Abstract
Blood flow in the brain is regulated by neurons and astrocytes. Knowledge of how these cells control blood flow is crucial for understanding how neural computation is powered, for interpreting functional imaging scans of brains, and for developing treatments for neurological disorders. It is now recognized that neurotransmitter-mediated signalling has a key role in regulating cerebral blood flow, that much of this control is mediated by astrocytes, that oxygen modulates blood flow regulation, and that blood flow may be controlled by capillaries as well as by arterioles. These conceptual shifts in our understanding of cerebral blood flow control have important implications for the development of new therapeutic approaches.
The human brain comprises only 2% of the body’s mass, but it consumes 20% of the energy that is produced when the body is in a resting state. This high consumption of energy is crucial for the normal functioning of the brain. The energy is mostly used to reverse the ion influxes that underlie synaptic potentials and action potentials1 (Fig. 1a). If there is an inadequate supply of blood glucose and oxygen to a region of the brain, then neurons and glia become injured or die. This occurs in disorders such as ischaemic stroke, vasospasm after sub-arachnoid haemorrhage, the secondary ischaemia that follows spinal-cord injury, and cerebral palsy after perinatal asphyxia. To sustain neuronal function, the brain has evolved ‘neurovascular coupling’ mechanisms to increase the flow of blood to regions in which neurons are active, a response termed functional hyperaemia. Different information coding strategies and neural algorithms require different increases in blood flow, depending on the extent to which they consume energy. An understanding of the mechanisms that generate functional hyperaemia is a prerequisite for developing therapies to correct defects in blood flow control that occur after disorders such as stroke2, hypertension3, spinal-cord injury4 and Alzheimer’s disease3.
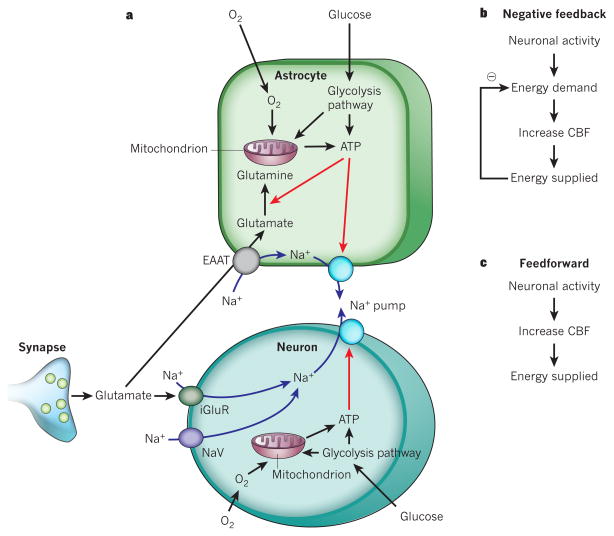
Figure 1. Energy supply, usage and blood flow regulation in the brain.
a, ATP is generated from glycolysis and mitochondrial oxidative phosphorylation in neurons and glia. ATP is mainly consumed (red arrows) by ion pumping in neurons, to maintain the ion gradients underlying synaptic and action potentials, following Na+ entry (blue arrows) through ionotropic glutamate receptors (iGluR) and voltage-gated Na+ channels (NaV). It is also used in glia for Na+-coupled neurotransmitter uptake by excitatory amino acid transporters (EAAT) and for metabolic processing (shown for conversion of glutamate to glutamine), and on maintaining the cells’ resting potentials. b, The negative-feedback control hypothesis for vascular energy supply, in which a fall in energy level induces an increased cerebral blood flow (CBF). c, The feedforward regulation hypothesis for vascular energy supply.
Concepts of how neuronal activity controls the vascular supply of glucose and O2 are changing rapidly. Traditionally, it was thought that active neurons generate a metabolic signal (a fall in O2 or glucose concentration, or a rise in carbon dioxide concentration), which triggers an increase in blood flow. This idea has recently been superseded, following the discovery that neurotransmitter-mediated signalling, particularly by glutamate, has a major role in regulating cerebral blood flow, and that much of this control is mediated by astrocytes. Glutamate-mediated signalling leads to the release of nitric oxide from neurons and of arachidonic acid derivatives from astrocytes (and possibly from neurons). These molecules can either increase or decrease blood flow, depending on the local O2 concentration, but how this switch occurs is debated. Furthermore, the relative importance of the different glutamate-released messengers varies between brain regions. Even the dogma that cerebral blood flow is controlled solely by arterioles has been challenged, with the finding that contractile cells called pericytes can control the diameter of capillaries, and that damage to these cells contributes to the long-lasting decrease in blood flow that occurs after stroke. These major conceptual shifts, which we discuss in this Review, provide a new understanding of how the brain regulates its energy supply in response to different information processing tasks. They also underpin the interpretation of data from functional imaging experiments, and offer new opportunities for developing therapeutic approaches to a range of disorders of the central nervous system.
Neurotransmitters increase cerebral blood flow
In this Review, we focus on the control of cerebral blood flow by local neuronal activity. We pay less attention to autoregulation, which, in the face of changes in systemic blood pressure, maintains an approximately constant blood flow to the brain.
Early researchers favoured the idea that blood flow is locally controlled by a negative-feedback system in which neural activity leads to energy demand, because ATP is used to restore ion gradients after the generation of synaptic and action potentials1. This ATP use was thought to produce a metabolic signal that increased blood flow and therefore provided more energy (Fig. 1b). This metabolic signal could be a lack of O2 or glucose, or the production of CO2 (which dilates cerebral vessels by being converted to H+ after combining with H2O)5.
However, manipulation of blood O2 (refs 6, 7) and glucose8 concentrations to test this negative-feedback hypothesis has shown that O2 and glucose do not regulate blood flow in this way. Furthermore, during neuronal activity, the local extracellular pH initially becomes alkaline, rather than becoming acidic as would be expected if arteriolar dilation were caused by the accumulation of CO2. This is partly because the increase in blood flow elicited by neural activity washes CO2 away rapidly, and partly because the neuronal Ca2+, H+-ATPase pump alkalinizes the extracellular space when neural activity raises the neuronal intracellular calcium concentration ([Ca2+]i)9,10. These findings do not support a local CO2 rise as the cause of the increased blood flow — despite the fact that exogenous CO2 does increase blood flow. However, the ‘metabolic messenger’ adenosine (which is produced when ATP is hydrolysed) does contribute to functional hyperaemia, because blocking adenosine receptors reduces the increase in blood flow evoked by neuronal activity11. Furthermore, another metabolic messenger, lactate (which is produced when pyruvate production by glycolysis outstrips pyruvate consumption by oxidative phosphorylation), also increases blood flow12. However, as explained below (see ‘O2 modulates neurovascular signalling’), this is mediated at least partly by a modulation of neurotransmitter-induced prostaglandin signalling to blood vessels.
More recent work has shown that control of the vascular energy supply by neural activity is largely mediated by feedforward mechanisms (Fig. 1c). In these processes, neurons either signal directly to blood vessels or activate astrocytes to release vasoactive agents onto the vessels. For both of these signalling routes, the coupling mechanisms involve neurotransmitter, particularly glutamate, signalling (Fig. 2).
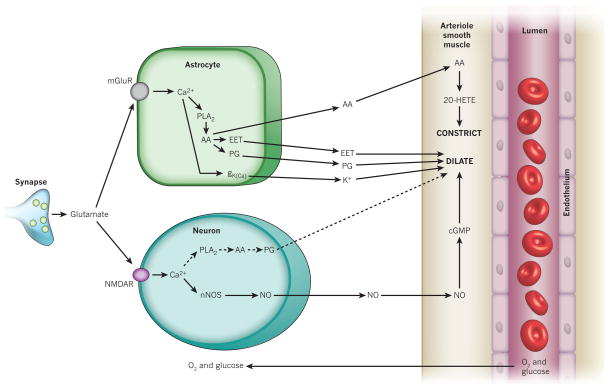
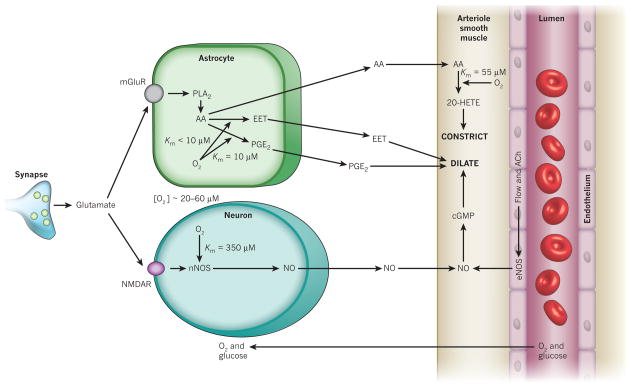
Figure 2. Major pathways by which glutamate regulates cerebral blood flow.
