Abstract
Neural stem cells (NSC) are cells that have the capacity to generate multiple types of differentiated brain cells. In conditions in which there is a loss of key functional cell groups, such as neurons, inducing or introducing neural stem cells to replace the function of those cells that were lost during the disease has the greatest potential therapeutic applications. Indeed, the achievement of one of the main objectives of various investigations is already on the horizon for some conditions, such as Alzheimer's disease. It is not known whether impaired neurogenesis contributes to neuronal depletion and cognitive dysfunction in Alzheimer’s disease (AD). The results of the different investigations are controversial; some studies have found that neurogenesis is increased in AD brains, but others have not.
Keywords: neurodegenerative disease, Alzheimer's disease, neurogenesis, neural stem cells, cell death
Introduction
One of the main dogmas of neuroscience in the last century was that central nervous system (CNS) regeneration is not possible in adulthood. However, various studies have demonstrated the existence of neurogenesis in adult brain regions [1]. Indeed, this has been confirmed by observing that new cells continue to be generated postnatally and throughout life [2,3].
The most active neurogenic regions in the brain are the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles [3,4,5]. In these two areas of the adult mammalian brain, cells with mitotic activity can be found [6,7]. These cells are called stem cells and have the capacity to generate multiple types of differentiated cells (multipotency) and undergo cell division, in which at least one of the daughter cells maintains its stem-cell potential and, thus, has a capacity for self-renewal [8,9].
Neuronal stem cells (NSCs) in the SVZ predominantly give rise to committed progenitor cells that migrate into the olfactory bulb (OB) via the rostral migratory stream and differentiate into local interneurons [10,11]; progenitors in the SGZ migrate into the granular cell layer of the hippocampus and also differentiate into neurons [12,13]. Since the discovery of these stem cells, the biggest controversy has been determining the nature of the precursor cells in the adult brain germinal zones. Studies have shown that a specific population of radial glia can give rise to neural precursors, which, in turn, produce neurons and glial cells [14,15].
The identification of new neurons in the brain has generated great expectations among scientists, particularly in the context of the conditions that result in the loss of functional cell groups, such as neurons. The potential therapeutic applications of inducing or introducing NSCs to replace the function of those lost in neurodegenerative diseases such as Alzheimer's disease (AD) has been one of the main objectives in recent years. As the structural and molecular mechanisms governing adult neurogenesis are important in AD, we will review the collective literature findings in this field with a focus on the findings from Alzheimer's mouse models.
Alzheimer's disease and the formation of new neural stem cells
Alzheimer's disease is a particular form of progressive dementia associated with distinct neuropathological changes [16,17]. It is characterized by memory loss and impairment in at least one other area of cognition [18,19,20]. At any time during the disease, patients may also experience changes in behavior or mood. Indeed, several lines of evidence suggest that AD may be a syndrome with overlapping causes that result in identical neuropathological changes, and, if this hypothesis is correct, there will likely be multiple treatment approaches, whose components will vary from patient to patient [21]. Several lines of investigation have proposed different treatment strategies in AD models, including the induction of neurogenesis.
Various evidence supports the idea that the disease symptoms of AD could partly be due to the impaired formation of new hippocampal neurons from endogenous NSCs in the SGZ, which are believed to contribute to mood regulation, learning and memory [22]. However, we still do not know what causes the disease.
In AD, different cellular alterations make the induction of neurogenesis more difficult than expected, and an improved understanding of the factors that govern NSC differentiation is needed before the stimulation or introduction of neural precursors into the brain becomes a viable option for the treatment of this disease. AD is extremely complex because the NSCs would have to be pre-differentiated in vitro into many different types of neuroblasts for subsequent implantation into a large number of brain areas. However, there has been evidence showing that endogenous neuronal precursors can proliferate in response to damage [23,24,25]. Neurogenesis was found to be increased in the brains of patients with AD, compared with the brains of age-matched control subjects [26], suggesting that compensatory mechanisms are directed to overcome the loss of function [27]. Currently, it is still unclear how the pathophysiological environment in the AD brain affects neural stem cell biology.
