Abstract
Long-term maintenance of tissue homeostasis relies on the accurate regulation of somatic stem cell activity. Somatic stem cells have to respond to tissue damage and proliferate according to tissue requirements, while avoiding over-proliferation. The regulatory mechanisms involved in these responses are now being unraveled in the intestinal epithelium of Drosophila, providing new insight into strategies and mechanisms of stem cell regulation in barrier epithelia. Here, we review these studies and highlight recent findings in vertebrate epithelia that indicate significant conservation of regenerative strategies between vertebrate and fly epithelia.
Introduction
Precise control of somatic stem cell activity is essential to the maintenance of tissue homeostasis in multicellular organisms. To ensure efficient replacement of damaged cells while limiting the potential for cancer, the proliferation rate of stem and progenitor cells has to be closely linked to tissue demands at any given time. This complex regulation, in which stem cells integrate local and systemic cues with cell-intrinsic maintenance mechanisms, is only beginning to be understood. Unraveling these signaling mechanisms is likely to not only provide insight into basic mechanisms of stem cell regulation, but also elucidate the molecular etiology of tissue dysfunction, including age-related degeneration and cancer (Radtke and Clevers, 2005; Rossi et al., 2008; Sharpless and DePinho, 2007). Indeed, characterization and genetic manipulation of selected stem cell populations in the mouse has demonstrated that the precise control of stem cell proliferation is crucial to prevent tumor formation (Barker et al., 2009; Lapouge et al., 2011; White et al., 2011; Youssef et al., 2010), and the identification of molecular similarities between cancer stem cells and tissue-specific stem cells further supports this notion (Merlos-Suarez et al., 2011).
Different stem and progenitor cell populations display remarkable diversity in their proliferative behavior. This diversity presumably reflects the different regenerative requirements of individual tissues, and allows classifying stem cells into distinct categories (Figure 1): (i) continuously cycling stem cells of high-turnover tissues, such as intestinal stem cells (Li and Clevers, 2010; Simons and Clevers, 2011; van der Flier and Clevers, 2009) and short-term hematopoietic stem cells (HSCs) (Fuchs, 2009), (ii) stem cells whose proliferative activity can be strongly induced by injury, including airway basal epithelial stem cells and muscle satellite cells (Abou-Khalil and Brack, 2010; Dhawan and Rando, 2005; Rock and Hogan, 2011), and (iii) stem cells with alternate quiescent and proliferative periods, such as hair follicle stem cells (Fuchs, 2009). While distinct, these categories may not necessarily reflect intrinsic qualitative differences in stem cell regulation, as all stem cell populations display significant proliferative plasticity. The mouse intestine, for example, has a very rapid turn-over rate, requiring lgr5+ stem cells in the small intestine to divide once every 24h to 48h (Barker et al., 2007; Snippert et al., 2010), but proliferation in the intestinal crypt can still be further increased in response to injury and infection (Liu et al., 2010c; Saleh and Elson, 2011; Seno et al., 2009). Similarly, in the bone marrow, long-term HSCs can undergo a reversible transition from quiescence to self-renewal (He et al., 2009; Wilson et al., 2008), while the mouse trachea and muscle satellite cells display a low turnover rate in homeostasis, but dramatically increase their proliferative activity in response to injury (Dhawan and Rando, 2005; Rock and Hogan, 2011; Shea et al., 2010). These similarities suggest that the proliferative plasticity of different stem cell populations may be regulated by common mechanisms.
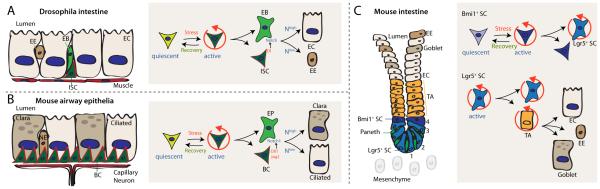
Figure 1. Similarities and differences between stem cell lineages in mouse and flies.
A: Drosophila intestinal stem cell (ISC) lineage. ISC proliferation can be induced by stress signals. ISCs express the Notch ligand Delta (Dl), and their division has an asymmetric outcome, generating a new ISC and an Enteroblast (EB), which differentiates into either an Enterocyte (EC; in response to high Notch activation) or an Enteroendocrine cell (EE, in response to low Notch activation).
B: Mouse airway epithelial stem cells (basal cells, BC) divide asymmetrically to give rise to an early progenitor (EP) cell and a new BC. Lineage decision between Clara cells and Ciliated cells is achieved by differential Notch signaling, similar to the fly ISC lineage (see Rock et al., 2011).
C. The crypt of the mammalian small intestine is composed of two different stem cell populations that can regenerate the full tissue. Lgr5+ cells appear to be continuously cycling and give rise to new Lgr5+ cells as well as transit amplifying (TA) cells that proliferate and differentiate into one of several cell fates (enterocytes, ECs; enteroendocrine cells, EEs; and Goblet cells). Differentiated Paneth cells serve as support cells in the basal crypt. Bmi1+ cells divide more rarely and have been proposed to serve as reservoir to replace Lgr5+ cells after injury. See Barker et al., 2007 and Tian et al., 2011.