Pathways from astrocytes and neurons (left) that regulate blood flow by sending messengers (arrows) to influence the smooth muscle around the arterioles that supply oxygen and glucose to the cells (right, shown as the vessel lumen surrounded by endothelial cells and smooth muscle). In neurons, synaptically released glutamate acts on N-methyl-D-aspartate receptors (NMDAR) to raise [Ca2+]i, causing neuronal nitric oxide synthase (nNOS) to release NO, which activates smooth muscle guanylate cyclase. This generates cGMP to dilate vessels. Raised [Ca2+]i may also (dashed line) generate arachidonic acid (AA) from phospholipase A2 (PLA2), which is converted by COX2 to prostaglandins (PG) that dilate vessels. Glutamate raises [Ca2+]i in astrocytes by activating metabotropic glutamate receptors (mGluR), generating arachidonic acid and thus three types of metabolite: prostaglandins (by COX1/3, and COX2 in pathological situations) and EETs (by P450 epoxygenase) in astrocytes, which dilate vessels, and 20-HETE (by ω-hydroxylase) in smooth muscle, which constricts vessels. A rise of [Ca2+]i in astrocyte endfeet may activate Ca2+-gated K+ channels (gK(Ca)), releasing K+, which also dilates vessels.
One cannot distinguish between metabolic feedback and neurotransmitter feedforward mechanisms simply by blocking neurotransmitter-mediated signalling. Although neurotransmitter antagonists block the increase in blood flow elicited by neural activity13–17, they also block the energy consumption evoked by synaptic signalling1,18,19. However, the roles of energy consumption and neurotransmitter-mediated signalling in regulating blood flow can be disentangled. Blocking the enzymes downstream of glutamate receptors that generate nitric oxide and arachidonic acid derivatives greatly reduces functional hyperaemia but has little effect on the energy use that is associated with neural activity18,20.
Neuronal signalling to blood vessels
Synaptic release of glutamate activates neuronal NMDA (N-methyl-D-aspartate) receptors, resulting in Ca2+ entry into neurons and activation of neuronal nitric oxide synthase (nNOS). This releases NO, which dilates vessels21, both in brain slices (which allow more refined mechanistic investigations, but lack intravascular pressure and blood flow that can release messengers that modulate the properties of vascular smooth muscle) and in vivo (Fig. 2). In the cortex, inhibition of nNOS reduces the increase in blood flow that is associated with neural activity22, suggesting a role for NO in neurovascular coupling. However, the response is restored by the addition of NO donors, which provide a constant concentration of NO. This indicates that, although the presence of NO is required, a dynamic rise of NO concentration in response to neuronal activity does not directly mediate neuron-to-vessel signalling23. Instead, NO might be needed to modulate the pathways in astrocytes which dilate and constrict blood vessels (see ‘NO modulates astrocyte signalling’). In the cerebellum, by contrast, NO donors do not reverse the reduction in functional hyperaemia caused by inhibiting nNOS, demonstrating that NO directly mediates a component of the response24,25. The activation of peptidergic interneurons can also dilate or constrict vessels in the cortex26, but the dilations that have been observed were usually irreversible (within the 7-min recording period). In addition, it is unclear whether these effects involve peptide release and whether they reflect direct signalling from neurons to vessels or indirect signalling by way of astrocytes (see ‘Astrocyte signalling to blood vessels’). Finally, the neurotransmitter GABA (γ-aminobutyric acid), acting through GABAA receptors, also mediates a component of the vasodilation produced in the cortex by basal forebrain stimulation27, but it is unclear whether this is a direct effect on the vasculature or is mediated by an effect on neurons or astrocytes.
Astrocyte signalling to blood vessels
Astrocytes are ideally situated to function as relay cells in neurovascular communication, as was suggested more than a century ago by Ramón y Cajal. They surround synapses and thus can be stimulated by neuronal activity, whereas their endfoot processes envelop blood vessels and can signal to the smooth muscle cells that control vessel diameter.
In theory, astrocytes can increase blood flow in response to neuronal activity by releasing potassium ions from their endfeet apposed to arterioles, because modest increases in extracellular K+ concentration (up to ~10 mM [K+]o) hyperpolarize smooth muscle cells. This occurs because a raised [K+]o increases the conductance of smooth muscle inward rectifier K+ channels, and this effect outweighs the positive shift of the K+ reversal potential, EK, produced by the raised [K+]o and leads to more outward current flowing (because the membrane potential is more positive than EK). This hyperpolarization reduces the influx of Ca2+ through voltage-gated channels and dilates the vessels28. The ‘K+ siphoning’ hypothesis holds that K+ released from active neurons depolarizes astrocytes, leading to K+ efflux from astrocyte endfeet29. This mechanism of neurovascular coupling has been tested by directly depolarizing glial cells while monitoring blood vessel diameter30. Depolarization fails to dilate vessels, demonstrating that K+ siphoning does not contribute significantly to vasodilation. However, astrocytes may dilate vessels through a different K+-based mechanism. When neuronal activity releases glutamate at synapses, some of the released glutamate escapes the synaptic cleft and activates metabotropic glutamate receptors (mGluRs) on astrocytes, thus increasing [Ca2+]i in astrocytes31 (Fig. 2). This increase in [Ca2+]i has been reported32 to lead to the opening of large-conductance Ca2+-activated K+ (BK) channels in astrocyte endfeet, releasing K+ onto vessels. A caveat is that these experiments used a thromboxane analogue to maintain vessel tone in the brain slices studied, and this analogue has been shown to stimulate the trafficking of BK channels to the surface membrane of astrocytes33. It remains to be determined whether this K+ release mechanism contributes to the regulation of blood flow in vivo.
There is strong evidence from brain slice and isolated retina preparations that astrocytes can control blood flow through the production and release of metabolites of arachidonic acid. When glutamate released from neurons activates astrocyte mGluRs (Fig. 2), the resultant rise in [Ca2+]i activates phospholipase A2, evoking the production of arachidonic acid from membrane phospholipids. The build-up of arachidonic acid leads, in turn, to the production of its metabolites, including prostaglandins and epoxyeicosatrienoic acids (EETs), which dilate nearby arterioles15,34–37. The prostaglandin involved is often suggested to be PGE2, although many studies rely on inhibiting only the first enzyme (cyclooxygenase) in the prostaglandin-synthesis pathway, and other prostaglandins may contribute to the dilation. (The main pathways by which arachidonic acid derivatives are produced are shown in Fig. 3.) PGE2 can relax vascular smooth muscle by binding to EP4 prostaglandin receptors38, which increase the activation of protein kinase A by cyclic AMP and thus decrease the phosphorylation of the myosin light chain39. The dilation produced by PGE2 and other arachidonic acid metabolites also partly reflects their activation of K+ channels in vascular smooth muscle cells40,41, making the membrane potential more negative and thus decreasing the entry of Ca2+ through voltage-gated channels. EETs may also elicit dilation by inhibiting receptors for thromboxane42, a vasoconstricting derivative of arachidonic acid. In vivo, a rise in [Ca2+]i in cortical astrocytes results in a vasodilation that is partly (~70%) mediated by prostaglandins43. The rest of the dilation could be mediated by EETs36, probably derived from astrocytes37, although this remains to be shown directly.
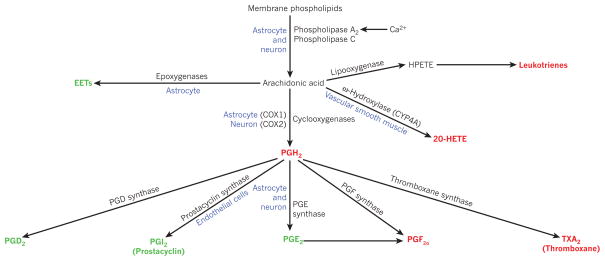
Figure 3. Arachidonic acid metabolites that may contribute to control of cerebral blood flow.
Arachidonic acid is formed from membrane phospholipids by Ca2+-dependent and Ca2+-independent lipases. Metabolites shown in green are vasodilators, red metabolites are vasoconstrictors, and blue denotes the location of some of the relevant enzymes. COX, cyclooxygenase; CYP, cytochrome P450 superfamily of enzymes; EET, epoxyeicosatrienoic acid; 20-HETE, 20-hydroxyeicosatetraenoic acid; HPETE, hydroperoxy-eicosatetraenoic acid.
