by Birch et al. (2010) in which lipoarabinomannan containing a truncated arabinan domain from an M. smegmatis AftC knockout mutant showed enhanced TLR2 signaling as compared with wild-type lipoarabinomannan. The positive effects of lipoarabinomannan in the earlier reports could be due to contamination of the lipoarabinomannan extract with lipopeptides (Nigou et al., 2008; Zahringer et al., 2008; Geurtsen et al., 2009; Birch et al., 2010;). Overall, the data indicate that lipomannan, and in a minor respect PIM6, are the only significant TLR2 ligands from this group of mycobacterial lipoglycans.
For TLR4, only a few mycobacterial lipoglycans have been reported as ligands. Using nonactivated Mϕ, only tri- and tetra-acylated lipomannan from M. tuberculosis and M. bovis BCG, respectively, show signaling via TLR4 (Gilleron et al., 2006; Doz et al., 2007;). In contrast to TLR2, which can only signal by the recruitment of adaptor proteins, Myeloid differentiation primary response gene (MyD)-88 and Toll-interleukin 1 receptor (TIR) domain-containing adapter protein (TIRAP), TLR4 can also signal via TIR-domain-containing adapter-inducing interferon-β and translocating chain-associating membrane protein (Akira & Takeda, 2004; Jo, 2008;). The secretion of proinflammatory cytokines, by Mϕ stimulated with lipomannan, is strongly dependent on the MyD88/TIRAP pathway (Doz et al., 2007). Most probably, cell wall-associated or soluble factors other than lipoglycans [e.g. heat shock proteins (Bulut et al., 2005)] stimulate TLR4 signaling in M. tuberculosis infection; while both live and ‘heat-killed’M. tuberculosis activate cells via TLR2, only live M. tuberculosis induces TLR4-dependent activation excluding a major role for the heat-stable glycolipids (Means et al., 1999). In Table 2, an overview is given of all TLR signaling observed for lipomannan using TLR1/2/4/6 knockout cells or antibodies against specific TLRs.
Table 2.
Overview of reports on the activation of TLR2 by LM
| LM type | Cells | Dependence shown for | Effect | References |
|---|---|---|---|---|
| Mc-LM, Mk-LM | THP-1 Mϕ | TLR2+CD14 | Release TNF and IL-8 | Vignal et al. (2003) |
| Mc-LM, Mk-LM, BCG-LM | BMDMϕ | TLR2 | Release TNF and NO | Quesniaux et al. (2004b) |
| Mc-LM, Mk-LM, BCG-LM | THP-1 Mϕ | TLR2 | expression MMP-9 | Elass et al. (2005) |
| BCG-LM | THP-1, BMDMϕ | TLR1/2 | Release TNF | Gilleron et al. (2006) |
| BCG-Ac1LM | THP-1, BMDMϕ | TLR1/2, TLR4 | Release TNF | |
| Mc-LM, Mk-LM, Ms-LM, Mtb-LM | human PBMC | TLR2 | Mϕ fusion into MGC in in vitro granuloma | Puissegur et al. (2007) |
| BCG-Ac1LM | BMDMϕ | TLR2, TLR4 | Release TNF and NO | Doz et al. (2007) |
| BCG-Ac2LM | BMDMϕ | TLR4 | Release TNF and NO | |
| Mtb-Ac1(2)LM | BMDMϕ | TLR4 | Release TNF | |
| Mtb-LM, BCG-LM | HEK/CD14/TLR2, THP-1 Mϕ | TLR1/2+CD14 | Activation NF-κB | Nigou et al. (2008) |
Ms, Mycobacterium smegmatis; Mk, Mycobacterium kansasii; Mc, Mycobacterium chelonae; Mtb, Mycobacterium tuberculosis; BCG, Mycobacterium bovis BCG; Ac1/Ac2LM, tri-/tetra-acylated LM (note: in some articles written as Ac3/Ac4LM); BMDMϕ, bone marrow-derived macrophages; PBMC, peripheral blood mononuclear cells; MGC, multinucleated giant cells.
A few studies report on an immunosuppressive effect of lipomannan, Ac1LM and PIMs on lipopolysaccharide-activated Mϕ (Quesniaux et al., 2004b; Doz et al., 2007, 2009). These inhibitory effects were independent of their signaling via TLR2, which partially compensated the decrease of proinflammatory cytokine secretion (Quesniaux et al., 2004b; Doz et al., 2007;). Which receptor-lipoglycan ligation is then responsible for the immunosupression is not yet known, but it is unlikely to be the MMR (Doz et al., 2007): in contrast to the higher-order PIMs, the MMR recognizes lipomannan and Ac1/Ac2PIM2 only with low affinity (Torrelles et al., 2006), while they show similar inhibitory effects on lipopolysaccharide-activated Mϕ (Doz et al., 2009). Importantly, neither lyso-PIM (monoacylated PIM) nor PI were inhibitory in this assay, indicating the requirement of mannosyl moiety and a diacylated MPI anchor (Doz et al., 2009). Of note, in all these experiments, lipopolysaccharide was used, which is a major ligand for TLR4. PIMs might interfere in the interaction between lipopolysaccharide and TLR4, thereby causing its inhibitory effect, but this seems unlikely (Doz et al., 2009). However, lipopolysaccharide is a nonmycobacterial ligand, which makes this assay system rather artificial for the comprehension of TLR-dependent immunomodulation by PIMs and related lipoglycans during mycobacterial infection. Finally, the immunomodulatory properties of Man-LAM on both lipopolysaccharide-activated Mϕ and DCs have been described as well (Geijtenbeek et al., 2003; Pathak et al., 2005;). These effects of Man-LAM can be attributed to its binding by the MMR and DC-SIGN, respectively, which will be discussed in the next sections.
In vivo studies using TLR knockout mice are not fully in agreement on whether TLRs are crucial for mycobacterial clearance and host defense (Drennan et al., 2004; Ferwerda et al., 2005;) or not (Sugawara et al., 2003a,b; Nicolle et al., 2004; Holscher et al., 2008;). Importantly, TLR signaling via the MyD88 pathway has been shown in multiple studies to be dispensable for the induction of an efficient adaptive immune response (Fremond et al., 2004; Nicolle et al., 2004; Ryffel et al., 2005; Holscher et al., 2008;). A critical role for TLRs then has been hypothesized in the early recognition and killing of mycobacteria (Quesniaux et al., 2004a; Ryffel et al., 2005; Jo, 2008;). However, this innate immune response mainly depends on MyD88 and may also involve a MyD88-mediated pathway different from via TLR (Feng et al., 2003; Holscher et al., 2008;), which is the type I interleukin-1 receptor (IL-1R1)-mediated signaling (Fremond et al., 2007; Reiling et al., 2008;). Alternatively, TLR2 activation may have a second function as a regulator by preventing an exaggerated inflammatory immune response in a later stage of mycobacterial infection (Drennan et al., 2004; Sutmuller et al., 2006; Jo, 2008; Harding & Boom, 2010;). Pattern recognition by multiple TLRs does contribute to the control of mycobacterial infection (Bafica et al., 2005; Ryffel et al., 2005; Jo et al., 2007;), but may play a less significant role than originally assigned (Reiling et al., 2008).
DC-SIGN
In contrast to the interaction between mycobacteria and Mϕ, which can be mediated by several different receptors (e.g. complement receptors, CD14, MMR, scavenger receptor) (Ernst, 1998), binding of mycobacteria to DCs is for the largest part mediated by DC-SIGN (CD209) (Tailleux et al., 2003). DC-SIGN is a type II transmembrane tetrameric C-type lectin containing one carbohydrate recognition domain per monomer. It was originally hypothesized that via interaction with DC-SIGN, mycobacteria modulate the immune response and thereby escape immune surveillance (Geijtenbeek et al., 2003; van Kooyk & Geijtenbeek, 2003;). However, the exact role of DC-SIGN in tuberculosis infection has not yet been clarified and is still under debate (Neyrolles et al., 2006; Ehlers, 2009; Tanne & Neyrolles, 2010;). Studies on the relation between the level of expression of DC-SIGN in human populations and susceptibility to tuberculosis are contradicting each other, varying from a protective effect to increased susceptibility to tuberculosis by high DC-SIGN expression or no correlation at all (Barreiro et al., 2006; Gomez et al., 2006; Ben-Ali et al., 2007; Vannberg et al., 2008;).
In a binding study using a DC-SIGN-expressing cell line, the lectin showed a high preference for the Mycobacterium spp. from the tuberculosis complex, while it bound little or not to strains from outside the complex (Pitarque et al., 2005). On the basis of differences in the mannose-capping degree of lipoarabinomannan between these species and the fact that DC-SIGN only recognizes lipoarabinomannan if it is mannose-capped, it was expected that Man-LAM would establish the interaction between mycobacteria and DC-SIGN as present on DCs (Geijtenbeek et al., 2003; Maeda et al., 2003;). Furthermore, DC-SIGN has a high-affinity α(1→2)-linked Manp residue, which increases with chain length (Koppel et al., 2004). The predominant mannose cap on Man-LAM of M. tuberculosis and M. bovis BCG consists of α(1→2)-linked dimannosides, and α(1→2)-linked trimannosides are also present (Pitarque et al., 2005). Surprisingly, an M. bovis BCG mutant, which only produces lipoarabinomannan without mannose cap, did not bind less to DC-SIGN and DCs as compared with wild-type BCG (Appelmelk et al., 2008); thus, ligands for DC-SIGN other than Man-LAM must be present in the cell wall.
Next to Man-LAM, PIMs have been examined for their binding to DC-SIGN as well (Torrelles et al., 2006; Driessen et al., 2009;). In particular, the higher-order PIMs (PIM5 and PIM6), with terminal α(1→2)-linked Manp residues reminiscent of the mannose cap of Man-LAM, were of interest. As expected, DC-SIGN recognizes the higher-order PIMs with high affinity as compared with the lower-order PIMs (Boonyarattanakalin et al., 2008; Driessen et al., 2009;). Besides PIMs, other potential ligands in the mycobacterial cell wall for DC-SIGN have been identified: lipomannan, Man-AM and mannosylated lipoproteins 19 and 45 kDa (Pitarque et al., 2005), and more recently, capsular α-glucan (Geurtsen et al., 2009) (a list is provided in Table 3). All these compounds have been shown to bind DC-SIGN and/or inhibit binding of M. tuberculosis to DC-SIGN. This suggests that binding of mycobacteria to DC-SIGN and DCs is not mediated by one dominant ligand, but that there is a redundancy in ligands for DC-SIGN present on the cell surface (Pitarque et al., 2005). This was supported by the observation that even a double knockout M. bovis BCG strain that neither produces the mannose cap on lipoarabinomannan nor higher-order PIMs did not show any reduction in binding to DC-SIGN and DCs as compared with its parent strain (Driessen et al., 2009). Moreover, this mutant strain did not induce an altered cytokine profile in DCs (Driessen et al., 2009). All of the reported ligands for DC-SIGN can be found in DC-SIGN nonbinding Mycobacterium spp. as well. Hence, other factors probably determine the binding to DC-SIGN instead of only the presence or the absence of one or a few ligands. The affinity of DC-SIGN for specific Mycobacterium spp. may be related to differences in the mannosylation pattern of potential ligands, the relative abundance of various compounds at the cell surface (in particular, α(1→2)-linked Manp-containing compounds), cell wall structure and/or capsule composition. Finally, not all potential ligands, when cell wall bound, may be accessible to DC-SIGN and thus of equal importance for the binding of mycobacteria to DC-SIGN.