To maintain homeostasis in mitotically active tissues, stem cell activity has to be controlled at several levels: (i) steady-state SC proliferation and self-renewal, as well as differentiation, to ensure long-term maintenance of a pluripotent SC pool, (ii) acute induction of SC proliferation in response to tissue damage, (iii) re-entry into a quiescent or non-proliferative state after the tissue has been repaired or regenerated. The complexity of somatic stem cell lineages in mammals often causes difficulties in definitively characterizing the regulation of these processes at the stem cell level in vivo, however, as transit amplifying cell populations exist in most regenerative tissues and as stem cells are not definitively identified in all organs. The lineage relationship between different groups of multipotent cell population(s) in the mouse intestinal epithelium, for example, remains under investigation. Two populations of cells, Bmi1-positive ‘+4’ cells and Lgr5-positive Crypt Base Columnar cells, were found to be able to fully regenerate the intestinal epithelium (Barker et al., 2007; Sangiorgi and Capecchi, 2008). A recent study demonstrates that a hierarchy exists in this lineage, and that Lgr5+ stem cells are dispensable for epithelial homeostasis in the villus. Interestingly, Bmi1+ cells, which constitute a more quiescent cell population, are capable of replenishing the Lgr5+ population in response to high regenerative demand (Tian et al., 2011). Whether the proliferation rate of these two SC populations is regulated by similar or distinct mechanisms remains largely unexplored.
Characterization of stem cell plasticity in simpler model organisms in which lineage relationships are clearly defined is thus expected to provide important conceptual and mechanistic insight into the maintenance of tissue homeostasis. Recent findings in Drosophila have provided such a model. Adult somatic stem cells have been identified in the fly gonad, the intestine and the malpighian tubules (Decotto and Spradling, 2005; Fox and Spradling, 2009; Gonczy and DiNardo, 1996; Margolis and Spradling, 1995; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Singh et al., 2007; Takashima et al., 2008). Among these stem cell populations, Intestinal Stem Cells (ISCs) of the posterior midgut display remarkable proliferative plasticity and similarity to mammalian epithelial stem cell populations (Casali and Batlle, 2009). Due to the advanced genetic tools available and the speed of genetic analysis, the exploration of molecular mechanisms regulating ISC function in flies has been extraordinarily rapid and comprehensive. An integrated model for epithelial stem cell regulation is thus emerging that is beginning to guide similar analysis in vertebrates. Here, we review the findings in the Drosophila system, highlighting emerging concepts as well as similarities and differences with vertebrate stem cell systems.
The Drosophila intestinal stem cell lineage
The Drosophila midgut displays functional and morphological similarities with the mammalian small intestine, as well as with other vertebrate barrier epithelia. It consists of a simple columnar epithelium that is surrounded by visceral muscle, but in contrast to the mammalian intestine is not organized in crypts and villi (Figure 1). Consistent with this simpler overall structure, it is also composed of a limited number of cell types: large and polyploid EnteroCytes (ECs), the main absorptive cells in the epithelium, several types of small diploid enteroendocrine cells (EE), which secrete different hormones (including tachykinin or allatostatin), as well as the common progenitors of these cells, the ISCs and their diploid daughter cells, EnteroBlasts (EBs). ISCs can be identified within the epithelium by their expression of the Notch ligand Delta (Dl) and the transcription factor escargot (esg). In young, healthy guts, EBs also express Esg, but not Dl, while expressing Notch signaling reporters. EEs, in turn, are the only intestinal cells expressing the transcription factor prospero (pros), and ECs express the transcription factor Pdm-1.
The ISC lineage was first characterized by Micchelli and Perrimon and by Ohlstein and Spradling (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Both labs employed somatic recombination to trace cell lineages in the adult posterior midgut, and thus conclusively demonstrated that ISCs represent a multipotent stem cell population that resides basally in the intestinal epithelium and that gives rise to all cell types of the epithelium. Importantly, these studies also showed that the Drosophila intestinal epithelium lacks a transit amplifying cell population, as EBs directly differentiate into EEs or ECs. This fact allows direct quantification of stem cell mitotic activity in this tissue (commonly detected using antibodies against phosphorylated Histone H3), as the only dividing cells detectable in the posterior midgut are ISCs.
Control of proliferative activity of ISCs
Homeostatic proliferation
Signaling events regulating homeostatic proliferation in epithelial tissues of mammals have been extensively studied, yet the cell-specific requirements for individual signaling events remain unclear. In the intestine, for example, Wnt signaling, Notch signaling and BMP signaling all promote proliferation, but also influence differentiation in the crypt (Crosnier et al., 2006; van der Flier and Clevers, 2009). Whether these effects are primarily a consequence of influencing stem cell proliferation or transit amplifying cell proliferation, however, remains unclear.
In flies, the availability of lineage-tracing techniques for lineages derived from homozygous mutant stem cells has allowed characterizing the signaling requirements for homeostatic proliferation of ISCs in detail, and has led to the emerging concept that signaling mechanisms ensuring homeostatic proliferation and signaling events inducing proliferation in response to injury are distinct.
Drosophila ISCs are mostly slow- or non-proliferating in young, unchallenged intestines, but become highly proliferative after an environmental challenge or tissue injury. The existence of a ‘quiescent’ state for ISCs has been under some debate: lineage tracing in ISCs had initially led to the impression that ISCs are continuously dividing cells, since once induced, clones appear to grow linearly, then enter a steady state where production of new cells seems to be in balance with the turnover rate of ECs (Ohlstein and Spradling, 2006). This interpretation is complicated, however, by mitotic and S-phase labeling experiments, which indicate long periods of very limited ISC proliferation in young, healthy guts. BrdU incorporation, for example, is observed in only 5-10 % of all ISCs in a 48 hour window (Hochmuth et al., 2011). A low basal level of tissue renewal has been revealed, on the other hand, by separate lineage tracing studies. In these, a ‘Flp-out’ strategy was used to induce heritable GFP expression in all ISCs and their progeny in adulthood, demonstrating that the whole tissue is turned over in about 2 weeks in females and in over a month in males (Jiang et al., 2009). This corresponds to total tissue turnover of about 4 times in females and twice in males over the whole lifespan of the animal.