In brain slices and in the isolated retina rises in [Ca2+]i in astrocytes can also constrict vessels34,44. This is mediated34 by the conversion of arachidonic acid to 20-hydroxy-eicosatetraenoic acid (20-HETE), probably by the enzyme CYP4A in vascular smooth muscle cells (Fig. 2). Whether a rise in astrocyte [Ca2+]i causes dilation or constriction may in part be determined by the pre-existing tone of the vessel45, but the O2 concentration also has a key role in determining this (see ‘O2 modulates neurovascular signalling’). In vivo, a rise in astrocyte [Ca2+]i produces vasoconstriction46, mediated by a phospholipase A2 derivative (presumably 20-HETE), during cortical spreading depression — a pathological wave of cell depolarization and refractoriness lasting minutes, after a brief period of increased excitability, which spreads across the cortex.
Modulation of these arachidonic acid metabolic pathways for therapeutic effect will depend on understanding the isoforms of the enzymes that synthesize the vasoactive messengers. For example, prostaglandins that mediate physiological vasodilation in response to a rise in astrocyte [Ca2+]i are mainly produced by cyclooxygenase 1 (COX1)43, which is expressed in astrocyte endfeet35, with a possible contribution by COX3 (ref. 47) or COX2 (refs 35, 48). However, in pathological conditions, expression of COX2 is upregulated in astrocytes48 and this might also contribute to prostaglandin synthesis. Neurons also express COX2 and PGE synthase, and this may account for a COX2 component of functional hyperaemia seen in the cortex49.
Another, conceptually distinct, pathway by which astrocytes may control cerebral blood flow involves neurotransmitter uptake. Although about half of the increase in blood flow that is induced by neural activity in the olfactory bulb is mediated by glutamate activating astrocyte mGluRs and releasing prostaglandins, a further one-third of the increase is mediated by the activation of glutamate transporters on astrocytes50. The glutamate transporter component did not involve a [Ca2+]i rise or prostaglandin release, and still occurred when glutamate receptors were blocked. In a related study in the visual cortex, blocking glutamate uptake prevented astrocyte [Ca2+]i increases and light-reflectance changes that are attributed to increased blood flow51. However, interpretation of these data is complicated because, first, blood flow was not measured directly, second, it was unclear whether the astrocyte [Ca2+]i rises were generated by mGluRs or by glutamate uptake, and third, blocking uptake will raise the extracellular glutamate concentration, which could desensitize glutamate receptors and have indirect effects. Similarly, in the olfactory bulb, co-transport of Na+ with GABA taken up into astrocytes raises [Na+]i. This in turn inhibits Na+/Ca2+ exchange, raising [Ca2+]i and constricting arterioles52, presumably by releasing arachidonic acid to generate 20-HETE (Fig. 2), although this was not tested.
The importance of astrocytes versus neurons
The relative importance of the neuronal and astrocytic vasodilating pathways remains a matter of debate, because synaptic glutamate release raises [Ca2+]i in both cell types53, with some astrocytes showing a fast rise in [Ca2+]i, similar to that in neurons54. Blood flow responses are strongly correlated with local field potentials that reflect synaptic and action potentials in neurons55,56, and ionotropic glutamate receptor antagonists significantly decrease both field potentials and blood flow responses to stimulation in the cerebellum, cortex and olfactory bulb16,17,55. This might indicate that a rise of [Ca2+]i in neuronal dendrites initiates much of the increase in blood flow53. However, the glutamate release that generates field potentials also activates astrocyte mGluRs, so field-potential amplitudes will also be correlated with astrocyte receptor activation. A correlation of blood flow changes with field potentials is therefore not sufficient to prove that postsynaptic neuronal signalling dominates the control of blood flow. Furthermore, experiments blocking ionotropic glutamate receptors to prevent the release of neuronal messengers will decrease neuronal firing, inhibiting glutamate release onto astrocytes and thus preventing the release of astrocyte messengers. The potential for astrocytes to control blood flow is demonstrated by the fact that neural activity raises [Ca2+] in astrocytes54,57, and that uncaging of Ca2+ in astrocytes dilates or constricts arterioles34,35,43,44.
If the simplified scheme shown in Fig. 2 were correct, then the relative contributions of neuronal and glial signalling could be dissected by blocking either the neuronal NO pathway or the astrocyte arachidonic acid pathways. Knocking out or blocking nNOS (the only form of NOS contributing to functional hyperaemia22) does not affect whisker-stimulation-induced neural field potentials, but reduces the resulting blood flow increases by 37–60% in the somatosensory cortex22,58. In the cerebellum, knockout or inhibition of nNOS decreases activity-induced blood flow by 50–90%13,24,25,59. The remaining blood flow increase may be mediated by adenosine14 or K+ (ref. 60), but the possible contribution of prostaglandins and EETs has not been investigated. Blocking prostaglandin production with cyclooxygenase inhibitors reduces functional hyperaemia in the cortex61,62 by 48–60% without affecting neuronal field potentials, whereas blocking EET production inhibits the response by 35–70%36. Thus, NO, cyclooxygenase and epoxygenase products are all important for generating functional hyperaemia in the cortex, whereas NO is more dominant in the cerebellum59.
However, we will see below (see ‘NO modulates astrocyte signalling’) that the different pathways interact at the level of the enzymes producing arachidonic acid derivatives. In addition, a saturating interaction at the level of the arteriole smooth muscle membrane potential is expected if EETs and prostaglandins both dilate arterioles by opening K+ channels, because once one pathway has hyperpolarized the cell out of the activation range of voltage-gated Ca2+ channels, then further opening of K+ channels will have no effect. Caution is therefore needed in interpreting the results of experiments in which single enzymes are blocked.
Pathway-specific signalling differences
Even if functional hyperaemia is largely driven by synaptic glutamate release, it does not follow that all excitatory synapses are equally influential in controlling blood flow, even if they impinge on cells in the same area. Indeed, activation of thalamocortical or transcallosal corticocortical inputs to the somatosensory cortex induces cerebral blood flow increases with a different dependence on the level of input activity56, monotonically increasing with activity for the transcallosal pathway and showing a maximum at an intermediate level of input for the thalamocortical input. These differences may be partly due to differential activation of interneurons that release vasoactive peptides56. However, they may also reflect differences in the functional anatomy of neurovascular coupling for the two input pathways. For example, some of the 8,000 synapses innervating a typical rodent neocortical pyramidal cell1 will release NO closer to arterioles, or induce a [Ca2+]i rise in astrocytes that is more effective at releasing vasodilatory messengers onto arterioles, than will other synapses. Similarly, in the cerebellar cortex, although cerebral blood flow increases evoked by activation of the parallel and climbing fibre inputs to Purkinje cells depend partly on NO, the blood flow response to parallel fibre stimulation also involves a rise in [K+]o, whereas the response to climbing fibre activation partly involves adenosine release60,63.
Thus, the relative importance of the neuronal and astrocyte pathways is likely to differ across brain areas, and even between different neural pathways in the same area. Consequently, the dependence of blood flow increases on the underlying neural activity will differ between different pathways, implying that functional imaging signals arising from these pathways will also reflect different aspects of neuronal function (see ‘What does functional imaging measure?’). Similarly, therapeutic modulation of the signalling pathways controlling cerebral blood flow may have different effects in different brain areas, and on different neuronal pathways in the same area.
NO modulates astrocyte signalling
Assessing the relative significance of, or interfering therapeutically with, the different signalling pathways producing functional hyperaemia is complicated by interactions between them. The effects of the NO, cyclooxygenase and epoxygenase pathways can occlude each other. In the cortex, for example, functional hyperaemia is inhibited ~50% by blocking NOS and ~50% by blocking cyclooxygenase, but blocking both produces only a 70% inhibition62. Similarly, blocking NOS, blocking EET production, or blocking both pathways together all produce a ~60% inhibition of functional hyperaemia37. These results may be explained by interactions between the NO and arachidonic acid pathways, which are shown in Fig. 4.
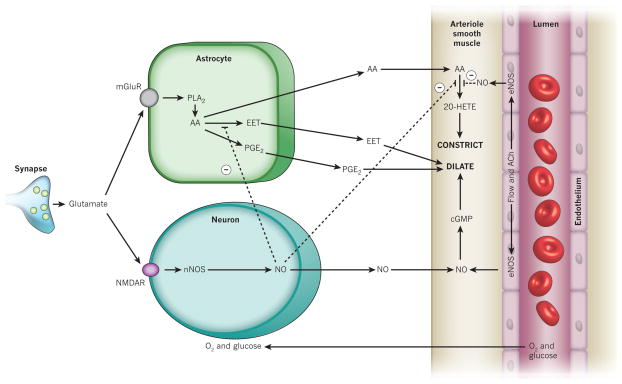
Figure 4. Nitric oxide inhibits the production of key arachidonic acid-derived messengers.