Table 3.
Overview of reported mycobacterial ligands for DC-SIGN
| Mycobacterial ligands for DC-SIGN | Discussed in |
|---|---|
| ManLAM | Geijtenbeek et al. (2003); Pitarque et al. (2005); Appelmelk et al. (2008) |
| ManAM | Pitarque et al. (2005) |
| LM | Pitarque et al. (2005) |
| (higher-order) PIMs | Torrelles et al. (2006); Boonyarattanakalin et al. (2008); Driessen et al. (2009) |
| 19- and 45-kDa antigen* | Pitarque et al. (2005) |
| Mannosylated proteins† | Driessen et al. (2009) |
| Capsular α-glucan | Geurtsen et al. (2009) |
Mannosylated lipoprotein (19 kDa) and mannosylated glycoprotein (45 kDa).
Unidentified proteins from Mycobacterium bovis BCG whole-cell lysates probed SDS-PAGE/immunoblot with a DC-SIGN-Fc construct. Note: a ConA affinity capture assay identified >30 putative mannosylated proteins in Mycobacterium tuberculosis culture filtrate (Gonzalez-Zamorano et al., 2009), which constitute potential ligands for DC-SIGN.
To investigate the DC-SIGN-dependent modulation of the immune response by mycobacteria or their mannosylated lipoglycans, several in vitro and in vivo studies have been performed. Purified mycobacterial Man-LAM, not Ara-LAM, inhibits both lipopolysaccharide- as well as BCG-induced maturation of DCs by reducing the expression of MHC class II and costimulatory molecules (CD80, CD83 and CD86) as compared with untreated lipopolysaccharide- or BCG-activated cells (Geijtenbeek et al., 2003). Furthermore, Man-LAM has been shown to induce the secretion of anti-inflammatory IL-10 in lipopolysaccharide-activated DCs (Geijtenbeek et al., 2003). Ligation of Man-LAM to DC-SIGN appears to interfere with lipopolysaccharide signaling via TLR4 by the activation of serine-threonine kinase Raf-1, which subsequently leads to acetylation of NF-κB subunit p65. Acylation of p65 prolongs and enhances the transcription of IL10, thereby increasing IL-10 production (Gringhuis et al., 2007). Together with a study on mice lacking SIGN receptor (SIGNR)-1, a murine homolog for DC-SIGN, which displayed a stronger T helper 1 response upon M. tuberculosis infection and a reduced level of IL-10 production (Wieland et al., 2007), this led to the initial thought that the interaction with DC-SIGN is beneficial for mycobacteria.
Recently, it has been shown that Man-LAM alters the cytokine profile in lipopolysaccharide-activated DCs by increasing the secretion of not only IL-10, but also of proinflammatory IL-12 and IL-6 (Gringhuis et al., 2009). Binding of Man-LAM or mannose-containing pathogens like M. tuberculosis induces the recruitment of effector proteins to a DC-SIGN signalosome, which is required for the activation of Raf-1, followed by enhancement of the transcription of the genes encoding IL-12 and IL-6 in a similar manner as for IL-10 (Gringhuis et al., 2009). Noteworthy, lipopolysaccharide is a nonmycobacterial ligand and both Man-LAM (Geijtenbeek et al., 2003) as well as intact mycobacteria (Driessen et al., 2009) do not induce IL-10 secretion in nonactivated DCs. In the context of mycobacterial infection, further research on interference of DC-SIGN signaling induced by Man-LAM with signaling via TLR2 by for example BCG-Ac1LM or the 19 kDa antigen would be of interest.
A study using mouse DCs showed a higher induction of suppressor of cytokine signaling-1 expression by SIGNR1 stimulation as compared with signaling via TLR2 (Srivastava et al., 2009). It is important to mention that seven murine homologs for human DC-SIGN are known. Each homolog differs in certain properties from the human DC-SIGN, among which are the specific mannose- and fucose-structures it recognizes, and this may alter their functions significantly (Powlesland et al., 2006; Tanne et al., 2009;). Strikingly, human DC-SIGN transgenic mice exhibited decreased pathology and prolonged survival during mycobacterial infection (Schaefer et al., 2008). A clear protective role has recently also been shown for the murine ortholog SIGNR3 (Tanne et al., 2009). Resistance to M. tuberculosis infection was impaired in SIGNR3-deficient mice, but not in mice lacking SIGNR1 and SIGNR5 (Tanne et al., 2009).
DC-SIGN specifically recognizes the pathogenic Mycobacterium spp. from the tuberculosis complex (Pitarque et al., 2005). By expressing many ligands for DC-SIGN, DC-SIGN is the most important uptake receptor for mycobacteria to infect DCs. However, concluding from these last studies, DC-SIGN-binding appeared not to be mainly beneficial for the pathogens, and DC-SIGN may function primarily in the protection of the host. How DC-SIGN signaling exactly optimizes the immune response against mycobacteria, i.e. by strengthening the proinflammatory response or by preventing excessive inflammation, remains to be established (Ehlers, 2009) (Fig. 7).
Fig. 7.
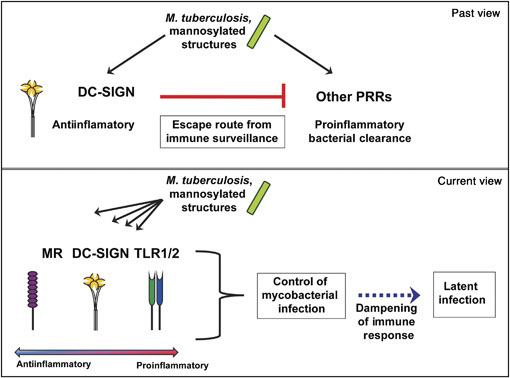
The role of DC-SIGN and other PRRs in the immune response against mycobacterial infection. Previously, it was hypothesized that binding to C-type lectin DC-SIGN forms an escape route for mycobacteria to immune surveillance by interfering with signaling via other PRRs. Currently, signaling via PRRs such as DC-SIGN, MMR and TLR2 has been suggested to have a dual function, with both a role in bacterial clearance or induction of proinflammatory cytokines, as well as in preventing an exaggerated immune response. While their main function is then in the protection of the host, Mycobacterium tuberculosis benefits from binding to or signaling via these PRRs as well, which may promote latent mycobacterial infection instead of complete bacterial clearance.
Mannose receptor
The MMR (CD206) is a type I transmembrane monomeric C-type lectin with eight carbohydrate recognition domains. In addition to the complement receptors, the MMR mediates for a large part in the phagocytic uptake of mycobacteria by Mϕ (Schlesinger, 1993). The MMR recognizes virulent M. tuberculosis strains Erdman and H37Rv, but not avirulent H37Ra, which has been suggested to be caused by subtle structural differences in Man-LAM (Schlesinger, 1993; Schlesinger et al., 1996;). Furthermore, the MMR also shows a low affinity for nontuberculosis mycobacteria M. smegmatis, M. phlei and M. kansassi, of which the first two species only bear lipoarabinomannan without a mannose cap (Astarie-Dequeker et al., 1999). Although the presence of a mannose cap on Man-LAM is essential for the recognition of lipoarabinomannan by the MMR (Schlesinger et al., 1994), it does not completely explain the differences in MMR-mediated uptake by Mϕ between the Mycobacterium spp. (Schlesinger et al., 1996; Astarie-Dequeker et al., 1999; Appelmelk et al., 2008;). Next to the recognition of the mannose cap on Man-LAM, the MMR furthers binds higher-order PIM5 and PIM6 with a preference of the triacylated species above the tetra-acylated ones, and glycopeptidolipids, but not to lower-order PIM2, lipomannan and Ara-LAM or PI-LAM (Schlesinger et al., 1994; Villeneuve et al., 2005; Torrelles et al., 2006;). Because of their structural similarities, mannosylated proteins and (phosphorylated) arabinomannans and mannan may be involved as well (Ortalo-Magnéet al., 1995; Dobos et al., 1996; Maes et al., 2007; Torrelles et al., 2008a;). Man-LAM and related lipoglycans all contribute to the MMR-mediated phagocytosis of mycobacteria (Villeneuve et al., 2005; Torrelles et al., 2008b;). Overexpression of Mt-ManB in M. smegmatis results in the overproduction of PIMs, lipomannan and lipoarabinomannan. Subsequent increased association with Mϕ is probably due to the higher amount of higher-order PIMs, as lipoarabinomannan from M. smegmatis does not have a mannose cap (McCarthy et al., 2005).
A role for the MMR has been implicated in many of the immunomodulatory effects by Man-LAM and the related glycans described above. Next to uptake of mycobacteria by Mϕ via the MMR, the MMR is also involved in the transport of glycolipids to uninfected bystanders cells and intracellular trafficking to the late endosomal compartments (Prigozy et al., 1997). However, in DCs, uptake of both mycobacteria as well as of the glycolipids is performed mainly by DC-SIGN, leaving a minor role for the MMR (Schaible et al., 2000; Geijtenbeek et al., 2003; Driessen et al., 2009;). Binding of Man-LAM to the MMR has been further linked to the delay of phagosome maturation (described in more detail in the corresponding section above) (Kang et al., 2005), the induction of MMP-9 by M. tuberculosis and Man-LAM similar to that observed for TLR2 activation by lipomannan (Rivera-Marrero et al., 2002) and the expansion of regulatory T cells (Tregs) (Garg et al., 2008). Furthermore, DCs from MMR knockout mice secrete more IL-12p40 upon infection with M. tuberculosis as compared with DCs from wild-type mice (Ehlers, 2009). Thus, the MMR seems to have an immunosuppressive function on DCs, which was earlier shown by cross-linking the MMR on DCs with anti-MMR antibodies and several natural ligands (Chieppa et al., 2003). Finally, in Mϕ, Man-LAM suppresses lipopolysaccharide-induced IL-12p40 secretion via the induction of IL-1 receptor-associated kinase (IRAK)-M expression (Pathak et al., 2005). IRAK-M negatively regulates TLR signaling (Kobayashi et al., 2002; Jo et al., 2007;) and its induction is probably triggered by binding of Man-LAM to the mannose receptor (Pathak et al., 2005).
Concluding, the MMR signaling may induce an anti-inflammatory response. A study by Torrelles et al. (2008b) reported, for clinical isolates of M. tuberculosis (HN885 and HN1554) with both truncated Man-LAM and a reduced amount of higher-order PIMs, a significant decrease in association with the MMR as compared with the Erdman strain, while uptake via the CR was not altered. Phagocytosis via the MMR may shape the nature of the immune response, and it was suggested that due to its immunosuppressive activity, uptake via the MMR may lead to a latent infection instead of active disease (Torrelles et al., 2008b). Related to this, an M. tuberculosis pimB-mutant strain (Rv0557/mgtA), which expresses less lipomannan and Man-LAM in its cell wall, showed an increased rate of macrophage death upon infection as compared with its parent strain (Torrelles et al., 2009). This does not necessarily mean increased virulence, but may even be advantageous for the host as the more virulent strains have been reported to inhibit apoptosis (Torrelles et al., 2009). Although the pimB-mutant phenotype could not be linked to reduced association with the MMR, the presence of high amounts of PIMs, lipomannan and Man-LAM results in a less inflammatory immune response and indicates adaptation of the mycobacteria to their host (Torrelles et al., 2009; Torrelles & Schlesinger, 2010;) (Fig. 7).