Postmortem analysis of the hippocampus in patients with AD has identified a significant increase in neurogenesis in patients with AD, compared with controls, with the most-severely affected patients displaying the greatest increase [26]. Other evidence has shown that the expression of proteins that are linked to the activation of cell cycle mechanisms and the regulation of chromosomal replication (MCM2, Ki67, and PCNA) are observed in glial cells and neurons in the hippocampus, entorhinal cortex, and white matter in elderly human brains with different extents of AD-type pathology. These proteins trend toward increased expression levels, which are associated with more-advanced Braak stages [28].
Mouse models of AD have provided controversial results. Some studies have demonstrated both increased and decreased hippocampal neurogenesis [29]. One important factor is the disease severity, with a compensatory increase in progenitor proliferation in the early stages and decreased proliferation and survival with in the advanced stages of the pathology [30,31].
Mechanisms that induce neurodegeneration in Alzheimer's disease and their effects on neurogenesis
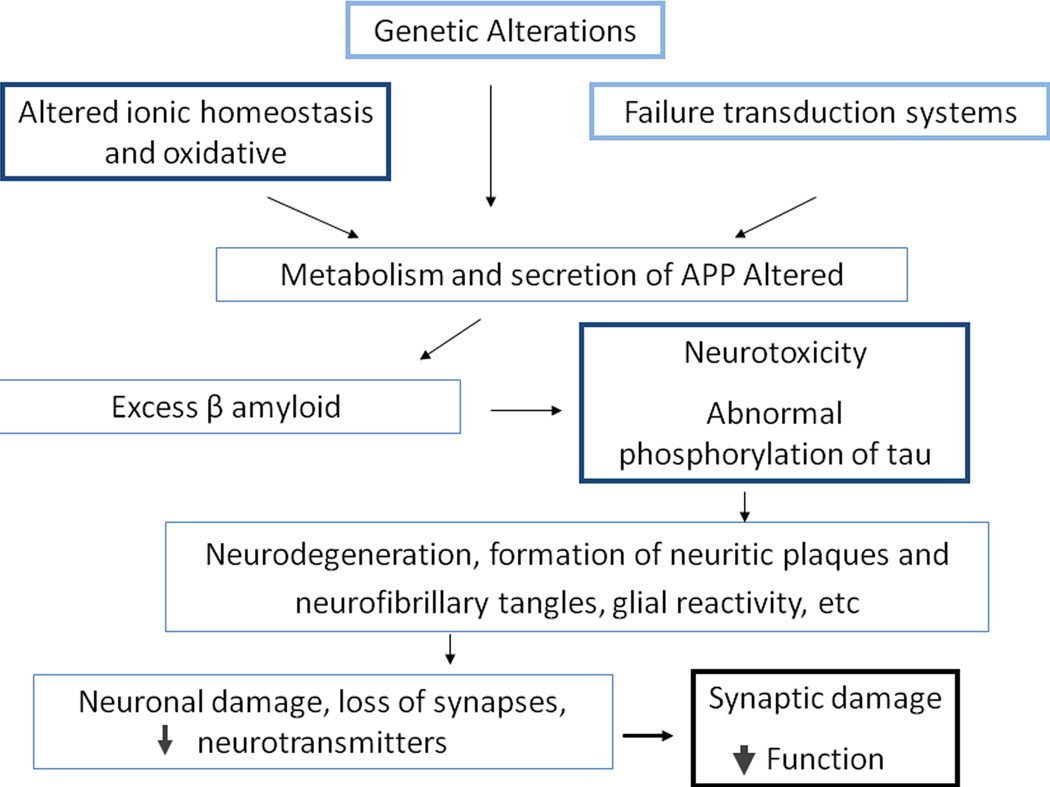
Alzheimer's disease is the primary cause of dementia in the elderly and begins with a hippocampal pathology [32,33]. This disease is characterized by dysfunctional intracellular and extracellular biochemical processes that result in neuronal death (Figure 1). Various evidence supports a role for the amyloid β(Aβ) peptide in AD [34,35]. When an autopsy is performed, the AD brain is characterized by a high density of amyloid plaques that are composed primarily of the Aβ peptide [36,37]. This histological finding is a necessary criterion for a conclusive diagnosis of the disease. The Aβ peptide isolated from AD brain tissue varies in length from 39–42 amino acids, and the predominant form found in the brains of AD patients is 42 amino acids (Aβ42) in length and has pathogenic importance because it can form toxic insoluble fibrils and accumulate in the neuritic plaques isolated from the brains of these patients [38,39]. Although the augmented levels of the pathogenic Aβ peptide assemblies likely contribute causally to AD [40], there is much debate about whether and how it affects adult neurogenesis in brain tissue.