NO inhibits (dashed lines) the production of both the vasoconstricting 20-HETE and the vasodilating EETs36,69. NO also weakly stimulates COX1 and inhibits COX2 (not shown)65. Endothelial nitric oxide synthase (eNOS) can be activated by flow-induced shear stress or by acetylcholine (ACh). Other abbreviations as in earlier figures.
Nitric oxide inhibits the enzymes that synthesize 20-HETE and EETs64. It also, much more weakly, inhibits prostaglandin production by COX2, but stimulates COX1 (ref. 65). The inhibition of the synthesis of the vasoconstrictor 20-HETE by NO is of particular interest for two reasons. First, this effect may underlie a significant fraction of the dilating effect of NO66 (20–60% in different vessels64), which is independent of the NO vasodilation mediated by cyclic GMP (Fig. 4). Second, it provides a probable explanation for why, in the neocortex, NO is required for functional hyperaemia to occur, but is not the primary mediator of the increase in blood flow23. If the main cause of the blood flow increase is the generation of arachidonic acid by astrocytes, then having NO present to inhibit 20-HETE formation will ensure that only the vasodilatory prostaglandin and EET derivatives of the arachidonic acid will affect arteriole diameter. In the retina, however, where arteriole vasodilations are produced by EETs34, the presence of NO promotes light-induced constrictions and inhibits dilations, suggesting that the production of EETs is more NO sensitive than is the production of 20-HETE34.
O2 modulates neurovascular signalling
Variations in O2 concentration in brain tissue alter neurovascular coupling in two ways. The O2 level affects the synthesis of the glial and neuronal messengers involved, and also alters the levels of lactate and adenosine that modulate the pathways by which these messengers regulate vascular tone.
O2 is needed for the synthesis of nitric oxide and the vasoactive messengers derived from arachidonic acid. Comparing in situ O2 levels with the O2 affinities of the O2-sensitive reactions (Fig. 5) suggests how changes in O2 concentration will affect neurovascular coupling. As O2 concentrations are decreased, the synthesis of NO by neurons is expected to be inhibited first (Michaelis constant (Km) ~ 350 μM at 25 °C67), followed by 20-HETE synthesis (Km ~ 55 μM at 37 °C68), whereas EET synthesis (Km < 10 μM at 37 °C68) and prostaglandin synthesis (Km ~ 10 μM at 24 °C69) should be maintained at much lower O2 concentrations. How do these values compare with the O2 levels found in brain tissue? Neurophysiologists often use 95–100% O2 (~1 mM, or 760 mm Hg) in solutions superperfusing isolated tissue, which lead to O2 concentrations of ~100–150 μM in brain slices. This is much higher than the in vivo values18,19 of 20–60 μM, which can even decrease by a further 13 μM during intense synaptic activity18,19. These in vivo values, which can be mimicked by superfusing brain slices with solution equilibrated with 20% O2 (ref. 70), are comparable to or lower than the Km values for O2 in the synthesis of 20-HETE and NO. Thus, at in vivo levels of O2, we expect NO and 20-HETE synthesis to be significantly limited by the amount of O2 available.
Figure 5. Oxygen differentially affects the synthesis of neurovascular messengers.
The concentration of O2 in the extracellular space is 20–60 μM. This is significantly higher than the effective Km for O2 activating the enzymes synthesizing EETs and prostaglandins, but is in a range in which changes in O2 concentration will modulate the production of NO and 20-HETE. Abbreviations as in earlier figures.
Consistent with this view, lowering the O2 concentration in solution superfusing brain slices from 95% to 20% (to lower the tissue [O2] from about 125 to 40 μM) has a dramatic effect on the vascular responses produced by uncaging Ca2+ in astrocytes35. With a supraphysiological O2 concentration in the tissue, raising astrocyte [Ca2+]i led to arteriolar constriction, whereas with a physiological [O2], vasodilation occurred. These changes are, in part, predicted from the modulatory effects of O2 and NO on the signalling pathways outlined above (in this and the preceding section). The suppression of 20-HETE formation by the lower O2 concentration is expected to reduce the vasoconstriction produced when arachidonic acid is generated in astrocytes (Fig. 5), and the lower [O2] will also result in less NO being present to inhibit the formation of vasodilatory EETs (Fig. 4) in tissues where these contribute to dilation.
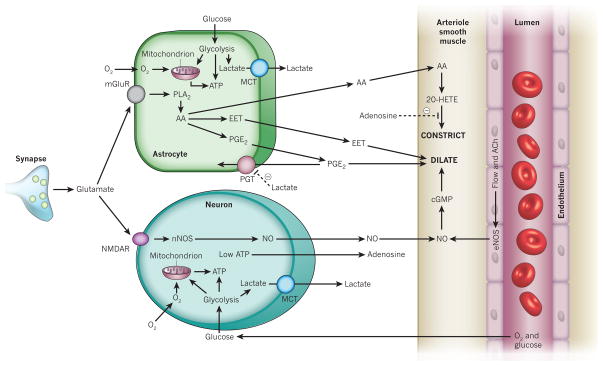
However, two other factors were also shown to contribute significantly to the effects of lowering [O2] (ref. 35; Fig. 6). As O2 concentrations decrease, the lack of energy for ATP synthesis causes an increase in the level of extracellular adenosine (Fig. 6), which binds to adenosine A2A receptors on vascular smooth muscle cells to depress vessel constriction. In addition, a decrease in the rate of oxidative phosphorylation relative to the rate of glycolysis results in lactate production (Fig. 6). Monocarboxylate transporters release the lactate into the extracellular space, where it reduces the clearance of extracellular PGE2 by the prostaglandin transporter (Fig. 6). Thus, when PGE2 is released from astrocytes, extracellular PGE2 increases to a greater degree, resulting in larger arteriole dilations35. This effect of lactate may partly explain why, in humans and rats in vivo, cerebral blood flow is regulated by the lactate/pyruvate concentration ratio and thus by the NADH/NAD+ ratio12. Interestingly, lactate is released into the extracellular space during synaptic activity71, which should promote vasodilation.
Figure 6. Lactate and adenosine affect neurovascular signalling at low [O2].
Low O2 concentrations lead to mitochondrial oxidative phosphorylation failing to consume all the pyruvate produced by glycolysis, resulting in an export of lactate by monocarboxylate transporters (MCTs). Extracellular lactate inhibits the reuptake of PGE2 by the prostaglandin transporter (PGT), promoting vasodilation35. Low energy levels also lead to the formation of adenosine, which inhibits 20-HETE-mediated arteriolar constriction by acting on adenosine A2A receptors35. Other abbreviations as in earlier figures.
Despite our understanding of how O2 levels regulate astrocyte-mediated neurovascular coupling in brain slices35, imposing artificially high O2 concentrations in vivo does not lead to smaller vasodilations or the emergence of vasoconstrictions7. This could reflect a potentiation of NO-mediated signalling from neurons to arterioles (relative to that occurring in brain slices superfused with 95% O2) by the high O2 level produced by hyperbaric O2 (ref. 7) (as NOS activity is potentiated by the higher [O2]; Fig. 5), which outweighs the effect of O2 on astrocyte-mediated signalling.
Control of blood flow at the capillary level
Until recently, it was assumed that neurovascular coupling is mediated solely by changes in the tone of the smooth muscle that forms a continuous layer around arterioles. This idea has been challenged by the discovery that pericytes — cells present at ~50-μm intervals along capillaries — can markedly alter capillary diameter, and thus potentially regulate cerebral blood flow at the capillary level.
Pericytes express contractile proteins, and their location on capillaries led to the suggestion, some 130 years ago, that they could constrict the microvasculature72. This idea was reinforced with observations that substances that alter arteriole diameter, including arachidonic acid derivatives and neurotransmitters, can contract and relax pericytes cultured on rubber membranes73. Importantly, in a series of papers, pericytes on isolated retinal capillaries were demonstrated to constrict or dilate in response to neurotransmitters, as a result of [Ca2+]i alterations74. It is assumed that the signalling pathways controlling pericyte constriction and dilation will be similar to those for arterioles shown in Fig. 2, but this still needs to be tested. In situ in brain slices, pericytes constrict in response to noradrenaline and dilate in response to glutamate75, and in the isolated retina, blocking ionotropic GABA receptors constricts capillaries, demonstrating that endogenous transmitter release can regulate capillary diameter75. Regulation of cerebral blood flow at the capillary level has yet to be demonstrated in vivo in physiological conditions72, although it does occur after ischaemia76.
Signals for contraction (presumed to be depolarization) and perhaps for dilation (hyperpolarization) can propagate from one pericyte to another74,75. This signal spread may occur through gap junctions between the interdigitating processes of the pericytes themselves, or through gap junctions with endothelial cells. Because active neurons are, on average, closer to pericytes than to arterioles77 (8–23 μm away versus 70–160 μm), this signal spread raises the theoretical possibility that vascular responses to changes in neuronal activity may be initiated by pericytes and then propagated to upstream arterioles.