This basal homeostatic proliferation and self-renewal of ISCs requires the activity of several growth factor signaling pathways (Figure 2). Using the MARCM method (Lee and Luo, 1999)(Textbox 1) to induce homozygosity for mutations in the EGF Receptor and the Insulin Receptor, it was shown that the growth factor response pathways activated by these receptors are essential for ISC proliferation under unstressed conditions (resulting in mutant clones consisting of mostly single stem cells) (Biteau and Jasper, 2011; Biteau et al., 2010; Jiang et al., 2011; Xu et al., 2011). Downstream mediators of these pathways include the Insulin Receptor Substrate, PI3Kinase, Akt, Ras and ERK and all these molecules have also been shown to be essential for ISC proliferation. Consistent with a general permissive role for these signaling pathways, activated ERK (dpERK) can be detected in all ISCs under normal conditions (Biteau and Jasper, 2011; Jiang et al., 2011; Xu et al., 2011). Interestingly, constitutive activation of EGFR/InR signaling components can also increase ISC proliferation rates, indicating that the level of RTK signaling activity modulates the proliferative state of ISCs (Biteau and Jasper, 2011; Jiang et al., 2011; Xu et al., 2011).
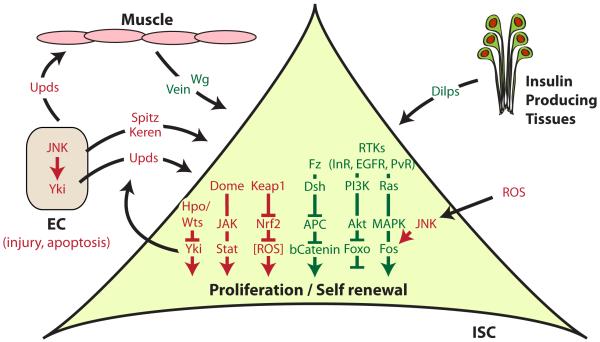
Figure 2. Signaling pathways regulating Intestinal Stem Cell proliferation and self-renewal in Drosophila.
ISCs integrate local and systemic cues with cell-intrinsic signals to adapt their proliferation rate to tissue demand. Signaling pathways required for homeostatic proliferation are represented in green, pathways required for stress and injury-induced ISC proliferation are represented in red.
In addition, the MAPK p38 is required for ISC proliferation under unstressed conditions (Park et al., 2009), and it was suggested that it acts as a mediator of the effect of the PDGF/VEGF-like receptor signaling pathway (composed of Pvf ligands and the PvR receptor) on ISC proliferation (Choi et al., 2008; Park et al., 2009). It has not yet been established, however, whether Pvf and PVR are also required for homeostatic proliferation.
Interestingly, ligands for the EGFR and the InR pathways are dynamically controlled in response to environmental challenges. Nutritional state can significantly affect insulin-like peptide (Dilp) expression, while oxidative stress or DNA damage results in repression of dilp expression (Geminard et al., 2009; Karpac et al., 2011; Slaidina et al., 2009; Wang et al., 2005). This regulation may thus allow adjusting ISC proliferation to systemic nutrient and stress levels (Amcheslavsky et al., 2009; McLeod et al., 2010). Nutrition also influences the activity of the TSC/Tor signaling pathway, and excessive Tor activation seems to have deleterious consequences for ISC activity (Amcheslavsky et al., 2011). Infection with pathogenic bacteria, on the other hand, induces EGF-like ligand expression in the gut (Buchon et al., 2010; Buchon et al., 2009b; Jiang et al., 2011; Xu et al., 2011). These ligands are secreted by both epithelial cells (including ISCs and ECs), as well as by the surrounding visceral muscle, displaying a remarkable redundancy and indicating that ISC maintenance and proliferation may be coordinated across the entire organ by ligands derived from multiple regions. Insulin and EGF-like growth factors thus serve as permissive signals for ISC proliferation, while also contributing to the proliferative response to stress.
Activation of stem cell proliferation by injury and stress
Recent studies in mammals support the idea that the mechanisms regulating SC proliferation in response to tissue injury are, at least partially, distinct from those essential for homeostatic regeneration. For example, the Hippo and Focal Adhesion Kinase pathways were recently shown to be required in the intestinal crypt for acute tissue regeneration in response to DSS or gamma irradiation, respectively, while being dispensible for homeostatic proliferation (Ashton et al., 2010; Cai et al., 2010). A comprehensive and detailed analysis of stress-induced SC proliferation is thus warranted and promises to provide important insight into the maintenance of tissue homeostasis under challenging conditions.
In Drosophila, upon stress or injury, ISCs respond by dramatically increasing their proliferative activity to replenish the epithelium with functional differentiated cells. This activation occurs in response to infection (Apidianakis et al., 2009; Buchon et al., 2009a; Chatterjee and Ip, 2009; Cronin et al., 2009; Jiang et al., 2009), oxidative stress (Biteau et al., 2008; Buchon et al., 2009a; Choi et al., 2008), DNA damage (Amcheslavsky et al., 2009) and heat stress, as well as other factors that damage the epithelium (such as DSS) and that cause EC apoptosis (Amcheslavsky et al., 2009; Jiang et al., 2009). Stress-induced activation of ISCs is essential for flies to survive these stresses (Buchon et al., 2009a; Cronin et al., 2009; Jiang et al., 2009).
