Abstract
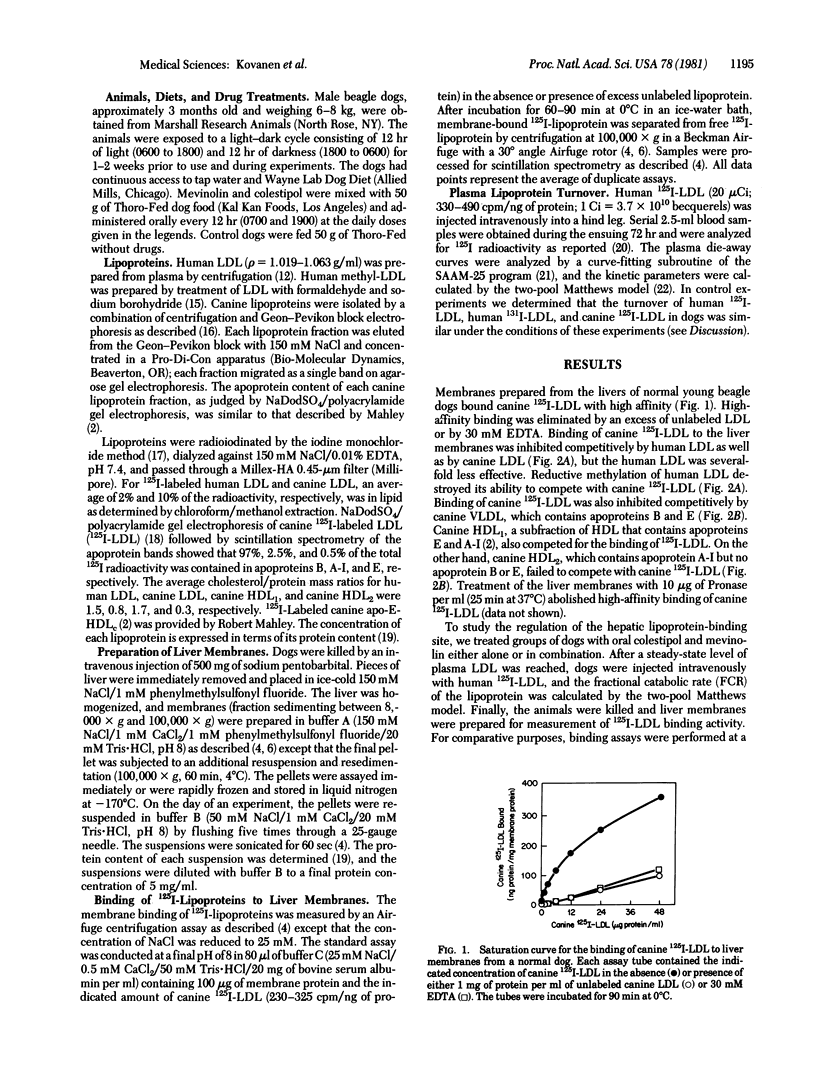
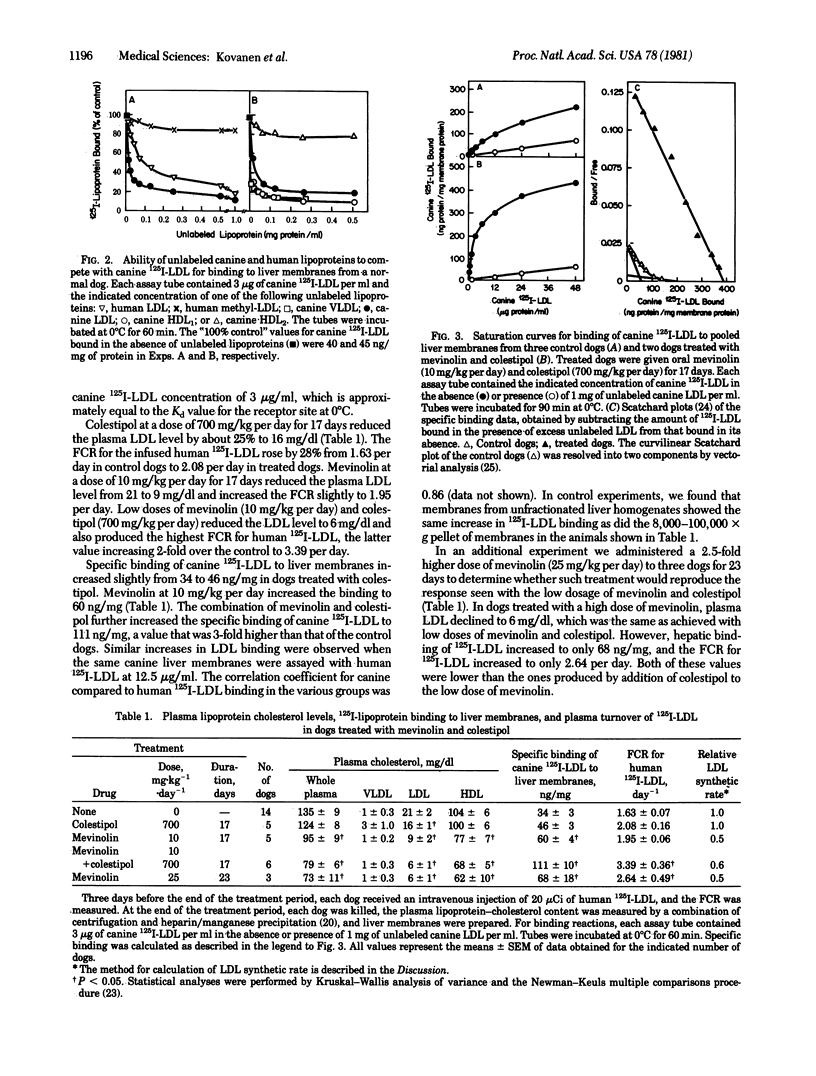
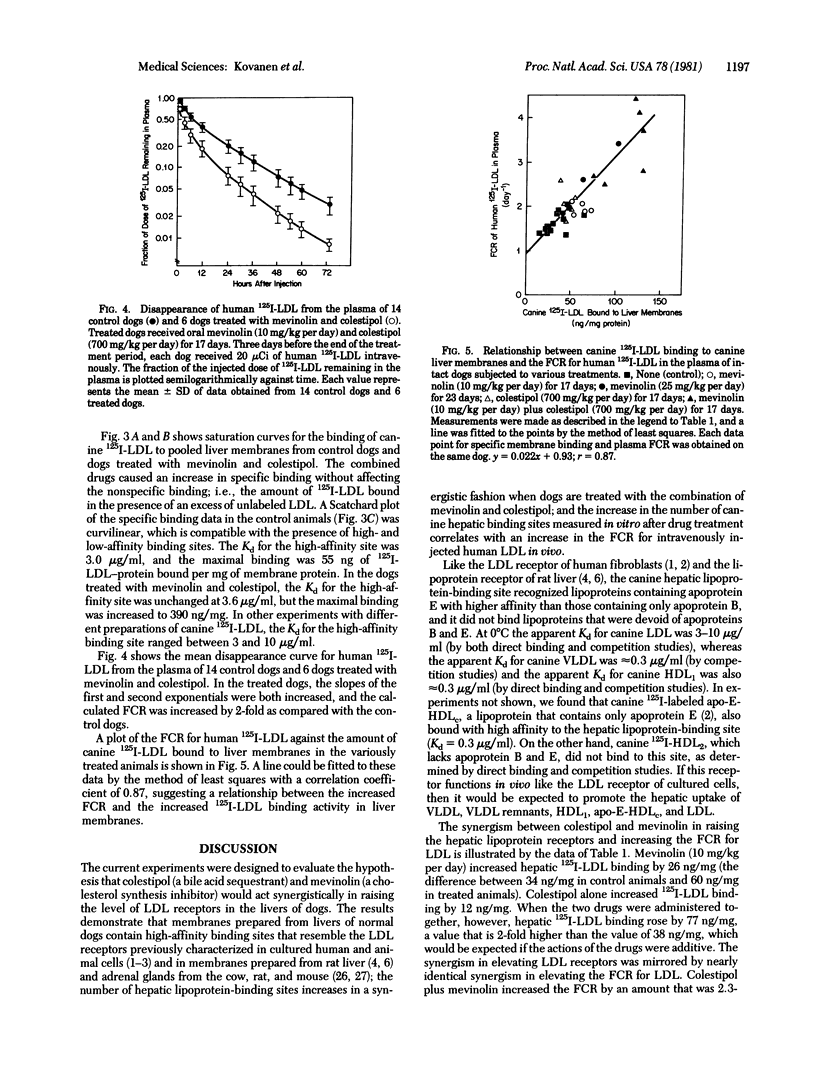
Liver membranes from young beagle dogs were found to possess binding sites that resemble the low density lipoprotein (LDL) receptors originally described in cultured human fibroblasts. Treatment of the dogs with colestipol (a bile acid sequestrant) and mevinolin (a cholesterol synthesis inhibitor) produced a 3-fold increase in LDL binding activity. This increase correlated with a 2-fold increase in the fractional catabolic rate for intravenously administered human or canine 125I-labeled LDL, suggesting that the increased hepatic receptors were responsible for the enhanced clearance of LDL from plasma. The hepatic lipoprotein receptors of control and drug-treated dogs resembled human fibroblast LDL receptors in that they bound apoprotein E-containing lipoproteins, such as very low density lipoproteins and a subfraction of high density lipoproteins (HDL1), with 10-fold higher affinity than the apoprotein B-containing lipoprotein LDL; failed to bind canine HDL2 and human HDL3, which are devoid of apoproteins B and E; failed to bind methylated LDL; required calcium; and were destroyed by Pronase. Treatment of dogs with mevinolin not only increased the fractional