The importance of capillary diameter control by pericytes for regulating cerebral blood flow will depend on the fraction of the haemodynamic resistance of the vascular network that the capillaries contribute. Models in the literature78,79 suggest that the capillary resistance is between 16% and 70% of the total vascular resistance (arterioles plus capillaries plus venules) within the brain parenchyma. If blood flow in capillaries obeys Poiseuille’s law of fluid dynamics (see below), with flow proportional to the fourth power of the vessel diameter, then these values lead to the conclusion that a 2.1-fold capillary dilation produced by superfused glutamate in the presence of noradrenaline75 could, in principle, increase blood flow 1.18- to 2.98-fold72, and thus contribute significantly to functional hyperaemia. However, this may overestimate the role of pericytes if endogenous noradrenaline release is less than that applied when probing the effect of glutamate75, or if the effect of superfused glutamate in brain slices overestimates dilations produced in perfused vessels in vivo by neurons releasing glutamate. On the other hand, Poiseuille’s law severely underestimates the power of pericytes to regulate blood flow, because red and white blood cells have to deform considerably to pass through capillaries that constrict below ~5 μm — or they may not be able to pass through at all, which becomes important when pericytes constrict capillaries after ischaemia76. This will result in a more non-linear dependence of flow on diameter, enhancing the influence of capillaries on blood flow. Experiments are needed to define the importance of pericytes for regulating blood flow in vivo.
Blood flow rises more than energy use
Traditional analyses of sensory systems assumed that external stimuli induce action potentials in neurons with receptive fields ‘tuned’ to particular features of the stimuli. On this basis, low levels of spontaneous neuronal activity merely provide a ‘noise’ background against which the stimulus must be detected, and sensory input should greatly increase neuronal activity and hence cortical energy use. Recently, however, it has been suggested that incoming sensory information produces only small changes to the ongoing activity level in cortical neurons80. Indeed, across a range of sensory systems, less than a 10% change of neuronal spiking is produced by perceptual tasks used in functional imaging experiments81, and as a result sensory input alters cortical energy use by a relatively small fraction82.
The fractional increase in blood flow induced by sustained neuronal activity is at least 4-fold greater than the increase in ATP consumption by the neurons82. This is consistent with blood flow being mainly regulated by feedforward neurotransmitter-mediated mechanisms rather than by a negative-feedback loop driven by energy demand (Fig. 1b, c), because a negative-feedback system could not produce a sustained increase in energy supply that is larger than the increase in energy consumption. As explained below (see ‘What does functional imaging measure?’), this disproportionate increase in blood flow is the basis of the blood-oxygen-level dependent (BOLD) functional imaging technique. It was initially thought that most of the energy used when neurons are activated came from glycolysis rather than oxidative phosphorylation, because neuronal activity increases glucose uptake much more than O2 uptake83. However, later studies found less of a difference in the glucose and O2 uptake rates84, and far more ATP is produced by oxidative phosphorylation than by glycolysis per glucose molecule. As a result, even with a much smaller increase in O2 uptake than in glucose uptake83, at least 60% of the extra ATP production would be occurring by oxidative phosphorylation85, and more recent work suggests that almost all of the ATP is generated by oxidative metabolism of glucose82.
A possible explanation for the large increase in blood flow induced by activity, relative to the increase in O2 uptake and ATP production, was based on modelling of O2 uptake: a large increase in blood flow (driven by glutamate release, not by O2 deficit) may be needed to generate even a small increase in O2 uptake86. However, pharmacologically blocking most of the increase in blood flow produced by activity has little effect on the increase in O2 consumption87, arguing against this explanation. At present, therefore, it seems that a large increase in blood flow (and glucose uptake) should not be needed to maintain energy supply during normal brain activity. Perhaps this large increase occurs as a by-product of a system that attempts to maintain blood flow during conditions of greater energy need that can occur pathologically (see ‘Spreading depression and neurovascular coupling’).
What does functional imaging measure?
BOLD functional magnetic resonance imaging (fMRI) of brain activity depends crucially on functional hyperaemia88. This technique detects a magnetic resonance radio signal emitted from proton spins. The strength of the signal is decreased by deoxyhaemoglobin, which is paramagnetic and makes the magnetic field less uniform. Without functional hyperaemia, the O2 consumption powering neuronal activity would increase the deoxyhaemoglobin level and thus decrease the MRI signal. In fact, BOLD images show an increase in intensity in active brain areas. This is because the increase in blood flow evoked by neuronal activity brings in excess oxygenated blood, so that the deoxyhaemoglobin level falls and the MRI signal is increased. Thus, simplistically, the size of the BOLD fMRI signal is determined by the difference between the amount of functional hyperaemia, which increases the signal, and the use of O2 by neurons, which reduces the signal (there is also a dependence on blood volume, which increases with the pressure increase driving the increased flow, which is ignored for simplicity here).
Because the effect of functional hyperaemia dominates, it follows that, to a large extent, BOLD fMRI signals reflect the causes of functional hyperaemia. When it was thought that activity-evoked blood flow increases were driven by energy use, fMRI images were believed to reflect energy use by active neurons. The discovery that functional hyperaemia is driven largely by glutamate release indicates, instead, that fMRI mainly images the neurovascular signalling consequences of synaptic activity89. Consistent with this, cortical BOLD signals correlate slightly better with field potentials reflecting stimulus-evoked synaptic currents than with stimulus-evoked action potentials90, but they still correlate well with action potential activity90 because cortical synaptic potentials are themselves well correlated with pyramidal cell spiking. It would be instructive to repeat this experiment in the cerebellum, where parallel fibre activity leads to a glutamate-mediated increase in blood flow, but to a decrease in principal (Purkinje) cell firing due to disynaptic inhibition55.
Because BOLD signals mainly reflect the causes of functional hyperaemia, if we assume that this is largely produced by astrocyte signalling, BOLD fMRI images will essentially reflect the activation of astrocyte mGluRs (Fig. 2). Conversely, if the neuronal NO pathway dominates, then BOLD essentially images the activation of postsynaptic nNOS. But BOLD may still give information on neuronal spiking activity, for two reasons. First, glial [Ca2+]i increases (and downstream generation of arachidonic acid derivatives) can be closely correlated with the activity of adjacent neurons51, presumably because the glia respond to glutamate released at synapses onto the neurons. Second, typical voxel sizes used in fMRI (~1.5–3 mm) include many output synapses from the local axon collaterals of pyramidal cells in the cortex91, and so the BOLD signal obtained, although originating from functional hyperaemia evoked by glutamate release, could nevertheless reflect the local pyramidal cell spiking activity that drives glutamate release at those synapses. For this to be the case, the effect on the vasculature of the axon collateral synapses would have to outweigh the effect of synapses carrying input to that part of the cortex from other brain areas.
The spatial resolution of BOLD fMRI has increased with the field strength used for imaging, and now approaches 200 μm in animals85. Even higher resolution can be obtained by optical imaging of changes in the oxy- and deoxyhaemoglobin concentrations within vessels92. The different spatial ranges of the vasodilatory mechanisms outlined in Fig. 2, that is, the distance that NO93 or arachidonic acid derivatives can diffuse and the distance that [Ca2+]i increases propagate through astrocytes, will limit the spatial resolution that functional imaging can potentially attain.
Spreading depression and neurovascular coupling
Functional hyperaemia could be particularly important for preventing neuronal death in pathological conditions in which energy use is raised, but it is in precisely these conditions that neurovascular coupling sometimes fails. One example is in cortical spreading depression, which occurs in migraine94, when it is relatively benign, and repeatedly after stroke, subarachnoid haemorrhage or brain trauma, when it is associated with delayed neurological deficits95–97. Spreading depression is associated with a notable failure of brain ion homeostasis98. The extracellular potassium concentration rises transiently from ~3 to ~50 mM, depolarizing neurons and astrocytes, and the extracellular glutamate concentration rises99 as a result of release from neurons and impairment of uptake100. Brain energy use increases101, presumably because the increase in glutamate concentration activates an influx of Na+ and Ca2+ ions that need to be pumped out, and the sodium pump is further activated by the rise in [K+]o. As a result, the cortex consumes more O2 and glucose than is provided by the blood, resulting in a decrease in their extracellular concentrations101–103. This increase in energy use is pronounced during the first few minutes of spreading depression, but persists at a lower level for hours101.