Figure 1.
Mechanisms of neurodegeneration in AD. Defective cellular and genetic processes can lead to different defects, synaptic damage and cell death.
Studies have provided evidence of the adverse effects of Aβ on the proliferation, differentiation, and survival of adult mouse neural progenitor cells (NPCs) in the SVZ. The direct exposure of the human embryonic cortical NPC to Aβ results in decreases cell proliferation, whereas the direct exposure of differentiating neurospheres to Aβ induces the apoptosis of the newly generated neurons [41].
According to the results of transgenic animal studies and cell culture experiments, β-amyloid may also play a role in regulating neurogenesis, although the results of these experimental studies are inconsistent. Different studies have reported that the amyloid protein either reduces [42,43,44] or induces neurogenesis in adult transgenic animals [26,45].
The exposure of cell cultures derived from the adult mouse SVZ to Aβ (25–35 and 1–42) have demonstrated that Aβ peptides can influence the fate (but not the proliferation) of NSCs by driving their differentiation toward a neuronal lineage [46]. Different results have also come from studies using bromodeoxyuridine (BrdU) labeling and neuronal or glial markers in different lines of transgenic mice expressing the human amyloid precursor protein [41,47,48,49].
The basis of the effects of Aβ on the proliferation, differentiation, and survival of NSCs remains to be fully determined. Multiple analyses have documented that this process is regulated by different growth factors that are abundant during development, dramatically decline with age, and can contribute to reduced neurogenic potential [50,51]. Other studies have postulated that Aβ induces an increase in GABAergic neurotransmission, or an imbalance between GABAergic and glutamatergic circuits may contribute to impairment in AD [52].
Neurofibrillary alterations in AD
The brains of AD patients are characterized by hyperphosphorylated tau and the formation of neurofibrillary lesions, which constitute the intracellular deposits that form neurofibrilary tangles (NFTs) in neuronal cell bodies and apical dendrites, neuropil threads in the distal dendrites and axons and the dystrophic neurites that are associated with neuritic plaques [53,54]. Tau hyperphosphorylation and aggregation appear to have distinct effects on cell differentiation and death. Although some reports have proposed a protective role for tau hyperphosphorylation [55,56], tau aggregation is postulated to induce neuronal death [57]. Tau hyperphosphorylation has been shown to be reversible, whereas tau aggregation is not [58]. Schindowski, et al. (2008) generated a novel transgenic mouse line (THY-Tau22, which has the typical biochemical phosphorylation pattern of human tau in AD) and demonstrated an increase in neurogenesis during tau hyperphosphorylation, cell cycle events during abnormal tau phosphorylation, and tau aggregation preceding neuronal death and neurodegeneration [59]. These authors stated that their findings have also been observed in other tau transgenic mouse models with the following tau mutations: P301S [60], P301L [61,62], V337M [63] and R406W [64,65].
Recent studies have suggested that tau phosphorylation is essential for hippocampal neurogenesis [66]. A set of different protein kinases, including glycogen synthase kinase 3b (GSK3b), MAP kinase, the cyclin-dependent kinase 5 (Cdk5) system and others [67], is involved in tau phosphorylation. These kinases might be sensitive to changes in their regulatory patterns or at the structural level and, thus, participate in the molecular pathway leading to neurodegeneration. In this context, there are evidences of mutations of the amyloid precursor protein provoke an increase in GSK3 activity that facilitate tau phosphorilation and cell toxicity [68].
Evidence suggests that the manipulation of tau phosphorylation may compensate for neuronal loss in neurological disorders, including AD. Other investigations have examined the expression of phosphorylated tau in the SVZ and its role in adult neurogenesis. They found that tau colocalized with some SVZ neural precursors. However, it is not known the implications of this findings and the mechanism that participate in the molecular pathway leading to tau and neural precursors.