A comprehensive picture of the signals mediating this proliferative response is emerging (Figure 2): ISCs respond to intrinsic challenges, such as oxidative stress through the Jun-N-terminal Kinase (JNK) pathway (Biteau et al., 2008; Choi et al., 2008), to local cues through the JAK/Stat signaling pathway (Buchon et al., 2009a; Cronin et al., 2009; Jiang et al., 2009), and to changes in tissue integrity through the hippo/yorkie pathway (Karpowicz et al., 2010; Ren et al., 2010; Shaw et al., 2010; Staley and Irvine, 2010). These inductive pathways are characterized by the fact that their activation is not required for ISC proliferation under homeostatic conditions, but is required and sufficient to increase ISC proliferative activity in response to stress. They are integrated in multiple ways: JAK/Stat signaling is activated in ISCs by locally derived interleukin 6 - like cytokines named Unpaired 1-3 (Upd1-3). These cytokines are secreted from damaged and dying ECs in response to infection (Buchon et al., 2009a; Cronin et al., 2009; Jiang et al., 2009). Artificial induction of JNK in ECs is sufficient to induce Upd expression (Jiang et al., 2009), potentially through the activation of the Yorkie transcription factor,(Staley and Irvine, 2010). However, JNK is not required in ECs for the proliferative response upon infection (Buchon et al., 2009a; Jiang et al., 2009). Yorkie also induces Upd expression in ISCs themselves, activating proliferation in an autocrine manner (Karpowicz et al., 2010). In addition, JAK/Stat signaling acts indirectly to promote ISC proliferation by increasing the expression of the EGF-like ligand Vein in the visceral muscle (Buchon et al., 2010). Through secreted Upd cytokines, injured or dying ECs thus initiate and promote regenerative activity in the epithelium by directly activating cell cycle progression in ISCs, while at the same time triggering increased growth factor secretion from the muscle. In addition to its potential role in dying ECs, the JNK pathway also autonomously activates ISC proliferation by phosphorylating the AP-1 transcription factor Fos (Biteau and Jasper, 2011). Fos is phosphorylated by JNK and the EGFR responsive MAPK kinase ERK on distinct sites, thus integrating both permissive and inductive signals (Ciapponi et al., 2001). Accordingly, Fos is required for both homeostatic ISC proliferation and for stress-induced activation of ISCs.
Redox state and stem cell function
An important question that remains unanswered in most stem cells lineages, is how exactly ISC proliferation is increased by stress signals. Two non-exclusive mechanisms can be envisioned (Figure 3A): Quiescent stem cells that do not divide under homeostatic conditions may be activated, leading to an overall increase in proliferating cells within the stem cell population. Alternatively, the cell cycle of actively cycling stem cells might be shortened, increasing the proliferation rate within an identical stem cell pool. Further studies of the proliferative response in the fly intestinal epithelum are required to differentiate between these possibilities, and are expected to generate testable hypotheses for the regulation of mammalian stem cell systems.
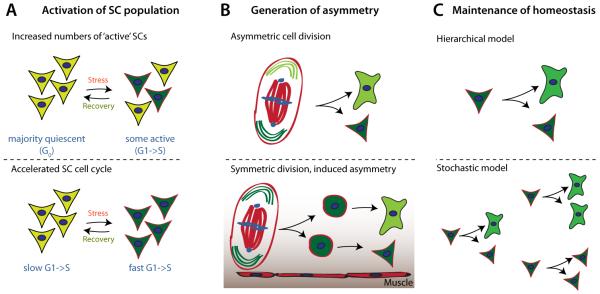
Figure 3. Controversies and open questions in somatic stem cell plasticity.
A: The mechanism of stem cell ‘activation’: Due to technical limitations, it is difficult to differentiate in vivo between a situation in which increased regeneration after a stimulus occurs by a transition of a number of stem cells from an ‘inactive’ quiescent state to an active state, and a mechanism in which the cell cycle of a majority of stem cells is simply accelerated. Extensive lineage tracing, preferentially in ex-vivo cultured tissues where individual stem cells can be followed might help resolve this question.
B: Generating asymmetry: In the Drosophila intestine, ISC divisions result in a consistently asymmetric outcome, it remains unclear, however, if ISC divisions are intrinsically asymmetric, or if daughter cells are asymmetrically specified by local cues.
C. Maintaining homeostasis: A stable pool of stem cells can be maintained within the tissue by either consistently asymmetric cell divisions (hierarchical model), or by mechanisms that control the overall number of stem cells generated by random symmetric/asymmetric divisions (stochastic model). While the Drosophila inestinal epithelium appears to be maintained primarily through the hierarchical model, stem cell homeostasis in the mouse crypt might be maintained by stochastic mechanisms (Snippert et al. 2010, but see also Tian et al., 2011).
In this context, one recent study supports the first possibility, namely the existence of quiescent cells within the ISC pool, and the stress-induced transition of these cells towards an actively dividing state (Hochmuth et al., 2011). Strikingly, this transition appears to be regulated primarily by changes in the intracellular redox state of ISCs, highlighting the emerging critical role of redox regulation in stem cell biology. A low intracellular concentration of reactive oxygen species (ROS) is increasingly recognized as a critical condition for stemness, self-renewal and pluripotency in both mammals and in Drosophila.