A failure of neurovascular coupling to provide a sufficient increase in blood flow during the increased energy consumption generated by spreading depression may underlie the neurological deficits associated with spreading depression after subarachnoid haemorrhage97. During spreading depression, cerebral blood flow is initially transiently increased101,102 (less so in migraine than in stroke or brain trauma94,104), sometimes with a preceding decrease in blood flow46,105. After a few minutes, however, blood flow is reduced94,101,102,105 by 20–30%. This decrease lasts for hours despite the increase in energy consumption during this period101.
The rise in [K+]o and associated cell depolarization during spreading depression raises [Ca2+]i in neurons and astrocytes46, leading to the release of messengers that evoke the observed blood flow changes. A release of calcitonin gene related peptide (CGRP) from trigeminal neurons innervating cerebral arteries, probably as a result of synaptic terminals depolarizing, contributes to the transient increase in blood flow induced by spreading depression104,106. CGRP may act on receptors on smooth muscle cells or on astrocytes to produce this effect, although blocking the production of prostaglandins or EETs does not reduce the blood flow increase104, indicating that a direct action on smooth muscle is more likely. Release of NO has also been suggested to contribute to the vasodilation105,106, although this is controversial104 and the effects of NOS blockers may reflect a need for NO to be present to suppress vasoconstriction mediated by 20-HETE (Fig. 4).
The sustained decrease in blood flow caused by spreading depression may be partly mediated by 20-HETE generated from astrocyte arachidonic acid. Reducing the increase in astrocyte [Ca2+]i that accompanies spreading depression (using thapsigargin to prevent Ca2+ accumulation in internal stores), or blocking phospholipase A2 to prevent arachidonic acid formation, both prevented the vasoconstriction46. This study, however, imaged only the early vasoconstriction occurring before the transient increase of blood flow, and not the long-lasting constriction that reduces blood flow for hours. During the prolonged constriction, normal functional hyperaemia is disrupted: vessels no longer dilate to physiological activation107. This is partly due to an impairment of the NO system105,108, which normally dilates vessels directly or suppresses 20-HETE-mediated constrictions (Fig. 4), and may reflect the fall in O2 concentration from ~24 to ~12 μM during this period101, which will approximately halve NO production (Fig. 5). Indeed, more generally, the fact that O2 supply by the blood depends partly on the release of the vasodilator NO, which requires O2 for its formation, suggests a potentially damaging feedback loop. An increase in energy consumption lowers the O2 concentration101, which decreases NO formation, lowering blood flow and reducing the O2 concentration further.
Neurovascular coupling after ischaemia
Following brain ischaemia, when an occluding intraluminal thrombus is cleared from a blood vessel either spontaneously or therapeutically using exogenous tissue plasminogen activator (tPA), after a brief period of hyperaemia there is a decrease in blood flow lasting several hours2,109–111. This inadequate matching of blood flow to neural activity may produce damage to neurons or glial cells beyond that caused by the initial ischaemia. The decreased flow, or ‘no-reflow phenomenon’, was attributed to a reduction in capillary diameter as a result of astrocyte endfoot swelling, causing capillary blockage by blood cells and fibrin109, but later work suggested that this effect had been overestimated112. A failure of arteriole vasodilation mechanisms (tested with CO2 and acetylcholine, which both release NO113 and arachidonic acid derivatives114) may underlie the long-lasting decrease in blood flow2,110,115. However, in studies measuring blood flow and not vessel diameter, defects in arteriole dilation would be hard to distinguish from a situation in which arteriole dilation occurs but fails to increase blood flow because some capillaries are blocked.
Recent evidence indicates an important role for capillary pericytes in the long-lasting blood flow decrease following ischaemia. Some pericytes constrict at the start of ischaemia75. This may be because their [Ca2+]i rises in the absence of ATP to pump Ca2+ out of the cell, although altered release of vasoactive messengers may also contribute (see below). Indeed, a decreased capillary diameter has been noted after ischaemia111. Interestingly, pericytes are also highly susceptible to damage in ischaemia76, when ATP levels are expected to be low. This raises the possibility that pericytes constrict capillaries at the start of a stroke, and then stay in rigor (because no ATP is available to relax their contractile filaments), causing the capillaries to remain too small for the passage of red blood cells. In agreement with this, pericytes remain constricted and prevent red blood cell passage even two hours after re-opening an occluded parent artery76. Suppression of oxidative and nitrosative stress prevents this pericyte constriction, restores the patency of capillaries, and improves tissue recovery76. The involvement of oxidative or nitrosative stress suggests another mechanism by which pericytes might constrict. Peroxynitrite inhibits the formation of vasodilating prostacyclin by endothelial cells116, resulting in the accumulation of vasoconstricting PGH2 (Fig. 3), and may also inhibit the formation of vasodilatory EETs117 and NO118, any of which could lead to pericyte constriction.
The therapeutic use of tPA may itself alter neurovascular coupling, in part because it may extravasate and gain access to the brain parenchyma when the blood–brain barrier becomes leaky in ischaemia (a process that may be enhanced by tPA upregulating119 matrix metalloprotease 9). There is debate about whether tPA is neurotoxic119, but endogenous tPA, which is released from neurons, cleaves the NR1 subunit of NMDA receptors and thus enhances NMDA receptor signalling120. Independent of this effect, endogenous tPA is essential for neurovascular coupling mediated by NMDA receptors and NO, because it regulates the phosphorylation of nNOS and thereby promotes NO release121. Accordingly, exogenous tPA promotes vessel dilation in an NO-dependent manner122, but it also enhances the failure of vasodilation that occurs after stroke123. To complicate the interpretation of these data, when used clinically tPA is co-packaged with a vehicle, a high concentration of L-arginine, which will promote NO formation by NOS. This will contribute to vasodilation and could possibly damage neurons.
Neurovascular coupling in Alzheimer’s disease
There is increasing evidence for vascular factors having a causal role in the development of Alzheimer’s disease3. Many patients with Alzheimer’s disease have regional cerebral hypoperfusion124, which correlates with cognitive decline125. Amyloid-β peptide decreases functional hyperaemia by promoting oxidative stress126, which inhibits the production of astrocytic and neuronal vasodilating messengers116–118. A raised basal [Ca2+]i and enhanced occurrence of spontaneous Ca2+ waves in the astrocytes of mice with amyloid-β plaques127, together with increased contractility of vascular smooth muscle cells128, will also disrupt the normal regulation of cerebral blood vessels by astrocytes and neurons. Such changes in neurovascular coupling may make it hard to interpret BOLD fMRI signals from older subjects129, just as inhibiting neurovascular coupling pharmacologically alters the relationship between neuronal activity and BOLD signals20. Similarly, the formation of an astrocytic scar after traumatic brain injury is likely to profoundly alter neurovascular coupling.
Prospects for new therapies
There have been four major conceptual shifts in our understanding of how cerebral blood flow is regulated. It is now thought that neurotransmitters, particularly glutamate, rather than energy use are the principal agents generating activity-induced blood flow; that astrocytes mediate a large part of this blood flow control; that O2 concentration regulates the relative importance of the signalling pathways involved; and that control of blood flow occurs at the capillary level as well as the arteriole level. These developments not only provide an increased understanding of how neural computation is powered, they also offer opportunities for developing therapies for treating disorders of cerebral blood flow.
In general, identifying the signalling pathways that regulate cerebral blood flow provides opportunities for manipulating those pathways therapeutically. This is conceptually straightforward for neurotransmitter-mediated signalling pathways that depend on well understood receptors and enzymes that can, in principle, be blocked. This could be done, for example, at the level of astrocyte mGluRs or the enzymes that generate vasoactive messengers such as cyclooxygenase, epoxygenase, CYP4A or NOS. The existence of many pathways regulating blood flow (Fig. 2) may allow subtle therapeutic modification of blood flow in different conditions or brain areas, so long as interactions between the different pathways (Fig. 4) are taken into account. The advent of new imaging techniques for rapid assessment of patients’ cerebral blood flow could also enable therapies to be given earlier, before neuronal damage has been initiated. In particular, the signalling changes that occur in spreading depression and stroke suggest new therapeutic approaches, as follows.
The decrease in blood flow that follows spreading depression101,102,105, which may contribute to cognitive decline after brain trauma, stroke or vasospasm after subarachnoid haemorrhage97, may be produced by excessive generation of vasoconstricting 20-HETE formed from astrocyte arachidonic acid. This could be tested using inhibitors of the CYP4A enzyme that generates 20-HETE. A role for pericyte constriction in this phenomenon should also be tested.