Reactive gliosis
In the last decade, evidence has converged regarding the roles of glial cells, alterations in their function and their implications for neuronal degeneration [69,70]. Reactive astrocytes have been found to be increased in the cortex and hippocampus of patients with AD. Although astrogliosis is an important neuropathological feature of AD, its significance is not completely clear. Some studies have suggested that the combined effects of cytokines derived from activated astroglial and microglial cells and Aβ mediate neuronal death. The generation of a cytokine milieu, which includes the up-regulation of tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), transforming growth factor β (TGFβ) and IL-6, at Aβ plaques in the brain potentially suppresses neurogenesis [71,72,73,74].
The increased expression of IL-6 in the aged brain is potentially significant, because this cytokine can be neurotoxic [75]. It has been shown that neurogenesis is decreased by 63% in the SGZ of adult transgenic mice whose astrocytes overexpress IL-6, and the proliferation, survival, and differentiation of neural progenitor cells labeled with thymidine are all reduced in the granule cell layers of these mice [76]. Embryonic cerebral precursor cells have also been shown to differentiate into astroglia when they are cultured in the presence of IL-6 or other cytokines; this mechanism is mediated by the Janus kinase signaling pathway [77]. Thus, IL-6 may restrain neurogenesis in the aged brain by redirecting progenitor cells toward a glial cell lineage [78].
TNFα is a key player in many pathological processes and is increased in some conditions, such as mild cognitive impairment and AD [79]. This cytokine and its receptors play important roles in neurogenesis in the adult brain [80,81]. Both receptors have been proposed to mediate distinct TNFα effects in the CNS, with TNF-R1 contributing to neuronal damage and TNF-R2 providing neuroprotection [82,83,84]. These results have been tested under both physiological and pathological conditions by activating these two receptors and observing their differential effects on proliferation and survival [72].
The cholinergic system in AD and its relationship with neurogenesis
Cholinergic deficits in AD are well established and include decreased levels of choline acetyltransferase (CAT), the biosynthetic enzyme for acetylcholine (ACh), decreased levels of acetylcholinesterase (AChE), the enzyme that degrades synaptic acetylcholine, and decreased levels of acetylcholine in the cortex [85]. These deficiencies are associated with a loss of neurons in the basal nucleus of Meynert [86]. A deficiency in cholinergic neurotransmission may account for some of the cognitive impairment observed in AD, and it correlates with the severity of dementia in multiple neocortical regions [88].
The cholinergic system plays an important role in neurogenesis because acetylcholine acts as growth regulatory signal in the brain. Ma et al. (2000) showed that ACh can stimulate the proliferation of NSCs and stem cell-derived progenitor cells during neural cell lineage progression in vitro [89]. In a transgenic mouse model, it has been shown that a diminution in the cholinergic innervation of the cortex is associated with different impairments in synaptic plasticity, and an acute increase in the availability of acetylcholine rescues these alterations in synaptic plasticity. The authors suggest that the cholinergic system mediates the impairment of cortical plasticity [90]. In addition, another study has provided in vivo evidence that the experience-dependent plasticity of the human auditory cortex is modulated by acetylcholine [91]. In spite of these results, few investigations have focused on clarifying the role of this system in neurogenesis and this condition.
Concluding remarks
One of the greatest challenges in elucidating the etiology of AD is the difficulty of studying the earliest changes in the neuronal function of the brain and correlating these changes with antemortem cognitive and behavioral function. Although suitable tissue specimens from patients with AD are very difficult to obtain, they are the most important and logical tools for understanding the causes of this disease. In this context, different mouse models of AD have been used to try to clarify the mechanism that induces this disease.
The discovery of neurogenesis in the adult brain and the regenerative potential of NSCs hold the promise of restoring the neural populations and regenerating the neural circuits that are necessary for cerebral function. Indeed, the core factors that drive neurogenesis in AD have not been elucidated. The induction of neurogenesis is of particular interest because the pathological manifestations of AD occur in the brain regions that are involved in learning and memory. Continued research in this area and the use of animal models are critical for evaluating whether neurogenesis-based therapeutic strategies will have the potential to aid those individuals with degenerative conditions.