Concluding remarks
The entire repertoire of enzymes and genes involved in the biogenesis of lipoarabinomannan and related glycoconjugates has almost been identified. However, unlike C. glutamicum, many of the genes involved in biosynthesis of these molecules in M. tuberculosis are essential for its survival (Sassetti et al., 2003; Kaur et al., 2009; Guerin et al., 2010; G.S. Besra, unpublished data) like its cousin M. smegmatis (Jackson et al., 2000; Kordulakova et al., 2002; Guerin et al., 2009; Skovierova et al., 2009;), and therefore represent excellent drug targets. Furthermore, the identification of enzymatic machinery is now leading to the biophysical analysis of the three-dimensional structures of these enzymes and the identification of inhibitors, which may develop into novel drugs against tuberculosis. In addition, very little is known about the regulation of the biosynthesis of these glycoconjugates and their respective presentation on the mycobacterial cell wall; such studies may lead to the identification of few more interesting and potential targets. To date, serine–threonine kinases have been suggested in the regulation process of mycobacterial glycosyltransferases (Alderwick et al., 2006b; Molle & Kremer, 2010;). However, further studies are required to unravel the detailed mechanism of regulation in terms of the species, composition, heterogeneity, size and length of these polymers.
PIMs, lipomannan and Man-LAM all display several immunomodulatory properties by interaction with different receptors of the immune system. Their activity depends on their degree of acylation and mannosylation. While lipomannan is mainly associated with TLR signalling, the higher-order PIMs and Man-LAM are recognized by the C-type lectins DC-SIGN and the MMR. Interestingly, alveolar Mϕ constitutively express the MMR (Wileman et al., 1986), but DC-SIGN expression is only induced in alveolar Mϕ in the lungs of tuberculosis-infected patients (Tailleux et al., 2005), which may influence the disease pattern in an important, but yet to be determined way. Both C-type lectins show comparable recognition specificities for the mannosylated glycolipids, and as receptors CD14 (Pugin et al., 1994) and pulmonary surfactant protein (SP)-A (Sidobre et al., 2000) and SP-D (Ferguson et al., 1999, 2006) also bind Man-LAM, the interactions of mycobacteria with all these PRRs may enhance or dampen inflammatory signals and thereby determine the nature of the immune response (Ernst, 1998; Torrelles et al., 2008a;). Anti-inflammatory signaling via the interaction of the glycolipids with the host immune system can be regarded as strategies of the mycobacteria to escape immune surveillance (Torrelles & Schlesinger, 2010), but may also be vital in the prevention of an exaggerated inflammatory response (Jo, 2008). As only 5–10% of the tuberculosis-infected persons develop the active disease, the glycolipids may play a crucial role in host adaptation of the mycobacteria and hence, a balanced immune response resulting in a latent tuberculosis infection (Fig. 7).
Acknowledgments
G.S.B. acknowledges support in the form of a Personal Research Chair from Mr James Bardrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Research Council and The Wellcome Trust (081569/Z/06/Z). The authors thank Drs Apoorva Bhatt and Jeroen Geurtsen for critically reading the manuscript.
Authors' contribution
A.K.M. and N.N.D. contributed equally to this work.
Statement
Re-use of this article is permitted in accordance with the Terms and Conditions set out at http://wileyonlinelibrary.com/onlineopen#OnlineOpen-Terms
References
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Alderwick LJ, Radmacher E, Seidel M, Gande R, Hitchen PG, Morris HR, Dell A, Sahm H, Eggeling L, Besra GS. Deletion of Cg-emb in Corynebacterineae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J Biol Chem. 2005;280:32362–32371. doi: 10.1074/jbc.M506339200. [DOI] [PubMed] [Google Scholar]
- Alderwick LJ, Dover LG, Seidel M, Gande R, Sahm H, Eggeling L, Besra GS. Arabinan-deficient mutants of Corynebacterium glutamicum and the consequent flux in decaprenylmonophosphoryl-d-arabinose metabolism. Glycobiology. 2006a;16:1073–1081. doi: 10.1093/glycob/cwl030. [DOI] [PubMed] [Google Scholar]
- Alderwick LJ, Molle V, Kremer L, Cozzone AJ, Dafforn TR, Besra GS, Futterer K. Molecular structure of EmbR, a response element of Ser/Thr kinase signaling in Mycobacterium tuberculosis. P Natl Acad Sci USA. 2006b;103:2558–2563. doi: 10.1073/pnas.0507766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderwick LJ, Seidel M, Sahm H, Besra GS, Eggeling L. Identification of a novel arabinofuranosyltransferase (AftA) involved in cell wall arabinan biosynthesis in Mycobacterium tuberculosis. J Biol Chem. 2006c;281:15653–15661. doi: 10.1074/jbc.M600045200. [DOI] [PubMed] [Google Scholar]
- Alderwick LJ, Lloyd GS, Lloyd AJ, Lovering AL, Eggeling L, Besra GS. Biochemical characterization of the Mycobacterium tuberculosis phosphoribosyl-1-pyrophosphate synthetase. Glycobiology. 2010;21:410–425. doi: 10.1093/glycob/cwq173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderwick LJ, Lloyd GS, Ghadbane H, May JW, Bhatt A, Eggeling L, Fütterer K, Besra GS. The C-terminal domain of the arabinosyltransferase Mycobacterium tuberculosis EmbC is a lectin-like carbohydrate binding module. PLoS Pathog. 2011;7:e1001299. doi: 10.1371/journal.ppat.1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberson JB, McMahon BT, Pinner M. A clinical trial of sanocrysin in pulmonary tuberculosis. Am Rev Tuberc. 1931;24:401–435. [Google Scholar]
- Appelmelk BJ, den Dunnen J, Driessen NN, et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium–host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarie-Dequeker C, N'Diaye EN, Le Cabec V, Rittig MG, Prandi J, Maridonneau-Parini I. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect Immun. 1999;67:469–477. doi: 10.1128/iai.67.2.469-477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhawat N, Mande SC. Identification of the INO1 gene of Mycobacterium tuberculosis H37Rv reveals a novel class of inositol-1-phosphate synthase enzyme. J Mol Biol. 1999;291:531–536. doi: 10.1006/jmbi.1999.2980. [DOI] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa L, Romero MM, Yokobori N, et al. Mycobacterium tuberculosis impairs dendritic cell response by altering CD1b DC-SIGN and MR profile. Immunol Cell Biol. 2010;88:716–726. doi: 10.1038/icb.2010.22. [DOI] [PubMed] [Google Scholar]
- Ballou CE, Lee YC. The structure of a myo-inositol mannoside from Mycobacterium tuberculosis Glycolipid. Biochemistry. 1964;3:682–685. doi: 10.1021/bi00893a014. [DOI] [PubMed] [Google Scholar]
- Ballou CE, Vilkas E, Lederer E. Structural studies on the myo-inositol phospholipids of Mycobacterium tuberculosis (var. bovis, strain BCG) J Biol Chem. 1963;238:69–76. [PubMed] [Google Scholar]
- Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7:929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- Barreiro LB, Neyrolles O, Babb CL, et al. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Coin L, Durbin R, et al. The Pfam protein families database. Nucleic Acids Res. 2004;32:D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt SM, Jabeen T, Mishra AK, Veerapen N, Krumbach K, Eggeling L, Besra GS, Fütterer K. Acceptor substrate discrimination in phosphatidyl-myo-inositol mannoside synthesis: structural and mutational analysis of mannosyltransferase Corynebacterium glutamicum PimB'. J Biol Chem. 2010;285:37741–37752. doi: 10.1074/jbc.M110.165407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ali M, Barreiro LB, Chabbou A, et al. Promoter and neck region length variation of DC-SIGN is not associated with susceptibility to tuberculosis in Tunisian patients. Hum Immunol. 2007;68:908–912. doi: 10.1016/j.humimm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Benaroch P, Yilla M, Raposo G, Ito K, Miwa K, Geuze HJ, Ploegh HL. How MHC class II molecules reach the endocytic pathway. EMBO J. 1995;14:37–49. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg S, Kaur D, Jackson M, Brennan PJ. The glycosyltransferases of Mycobacterium tuberculosis-roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology. 2007;17:35–56R. doi: 10.1093/glycob/cwm010. [DOI] [PubMed] [Google Scholar]
- Besra GS, Brennan PJ. The mycobacterial cell wall: biosynthesis of arabinogalactan and lipoarabinomannan. Biochem Soc T. 1997;25:845–850. doi: 10.1042/bst0250845. [DOI] [PubMed] [Google Scholar]
- Besra GS, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. A new interpretation of the structure of the mycolyl-arabinogalactan complex of Mycobacterium tuberculosis as revealed through characterization of oligoglycosylalditol fragments by fast-atom bombardment mass spectrometry and 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1995;34:4257–4266. doi: 10.1021/bi00013a015. [DOI] [PubMed] [Google Scholar]
- Besra GS, Morehouse CB, Rittner CM, Waechter CJ, Brennan PJ. Biosynthesis of mycobacterial lipoarabinomannan. J Biol Chem. 1997;272:18460–18466. doi: 10.1074/jbc.272.29.18460. [DOI] [PubMed] [Google Scholar]
- Bhatt A, Molle V, Besra GS, Jacobs WR, Jr, Kremer L. The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Mol Microbiol. 2007;64:1442–1454. doi: 10.1111/j.1365-2958.2007.05761.x. [DOI] [PubMed] [Google Scholar]
- Bhowruth V, Dover LG, Besra GS. Tuberculosis chemotherapy: recent developments and future perspectives. Progr Med Chem. 2007;45:169–203. doi: 10.1016/S0079-6468(06)45504-1. [DOI] [PubMed] [Google Scholar]
- Birch HL, Alderwick LJ, Bhatt A, Rittmann D, Krumbach K, Singh A, Bai Y, Lowary TL, Eggeling L, Besra GS. Biosynthesis of mycobacterial arabinogalactan: identification of a novel α(1→3) arabinofuranosyltransferase. Mol Microbiol. 2008;69:1191–1206. doi: 10.1111/j.1365-2958.2008.06354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch HL, Alderwick LJ, Appelmelk BJ, Maaskant J, Bhatt A, Singh A, Nigou J, Eggeling L, Geurtsen J, Besra GS. A truncated lipoglycan from mycobacteria with altered immunological properties. P Natl Acad Sci USA. 2010;107:2634–2639. doi: 10.1073/pnas.0915082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyarattanakalin S, Liu X, Michieletti M, Lepenies B, Seeberger PH. Chemical synthesis of all phosphatidylinositol mannoside (PIM) glycans from Mycobacterium tuberculosis. J Am Chem Soc. 2008;130:16791–16799. doi: 10.1021/ja806283e. [DOI] [PubMed] [Google Scholar]
- Brennan P, Ballou CE. Biosynthesis of mannophosphoinositides by Mycobacterium phlei. The family of dimannophosphoinositides. J Biol Chem. 1967;242:3046–3056. [PubMed] [Google Scholar]
- Brennan P, Ballou CE. Biosynthesis of mannophosphoinositides by Mycobacterium phlei. Enzymatic acylation of the dimannophosphoinositides. J Biol Chem. 1968a;243:2975–2984. [PubMed] [Google Scholar]