The long-lasting decrease in blood flow observed after experimental ischaemia2,109–111, produced in part by pericyte constriction75,76, indicates that even rapid dissolution of a clot using tPA may be limited in its effects on restoring perfusion. This suggests that, in combination with tPA application, it would be worth trying to preserve normal pericyte function. Pericyte constriction and death after ischaemia are mediated by oxidative or nitrosative stress76, so infusing antioxidants at the same time as tPA may be a useful adjuvant approach. Preclinical studies assessing how pericyte properties vary with age and gender, and whether pericyte contraction contributes to pathology in hypertension (which is associated with neurovascular dysfunction3) or hyperglycaemia72,74,130, will also be valuable.
Acknowledgments
We apologize to those whose work we have not cited because of space constraints. We thank the following for useful discussion: K. Caesar, A. Gjedde, C. Hall, A. Mishra, G. Rees and A. Roth. Work in our laboratories is supported by the Fondation Leducq, the European Research Council, the Wellcome Trust, the UK Medical Research Council, the Dunhill Medical Trust, the Biomedical Research Centres of the UK National Institute for Health Research, the European Commission’s Sixth Framework Programme, the Human Frontier Science Program, the Danish Medical Research Council, the Lundbeck Foundation, the Nordea Foundation Centre for Healthy Aging, the Novo Nordisk Foundation, the Canadian Institutes of Health Research, the Canada Research Chair in Neuroscience, and the US National Institutes of Health (National Eye Institute).
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
References
- 1.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Leffler CW, Busija DW, Mirro R, Armstead WM, Beasley DG. Effects of ischemia on brain blood flow and oxygen consumption of newborn pigs. Am J Physiol. 1989;257:H1917–H1926. doi: 10.1152/ajpheart.1989.257.6.H1917. [DOI] [PubMed] [Google Scholar]
- 3.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 4.Baptiste DC, Fehlings M. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 5.Tian R, et al. Role of extracellular and intracellular acidosis for hypercapnia-induced inhibition of tension of isolated rat cerebral arteries. Circ Res. 1995;76:269–275. doi: 10.1161/01.res.76.2.269. [DOI] [PubMed] [Google Scholar]
- 6.Mintun MA, et al. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci USA. 2001;98:6859–6864. doi: 10.1073/pnas.111164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindauer U, et al. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J Cereb Blood Flow Metab. 2010;30:757–768. doi: 10.1038/jcbfm.2009.259. Challenges brain slice data showing that high [O2] converts dilations seen at physiological [O2] into constrictions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers WJ, Hirsch IB, Cryer PE. Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation. Am J Physiol. 1996;270:H554–H559. doi: 10.1152/ajpheart.1996.270.2.H554. [DOI] [PubMed] [Google Scholar]
- 9.Astrup J, et al. Evidence against H+ and K+ as main factors for the control of cerebral blood flow: a microelectrode study. Ciba Found Symp. 1978;56:313–337. doi: 10.1002/9780470720370.ch16. [DOI] [PubMed] [Google Scholar]
- 10.Makani S, Chesler M. Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J Neurophysiol. 2010;103:667–676. doi: 10.1152/jn.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko KR, Ngai AC, Winn HR. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am J Physiol Heart Circ Physiol. 1990;259:H1703–H1708. doi: 10.1152/ajpheart.1990.259.6.H1703. [DOI] [PubMed] [Google Scholar]
- 12.Ido Y, Chang K, Woolsey TA, Williamson JR. NADH: sensor of blood flow need in brain, muscle and other tissues. FASEB J. 2001;15:1419–1421. doi: 10.1096/fj.00-0652fje. [DOI] [PubMed] [Google Scholar]
- 13.Akgören N, Fabricius M, Lauritzen M. Importance of nitric oxide for local increases of blood flow in rat cerebellar cortex during electrical stimulation. Proc Natl Acad Sci USA. 1994;91:5903–5907. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Iadecola C. Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharmacology. 1994;33:1453–1461. doi: 10.1016/0028-3908(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 15.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen AN, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaigneau E, et al. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci. 2007;27:6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Offenhauser N, Thomsen K, Caesar K, Lauritzen M. Activity induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J Physiol. 2005;565:279–294. doi: 10.1113/jphysiol.2005.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecoq J, et al. Odor-evoked oxygen consumption by action potential and synaptic transmission in the olfactory bulb. J Neurosci. 2009;29:1424–1433. doi: 10.1523/JNEUROSCI.4817-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Lawrence KS, Ye FQ, Lewis BK, Frank JA, McLaughlin AC. Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med. 2003;50:99–106. doi: 10.1002/mrm.10502. [DOI] [PubMed] [Google Scholar]
- 21.Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-D-aspartate in cerebral cortex. Brain Res Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma J, Ayata C, Huang PL, Fishman MC, Moskowitz MA. Regional cerebral blood flow response to vibrissal stimulation in mice lacking type I NOS gene expression. Am J Physiol. 1996;270:H1085–H1090. doi: 10.1152/ajpheart.1996.270.3.H1085. [DOI] [PubMed] [Google Scholar]
- 23.Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol Heart Circ Physiol. 1999;277:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- 24.Akgören N, Dalgaard P, Lauritzen M. Cerebral blood flow increases evoked by electrical stimulation of rat cerebellar cortex: relation to excitatory synaptic activity and nitric oxide synthesis. Brain Res. 1996;710:204–214. doi: 10.1016/0006-8993(95)01354-7. [DOI] [PubMed] [Google Scholar]
- 25.Yang G, Zhang Y, Ross ME, Iadecola C. Attenuation of activity-induced increases in cerebellar blood flow in mice lacking neuronal nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2003;285:H298–H304. doi: 10.1152/ajpheart.00043.2003. [DOI] [PubMed] [Google Scholar]
- 26.Cauli B, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab. 2008;28:221–231. doi: 10.1038/sj.jcbfm.9600558. [DOI] [PubMed] [Google Scholar]
- 28.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filosa JA, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nature Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 33.Ou JW, et al. Ca2+- and thromboxane-dependent distribution of MaxiK channels in cultured astrocytes: from microtubules to the plasma membrane. Glia. 2009;57:1280–1295. doi: 10.1002/glia.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon GRJ, et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. Shows that O2 level profoundly affects vascular response to neuronal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng X, et al. Suppression of functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–H2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 37.Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Davis RJ, et al. EP4 prostanoid receptor-mediated vasodilation of human middle cerebral arteries. Br J Pharmacol. 2004;141:580–585. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takata F, et al. Adrenomedullin-induced relaxation of rat brain pericytes is related to the reduced phosphorylation of myosin light chain through the cAMP/PKA signaling pathway. Neurosci Lett. 2009;449:71–75. doi: 10.1016/j.neulet.2008.10.082. [DOI] [PubMed] [Google Scholar]
- 40.Serebryakov V, Zakharenko S, Snetkov V, Takeda K. Effects of prostaglandins E1 and E2 on cultured smooth muscle cells and strips of rat aorta. Prostaglandins. 1994;47:353–365. doi: 10.1016/0090-6980(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 41.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 42.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther. 2009;328:231–239. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 43.Takano T, et al. Astrocyte mediated control of cerebral blood flow. Nature Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. Extends, to the in vivo situation, the Zonta et al. (2003) result that astrocytes control cerebral blood flow. [DOI] [PubMed] [Google Scholar]
- 44.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 45.Blanco VM, Stern JE, Filosa J. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci. 2007;27:4036–4044. doi: 10.1523/JNEUROSCI.0721-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kis B, Snipes JA, Isse T, Nagy K, Busija DW. Putative cyclooxygenase-3 expression in rat brain cells. J Cereb Blood Flow Metab. 2003;23:1287–1292. doi: 10.1097/01.WCB.0000090681.07515.81. [DOI] [PubMed] [Google Scholar]
- 48.Hirst WD, et al. Expression of COX-2 by normal and reactive astrocytes in the adult rat central nervous system. Mol Cell Neurosci. 1999;13:57–68. doi: 10.1006/mcne.1998.0731. [DOI] [PubMed] [Google Scholar]
- 49.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 52.Doengi M, et al. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc Natl Acad Sci USA. 2009;106:17570–17575. doi: 10.1073/pnas.0809513106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nature Rev Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- 54.Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathiesen C, Caesar K, Akgören N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512:555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enager P, et al. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 58.Lindauer U, Megow D, Schultze J, Weber JR, Dirnagl U. Nitric oxide synthase inhibition does not affect somatosensory evoked potentials in the rat. Neurosci Lett. 1996;216:207–210. doi: 10.1016/0304-3940(96)13044-5. [DOI] [PubMed] [Google Scholar]