Acknowledgements
R.E.G-C was supported by COECyTJAL PS-2009-827, PROMEP 103.5/09/743C. O.G-P was supported by CONACyT’s grant (CB-2008-101476) and NIH/NINDS (R01 NS070024-02)
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 3.Gould E, Reeves AJ, Graziano MS, et al. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 4.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursor to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 6.Emsley JG, Mitchell BD, Kempermann G, et al. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Namba T, Mochizucki H, Onodera M, et al. Postnatal neurogenesis in hippocampal slices cultures: early in vitro labeling of neural precursor cells leadas to efficient neuronal production. J Neurosci Res. 2007;85:1702–1712. doi: 10.1002/jnr.21295. [DOI] [PubMed] [Google Scholar]
- 8.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 9.Seri B, Garcia-Verdugo JM, McEwen BS, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gritti A, Bonfanti L, Doetsch F, et al. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coskun V, Luskin MB, et al. Intrinsic and extrinsic regulation of the proliferation and differentiation of cells in the rodent rostral migratory stream. J Neurosci Res. 2002;69:795–802. doi: 10.1002/jnr.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 13.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Buylla, Garcia-Verdugo JM, Tramontin AD. A unified hipótesis on the lineage of neural stem cells. Nat Rev Neurosci. 2005;25:10–18. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 15.Spassky N, Merkle FT, Flames N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maccioni RB, Munoz JP, Barbeito L. The molecular bases of Alzheimer´s disease and other neurodegenerative disorders. Arch Med Res. 2001;32:367–381. doi: 10.1016/s0188-4409(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 17.Bird TD. Genetic aspects of Alzheimer´s Disease. Genet Med. 2008;10:231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storey E, Kinsella GJ, Slavin MJ, et al. The neuropsychological diagnosis of Alzheimer´s disease. J Alzheimer´s Dis. 2001;3:261–285. doi: 10.3233/jad-2001-3302. [DOI] [PubMed] [Google Scholar]
- 19.Corey-Bloom J. The ABC of Alzheimer´s disease: cognitive changes and therir management in Alzheimer´s disease and related dementias. Int Psychogeriatr. 2002;14:51–75. doi: 10.1017/s1041610203008664. [DOI] [PubMed] [Google Scholar]
- 20.Giannakopoulos P, Kövari E, Gold G. Pathological substrates of cognitive decline in Alzheimer´s Disease. Front Neurol Neurosci. 2009;24:20–29. doi: 10.1159/000197881. [DOI] [PubMed] [Google Scholar]
- 21.Rice DP, Fillit HM, Max W, et al. Prevalence, costs, and treatment of Alzheimer´s Disease and related dementia: a managed care perspective. Am J Manag Care. 2001;7:809–818. [PubMed] [Google Scholar]
- 22.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Fallon J, Reid S, Kinyamu R, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci USA. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldauf K, Reymann KG. Influence on proliferation, early neurogenesis and infart volume after transient focal ischemia. Brain Res. 2005;1056:158–167. doi: 10.1016/j.brainres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientis. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- 26.Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to cites of CNS injury by the stromal cell derived factor 1 alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wharton SB, Williams GH, Stoeber K, et al. Expression of Ki67, PCNA and the chromosome replication licensing protein Mcm2 in glial cells of the ageing human hippocampus increases with the burden of Alzheimer- type pathology. Neurosci Lett. 2005;383:33–38. doi: 10.1016/j.neulet.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Kempermann G. Adult Neurogenesis: Stem cells and Neuronal development in adult brain. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 30.Gan L, Qiao S, Lan X, et al. Neurogenic responses to amyloid-beta plaques in the brain of Alzhimer´s disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiol Dis. 2008;29:71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biscaro B, Lindvall O, Hock C, et al. AB immunotherapy protect morphology and survival of adult born neurons in doubly transgenic APP/PS1 mice. J Neurosci. 2009;29:14108–14119. doi: 10.1523/JNEUROSCI.2055-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Alafuzoff, Arzberger T, et al. Staging of Alzheimer´s disease-associated neurofibrillary pathology using paraffin sections and immunocytochemestry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson D, Castaño E, Kokjohn TA, et al. Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer´s disease. Neurol Res. 2005;27:869–881. doi: 10.1179/016164105X49436. [DOI] [PubMed] [Google Scholar]