- Brennan P, Ballou CE. Phosphatidylmyoinositol monomannoside in Propionibacterium shermanii. Biochem Bioph Res Co. 1968b;30:69–75. doi: 10.1016/0006-291x(68)90714-6. [DOI] [PubMed] [Google Scholar]
- Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 2003;83:91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Crick DC. The cell-wall core of Mycobacterium tuberculosis in the context of drug discovery. Curr Top Med Chem. 2007;7:475–488. doi: 10.2174/156802607780059763. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Lehane DP. The phospholipids of corynebacteria. Lipids. 1971;6:401–409. doi: 10.1007/BF02531377. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- Brown AK, Meng G, Ghadbane H, Scott DJ, Dover LG, Nigou J, Besra GS, Fütterer K. Dimerization of inositol monophosphatase Mycobacterium tuberculosis SuhB is not constitutive, but induced by binding of the activator Mg2+ BMC Struct Biol. 2007;28:55. doi: 10.1186/1472-6807-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 2005;280:20961–20967. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Chan ED, Iseman MD. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis. 2008;21:587–595. doi: 10.1097/QCO.0b013e328319bce6. [DOI] [PubMed] [Google Scholar]
- Chargaff E, Schaefer W. A specific polysaccharide from the Bacillus Calmette-Guerin (BCG) J Biol Chem. 1935;112:393–405. [Google Scholar]
- Chatterjee D, Khoo KH. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Bozic CM, McNeil M, Brennan PJ. Structural features of the arabinan component of the lipoarabinomannan of Mycobacterium tuberculosis. J Biol Chem. 1991;266:9652–9660. [PubMed] [Google Scholar]
- Chatterjee D, Hunter SW, McNeil M, Brennan PJ. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J Biol Chem. 1992a;267:6228–6233. [PubMed] [Google Scholar]
- Chatterjee D, Lowell K, Rivoire B, McNeil MR, Brennan PJ. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem. 1992b;267:6234–6239. [PubMed] [Google Scholar]
- Chatterjee D, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology. 1993;3:497–506. doi: 10.1093/glycob/3.5.497. [DOI] [PubMed] [Google Scholar]
- Chieppa M, Bianchi G, Doni A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- Christophe T, Jackson M, Jeon HK, et al. High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog. 2009;5:e1000645. doi: 10.1371/journal.ppat.1000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua J, Deretic V. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J Biol Chem. 2004;279:36982–36992. doi: 10.1074/jbc.M405082200. [DOI] [PubMed] [Google Scholar]
- Clemens DL, Horwitz MA. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med. 1996;184:1349–1355. doi: 10.1084/jem.184.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ST, Barrell BG. Analysis of the genome of Mycobacterium tuberculosis H37Rv. Novart Fdn Symp. 1998;217:160–172. discussion 172–177. [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Crellin PK, Kovacevic S, Martin KL, Brammananth R, Morita YS, Billman-Jacobe H, McConville MJ, Coppel RL. Mutations in pimE restore lipoarabinomannan synthesis and growth in a Mycobacterium smegmatislpqW mutant. J Bacteriol. 2008;190:3690–3699. doi: 10.1128/JB.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo PM, Torres Demichelis V, Daniotti JL. Neobiosynthesis of glycosphingolipids by plasma membrane-associated glycosyltransferases. J Biol Chem. 2010;285:29179–29190. doi: 10.1074/jbc.M110.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffé M, Etienne G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tubercle Lung Dis. 1999;79:153–169. doi: 10.1054/tuld.1998.0200. [DOI] [PubMed] [Google Scholar]
- Daffé M, Brennan PJ, McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J Biol Chem. 1990;265:6734–6743. [PubMed] [Google Scholar]
- de la Salle H, Mariotti S, Angenieux C, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- de Libero G, Mori L. How the immune system detects lipid antigens. Prog Lipid Res. 2009;42:120–127. doi: 10.1016/j.plipres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Delmas C, Gilleron M, Brando T, Vercellone A, Gheorghui M, Riviere M, Puzo G. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology. 1997;7:811–817. doi: 10.1093/glycob/7.6.811. [DOI] [PubMed] [Google Scholar]
- Dinadayala P, Kaur D, Berg S, Amin AG, Vissa VD, Chatterjee D, Brennan PJ, Crick DC. Genetic basis for the synthesis of the immune-modulatory mannose caps of lipoarabinomannan in Mycobacterium tuberculosis. J Biol Chem. 2006;281:20027–20035. doi: 10.1074/jbc.M603395200. [DOI] [PubMed] [Google Scholar]
- Dobos KM, Khoo KH, Swiderek KM, Brennan PJ, Belisle JT. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. J Bacteriol. 1996;178:2498–2506. doi: 10.1128/jb.178.9.2498-2506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover LG, Cerdeno-Tarraga AM, Pallen MJ, Parkhill J, Besra GS. Comparative cell wall core biosynthesis in the mycolated pathogens, Mycobacterium tuberculosis and Corynebacterium diphtheriae. FEMS Microbiol Rev. 2004;28:225–250. doi: 10.1016/j.femsre.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Dover LG, Bhatt A, Bhowruth V, Willcox BE, Besra GS. New drugs and vaccines for drug-resistant Mycobacterium tuberculosis infections. Expert Rev Vaccines. 2008;7:481–497. doi: 10.1586/14760584.7.4.481. [DOI] [PubMed] [Google Scholar]
- Doz E, Rose S, Nigou J, Gilleron M, Puzo G, Erard F, Ryffel B, Quesniaux VF. Acylation determines the toll-like receptor (TLR)-dependent positive vs. TLR2- mannose receptor- and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J Biol Chem. 2007;282:26014–26025. doi: 10.1074/jbc.M702690200. [DOI] [PubMed] [Google Scholar]
- Doz E, Rose S, Court N, et al. Mycobacterial phosphatidylinositol mannosides negatively regulate host Toll-like receptor 4 MyD88-dependent proinflammatory cytokines and TRIF-dependent co-stimulatory molecule expression. J Biol Chem. 2009;284:23187–23196. doi: 10.1074/jbc.M109.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan MB, Nicolle D, Quesniaux VJF, et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen NN, Ummels R, Maaskant JJ, et al. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun. 2009;77:4538–4547. doi: 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen NN, Stoop EJ, Ummels R, et al. Mycobacterium marinum MMAR_2380, a predicted transmembrane acyltransferase, is essential for the presence of the mannose cap on lipoarabinomannan. Microbiology. 2010;156:3492–3502. doi: 10.1099/mic.0.037507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison. Eur J Cell Biol. 2009;89:95–101. doi: 10.1016/j.ejcb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Elass E, Aubry L, Masson M, Denys A, Guerardel Y, Maes E, Legrand D, Mazurier J, Kremer L. Mycobacterial lipomannan induces matrix metalloproteinase-9 expression in human macrophagic cells through a Toll-like receptor 1 (TLR1) Infect Immun. 2005;73:7064–7068. doi: 10.1128/IAI.73.10.7064-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst WA, Maher J, Cho S, et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity. 1998;8:331–340. doi: 10.1016/s1074-7613(00)80538-5. [DOI] [PubMed] [Google Scholar]
- Felio K, Nguyen H, Dascher CC, et al. CD1-restricted adaptive immune responses to mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. 2009;206:2497–2509. doi: 10.1084/jem.20090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, Sher A. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacteriumavium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003;171:4758–4764. doi: 10.4049/jimmunol.171.9.4758. [DOI] [PubMed] [Google Scholar]
- Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol. 1999;163:312–321. [PubMed] [Google Scholar]
- Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, Crouch EC, Schlesinger LS. Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun. 2006;74:7005–7009. doi: 10.1128/IAI.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda G, Girardin SE, Kullberg BJ, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Scotet E, Niemeyer M, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. P Natl Acad Sci USA. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Chua J, Deretic V. Induction of p38 mitogen-activated protein kinase reduces early endosome autoantigen 1 (EEA1) recruitment to phagosomal membranes. J Biol Chem. 2003a;278:46961–46967. doi: 10.1074/jbc.M305225200. [DOI] [PubMed] [Google Scholar]
- Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. P Natl Acad Sci USA. 2003b;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- Gagliardi MC, Lemassu A, Teloni R, Mariotti S, Sargentini V, Pardini M, Daffé M, Nisini R. Cell wall-associated α-glucan is instrumental for Mycobacterium tuberculosis to block CD1 molecule expression and disable the function of dendritic cell derived from infected monocyte. Cell Microbiol. 2007;9:2081–2092. doi: 10.1111/j.1462-5822.2007.00940.x. [DOI] [PubMed] [Google Scholar]
- Gagliardi MC, Teloni R, Giannoni F, et al. Mycobacteria exploit p38 signaling to affect CD1 expression and lipid antigen presentation by human dendritic cells. Infect Immun. 2009;77:4947–4952. doi: 10.1128/IAI.00607-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Barnes PF, Roy S, Quiroga MF, Wu S, Garcia VE, Krutzik SR, Weis SE, Vankayalapati R. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008;38:459–469. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk BJ, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurtsen J, Chedammi S, Mesters J, et al. Identification of mycobacterial α-glucan as a novel ligand for DC-SIGN: involvement of mycobacterial capsular polysaccharides in host immune modulation. J Immunol. 2009;183:5221–5231. doi: 10.4049/jimmunol.0900768. [DOI] [PubMed] [Google Scholar]