In mice, elevated ROS levels result in reduced regenerative potential and self-renewal in a wide range of stem cell populations, including neuronal and glial progenitors and HSCs (Diehn et al., 2009; Ito et al., 2004; Le Belle et al., 2011; Liu et al., 2009; Miyamoto et al., 2007; Miyamoto et al., 2008; Smith et al., 2000; Tothova and Gilliland, 2007; Tothova et al., 2007; Tsatmali et al., 2005). The reason and consequence of the correlation between redox state and stem cell behavior remains enigmatic, but recent findings in flies have begun unraveling signaling mechanisms mediating this effect. Increased ROS concentration primes hematopoietic progenitors of the larval lymphgland for differentiation (Owusu-Ansah and Banerjee, 2009) and promotes ISC proliferation in the adult gut (Biteau et al., 2008; Buchon et al., 2009a; Choi et al., 2008; Hochmuth et al., 2011). In the intestine, high levels of ROS are produced by ECs to control commensal and pathogenic bacterial populations, and increased ROS production is a likely cause of the increased ISC proliferation observed in aging animals (Biteau et al., 2008; Buchon et al., 2009a; Choi et al., 2008; Ha et al., 2009; Ha et al., 2005; Hochmuth et al., 2011). A central regulator of the intracellular redox state in vertebrates and invertebrates is Nrf2, a member of the ‘cap-and-collar’ (Cnc) family of transcription factors. By influencing the stem cell redox state, the Drosophila homologue of Nrf2, CncC, and its negative regulator Keap1 control ISC proliferation rates, and this regulation is required to limit ISC hyperproliferation and intestinal degeneration in aging flies (Hochmuth et al., 2011). Interestingly, Keap1 mutant mice show significant hyperkeratosis of the esophageal epithelium, suggesting that Keap1/Nrf2 also influences cell differentiation and proliferation in mammalian barrier epithelia (Wakabayashi et al., 2003). The Keap1/CncC regulatory module thus emerges as central in the control of ISC proliferation and intestinal regeneration.
How the changes in redox state induced by Nrf2 activity might be translated into specific stem cell responses remains unclear. The control of hematopoietic progenitor differentiation by high ROS levels suggest an interesting role for the JNK pathway, however, as it acts to induce Foxo activity and promote Polycomb complex downregulation in this system (Owusu-Ansah and Banerjee, 2009). In the intestine, JNK activation induces proliferation of ISCs, but Foxo activation suppresses proliferation (Biteau et al., 2008; Biteau et al., 2010). While a similar JNK response thus might play a role downstream of Nrf2 in ISCs, the mechanistic details of this response are likely to be substantially distinct from the hematopoietic system.
Proliferative plasticity is required for tissue maintenance and longevity
It is of interest that all the signaling pathways described above have been implicated in tumor formation in vertebrates (Radtke and Clevers, 2005). For example, a role of AP-1 transcription factors and JNK signaling in the regulation of intestinal stem cell proliferation and intestinal cancer has been reported in mice: JNK can induce cell proliferation in the intestinal crypt and increases tumor incidence and growth in an inflammation-induced colon cancer model (Sancho et al., 2009). It remains unclear, however, if this proliferative response is initiated in Lgr5+ or Bmi1+ stem cells, or rather in the transit amplifying cell population. The Fos binding partner c-Jun may mediate these effects, as indicated by the fact that knock-in of non-phosphorylatable c-Jun mutants into the c-Jun locus reduces tumorigenesis in the intestine caused by loss of APC (Nateri et al., 2005). Further illustrating the conserved function of Fos in the regulation of stem cell function, increased Fos activity has also been found to be sufficient to promote hematopoietic stem cell self-renewal in mice (Deneault et al., 2009). In the fly, ISC-specific activation of Wnt signaling, by mutating APC or expressing an active form of b-catenin or wingless itself, is also sufficient to induce the formation of tumor-like stem cell clusters (Cordero et al., 2009; Lee et al., 2009; Lin et al., 2008). Whether Wnt signaling also interacts with JNK and Jun/Fos in Drosophila ISCs remains to be tested, yet regulation of stem cell proliferation by JNK signaling and AP-1 appears to be a broadly conserved phenomenon.
The precise role of stem cell specific stress signaling in the development of tumors remains unclear, however, and the detailed analysis of stem cell activity in the Drosophila intestine is providing important new insight into the requirements for dynamic stem cell control to prevent loss of epithelial homeostasis. The emerging model highlights the critical role for negative feedback regulation of inductive signaling to ensure an appropriate but limited burst of ISC proliferation following injury. Thus, when stimuli are removed, ISC proliferation is rapidly decreased after the initial induction of proliferation (Buchon et al., 2010; Jiang et al., 2009). Chronic and excessive activation of JNK signaling in the ISC lineage, in turn, causes intestinal dysplasia (Biteau et al., 2008), while over-expression of tumorigenic RasV12 predisposes ISCs to tumor formation when exposed to a stimulus (Apidianakis et al., 2009). It is likely that the down-regulation of proliferative activity is the consequence of the induction of multiple negative regulators of inductive signaling pathways, such as Socs36E (inhibitor of JAK/Stat signaling), Puckered (inhibitory phosphatase of JNK) or Argos (negative regulator of EGFR) (Biteau et al., 2008; Buchon et al., 2009a; Buchon et al., 2009b; Jiang et al., 2009). However, this hypothesis remains to be validated, and additional mechanisms have to be tested. For example, reactivation of the Nrf2 pathway may contribute to the return of ISCs to a non-proliferative state. Strikingly, a recent study in mouse muscle satellite cells demonstrate the essential role of an inhibitor of EGFR signaling in the reversible quiescence of this stem cell popuation, demonstrating the potential conservation of the mechanisms proposed in the Drosophila intestine (Shea et al., 2010).