- 35.Selkoe DJ. Alzheimer´s disease results from the cerebral accumulation and citotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:95–99. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 36.Amatsubo T, Yanagisawa D, Morikawa S, et al. Amyloid imaging using high field magnetic Resonance. Magn Reson Med. 2010;9:95–99. doi: 10.2463/mrms.9.95. [DOI] [PubMed] [Google Scholar]
- 37.Cerpa W, Dinamarca MC, Inestrosa NC, et al. Structure-function implications in Alzheimer´s disease: effect of beta oligomers at central synapses. Curr Alzheimer Res. 2008;5:233–243. doi: 10.2174/156720508784533321. [DOI] [PubMed] [Google Scholar]
- 38.LaFerla FM, Oddo S. Alzheimer´s disease: Abeta, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Kar S, Slowikowski SP, Westaway D, et al. Interactions between β amyloid and central cholinergic neurons: implications for Alzheimer´s disease. J Psychiatry. 2004;29:427–441. [PMC free article] [PubMed] [Google Scholar]
- 40.Tanzi RE, Bertram L. Twenty years of the Alzheimers disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Haughey NJ, Liu D, Nath A, et al. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta- peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med. 2002;1:125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- 42.Haughey NJ, Nath A, Chan SL, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J. Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 43.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Li B, Yamamori H, Tatebayashi Y, et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67:78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta- amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calafiore M, Battaglia G, Zappala A. Progenitor cells from the adult mouse brain acquire a neuronal phenotype in response to beta amyloid. Neurobiol Agin. 2006;27:606–613. doi: 10.1016/j.neurobiolaging.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Donovan MH, Yazdani U, Norris RD, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind) J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- 49.Verret L, Trouche S, Zerwas M, et al. Hippocampal neu- rogenesis during normal and pathological aging. Psychoneuroendocrinology. 2007;32:S26–S30. doi: 10.1016/j.psyneuen.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Halbach OB. Involvement of BDNF in age dependent alterations in the hippocampus. Front Aging Neurosci. 2010;2:1–11. doi: 10.3389/fnagi.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bath KG, Lee FS. Neurotrophic factor control of adult SVZ neurogenesis. Dev Neurobiol. 2010;70:339–349. doi: 10.1002/dneu.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun B, Halabisky B, Zhou Y, et al. Imbalance between GABAergic and Glutamatergic Transmission impairs Adult neurogenesis in an animal model of Alzheimer´s Disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Igbal K, Novak M. From tangles to tau protein. Bratisl Lek Listy. 2006;107:341–342. [PubMed] [Google Scholar]
- 54.Corbo CP, Alonso AC. Therapeutic targets in Alzheimer´s disease and related proteins. Prog Mol Biol Transl Sci. 2011;98:47–83. doi: 10.1016/B978-0-12-385506-0.00002-8. [DOI] [PubMed] [Google Scholar]
- 55.Hamdane M, Bretteville A, Sambo AV, et al. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J Cell Sci. 2005;118:1291–1298. doi: 10.1242/jcs.01724. [DOI] [PubMed] [Google Scholar]
- 56.Lee HG, Perry G, Moreira PI, et al. Tau phosphorylation in Alzheimer’s disease: pathogen or protector. Trends Mol Med. 2005;11:164–169. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Ramsden M, Kotilinek L, Forster C, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schindowski K, Belarbi K, Bretteville AA. Neurogenesis and cell cycle-reactivated neuronal death during pathogenic tau aggregation. Genes, Brain and Behaviour. 2008;7:92–100. doi: 10.1111/j.1601-183X.2007.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen B, Ingram E, Takao M, et al. Abundant tau filaments and nonapoptotic neurodegen- eration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gotz J, Chen F, Barmettler R, et al. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- 62.Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 63.Tanemura K, Murayama M, Akagi T, et al. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikeda M, Shoji M, Kawarai T, et al. Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am J Pathol. 2005;166:521–531. doi: 10.1016/S0002-9440(10)62274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim F, Hernandez F, Lucas JJ, et al. FTDP-17 mutations in tau transgenic mice provoke lysosomal abnormalities and Tau filaments in forebrain. Mol Cell Neurosci. 2001;18:702–714. doi: 10.1006/mcne.2001.1051. [DOI] [PubMed] [Google Scholar]