- Gibson KJ, Eggeling L, Maughan WN, Krumbach K, Gurcha SS, Nigou J, Puzo G, Sahm H, Besra GS. Disruption of Cg-ppm1, a polyprenyl monophosphomannose synthase, and the generation of lipoglycan-less mutant in Corynebacterium glutamicum. J Biol Chem. 2003;278:40842–40850. doi: 10.1074/jbc.M307988200. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Bala L, Brando T, Vercellone A, Puzo G. Mycobacterium tuberculosis H37Rv parietal and cellular lipoarabinomannans. Characterization of the acyl- and glyco-forms. J Biol Chem. 2000;275:677–684. doi: 10.1074/jbc.275.1.677. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Ronet C, Mempel M, Monsarrat B, Gachelin G, Puzo G. Acylation state of the phosphatidylinositol mannosides from Mycobacterium bovis Bacillus Calmette Guérin and ability to induce granuloma and recruit natural killer T cells. J Biol Chem. 2001;276:34896–34904. doi: 10.1074/jbc.M103908200. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Quesniaux VF, Puzo G. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette-Guérin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J Biol Chem. 2003;278:29880–29889. doi: 10.1074/jbc.M303446200. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Nigou J, Nicolle D, Quesniaux V, Puzo G. The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem Biol. 2006;13:39–47. doi: 10.1016/j.chembiol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Gomez LM, Anaya JM, Sierra-Filardi E, Cadena J, Corbi A, Martin J. Analysis of DC-SIGN (CD209) functional variants in patients with tuberculosis. Hum Immunol. 2006;67:808–811. doi: 10.1016/j.humimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zamorano M, Mendoza-Hernandez G, Xolalpa W, Parada C, Vallecillo AJ, Bigi F, Espitia C. Mycobacterium tuberculosis glycoproteomics based on ConA-lectin affinity capture of mannosylated proteins. J Proteome Res. 2009;8:721–733. doi: 10.1021/pr800756a. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van het Hof B, van Kooyk Y, Geijtenbeek TBH. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TBH. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- Guérardel Y, Maes E, Elass E, Leroy Y, Timmerman P, Besra GS, Locht C, Strecker G, Kremer L. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with α1,3-mannopyranose side chains. J Biol Chem. 2003;277:30635–30648. doi: 10.1074/jbc.M204398200. [DOI] [PubMed] [Google Scholar]
- Guerin ME, Kordulakova J, Schaeffer F, Svetlikova Z, Buschiazzo A, Giganti D, Gicquel B, Mikusova K, Jackson M, Alzari PM. Molecular recognition and interfacial catalysis by the essential phosphatidylinositol mannosyltransferase PimA from mycobacteria. J Biol Chem. 2007;282:20705–20714. doi: 10.1074/jbc.M702087200. [DOI] [PubMed] [Google Scholar]
- Guerin ME, Kaur D, Somashekar BS, Gibbs S, Gest P, Chatterjee D, Brennan PJ, Jackson M. New insights into the early steps of phosphatidylinositol mannoside biosynthesis in mycobacteria: PimB' is an essential enzyme of Mycobacterium smegmatis. J Biol Chem. 2009;284:25687–25696. doi: 10.1074/jbc.M109.030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin ME, Kordulakova J, Alzari PM, Brennan PJ, Jackson M. Molecular basis of phosphatidylinositol mannoside biosynthesis and regulation in mycobacteria. J Biol Chem. 2010;285:33577–33583. doi: 10.1074/jbc.R110.168328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurcha SS, Baulard AR, Kremer L, Locht C, Moody DB, Muhlecker W, Costello CE, Crick DC, Brennan PJ, Besra GS. Ppm1, a novel polyprenol monophosphomannose synthase from Mycobacterium tuberculosis. Biochem J. 2002;365:441–450. doi: 10.1042/BJ20020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haites RE, Morita YS, McConville MJ, Billman-Jacobe H. Function of phosphatidylinositol in mycobacteria. J Biol Chem. 2005;280:10981–10987. doi: 10.1074/jbc.M413443200. [DOI] [PubMed] [Google Scholar]
- Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8:296–307. doi: 10.1038/nrmicro2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa E, Tokumasu F, Nardone GA, Jin AJ, Hackley VA, Dvorak JA. A Mycobacterium tuberculosis-derived lipid inhibits membrane fusion by modulating lipid membrane domains. Biophys J. 2007;93:4018–4030. doi: 10.1529/biophysj.107.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann SJ, Sell R, Brewer TF. The influence of program acceptability on the effectiveness of public health policy: a study of directly observed therapy for tuberculosis. Am J Public Health. 1998;88:442–445. doi: 10.2105/ajph.88.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Ballou CE. Biosynthesis of mannophospholipids by Mycobacterium phlei. J Biol Chem. 1966;241:895–902. [PubMed] [Google Scholar]
- Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. P Natl Acad Sci USA. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, Reiling N, Schaible UE, et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2 -4 and -9. Eur J Immunol. 2008;38:680–694. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- Huang H, Scherman MS, D'Haeze W, Vereecke D, Holsters M, Crick DC, McNeil MR. Identification and active expression of the Mycobacterium tuberculosis gene encoding 5-phospho-α-d-ribose-1-diphosphate: decaprenyl-phosphate 5-phosphoribosyltransferase, the first enzyme committed to decaprenylphosphoryl-d-arabinose synthesis. J Biol Chem. 2005;280:24539–24543. doi: 10.1074/jbc.M504068200. [DOI] [PubMed] [Google Scholar]
- Hunter SW, Brennan PJ. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990;265:9272–9279. [PubMed] [Google Scholar]
- Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- Ilangumaran S, Arni S, Poincelet M, Theler JM, Brennan PJ, Nasir-ud-Din, Hoessli DC. Integration of mycobacterial lipoarabinomannans into glycosylphosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J Immunol. 1995;155:1334–1342. [PubMed] [Google Scholar]
- Jackson M, Brennan PJ. Polymethylated polysaccharides from Mycobacterium species revisited. J Biol Chem. 2009;284:1949–1953. doi: 10.1074/jbc.R800047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Crick DC, Brennan PJ. Phosphatidylinositol is an essential phospholipid of mycobacteria. J Biol Chem. 2000;275:30092–30099. doi: 10.1074/jbc.M004658200. [DOI] [PubMed] [Google Scholar]
- Jo EK. Mycobacterial interaction with innate receptors: TLRs C-type lectins and NLRs. Curr Opin Infect Dis. 2008;21:279–286. doi: 10.1097/QCO.0b013e3282f88b5d. [DOI] [PubMed] [Google Scholar]
- Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signaling cascades regulating innate immune responses to mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Joe M, Sun D, Taha H, Completo GC, Croudace JE, Lammas DA, Besra GS, Lowary TL. The 5-deoxy-5-methylthio-xylofuranose residue in mycobacterial lipoarabinomannan. absolute stereochemistry, linkage position, conformation, and immune-modulatory activity. J Am Chem Soc. 2006;128:5059–5072. doi: 10.1021/ja057373q. [DOI] [PubMed] [Google Scholar]
- Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, Fenton MJ. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukocyte Biol. 2001;69:1036–1044. [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Nojima S. The phospholipid compositions of some Actinomycetes. Biochim Biophys Acta. 1967;144:681–683. doi: 10.1016/0005-2760(67)90058-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Schaible UE. Antigen presentation and recognition in bacterial infections. Curr Opin Immunol. 2005;17:79–87. doi: 10.1016/j.coi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Kaur D, Berg S, Dinadayala P, Gicquel B, Chatterjee D, McNeil MR, Vissa VD, Crick DC, Jackson M, Brennan PJ. Biosynthesis of mycobacterial lipoarabinomannan: role of a branching mannosyltransferase. P Natl Acad Sci USA. 2006;103:13664–13669. doi: 10.1073/pnas.0603049103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, McNeil MR, Khoo KH, Chatterjee D, Crick DC, Jackson M, Brennan PJ. New insights into the biosynthesis of mycobacterial lipomannan arising from deletion of a conserved gene. J Biol Chem. 2007;282:27133–27140. doi: 10.1074/jbc.M703389200. [DOI] [PubMed] [Google Scholar]
- Kaur D, Obregon-Henao A, Pham H, Chatterjee D, Brennan PJ, Jackson M. Lipoarabinomannan of Mycobacterium: mannose capping by a multifunctional terminal mannosyltransferase. P Natl Acad Sci USA. 2008;105:17973–17977. doi: 10.1073/pnas.0807761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Guerin ME, Skovierová H, Brennan PJ, Jackson M. Chapter 2: biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv Appl Microbiol. 2009;69:23–78. doi: 10.1016/S0065-2164(09)69002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley VA, Schorey JS. Mycobacterium's arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol Biol Cell. 2003;14:3366–3377. doi: 10.1091/mbc.E02-12-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo KH, Dell A, Morris HR, Brennan PJ, Chatterjee D. Structural definition of acylated phosphatidylinositol mannosides from Mycobacterium tuberculosis: definition of a common anchor for lipomannan and lipoarabinomannan. Glycobiology. 1995a;5:117–127. doi: 10.1093/glycob/5.1.117. [DOI] [PubMed] [Google Scholar]
- Khoo KH, Dell A, Morris HR, Brennan PJ, Chatterjee D. Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J Biol Chem. 1995b;270:12380–12389. doi: 10.1074/jbc.270.21.12380. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Hmama Z, Herrera-Velit P, Rochford R, Reiner NE. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. Role of the Src homology 2 containing tyrosine phosphatase 1. J Biol Chem. 1998;273:645–652. doi: 10.1074/jbc.273.1.645. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- Koppel EA, Ludwig IS, Hernandez MS, et al. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology. 2004;209:117–127. doi: 10.1016/j.imbio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Kordulakova J, Gilleron M, Mikusova K, Puzo G, Brennan PJ, Gicquel B, Jackson M. Definition of the first mannosylation step in phosphatidylinositol mannoside synthesis. PimA is essential for growth of mycobacteria. J Biol Chem. 2002;277:31335–31344. doi: 10.1074/jbc.M204060200. [DOI] [PubMed] [Google Scholar]
- Kordulakova J, Gilleron M, Puzo G, Brennan PJ, Gicquel B, Mikusova K, Jackson M. Identification of the required acyltransferase step in the biosynthesis of the phosphatidylinositol mannosides of Mycobacterium species. J Biol Chem. 2003;278:36285–36295. doi: 10.1074/jbc.M303639200. [DOI] [PubMed] [Google Scholar]
- Kovacevic S, Anderson D, Morita YS, Patterson J, Haites R, McMillan BN, Coppel R, McConville MJ, Billman-Jacobe H. Identification of a novel protein with a role in lipoarabinomannan biosynthesis in mycobacteria. J Biol Chem. 2006;281:9011–9017. doi: 10.1074/jbc.M511709200. [DOI] [PubMed] [Google Scholar]
- Kowalska H, Pastuszak I, Szymona M. A mannoglucokinese of Mycobacterium tuberculosis H37Ra. Acta Microbiol Pol. 1980;29:249–257. [PubMed] [Google Scholar]
- Kremer L, Gurcha SS, Bifani P, Hitchen PG, Baulard A, Morris HR, Dell A, Brennan PJ, Besra GS. Characterization of a putative α-mannosyltransferase involved in phosphatidylinositol trimannoside biosynthesis in Mycobacterium tuberculosis. Biochem J. 2002;363:437–447. doi: 10.1042/0264-6021:3630437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Sullivan BA. Acid test: lipid antigens get into the groove. Immunity. 2008;28:727–729. doi: 10.1016/j.immuni.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Lea-Smith DJ, Martin KL, Pyke JS, Tull D, McConville MJ, Coppel RL, Crellin PK. Analysis of a new mannosyltransferase required for the synthesis of phosphatidylinositol mannosides and lipoarbinomannan reveals two lipomannan pools in Corynebacterineae. J Biol Chem. 2008;283:6773–6782. doi: 10.1074/jbc.M707139200. [DOI] [PubMed] [Google Scholar]
- Lee YC, Ballou CE. Structural studies on the myo-Inositol mannodides from the glycolipids of Mycobacterium tuberculosis and Mycobacterium phlei. J Biol Chem. 1964;239:1316–1327. [PubMed] [Google Scholar]
