Abstract
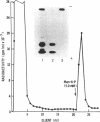
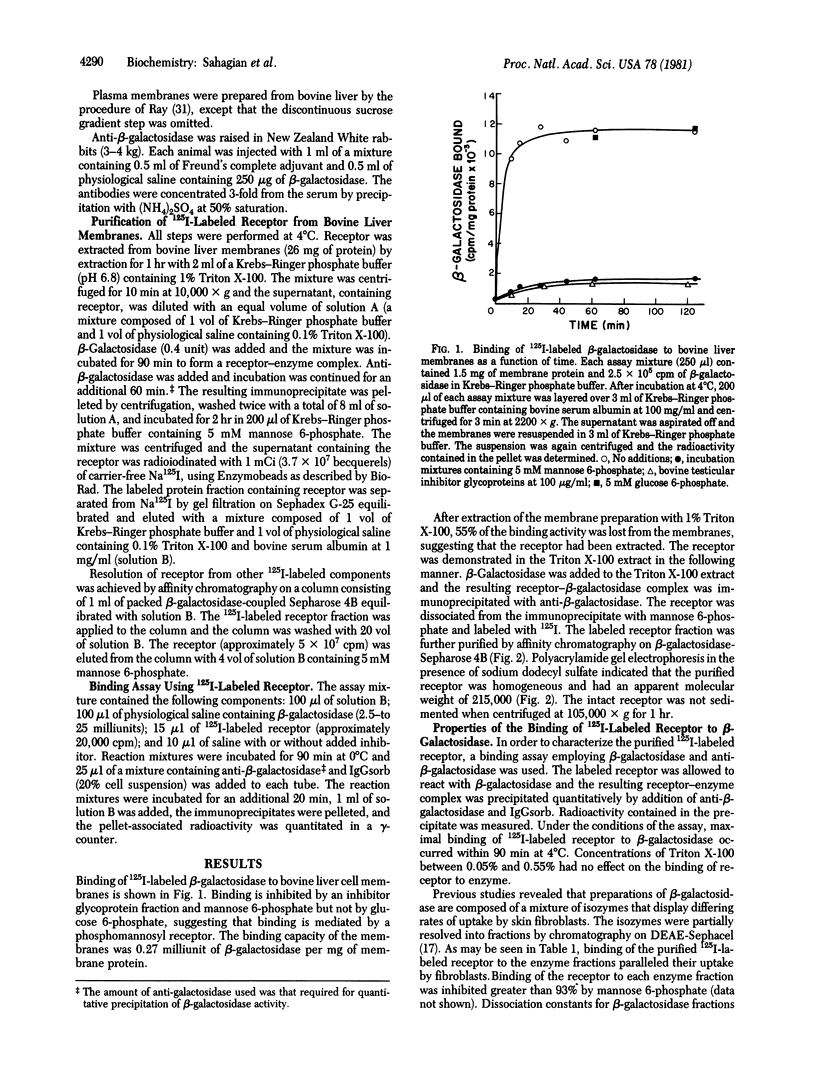
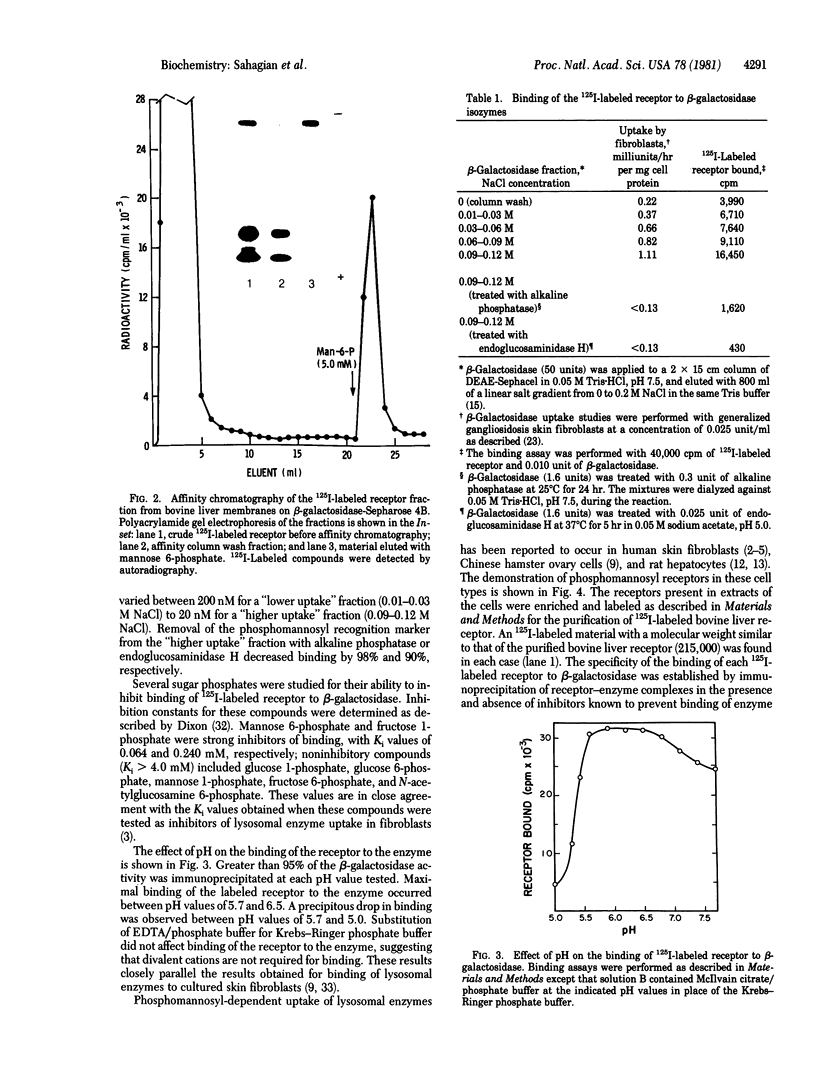
A receptor that binds the phosphomannosyl recognition marker of bovine testicular beta-galactosidase (beta-D-galactoside galactohydrolase, EC 3.2.1.23) was isolated from bovine liver membranes. The receptor was extracted from crude plasma membrane preparations with Triton X-100 and immunoprecipitated as a receptor--beta-galactosidase complex with anti-beta-galactosidase. The receptor was dissociated from the precipitate with mannose 6-phosphate, labeled with 125I, and purified on a beta-galactosidase-Sepharose 4B affinity matrix. A quantitative binding assay employing anti-beta-galactosidase and IgGsorb (formalin-fixed Staphylococcus aureus) was devised to study the binding of 125I-labeled receptor to beta-galactosidase. Maximal binding of receptor to enzyme occurred at pH values between 5.7 and 6.5. Divalent cations were not required for binding. The values of the dissociation constant obtained for beta-galactosidase varied between 200 nM observed with "lower uptake" forms and 20 nM for "higher uptake" forms of the enzyme. A number of phosphorylated monosaccharides were tested as inhibitors of binding of enzyme to receptor; mannose 6-phosphate and fructose 1-phosphate served as inhibitors and exhibited Ki values of 0.064 mM and 0.24 mM, respectively. The receptor has a subunit molecular weight of 215,000. Similar receptors were also demonstrated in Triton X-100 extracts of human skin fibroblasts, Chinese hamster ovary cells, and rat hepatocytes. These cell types are known to assimilate lysosomal enzymes containing covalently bound mannose 6-phosphate residues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DISTLER J. J., MERRICK J. M., ROSEMAN S. Glucosamine metabolism. III. Preparation and N-acetylation of crystalline D-glucosamine- and D-galactosamine-6-phosphoric acids. J Biol Chem. 1958 Jan;230(1):497–509. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler J. J., Jourdian G. W. beta-Galactosidase from bovine testes. Methods Enzymol. 1978;50:514–520. doi: 10.1016/0076-6879(78)50055-4. [DOI] [PubMed] [Google Scholar]