- 66.Hong XP, Peng CX, Wei-w, et al. Essential role of tau phosphorylation in adult hippocampal neurogenesis. Hippocampus. 2010;20:1339–1349. doi: 10.1002/hipo.20712. [DOI] [PubMed] [Google Scholar]
- 67.Maccioni RB, Otth C, Concha II. The protein Kinase CdK5 structural aspects, roles in neurogenesis and involvement in Alzheimer´s disease. Eur J Biochem. 2001;268:1518–1527. doi: 10.1046/j.1432-1033.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- 68.Delacourte A, David JP, Seargeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer´s disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 69.Saez TE, Pehar M, Vargas M, et al. Astrocytic nitric oxide triggers tau hyperphosphorylation in hippocampal neurons. In vivo. 2004;18:275–280. [PubMed] [Google Scholar]
- 70.Saez ET, Pehar M, Vargas MR, et al. Production of nerve grow factor by beta-amyloid-stimulated astrocytes induces p75 NTR-dependent tau hyperphosphorylation in cultured hippocampal neurons. J Neurosci Res. 2006;84:1098–1106. doi: 10.1002/jnr.20996. [DOI] [PubMed] [Google Scholar]
- 71.Buckwalter MS, Yamane M, Coleman BS, et al. Chronically increased transforming growth factor-β1 strongly inhibits hip- pocampal neurogenesis in aged mice. Am J Pathol. 2006;169:154–164. doi: 10.2353/ajpath.2006.051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iosif RE, Christine T, Dahl EK, et al. Tumor Necrosis Factor Receptor 1 is a negative Regulator of progenitor Proliferation in Adult Hippocampal Neurogenesis. The Journal of Neuroscience. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaneko N, Kudo K, Mabuchi T, et al. Suppression of cell proliferation by interferon-α through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31:2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- 74.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 75.Papanicolaou DA, Wilder RL, Manolagas SC, et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 76.Vallieres L, Campbell IL, Gage FH, et al. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonni A, Sun Y, Nadal-Vicens M, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 78.Godbout JP, Johnson RW. Interleukin-6 in the aging brain. Journal of Neuroimmunology. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 79.Tarkowski E, Liljeroth AM, Minthon L, et al. Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res Bull. 2003;61:255–260. doi: 10.1016/s0361-9230(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 80.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 81.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 82.Fontaine V, Mohand-Said S, Hanoteau N, et al. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-07-j0001.2002. RC216(1–7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang L, Lindholm K, Konishi Y, et al. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction path- ways. J Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchetti L, Klein M, Schlett K, et al. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate- induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- 85.Mufson EJ, Ginsberg SD, Ikonomovic MD, et al. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 86.Lehéricy S, Hirsch EC, Cervera-Piérot P, et al. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer´s disease. J Comp Neurol. 1993;330:15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- 87.Auld DS, Kornecook TJ, Bastianetto S, et al. Alzheimer´s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 88.Bierer L, Haroutunian V, Gabriel S, et al. Neurochemical correlatos of dementia severity in Alzheimer´s disease: relative importante of the cholinergic déficits. J Neurochem. 1995;64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- 89.Ma W, Maric D, Li BS, et al. Acetyilcholine stimulates cortical precursor cell proliferation in Vitro via muscarinic receptor activation and MAP Kinase phosphorylation. Eur J Neurosci. 2000;12:1227–1240. doi: 10.1046/j.1460-9568.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 90.Pesavento E, Capsoni S, Domenici L, et al. Acute cholinergic rescue of synaptic plasticity in the neurodegenerating cortex o fanti nerve growth factor mice. Eur J Neuroci. 2000;15:1030–1036. doi: 10.1046/j.1460-9568.2002.01937.x. [DOI] [PubMed] [Google Scholar]
- 91.Thiel CM, Friston KJ. Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron. 2002;35:567–574. doi: 10.1016/s0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]