- Lee YC, Ballou CE. Complete structures of the glycophospholipids of mycobacteria. Biochemistry. 1965;4:1395–1404. doi: 10.1021/bi00883a026. [DOI] [PubMed] [Google Scholar]
- Lemassu A, Daffé M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J. 1994;297:351–357. doi: 10.1042/bj2970351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Mushegian A. Three monophyletic superfamilies account for the majority of the known glycosyltransferases. Protein Sci. 2003;12:1418–1431. doi: 10.1110/ps.0302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Stern RJ, Scherman MS, Vissa VD, Yan W, Jones VC, Zhang F, Franzblau SG, Lewis WH, McNeil MR. Drug targeting Mycobacterium tuberculosis cell wall synthesis: genetics of dTDP-rhamnose synthetic enzymes and development of a microtiter plate-based screen for inhibitors of conversion of dTDP-glucose to dTDP-rhamnose. Antimicrob Agents Ch. 2001;45:1407–1416. doi: 10.1128/AAC.45.5.1407-1416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Nigou J, Herrmann JL, Jackson M, Amara A, Lagrange PH, Puzo G, Gicquel B, Neyrolles O. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- Maes E, Coddeville B, Kremer L, Guerardel Y. Polysaccharide structural variability in mycobacteria: identification and characterization of phosphorylated mannan and arabinomannan. Glycoconjugate J. 2007;24:439–448. doi: 10.1007/s10719-007-9036-1. [DOI] [PubMed] [Google Scholar]
- Makarov V, Manina G, Mikusova K, et al. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science. 2009;324:801–804. doi: 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome–lysosome fusion and increased survival within human macrophages. J Exp Med. 2000;191:287–302. doi: 10.1084/jem.191.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik ZA, Iyer SS, Kusner DJ. Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. J Immunol. 2001;166:3392–3401. doi: 10.4049/jimmunol.166.5.3392. [DOI] [PubMed] [Google Scholar]
- Marks PA. A newer pathway of carbohydrate metabolism; the pentose phosphate pathway. Diabetes. 1956;5:276–283. doi: 10.2337/diab.5.4.276. [DOI] [PubMed] [Google Scholar]
- Marland Z, Beddoe T, Zaker-Tabrizi L, Lucet IS, Brammananth R, Whisstock JC, Wilce MC, Coppel RL, Crellin PK, Rossjohn J. Hijacking of a substrate-binding protein scaffold for use in mycobacterial cell wall biosynthesis. J Mol Biol. 2006;359:983–997. doi: 10.1016/j.jmb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Masucci P, McAlpine IL, Glenn JT. Biochemical studies of bacterial derivatives. XII. The preparation of human tubercle bacillus polysaccharide MB-200 and some of its biological properties. Am Rev Tuberc. 1930;22:669–677. [Google Scholar]
- McCarthy TR, Torrelles JB, MacFarlane AS, Katawczik M, Kutzbach B, Desjardin LE, Clegg S, Goldberg JB, Schlesinger LS. Overexpression of Mycobacterium tuberculosismanB, a phosphomannomutase that increases phosphatidylinositol mannoside biosynthesis in Mycobacterium smegmatis and mycobacterial association with human macrophages. Mol Microbiol. 2005;58:774–790. doi: 10.1111/j.1365-2958.2005.04862.x. [DOI] [PubMed] [Google Scholar]
- McNeil M, Daffé M, Brennan PJ. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J Biol Chem. 1990;265:18200–18206. [PubMed] [Google Scholar]
- McNeil M, Daffé M, Brennan PJ. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J Biol Chem. 1991;266:13217–13223. [PubMed] [Google Scholar]
- McNeil MR, Robuck KG, Harter M, Brennan PJ. Enzymatic evidence for the presence of a critical terminal hexa-arabinoside in the cell walls of Mycobacterium tuberculosis. Glycobiology. 1994;4:165–173. doi: 10.1093/glycob/4.2.165. [DOI] [PubMed] [Google Scholar]
- Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- Mikusová K, Huang H, Yagi T, Holsters M, Vereecke D, D'Haeze W, Scherman MS, Brennan PJ, McNeil MR, Crick DC. Decaprenylphosphoryl arabinofuranose, the donor of the d-arabinofuranosyl residues of mycobacterial arabinan, is formed via a two-step epimerization of decaprenylphosphoryl ribose. J Bacteriol. 2005;187:8020–8025. doi: 10.1128/JB.187.23.8020-8025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin DE, Kremer L, Dover LG, Besra GS. The methyl-branched fortifications of Mycobacterium tuberculosis. Chem Biol. 2002;9:545–553. doi: 10.1016/s1074-5521(02)00142-4. [DOI] [PubMed] [Google Scholar]
- Misaki A, Yukawa S. Studies on cell walls of mycobacteria. II. Constitution of polysaccharides from BCG cell walls. J Biochem. 1966;59:511–520. doi: 10.1093/oxfordjournals.jbchem.a128335. [DOI] [PubMed] [Google Scholar]
- Misaki A, Azuma I, Yamamura Y. Structural and immunochemical studies on d-arabino-d-mannans and d-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J Biochem. 1977;82:1759–1770. doi: 10.1093/oxfordjournals.jbchem.a131874. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Alderwick LJ, Rittmann D, Tatituri RV, Nigou J, Gilleron M, Eggeling L, Besra GS. Identification of an α(1→6) mannopyranosyltransferase (MptA), involved in Corynebacterium glutamicum lipomannan biosynthesis, and identification of its orthologue in Mycobacterium tuberculosis. Mol Microbiol. 2007;65:1503–1517. doi: 10.1111/j.1365-2958.2007.05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Alderwick LJ, Rittmann D, Wang C, Bhatt A, Jacobs WR, Jr, Takayama K, Eggeling L, Besra GS. Identification of a novel α(1→6) mannopyranosyltransferase MptB from Corynebacterium glutamicum by deletion of a conserved gene, NCgl1505, affords a lipomannan- and lipoarabinomannan-deficient mutant. Mol Microbiol. 2008a;68:1595–1613. doi: 10.1111/j.1365-2958.2008.06265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Klein C, Gurcha SS, Alderwick LJ, Babu P, Hitchen PG, Morris HR, Dell A, Besra GS, Eggeling L. Structural characterization and functional properties of a novel lipomannan variant isolated from a Corynebacterium glutamicum pimB' mutant. Antonie van Leeuwenhoek. 2008b;94:277–287. doi: 10.1007/s10482-008-9243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Batt S, Krumbach K, Eggeling L, Besra GS. Characterization of the Corynebacterium glutamicumΔpimB'ΔmgtA double deletion mutant and the role of Mycobacterium tuberculosis orthologues Rv2188c and Rv0557 in glycolipid biosynthesis. J Bacteriol. 2009;191:4465–4472. doi: 10.1128/JB.01729-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Krumbach K, Rittmann D, Appelmelk B, Pathak V, Pathak AK, Nigou J, Geurtsen J, Eggeling L, Besra GS. Lipoarbinomannan biosynthesis in Corynebacterineae: the interplay of two α(1→2)-mannopyranosyltransferases MptC and MptD in mannan branching. Mol Microbiol. 2011 doi: 10.1111/j.1365-2958.2011.07640.x. DOI: 10.1111/j.1365-2958.2011.07640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle V, Kremer L. Division and cell envelope regulation by Ser/Thr phosphorylation: Mycobacterium shows the way. Mol Microbiol. 2010;75:1064–1077. doi: 10.1111/j.1365-2958.2009.07041.x. [DOI] [PubMed] [Google Scholar]
- Moody DB. TLR gateways to CD1 function. Nat Immunol. 2006;7:811–817. doi: 10.1038/ni1368. [DOI] [PubMed] [Google Scholar]
- Moody DB, Reinhold BB, Guy MR, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- Morita YS, Patterson JH, Billman-Jacobe H, McConville MJ. Biosynthesis of mycobacterial phosphatidylinositol mannosides. Biochem J. 2004;378:589–597. doi: 10.1042/BJ20031372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita YS, Velasquez R, Taig E, Waller RF, Patterson JH, Tull D, Williams SJ, Billman-Jacobe H, McConville MJ. Compartmentalization of lipid biosynthesis in mycobacteria. J Biol Chem. 2005;280:21645–21652. doi: 10.1074/jbc.M414181200. [DOI] [PubMed] [Google Scholar]
- Morita YS, Sena CB, Waller RF, et al. PimE is a polyprenol-phosphate-mannose-dependent mannosyltransferase that transfers the fifth mannose of phosphatidylinositol mannoside in mycobacteria. J Biol Chem. 2006;281:25143–25155. doi: 10.1074/jbc.M604214200. [DOI] [PubMed] [Google Scholar]
- Movahedzadeh F, Smith DA, Norman RA, et al. The Mycobacterium tuberculosisino1 gene is essential for growth and virulence. Mol Microbiol. 2004;51:1003–1014. doi: 10.1046/j.1365-2958.2003.03900.x. [DOI] [PubMed] [Google Scholar]
- Movahedzadeh F, Wheeler PR, Dinadayala P, Av-Gay Y, Parish T, Daffé M, Stoker NG. Inositol monophosphate phosphatase genes of Mycobacterium tuberculosis. BMC Microbiol. 2010;10:50. doi: 10.1186/1471-2180-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrolles O, Gicquel B, Quintana-Murci L. Towards a crucial role for DC-SIGN in tuberculosis and beyond. Trends Microbiol. 2006;14:383–387. doi: 10.1016/j.tim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Pieters J. The Trojan horse: survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol. 2005;15:269–276. doi: 10.1016/j.tcb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Nicolle D, Fremond C, Pichon X, Bouchot A, Maillet I, Ryffel B, Quesniaux VJ. Long-term control of Mycobacterium bovis BCG infection in the absence of Toll-like receptors (TLRs): investigation of TLR2-, TLR6-, or TLR2-TLR4-deficient mice. Infect Immun. 2004;72:6994–7004. doi: 10.1128/IAI.72.12.6994-7004.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigou J, Besra GS. Characterization and regulation of inositol monophosphatase activity in Mycobacterium smegmatis. Biochem J. 2002a;361:385–390. doi: 10.1042/bj3610385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigou J, Besra GS. Cytidine diphosphate-diacylglycerol synthesis in Mycobacterium smegmatis. Biochem J. 2002b;367:157–162. doi: 10.1042/BJ20020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigou J, Gilleron M, Cahuzac B, Bounery JD, Herold M, Thurnher M, Puzo G. The phosphatidyl-myo-inositol anchor of the lipoarabinomannans from Mycobacterium bovis bacillus Calmette-Guérin. Heterogeneity, structure, and role in the regulation of cytokine secretion. J Biol Chem. 1997;272:23094–23103. doi: 10.1074/jbc.272.37.23094. [DOI] [PubMed] [Google Scholar]
- Nigou J, Gilleron M, Rojas M, Garcia LF, Thurnher M, Puzo G. Mycobacterial lipoarabinomannans: modulators of dendritic cell function and the apoptotic response. Microbes Infect. 2002;4:945–953. doi: 10.1016/s1286-4579(02)01621-0. [DOI] [PubMed] [Google Scholar]
- Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003;85:153–166. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- Nigou J, Vasselon T, Ray A, Constant P, Gilleron M, Besra GS, Sutcliffe I, Tiraby G, Puzo G. Mannan chain length controls lipoglycans signaling via and binding to TLR2. J Immunol. 2008;180:6696–6702. doi: 10.4049/jimmunol.180.10.6696. [DOI] [PubMed] [Google Scholar]
- Ning B, Elbein AD. Purification and properties of mycobacterial GDP-mannose pyrophosphorylase. Arch Biochem Biophys. 1999;362:339–345. doi: 10.1006/abbi.1998.1053. [DOI] [PubMed] [Google Scholar]
- Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- Ortalo-Magné A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffé M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141:1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- Ortalo-Magné A, Andersen AB, Daffé M. The outermost capsular arabinomannans and other mannoconjugates of virulent and avirulent tubercle bacilli. Microbiology. 1996a;142:927–935. doi: 10.1099/00221287-142-4-927. [DOI] [PubMed] [Google Scholar]
- Ortalo-Magné A, Lemassu A, Laneelle MA, Bardou F, Silve G, Gounon P, Marchal G, Daffé M. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J Bacteriol. 1996b;178:456–461. doi: 10.1128/jb.178.2.456-461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RM, Hsu FF, VanderVen BC, et al. M. tuberculosis Rv2252 encodes a diacylglycerol kinase involved in the biosynthesis of phosphatidylinositol mannosides (PIMs) Mol Microbiol. 2006;60:1152–1163. doi: 10.1111/j.1365-2958.2006.05174.x. [DOI] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. P Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish T, Liu J, Nikaido H, Stoker NG. A Mycobacterium smegmatis mutant with a defective inositol monophosphate phosphatase gene homolog has altered cell envelope permeability. J Bacteriol. 1997;179:7827–7833. doi: 10.1128/jb.179.24.7827-7833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak SK, Basu S, Bhattacharyya A, Pathak S, Kundu M, Basu J. Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor-dependent interleukin-12p40 production in macrophages. J Biol Chem. 2005;280:42794–42800. doi: 10.1074/jbc.M506471200. [DOI] [PubMed] [Google Scholar]
- Patterson JH, Waller RF, Jeevarajah D, Billman-Jacobe H, McConville MJ. Mannose metabolism is required for mycobacterial growth. Biochem J. 2003;372:77–86. doi: 10.1042/BJ20021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50:S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarque S, Herrmann JL, Duteyrat JL, et al. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem J. 2005;392:615–624. doi: 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarque S, Larrouy-Maumus G, Payre B, Jackson M, Puzo G, Nigou J. The immune-modulatory lipoglycans lipoarabinomannan and lipomannan are exposed at the mycobacterial cell surface. Tuberculosis (Edinburgh) 2008;88:560–565. doi: 10.1016/j.tube.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli SA, Segelke BW, Sugita M, Wilson IA, Brenner MB. The CD1 family of lipid antigen-presenting molecules. Immunol Today. 1998;19:362–368. doi: 10.1016/s0167-5699(98)01289-4. [DOI] [PubMed] [Google Scholar]
- Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, Brenner MB, Modlin RL, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, Glauser MP, Tobias PS, Ulevitch RJ. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Puissegur MP, Lay G, Gilleron M, et al. Mycobacterial lipomannan induces granuloma macrophage fusion via a TLR2-dependent ADAM9- and β1 integrin-mediated pathway. J Immunol. 2007;178:3161–3169. doi: 10.4049/jimmunol.178.5.3161. [DOI] [PubMed] [Google Scholar]
- Quesniaux V, Fremond C, Jacobs M, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004a;6:946–959. doi: 10.1016/j.micinf.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004b;172:4425–4434. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- Reiling N, Ehlers S, Holscher C. MyDths and un-TOLLed truths: sensor instructive and effector immunity to tuberculosis. Immunol Lett. 2008;116:15–23. doi: 10.1016/j.imlet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Relloso M, Cheng TY, Im JS, et al. pH-dependent interdomain tethers of CD1b regulate its antigen capture. Immunity. 2008;28:774–786. doi: 10.1016/j.immuni.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades E, Hsu F, Torrelles JB, Turk J, Chatterjee D, Russell DG. Identification and macrophage-activating activity of glycolipids released from intracellular Mycobacterium bovis BCG. Mol Microbiol. 2003;48:875–888. doi: 10.1046/j.1365-2958.2003.03473.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Marrero CA, Schuyler W, Roser S, Ritzenthaler JD, Newburn SA, Roman J. M. tuberculosis induction of matrix metalloproteinase-9: the role of mannose and receptor-mediated mechanisms. Am J Physiol Lung C. 2002;282:L546–L555. doi: 10.1152/ajplung.00175.2001. [DOI] [PubMed] [Google Scholar]
- Roos AK, Andersson CE, Bergfors T, Jacobsson M, Karlen A, Unge T, Jones TA, Mowbray SL. Mycobacterium tuberculosis ribose-5-phosphate isomerase has a known fold, but a novel active site. J Mol Biol. 2004;335:799–809. doi: 10.1016/j.jmb.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Roos AK, Burgos E, Ericsson DJ, Salmon L, Mowbray SL. Competitive inhibitors of Mycobacterium tuberculosis ribose-5-phosphate isomerase B reveal new information about the reaction mechanism. J Biol Chem. 2005;280:6416–6422. doi: 10.1074/jbc.M412018200. [DOI] [PubMed] [Google Scholar]
- Roura-Mir C, Wang L, Cheng TY, Matsunaga I, Dascher CC, Peng SL, Fenton MJ, Kirschning C, Moody DB. Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. J Immunol. 2005;175:1758–1766. doi: 10.4049/jimmunol.175.3.1758. [DOI] [PubMed] [Google Scholar]
- Russell DG. TB comes to a sticky beginning. Nat Med. 2001;7:894–895. doi: 10.1038/90926. [DOI] [PubMed] [Google Scholar]
- Ryffel B, Fremond C, Jacobs M, Parida S, Botha T, Schnyder B, Quesniaux V. Innate immunity to mycobacterial infection in mice: critical role for toll-like receptors. Tuberculosis (Edinburgh) 2005;85:395–405. doi: 10.1016/j.tube.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Salman M, Lonsdale JT, Besra GS, Brennan PJ. Phosphatidylinositol synthesis in mycobacteria. Biochim Biophys Acta. 1999;1436:437–450. doi: 10.1016/s0005-2760(98)00151-9. [DOI] [PubMed] [Google Scholar]
- Sani M, Houben ENG, Geurtsen J, et al. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog. 2010;6:e1000794. doi: 10.1371/journal.ppat.1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Reiling N, Fessler C, et al. Decreased pathology and prolonged survival of human DC-SIGN transgenic mice during mycobacterial infection. J Immunol. 2008;180:6836–6845. doi: 10.4049/jimmunol.180.10.6836. [DOI] [PubMed] [Google Scholar]
- Schaeffer ML, Khoo KH, Besra GS, Chatterjee D, Brennan PJ, Belisle JT, Inamine JM. The pimB gene of Mycobacterium tuberculosis encodes a mannosyltransferase involved in lipoarabinomannan biosynthesis. J Biol Chem. 1999;274:31625–31631. doi: 10.1074/jbc.274.44.31625. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Hagens K, Fischer K, Collins HL, Kaufmann SH. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J Immunol. 2000;164:4843–4852. doi: 10.4049/jimmunol.164.9.4843. [DOI] [PubMed] [Google Scholar]
- Scherman H, Kaur D, Pham H, Skovierova H, Jackson M, Brennan PJ. Identification of a polyprenylphosphomannosyl synthase involved in the synthesis of mycobacterial mannosides. J Bacteriol. 2009;191:6769–6772. doi: 10.1128/JB.00431-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherman MS, Kalbe-Bournonville L, Bush D, Xin Y, Deng L, McNeil M. Polyprenylphosphate-pentoses in mycobacteria are synthesized from 5-phosphoribose pyrophosphate. J Biol Chem. 1996;271:29652–29658. doi: 10.1074/jbc.271.47.29652. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–4575. [PubMed] [Google Scholar]
- Seibert FB, Watson DW. Isolation of the polysaccharides and nucleic acid of tuberculin by electrophoresis. J Biol Chem. 1941;140:55–69. [Google Scholar]
- Seidel M, Alderwick LJ, Birch HL, Sahm H, Eggeling L, Besra GS. Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in Corynebacterineae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J Biol Chem. 2007;282:14729–14740. doi: 10.1074/jbc.M700271200. [DOI] [PubMed] [Google Scholar]
- Sena CB, Fukuda T, Miyanagi K, Matsumoto S, Kobayashi K, Murakami Y, Maeda Y, Kinoshita T, Morita YS. Controlled expression of branch-forming mannosyltransferase is critical for mycobacterial lipoarabinomannan biosynthesis. J Biol Chem. 2010;285:13326–13336. doi: 10.1074/jbc.M109.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severn WB, Furneaux RH, Falshaw R, Atkinson PH. Chemical and spectroscopic characterisation of the phosphatidylinositol manno-oligosaccharides from Mycobacterium bovis AN5 and WAg201 and Mycobacterium smegmatis mc2 155. Carbohyd Res. 1998;308:397–408. doi: 10.1016/s0008-6215(98)00108-6. [DOI] [PubMed] [Google Scholar]
- Shabaana AK, Kulangara K, Semac I, Parel Y, Ilangumaran S, Dharmalingam K, Chizzolini C, Hoessli DC. Mycobacterial lipoarabinomannans modulate cytokine production in human T helper cells by interfering with raft/microdomain signaling. Cell Mol Life Sci. 2005;62:179–187. doi: 10.1007/s00018-004-4404-5. [DOI] [PubMed] [Google Scholar]
- Shi L, Berg S, Lee A, Spencer JS, Zhang J, Vissa V, McNeil MR, Khoo KH, Chatterjee D. The carboxy terminus of EmbC from Mycobacterium smegmatis mediates chain length extension of the arabinan in lipoarabinomannan. J Biol Chem. 2006;281:19512–19526. doi: 10.1074/jbc.M513846200. [DOI] [PubMed] [Google Scholar]
- Sidobre S, Nigou J, Puzo G, Riviere M. Lipoglycans are putative ligands for the human pulmonary surfactant protein A attachment to mycobacteria. Critical role of the lipids for lectin-carbohydrate recognition. J Biol Chem. 2000;275:2415–2422. doi: 10.1074/jbc.275.4.2415. [DOI] [PubMed] [Google Scholar]
- Sieling PA, Chatterjee D, Porcelli SA, et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- Sieling PA, Jullien D, Dahlem M, Tedder TF, Rea TH, Modlin RL, Porcelli SA. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Gaullier JM, D'Arrigo A, Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Skovierova H, Larrouy-Maumus G, Zhang J, et al. AftD, a novel essential arabinofuranosyltransferase from mycobacteria. Glycobiology. 2009;19:1235–1247. doi: 10.1093/glycob/cwp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovierova H, Larrouy-Maumus G, Pham G, et al. Biosynthetic origin of the galactosamine substituent of arabinogalactan in Mycobacterium tuberculosis. J Biol Chem. 2010;285:41348–41355. doi: 10.1074/jbc.M110.188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevatsan S, Stockbauer KE, Pan X, Kreiswirth BN, Moghazeh SL, Jacobs WR, Jr, Telenti A, Musser JM. Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob Agents Ch. 1997;41:1677–1681. doi: 10.1128/aac.41.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V, Manchanda M, Gupta S, Singla R, Behera D, Das G, Natarajan K. Toll-like receptor 2 and DC-SIGNR1 differentially regulate suppressors of cytokine signaling 1 in dendritic cells during Mycobacterium tuberculosis infection. J Biol Chem. 2009;284:25532–25541. doi: 10.1074/jbc.M109.006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- Sugawara I, Yamada H, Li C, Mizuno S, Takeuchi O, Akira S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003a;47:327–336. doi: 10.1111/j.1348-0421.2003.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Sugawara I, Yamada H, Mizuno S, Takeda K, Akira S. Mycobacterial infection in MyD88-deficient mice. Microbiol Immunol. 2003b;47:841–847. doi: 10.1111/j.1348-0421.2003.tb03450.x. [DOI] [PubMed] [Google Scholar]