- Distler J., Hieber V., Sahagian G., Schmickel R., Jourdian G. W. Identification of mannose 6-phosphate in glycoproteins that inhibit the assimilation of beta-galactosidase by fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4235–4239. doi: 10.1073/pnas.76.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S. Beta-glucuronidase binding to human fibroblast membrane receptors. J Biol Chem. 1980 Jun 10;255(11):5069–5074. [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieber V., Distler J., Myerowitz R., Schmickel R. D., Jourdian G. W. Selective noncompetitive assimilation of bovine testicular beta-galactosidase and bovine liver beta-glucuronidase by generalized gangliosidosis fibroblasts. J Clin Invest. 1980 Apr;65(4):879–884. doi: 10.1172/JCI109740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Fischer D., Achord D., Sly W. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Invest. 1977 Nov;60(5):1088–1093. doi: 10.1172/JCI108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine, and closely related analogs. J Biol Chem. 1980 May 10;255(9):4011–4019. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Natowicz M. R., Chi M. M., Lowry O. H., Sly W. S. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4322–4326. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E. F., Lim T. W., Shapiro L. J. Inherited disorders of lysosomal metabolism. Annu Rev Biochem. 1975;44:357–376. doi: 10.1146/annurev.bi.44.070175.002041. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F., Sando G. N., Garvin A. J., Rome L. H. The transport of lysosomal enzymes. J Supramol Struct. 1977;6(1):95–101. doi: 10.1002/jss.400060108. [DOI] [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]