- 59.Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol. 1999;277:R1760–R1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- 60.Caesar K, Akgören N, Mathiesen C, Lauritzen M. Modification of activity-dependent increases in cerebellar blood flow by extracellular potassium in anaesthetized rats. J Physiol. 1999;520:281–292. doi: 10.1111/j.1469-7793.1999.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Golanov EV, Reis DJ. Nitric oxide and prostanoids participate in cerebral vasodilation elicited by electrical stimulation of the rostral ventrolateral medulla. J Cereb Blood Flow Metab. 1994;14:492–502. doi: 10.1038/jcbfm.1994.61. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J Cereb Blood Flow Metab. 2007;27:575–587. doi: 10.1038/sj.jcbfm.9600372. [DOI] [PubMed] [Google Scholar]
- 63.Akgören N, Mathiesen C, Rubin I, Lauritzen M. Laminar analysis of activity-dependent increases of CBF in rat cerebellar cortex: dependence on synaptic strength. Am J Physiol. 1997;273:H1166–H1176. doi: 10.1152/ajpheart.1997.273.3.H1166. [DOI] [PubMed] [Google Scholar]
- 64.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 65.Fujimoto Y, Uno E, Sakuma S. Effect of reactive oxygen and nitrogen species on cyclooygenase-1 and -2 activities. Prostaglandins Leukot Essent Fatty Acids. 2004;71:335–340. doi: 10.1016/j.plefa.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Sun CW, Falck JR, Okamoto H, Harder DR, Roman RJ. Role of cGMP versus 20-HETE in the vasodilator response to nitric oxide in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2000;279:H339–H350. doi: 10.1152/ajpheart.2000.279.1.H339. [DOI] [PubMed] [Google Scholar]
- 67.Stuehr DJ, Santolini J, Wang Z, Wei C, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 68.Harder DR, et al. Identification of a putative microvascular oxygen sensor. Circ Res. 1996;79:54–61. doi: 10.1161/01.res.79.1.54. [DOI] [PubMed] [Google Scholar]
- 69.Juránek I, Suzuki H, Yamamoto S. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta. 1999;1436:509–518. [Google Scholar]
- 70.Hall CN, Attwell D. Assessing the physiological concentration and targets of nitric oxide in brain tissue. J Physiol. 2008;586:3597–3615. doi: 10.1113/jphysiol.2008.154724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caesar K, et al. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:5. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 74.Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- 75.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yemişçi M, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nature Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. Shows that pericyte constriction decreases blood flow after stroke. [DOI] [PubMed] [Google Scholar]
- 77.Lovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience. 1999;92:47–60. doi: 10.1016/s0306-4522(98)00737-4. [DOI] [PubMed] [Google Scholar]
- 78.Lu K, et al. Cerebral autoregulation and gas exchange studied using a human cardiopulmonary model. Am J Physiol Heart Circ Physiol. 2004;286:H584–H601. doi: 10.1152/ajpheart.00594.2003. [DOI] [PubMed] [Google Scholar]
- 79.Boas DA, Jones SR, Devor A, Huppert TJ, Dale AM. A vascular anatomical network model of the spatio-temporal response to brain activation. Neuroimage. 2008;40:1116–1129. doi: 10.1016/j.neuroimage.2007.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- 81.Schölvinck M, Howarth C, Attwell D. The cortical energy needed for conscious perception. Neuroimage. 2008;40:1460–1468. doi: 10.1016/j.neuroimage.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin AL, Fox PT, Hardies J, Duong TQ, Gao JH. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci USA. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. Important quantification of the relative magnitudes of stimulus-induced changes in blood flow, O2 use and ATP generation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activation. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 84.Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 85.Mangia S, et al. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab. 2009;29:441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 87.Leithner C, et al. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J Cereb Blood Flow Metab. 2010;30:311–322. doi: 10.1038/jcbfm.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uğurbil K, et al. Magnetic resonance studies of brain function and neurochemistry. Annu Rev Biomed Eng. 2000;2:233–260. doi: 10.1146/annurev.bioeng.2.1.633. [DOI] [PubMed] [Google Scholar]
- 89.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 90.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:1517–1531. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 91.Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hillman EM, et al. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- 95.Fabricius M, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- 96.Dohmen C, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- 97.Dreier JP, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 98.Hansen AJ, Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand. 1981;113:437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 99.Van Harreveld A, Kooiman M. Amino acid release from the cerebral cortex during spreading depression and asphyxiation. J Neurochem. 1965;12:431–439. doi: 10.1111/j.1471-4159.1965.tb04243.x. [DOI] [PubMed] [Google Scholar]
- 100.Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988;335:433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- 101.Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29:1517–1527. doi: 10.1038/jcbfm.2009.73. Quantifies changes in energy use, blood flow and neurovascular coupling after spreading depression. [DOI] [PubMed] [Google Scholar]
- 102.Takano T, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nature Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- 103.Hashemi P, et al. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leão’s spreading depression. J Cereb Blood Flow Metab. 2009;29:166–175. doi: 10.1038/jcbfm.2008.108. [DOI] [PubMed] [Google Scholar]
- 104.Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol. 2008;86:379–395. doi: 10.1016/j.pneurobio.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fabricius M, Akgören N, Lauritzen M. Arginine–nitric oxide pathway and cerebrovascular regulation in cortical spreading depression. Am J Physiol. 1995;269:H23–H29. doi: 10.1152/ajpheart.1995.269.1.H23. [DOI] [PubMed] [Google Scholar]
- 106.Wahl M, Schilling L, Parsons AA, Kaumann A. Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression. Brain Res. 1994;637:204–210. doi: 10.1016/0006-8993(94)91234-3. [DOI] [PubMed] [Google Scholar]
- 107.Wahl M, Lauritzen M, Schilling L. Changes of cerebrovascular reactivity after cortical spreading depression in cats and rats. Brain Res. 1987;411:72–80. doi: 10.1016/0006-8993(87)90682-2. [DOI] [PubMed] [Google Scholar]
- 108.Scheckenbach KE, Dreier JP, Dirnagl U, Lindauer U. Impaired cerebrovascular reactivity after cortical spreading depression in rats: restoration by nitric oxide or cGMP. Exp Neurol. 2006;202:449–455. doi: 10.1016/j.expneurol.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 109.Ames A, III, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischaemia II The no-reflow phenomenon. Am J Pathol. 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- 110.Nelson CW, Wei EP, Povlishock JT, Kontos HA, Moskowitz MA. Oxygen radicals in cerebral ischemia. Am J Physiol. 1992;263:H1356–H1362. doi: 10.1152/ajpheart.1992.263.5.H1356. [DOI] [PubMed] [Google Scholar]
- 111.Hauck EF, Apostel S, Hoffmann JF, Heimann A, Kempski O. Capillary flow and diameter changes during reperfusion after global cerebral ischemia studied by intravital video microscopy. J Cereb Blood Flow Metab. 2004;24:383–391. doi: 10.1097/00004647-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Theilen H, Schröck H, Kuschinsky W. Gross persistence of capillary plasma perfusion after middle cerebral artery occlusion in the rat brain. J Cereb Blood Flow Metab. 1994;14:1055–1061. doi: 10.1038/jcbfm.1994.138. [DOI] [PubMed] [Google Scholar]
- 113.Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol. 1994;266:R546–R552. doi: 10.1152/ajpregu.1994.266.2.R546. [DOI] [PubMed] [Google Scholar]
- 114.Wagerle LC, Mishra OP. Mechanism of CO2 response in cerebral arteries of the newborn pig: role of phospholipase, cyclooxygenase, and lipoxygenase pathways. Circ Res. 1988;62:1019–1026. doi: 10.1161/01.res.62.5.1019. [DOI] [PubMed] [Google Scholar]
- 115.Kågström E, Smith ML, Siesjö BK. Cerebral circulatory responses to hypercapnia and hypoxia in the recovery period following complete and incomplete cerebral ischemia in the rat. Acta Physiol Scand. 1983;118:281–291. doi: 10.1111/j.1748-1716.1983.tb07272.x. [DOI] [PubMed] [Google Scholar]
- 116.Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol. 1999;154:1359–1365. doi: 10.1016/S0002-9440(10)65390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s) Pharmacol Res. 2004;49:525–533. doi: 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 118.Sun J, Druhan LJ, Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys. 2008;471:126–133. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 120.Nicole O, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nature Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 121.Park L, et al. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. Suggests that tPA, as used clinically to clear clots from blocked vessels, has a role in neurovascular coupling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Armstead WM, Cines DB, Al-Roof Higazi A. Altered NO function contributes to impairment of uPA and tPA cerebrovasodilation after brain injury. J Neurotrauma. 2004;21:1204–1211. doi: 10.1089/neu.2004.21.1204. [DOI] [PubMed] [Google Scholar]
- 123.Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- 124.Johnson NA, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruitenberg A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 126.Park L, et al. Aβ-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–342. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- 127.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chow N, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci USA. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nature Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 130.Geraldes P, et al. Activation of PKC-δ and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nature Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]