- Sugita M, Grant EP, van Donselaar E, Hsu VW, Rogers RA, Peters PJ, Brenner MB. Separate pathways for antigen presentation by CD1 molecules. Immunity. 1999;11:743–752. doi: 10.1016/s1074-7613(00)80148-x. [DOI] [PubMed] [Google Scholar]
- Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Sweet L, Singh PP, Azad AK, Rajaram MV, Schlesinger LS, Schorey JS. Mannose receptor-dependent delay in phagosome maturation by Mycobacteriumavium glycopeptidolipids. Infect Immun. 2009;78:518–526. doi: 10.1128/IAI.00257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaud H, Tisnovska H, Vilkas E. Phoppholipids and glycolipids of a Micromonospora strain. Biochimie. 1971;53:55–61. doi: 10.1016/s0300-9084(71)80083-4. [DOI] [PubMed] [Google Scholar]
- Tailleux L, Schwartz O, Herrmann JL, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, et al. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005;2:e381. doi: 10.1371/journal.pmed.0020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Goldman DS. Enzymatic synthesis of mannosyl-1-phosphoryl-decaprenol by a cell-free system of Mycobacterium tuberculosis. J Biol Chem. 1970;245:6251–6257. [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- Tanne A, Neyrolles O. C-type lectins in immune defense against pathogens: the murine DC-SIGN homologue SIGNR3 confers early protection against Mycobacterium tuberculosis infection. Virulence. 2010;1:285–290. doi: 10.4161/viru.1.4.11967. [DOI] [PubMed] [Google Scholar]
- Tanne A, Ma B, Boudou F, et al. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med. 2009;206:2205–2220. doi: 10.1084/jem.20090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatituri RV, Illarionov PA, Dover LG, et al. Inactivation of Corynebacterium glutamicum NCgl0452 and the role of MgtA in the biosynthesis of a novel mannosylated glycolipid involved in lipomannan biosynthesis. J Biol Chem. 2007a;282:4561–4572. doi: 10.1074/jbc.M608695200. [DOI] [PubMed] [Google Scholar]
- Tatituri RV, Alderwick LJ, Mishra AK, et al. Structural characterization of a partially arabinosylated lipoarabinomannan variant isolated from a Corynebacterium glutamicum ubiA mutant. Microbiology. 2007b;153:2621–2629. doi: 10.1099/mic.0.2007/008078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekaia F, Gordon SV, Garnier T, Brosch R, Barrell BG, Cole ST. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tubercle Lung Dis. 1999;79:329–342. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- Telenti A, Philipp WJ, Sreevatsan S, Bernasconi C, Stockbauer KE, Wieles B, Musser JM, Jacobs WR. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat Med. 1997;3:567–570. doi: 10.1038/nm0597-567. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinburgh) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. Role of C-type lectins in mycobacterial infections. Curr Drug Targets. 2008a;9:102–112. doi: 10.2174/138945008783502467. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Knaup R, Kolareth A, et al. Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem. 2008b;283:31417–31428. doi: 10.1074/jbc.M806350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, DesJardin LE, MacNeil J, et al. Inactivation of Mycobacterium tuberculosis mannosyltransferase pimB reduces the cell wall lipoarabinomannan and lipomannan content and increases the rate of bacterial-induced human macrophage cell death. Glycobiology. 2009;19:743–755. doi: 10.1093/glycob/cwp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treumann A, Xidong F, McDonnell L, Derrick PJ, Ashcroft AE, Chatterjee D, Homans SW. 5-Methylthiopentose: a new substituent on lipoarabinomannan in Mycobacterium tuberculosis. J Mol Biol. 2002;316:89–100. doi: 10.1006/jmbi.2001.5317. [DOI] [PubMed] [Google Scholar]
- Turnbull WB, Shimizu KH, Chatterjee D, Homans SW, Treumann A. Identification of the 5-methylthiopentosyl substituent in Mycobacterium tuberculosis lipoarabinomannan. Angew Chem Int Edit. 2004;43:3918–3922. doi: 10.1002/anie.200454119. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. P Natl Acad Sci USA. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVen BC, Harder JD, Crick DC, Belisle JT. Export-mediated assembly of mycobacterial glycoproteins parallels eukaryotic pathways. Science. 2005;309:941–943. doi: 10.1126/science.1114347. [DOI] [PubMed] [Google Scholar]
- van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Geijtenbeek TBH. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- Vannberg FO, Chapman SJ, Khor CC, et al. CD209 genetic polymorphism and tuberculosis disease. PLoS One. 2008;3:e1388. doi: 10.1371/journal.pone.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venisse A, Berjeaud JM, Chaurand P, Gilleron M, Puzo G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J Biol Chem. 1993;268:12401–12411. [PubMed] [Google Scholar]
- Venkataswamy MM, Baena A, Goldberg MF, et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guérin. J Immunol. 2009;183:1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellone A, Nigou J, Puzo G. Relationships between the structure and the roles of lipoarabinomannans and related glycoconjugates in tuberculosis pathogenesis. Front Biosci. 1998;3:e149–e163. doi: 10.2741/a372. [DOI] [PubMed] [Google Scholar]
- Vergne I, Chua J, Deretic V. Mycobacterium tuberculosis phagosome maturation arrest: selective targeting of PI3P-dependent membrane trafficking. Traffic. 2003a;4:600–606. doi: 10.1034/j.1600-0854.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003b;198:653–659. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Singh SB, Deretic V. Cell biology of Mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Bi. 2004a;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- Vergne I, Fratti RA, Hill PJ, Chua J, Belisle J, Deretic V. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell. 2004b;15:751–760. doi: 10.1091/mbc.E03-05-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. P Natl Acad Sci USA. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal C, Guerardel Y, Kremer L, Masson M, Legrand D, Mazurier J, Elass E. Lipomannans but not lipoarabinomannans purified from Mycobacteriumchelonae and Mycobacteriumkansasii induce TNF-α and IL-8 secretion by a CD14-toll-like receptor 2-dependent mechanism. J Immunol. 2003;171:2014–2023. doi: 10.4049/jimmunol.171.4.2014. [DOI] [PubMed] [Google Scholar]
- Vilkas E, Lederer E. Isolation of a phosphatidyl-inosito-di-d-mannoside from a mycobacterial phosphatide. B Soc Chim Biol (Paris) 1956;38:111–121. [PubMed] [Google Scholar]
- Villeneuve C, Gilleron M, Maridonneau-Parini I, Daffé M, Astarie-Dequeker C, Etienne G. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J Lipid Res. 2005;46:475–483. doi: 10.1194/jlr.M400308-JLR200. [DOI] [PubMed] [Google Scholar]
- Walburger A, Koul A, Ferrari G, et al. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304:1800–1804. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- Wang C, Hayes B, Vestling MM, Takayama K. Transposome mutagenesis of an integral membrane transporter in Corynebacterium matruchotii. Biochem Bioph Res Co. 2006;340:953–960. doi: 10.1016/j.bbrc.2005.12.097. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Watari E, Matsunaga I, et al. BCG vaccine elicits both T-cell mediated and humoral immune responses directed against mycobacterial lipid components. Vaccine. 2006;24:5700–5707. doi: 10.1016/j.vaccine.2006.04.049. [DOI] [PubMed] [Google Scholar]
- Welin A, Winberg ME, Abdalla H, Sarndahl E, Rasmusson B, Stendahl O, Lerm M. Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infect Immun. 2008;76:2882–2887. doi: 10.1128/IAI.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO report 2009: WHO/HTM/TB/2009.411. Geneva: World Health Organization; 2009. Global tuberculosis control – epidemiology, strategy, financing. [Google Scholar]
- Wieland CW, Knapp S, Florquin S, de Vos AF, Takeda K, Akira S, Golenbock DT, Verbon A, van der Poll T. Non-mannose-capped lipoarabinomannan induces lung inflammation via toll-like receptor 2. Am J Resp Crit Care. 2004;170:1367–1374. doi: 10.1164/rccm.200404-525OC. [DOI] [PubMed] [Google Scholar]
- Wieland CW, Koppel EA, den Dunnen J, Florquin S, McKenzie AN, van Kooyk Y, van der Poll T, Geijtenbeek TBH. Mice lacking SIGNR1 have stronger T helper 1 responses to Mycobacterium tuberculosis. Microbes Infect. 2007;9:134–141. doi: 10.1016/j.micinf.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. P Natl Acad Sci USA. 1986;83:2501–2505. doi: 10.1073/pnas.83.8.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolucka BA. Biosynthesis of d-arabinose in mycobacteria – a novel bacterial pathway with implications for antimycobacterial therapy. FEBS J. 2008;275:2691–2711. doi: 10.1111/j.1742-4658.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, de Hoffmann E. Isolation and characterization of the major form of polyprenyl-phospho-mannose from Mycobacterium smegmatis. Glycobiology. 1998;8:955–962. doi: 10.1093/glycob/8.10.955. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, McNeil MR, de Hoffmann E, Chojnacki T, Brennan PJ. Recognition of the lipid intermediate for arabinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria. J Biol Chem. 1994;269:23328–23335. [PubMed] [Google Scholar]
- Wright A, Zignol M, Van Deun A, et al. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–1873. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell DG. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacteriumavium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- Yano I, Furukawa Y, Kusunose M. Phospholipids of Nocardia coeliaca. J Bacteriol. 1969;98:124–130. doi: 10.1128/jb.98.1.124-130.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DC, Moody DB. T-cell recognition of glycolipids presented by CD1 proteins. Glycobiology. 2006;16:103R–112R. doi: 10.1093/glycob/cwj111. [DOI] [PubMed] [Google Scholar]
- Young M, Artsatbanov V, Beller HR, et al. Genome sequence of the Fleming strain of Micrococcus luteus, a simple free-living Actinobacterium. J Bacteriol. 2010;192:841–860. doi: 10.1128/JB.01254-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahringer U, Lindner B, Inamura S, Heine H, Alexander C. TLR2 – promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Zajonc DM, Ainge GD, Painter GF, Severn WB, Wilson IA. Structural characterization of mycobacterial phosphatidylinositol mannoside binding to mouse CD1d. J Immunol. 2006;177:4577–4583. doi: 10.4049/jimmunol.177.7.4577. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- Zhang N, Torrelles JB, McNeil MR, Escuyer VE, Khoo KH, Brennan PJ, Chatterjee D. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N-terminal recognition region and a C-terminal synthetic region. Mol Microbiol. 2003;50:69–76. doi: 10.1046/j.1365-2958.2003.03681.x. [DOI] [PubMed] [Google Scholar]