catabolic rate for LDL but also reduced the synthetic rate for the lipoprotein. The current data suggest that the liver of dogs contains functional LDL receptors that are susceptible to metabolic regulation and that a drug-induced increase in the activity of these receptors can contribute to a lowering of plasma levels of LDL-cholesterol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Brown M. S. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975 Dec;56(6):1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y. S., Jones A. L., Hradek G. T., Windler E. E., Havel R. J. Autoradiographic localization of the sites of uptake, cellular transport, and catabolism of low density lipoproteins in the liver of normal and estrogen-treated rats. Proc Natl Acad Sci U S A. 1981 Jan;78(1):597–601. doi: 10.1073/pnas.78.1.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- Kovanen P. T., Basu S. K., Goldstein J. L., Brown M. S. Low density lipoprotein receptors in bovine adrenal cortex. II. Low density lipoprotein binding to membranes prepared from fresh tissue. Endocrinology. 1979 Mar;104(3):610–616. doi: 10.1210/endo-104-3-610. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Kovanen P. T., Goldstein J. L., Chappell D. A., Brown M. S. Regulation of low density lipoprotein receptors by adrenocorticotropin in the adrenal gland of mice and rats in vivo. J Biol Chem. 1980 Jun 25;255(12):5591–5598. [PubMed] [Google Scholar]
- Kovanen P. T., Schneider W. J., Hillman G. M., Goldstein J. L., Brown M. S. Separate mechanisms for the uptake of high and low density lipoproteins by mouse adrenal gland in vivo. J Biol Chem. 1979 Jun 25;254(12):5498–5505. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H. Canine lipoproteins and atherosclerosis. I. Isolation and characterization of plasma lipoproteins from control dogs. Circ Res. 1974 Nov;35(5):713–721. doi: 10.1161/01.res.35.5.713. [DOI] [PubMed] [Google Scholar]
- Moutafis C. D., Myant N. B. The metabolism of cholesterol in two hypercholesterolaemic patients treated with cholestyramine. Clin Sci. 1969 Oct;37(2):443–454. [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Packard C. J., Bicker S., Lawrie T. D., Morgan H. G. Cholestyramine promotes receptor-mediated low-density-lipoprotein catabolism. N Engl J Med. 1980 May 29;302(22):1219–1222. doi: 10.1056/NEJM198005293022202. [DOI] [PubMed] [Google Scholar]
- Tsujita Y., Kuroda M., Tanzawa K., Kitano N., Endo A. Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Atherosclerosis. 1979 Mar;32(3):307–313. doi: 10.1016/0021-9150(79)90174-6. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]