The importance of the transient aspect of the proliferative response is particularly highlighted by the deleterious consequences of constant ISC proliferation in aging flies. In older animals, an increase in bacterial load, and subsequent oxidative stress, in the intestine results in chronic activation of the regenerative response, including JNK, Upd/JAK/Stat and Pvf/p38 activities (Biteau et al., 2008; Buchon et al., 2009a; Choi et al., 2008; Park et al., 2009). This leads to sustained ISC proliferation and loss of tissue homeostasis, as non-functional supernumerary cells accumulate in the epithelium. This deregulation impairs metabolic homeostasis and limits Drosophila longevity (Biteau et al., 2010).
Maintenance of stem cell identity and differentiation
Proliferative plasticity of somatic stem cells thus allows adapting the number of newly produced daughter cells to tissue demand. At the same time, self-renewal of stem cells is essential to maintain the stem cell population itself, and differentiation cues have to be regulated to ensure proper daughter cell differentiation but prevent stem cell differentiation.
Maintenance of stem cell populations
The exact mechanisms ensuring maintenance of an appropriate stem cell population are currently under intense investigation. An elegant recent study has suggested that the stem cell population in the mouse crypt is maintained by population asymmetry (termed ‘stochastic model’), rather than fixed intrinsic asymmetry of stem cell divisions (‘hierarchical model’) (Figure 3C)(Snippert et al., 2010). Snippert et al used multi-color lineage tracing to distinguish between the stochastic and hierarchical model in the maintenance of crypt lineages by Lgr5+ stem cells. Since mosaically labeled crypts become mostly ‘monochrome’ over a period of 2 months, the authors infer that this tissue is maintained by population asymmetry rather than by fixed asymmetry of the stem cell division (in which case each individually induced lineage is expected to be stable over time, preserving mosaicism). It remained unclear, however, if the selective survival of individual lineages might reflect an unknown stem cell hierarchy in the crypt, in which a single stem cell is responsible for the long-term maintenance and replacement of all other ‘secondary’ stem cells in this tissue. This second possible interpretation of Snippert et al’s results has become more likely due to a new study, in which a hierarchy between Lgr5+ and Bmi1+ stem cells in the crypt has been identified (Tian et al., 2011). Strikingly, complete ablation of the Lgr5+ cell population in the intestine does not affect the integrity and homeostasis of the intestinal epithelium, while Bmi1+ stem cells can give rise to all structures of the crypt, including Lgr5+ stem cells (Quyn et al., 2010; Tian et al., 2011). One interpretation of Snippert et al’s results is therefore that crypts drift toward clonality over time because the Lgr5+ stem cell population is being replenished from individual Bmi1+ stem cells. Furthermore, the spindle orientation of dividing stem cells in the crypt indicates a predominance of asymmetric divisions, supporting a hierarchical model, as in a stochastic model divisions are expected to be to a large extent symmetric (Quyn et al., 2010).
The simpler organization of the Drosophila ISC lineage has allowed characterizing maintenance strategies in this system more definitively, but important questions remain. ISCs and their function are maintained throughout the life of the organism, as illustrated by the fact that clone numbers observed in individual guts are in effect stable until late in life (i.e. for 4-6 weeks; (Lin et al., 2008; Ohlstein and Spradling, 2006). The mechanisms ensuring ISC self-renewal are starting to be characterized, but it remains unclear if ISCs that have been specified during development simply survive throughout life of the animal, or if there are active replenishment processes in which ISCs are regenerated through symmetric divisions of older cells (Figure 3B). ISC nests display an intrinsic asymmetry, as the ISC is basally localized and closely contacts the basement membrane and the visceral muscle, while the EB resides more apically. Furthermore, these cells can be clearly distinguished by the asymmetric distribution of the Notch ligand Delta (Dl), which is detected exclusively in the ISC, and of Notch signaling reporters, which are invariably active in EBs but not ISCs. The outcome of ISC divisions thus seems to be consistently asymmetric in young, healthy guts. The asymmetry of the ISC division itself has been debated (Hou, 2010; Simons and Clevers, 2011), however, as no asymmetric localization of intracellular signaling components prior to division has been described in ISCs to date (a hallmark of asymmetric divisions observed for example in Drosophila neuroblasts (Knoblich, 2010)). Supporting the model of an initially symmetric cell division, vesicles containing the Dl protein are equally segregated during ISC cell division (but Dl expression is rapidly inhibited in the EB), and rare nests containing two delta-positive cells can be observed (Ohlstein and Spradling, 2007).