- Robbins A. R. Isolation of lysosomal alpha-mannosidase mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1911–1915. doi: 10.1073/pnas.76.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. H., Miller J. Butanedione treatment reduces receptor binding of a lysosomal enzyme to cells and membranes. Biochem Biophys Res Commun. 1980 Feb 12;92(3):986–993. doi: 10.1016/0006-291x(80)90799-8. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Weissmann B., Neufeld E. F. Direct demonstration of binding of a lysosomal enzyme, alpha-L-iduronidase, to receptors on cultured fibroblasts. Proc Natl Acad Sci U S A. 1979 May;76(5):2331–2334. doi: 10.1073/pnas.76.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Ullrich K., Basner R., Gieselmann V., Von Figura K. Recognition of human urine alpha-N-acetylglucosaminidase by rat hepatocytes. Involvement of receptors specific for galactose, mannose 6-phosphate and mannose. Biochem J. 1979 May 15;180(2):413–419. doi: 10.1042/bj1800413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Fleischer M., von Figura K. Epithelial rat liver cells have cell surface receptors recognizing a phosphorylated carbohydrate on lysosomal enzymes. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1591–1598. doi: 10.1515/bchm2.1978.359.2.1591. [DOI] [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Weber E., Von Figura K. Evidence for lysosomal enzyme recognition by human fibroblasts via a phosphorylated carbohydrate moiety. Biochem J. 1978 Mar 15;170(3):643–650. doi: 10.1042/bj1700643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Klein U. Isolation and characterization of phosphorylated oligosaccharides from alpha-N-acetylglucosaminidase that are recognized by cell-surface receptors. Eur J Biochem. 1979 Mar;94(2):347–354. doi: 10.1111/j.1432-1033.1979.tb12900.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Weber E. An alternative hypothesis of cellular transport of lysosomal enzymes in fibroblasts. Effect of inhibitors of lysosomal enzyme endocytosis on intra- and extra-cellular lysosomal enzyme activities. Biochem J. 1978 Dec 15;176(3):943–950. doi: 10.1042/bj1760943. [DOI] [PMC free article] [PubMed] [Google Scholar]