On the other hand, similar to what is observed in the mouse and human intestinal stem cells (Quyn et al., 2010), the mitotic plane in dividing ISCs is oriented at an invariable angle with respect to the basement membrane, with the prospective EB located apically (Ohlstein and Spradling, 2007). This observation might indicate an imposed orientation in the division of ISCs that is derived from local cues and guarantees that the cell located basally retains stem cell identity, by preventing N activation. To date, such signals have not been described. Interestingly, however, the visceral muscle that surrounds the intestinal epithelium does secrete cytokines (the Wnt-like molecule wingless) and growth factors (the EGF ligand vein) that may be acting in a concentration gradient on ISCs (Biteau and Jasper, 2011; Buchon et al., 2010; Jiang et al., 2011; Lin et al., 2008; Xu et al., 2011), thus potentially providing cues for the positioning of the division plane. Indeed, EGFR and Frizzled mutant clones were shown to be eliminated from the intestinal epithelium, suggesting that ISCs lacking these receptors lose functionality and/or identity (Lin et al., 2008; Xu et al., 2011). However, an effect of these mutations on cell survival cannot be ruled out. Furthermore, while knocking down Vein in the muscle by RNAi limits the proliferative response of ISCs to stress (Biteau and Jasper, 2011; Buchon et al., 2010; Jiang et al., 2011; Lin et al., 2008; Xu et al., 2011), it remains to be clearly established whether muscle-derived ligands are critical for long-term maintenance of ISCs, or whether they might serve a supporting role while ligands derived from EBs, EEs, or ECs contribute to the ISC ‘niche’. Interestingly, Paneth cells, which constitute the stem cell niche in the mouse intestinal crypt, express the ligands Wnt3, Wnt11 and EGF and are essential to promote stem cell function in vitro and in vivo (Sato et al., 2011). Some of the support mechanisms by which the niche influences stem cell maintenance may thus be conserved between fly and mouse intestines.
It is possible that stem cell division in the Drosophila intestinal epithelium can switch from a consistently asymmetric outcome to symmetric division in response to certain challenges. Supporting this model, recent reports indicate a potential increase in ISC numbers after starvation and refeeding (McLeod et al., 2010), but no direct evidence identifying completely symmetric divisions in the posterior midgut has been reported. In addition, infection, oxidative stress and aging result in the formation of ectopic Dl expressing cells in the posterior midgut. Under these exceptional hyperproliferative conditions, the ISC population might thus resort to symmetric divisions to adjust ISC numbers to tissue requirements. It is important to note, however, that under these conditions ISC daughter cells are also formed the are polyploid and express the ISC/EB marker esg in combination with Dl, while also showing Notch activation. These cells are mis-differentiated and accumulate on the basement membrane, disrupting the epithelium and complicating the analysis of stem cell population size under such conditions (Biteau et al., 2008). A careful analysis using lineage tracing approaches that individually label both stem and daughter cells will be required to confirm a possible switch from asymmetric to symmetric divisions in ISCs (Griffin et al., 2009; Yu et al., 2009).
Self renewal and Differentiation
As suggested by the differential activation of Notch signaling between ISC and EBs, this pathway regulates the balance between self-renewal/stem cell identity and differentiation, both in mammals and flies. In mouse, the effect of Notch activation on stem and progenitor cell populations is highly context dependent (for review see (Liu et al., 2010a)). In hematopoietic, neuronal, and intestinal lineages, Notch is required for self-renewal and promotes proliferation (Aguirre et al., 2010; Duncan et al., 2005; Fre et al., 2005; Hitoshi et al., 2002; Imayoshi et al., 2010; van Es et al., 2005), whereas activation of the Notch pathway induces differentiation of SC populations in the epidermis and the mammary epithelium (Blanpain et al., 2006; Bouras et al., 2008). In depth characterization of the exact mechanisms by which Notch regulates stem cell identity and differentiation, as well as its interactions with other signaling pathways, will be required to fully understand the control of stem cell self-renewal and differentiation. Studies in flies, where Notch promotes differentiation and regulates cell fate decision of ISC daughter cells, have already shed new light on these processes, providing new insight also into the regulation of mammalian stem cell systems: Notch signaling was recently shown to regulate cell-fate decisions in the regenerating airway epithelium in a manner reminiscent of the Drosophila ISC lineage (Rock et al., 2011). Dl/N signaling in the fly ISC lineage promotes differentiation of EBs into either EEs or ECs. The cell fate decision between ECs and EEs seems to be regulated by the intensity of the Dl signal, i.e. high levels of N activity in EBs result in EC differentiation, while the absence of N activation promotes EE differentiation (Ohlstein and Spradling, 2007). This regulation is conserved in vertebrate airway epithelia (Rock et al., 2011). Similar to fly ISCs, basal stem cells (BCs) express the Notch ligands Dll1 and Jagged2, activating N in their daughter cells. N promotes BC differentiation, and it has been proposed that high N activation promotes differentiation into the secretory fate, while lower activation of N results in differentiation into ciliated cells (Rock et al., 2011). Both in flies and in mice it remains unclear, however, how the intensity of Notch activation in daughter cells, and thus their lineage decision, is regulated.
Other studies have provided important insight into the mechanisms controlling ISC daughter cell differentiation: Maintaining the close proximity of ISCs and EBs through ECadherin-mediated cell adhesion is crucial for successful EB differentiation (Maeda et al., 2008). Dl-mediated N activation in EBs increases the activity of the Suppressor of Hairless (Su(H)) transcription factor, presumably by replacing the Hairless transcriptional repressor from Enhancer of Split (E(spl)) complex promoters with the Notch intracellular domain (NICD) (Bardin et al., 2010). Differentiation of EBs into the EC lineage also requires an active JAK/Stat signaling pathway (Beebe et al., 2010; Jiang et al., 2009). However, the exact role of this pathway in the control of ISC proliferation, maintenance and EB cell fate decision, as well as its integration with the Notch signaling pathway remain uncertain due to conflicting reports (Beebe et al., 2010; Jiang et al., 2009; Lin et al., 2010; Liu et al., 2010b).
Conclusion
A detailed picture of regenerative responses in the Drosophila intestinal epithelium is emerging, in which ISCs integrate multiple signals to ensure appropriate proliferative activity. It is evident that these control mechanisms break down in aging flies, highlighting the importance of understanding the intricate control mechanisms regulating this cell population in order to gain insight into stem-cell based pathologies in humans. Many questions remain, including: How are the multiple signaling pathways that regulate SC proliferation coordinated to adapt the number of newly produced cells to tissue demand? Do these signaling pathways directly regulate the cell cycle of these cells and/or the balance between quiescent and active stem cells? How is the proper number of stem cells maintained in its microenvironement? How is the cell fate decision made in differentiating cells to ensure that the proper proportion of each cell type is maintained in the tissue? What are the consequences of aging on all these processes? The evolutionary conservation of the involved regulators and signaling pathways indicates that studies in flies will serve as a guiding principle for future work in mammalian systems.
Acknowledgement
This work was supported by the National Institute on Aging (NIH RO1 AG028127), NYSTEM (grant # N08G-048), and the Ellison Medical Foundation (AG-SS-2224-08) to H.J, as well as an AFAR/Ellison Medical Foundation postdoctoral fellowship to B.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Khalil R, Brack AS. Muscle stem cells and reversible quiescence: the role of sprouty. Cell Cycle. 2010;9:2575–2580. doi: 10.4161/cc.9.13.12149. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Ito N, Jiang J, Ip YT. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J Cell Biol. 2011 doi: 10.1083/jcb.201103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell stem cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Pitsouli C, Perrimon N, Rahme L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development (Cambridge, England) 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell stem cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development (Cambridge, England) 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS genetics. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes & development. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell stem cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes & development. 2009a;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell host & microbe. 2009b;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes & development. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell stem cell. 2009;4:124–127. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. Journal of cellular physiology. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapponi L, Jackson DB, Mlodzik M, Bohmann D. Drosophila Fos mediates ERK and JNK signals via distinct phosphorylation sites. Genes & development. 2001;15:1540–1553. doi: 10.1101/gad.886301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero J, Vidal M, Sansom O. APC as a master regulator of intestinal homeostasis and transformation: from flies to vertebrates. Cell Cycle. 2009;8:2926–2931. [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science (New York, NY. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Deneault E, Cellot S, Faubert A, Laverdure JP, Frechette M, Chagraoui J, Mayotte N, Sauvageau M, Ting SB, Sauvageau G. A functional screen to identify novel effectors of hematopoietic stem cell activity. Cell. 2009;137:369–379. doi: 10.1016/j.cell.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell stem cell. 2009;5:290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Gonczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development (Cambridge, England) 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Griffin R, Sustar A, Bonvin M, Binari R, del Valle Rodriguez A, Hohl AM, Bateman JR, Villalta C, Heffern E, Grunwald D, et al. The twin spot generator for differential Drosophila lineage analysis. Nat Methods. 2009;6:600–602. doi: 10.1038/nmeth.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat Immunol. 2009;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science (New York, NY. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes & development. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell stem cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou SX. Intestinal stem cell asymmetric division in the Drosophila posterior midgut. Journal of cellular physiology. 2010;224:581–584. doi: 10.1002/jcp.22194. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell stem cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Younger A, Jasper H. Dynamic coordination of innate immune signaling and insulin signaling regulates systemic responses to localized DNA damage. Dev Cell. 2011;20:841–854. doi: 10.1016/j.devcel.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development (Cambridge, England) 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nature reviews. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, Blanpain C. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci U S A. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell stem cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development (Cambridge, England) 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science (New York, NY. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. Journal of molecular cell biology. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010a;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010b;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lu R, Wu S, Sun J. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett. 2010c;584:911–916. doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells. 2008;13:1219–1227. doi: 10.1111/j.1365-2443.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development (Cambridge, England) 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Munoz P, et al. The Intestinal Stem Cell Signature Identifies Colorectal Cancer Stem Cells and Predicts Disease Relapse. Cell stem cell. 2011 doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell stem cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, Suda T. FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood. 2008;112:4485–4493. doi: 10.1182/blood-2008-05-159848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science (New York, NY. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kim YS, Yoo MA. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging (Albany NY) 2009;1:637–651. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, Ridgway RA, Sansom OJ, Nathke IS. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell stem cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science (New York, NY. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell stem cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Hogan BL. Epithelial Progenitor Cells in Lung Development, Maintenance, Repair, and Disease. Annu Rev Cell Dev Biol. 2011 doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL, editors. Stems cells and the pathways to aging and cancer. 2008. [DOI] [PubMed] [Google Scholar]
- Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34:293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, Behrens A. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. The EMBO journal. 2009;28:1843–1854. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nature reviews. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development (Cambridge, England) 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell stem cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell stem cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011 doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell stem cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–150. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, Lowry WE. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci U S A. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, Sotiropoulou PA, Blanpain C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- Yu HH, Chen CH, Shi L, Huang Y, Lee T. Twin-spot MARCM to reveal the developmental origin and identity of neurons. Nat Neurosci. 2009;12:947–953. doi: 10.1038/nn.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Chauhan C, Hou SX. Characterization of midgut stem cell- and enteroblast- specific Gal4 lines in drosophila. Genesis. 2010:1–6. doi: 10.1002/dvg.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]