Abstract
Most avian influenza A viruses, which preferentially replicate at the high temperatures found in the digestive tract of birds, have a glutamic acid at residue 627 of the viral RNA polymerase PB2 subunit (Glu-627), whereas the human viruses, which optimally replicate at the low temperatures observed in the human respiratory tract, have a lysine (Lys-627). The mechanism of action for this mutation is still not understood, although interaction with host factors has been proposed to play a major role. In this study, we explored an alternative, yet related, hypothesis that this PB2 mutation may alter the temperature-dependent enzymatic polymerase activity of the viral polymerase. First, the avian polymerase protein, which was purified from baculovirus expression system, indeed remained significantly active at higher temperatures (i.e. 37 and 42 °C), whereas the human E627K mutant drastically lost activity at these high temperatures. Second, our steady-state kinetics data revealed that the human E627K mutant polymerase is catalytically more active than the avian Glu-627 polymerase at 34 °C. Importantly, the E627K mutation elevates apparent Kcat at low temperatures with little effect on Km, suggesting that the E627K mutation alters the biochemical steps involved in enzyme catalysis rather than the interaction with the incoming NTP. Third, this temperature-dependent kinetic impact of the human E627K mutation was also observed with different RNA templates, with different primers and also in the presence of nucleoprotein. In conclusion, our study suggests that the amino acid sequence variations at residue 627 of PB2 subunit can directly alter the enzyme kinetics of influenza polymerase.
Keywords: Enzyme Kinetics, Enzyme Mutation, Evolution, RNA Viruses, Viral Polymerase, Host Adaptation, Influenza A Virus
Introduction
Influenza A viruses infect a wide range of host species including humans, pigs, horses, seals, and birds (1). Aquatic avian species are the main reservoir of different antigenic variants of influenza A virus. Each of the 16 serologically distinct hemagglutinins and nine neuraminidases have been detected in viruses recovered from birds (1). Host switch of avian viruses to humans may occur either through genetic reassortment or direct transmission caused by point mutations, which may result in influenza pandemics (2). One important influenza A virus phenotype tightly associated with viral host switch is temperature-dependent viral growth. Although human influenza A virus strains replicate in the upper respiratory tract at a temperature close to 33 °C and induce an acute respiratory illness (3), avian influenza A virus strains replicate in the intestinal tract of infected birds at a temperature close to 42 °C, and the infection is usually asymptomatic (4). However, the molecular bases for host specificity, temperature-dependent growth, and virulence of influenza A viruses are only partly understood.
The major determinants of species tropism are the surface proteins, hemagglutinins, and neuraminidases because of different receptor specificities between avian and human viruses (5–7). In addition, it is increasingly apparent that gene segments encoding internal proteins, especially the PB2 segment, which is one of three subunits of influenza A virus RNA polymerase complex, also carry determinants for viral host range (8–10). Studies on reassortment viruses have clearly shown that the amino acid at residue 627 of PB2 subunit is an important determinant of host range and virulence of influenza A viruses (10, 11). This residue is almost exclusively glutamic acid in viruses that are adapted to avian species and lysine in viruses adapted to humans. The presence of a lysine at residue 627 enhances polymerase (Pol)2 activity, viral replication, and, in certain cases, pathogenicity in mammals (12, 13). Specifically, the presence of a lysine at amino acid 627 has been reported to allow a higher level of replication to take place at 33 °C, whereas the presence of a glutamic acid at this position appears to confer optimal activity at 41 °C (11), and this correlates well with the temperature at the site of infection in these two species (the airway and the gut, respectively).
Even though the importance of the PB2 627 residue is well established for host specificity, little is known about the functional mechanism. The major hypothesis that has been extensively investigated is that residue 627 interacts with essential host factors or small molecules that differ between mammalian and avian species (8, 14). Over the last few years, a number of proteins have been proposed as potential candidate host factors, although none have been specifically found to be associated with the 627 position (15, 16). Another study reported that PB2 K627E binds influenza nucleoprotein (NP) in human cells when expressed alone, but not when assembled into the trimeric polymerase complex, suggesting that this mutation might induce species-specific conformational alterations that disrupt ribonucleoprotein assembly (17). Thus, these hypotheses are based on the existence of host factors that currently remain elusive. Yet another study suggests that this residue is involved in RNA binding, and differences in this property may correlate with differences in pathogenicity across different species (18).
Recently, the crystal structure of the C-terminal portion of PB2 was solved, which suggests that residue 627 lies on the surface of a discrete domain of PB2 (19, 20). Although the mutation K627E shows no gross structural differences, it does, however, completely reverse the basic charge on the domain surface (19). The crystal structure of this domain does not resemble any known protein domains; therefore, the structure alone is not sufficient to explain the effect of temperature on viral replication between the Glu and Lys variants. However, it is possible that this mutation affects some aspect of overall polymerase function, possibly by promoting interactions with other domains and affecting polymerase activity at different temperatures.
Here, we tested whether the two amino acid sequence variations at residue 627 of PB2 subunit, which are tied to different temperature-dependent growth phenotypes of avian and human strains, directly alter biochemical enzyme kinetics of influenza virus RNA polymerase complex. For this test, we determined the temperature-dependent enzyme activity using purified avian H3N2 Nanchang polymerase with either avian PB2 Glu-627 or human PB2 E627K by steady-state in vitro primer extension assays. The H3N2 Nanchang influenza A virus is a well characterized avian strain that was directly isolated from birds (21–23). Because we are using a baculovirus-expressed polymerase complex, we can determine the intrinsic effects of this mutation in the absence of avian or human host factors. Indeed, we report that a single mutation in the 627 residue of PB2 subunit can impact the temperature-dependent polymerase kinetics of influenza A virus polymerase complex.
EXPERIMENTAL PROCEDURES
Expression and Purification of Influenza A Virus RNA Polymerase Complex and NP
The polymerases used in this study were expressed using the baculovirus expression system as described in our previous study (24). The PA (codon-optimized, Geneart), PB1, and PB2 genes were amplified by reverse transcription-PCR from the H3N2 influenza strain (A/chicken/Nanchang/3-120/01) (21), obtained from Dr. R. Webster (St. Jude Children's Research Hospital, Memphis, TN). The genes were then cloned to the baculovirus expression vector pVL1392 (Invitrogen) between the BglII and XbaI sites. The PA gene was tagged at the C terminus with the tandem affinity purification (TAP) tag, which consisted of a thrombin cleavage site followed by His6 tag, tobacco etch virus cleavage site, and finally an IgG-binding domain. To create a “humanized” PB2, a single mutation was made at residue 627 (E627K) by site-directed mutagenesis. Each of the four pVL1392 plasmids (PA-TAP, PB1, Glu-627 PB2, and E627K PB2) were individually transfected into Sf9 insect cells for the production of recombinant viruses in SF900II SFM media (Invitrogen), following the protocols provided by the vendor (BD Biosciences Pharmingen). To prepare the purified trimeric polymerase, the three recombinant viruses (PA-TAP, PB1, and Glu-627 PB2) were used to coinfect Tni insect cells grown in Express Five SFM medium supplemented with l-glutamine (Invitrogen). Similarly, for polymerase with the E627K PB2 mutation, PA-TAP, PB1, and E627K PB2 recombinant viruses were used. The cells were harvested 72 h after infection, and the lysates obtained were purified by the TAP purification technique as described previously (24, 25). Typically, 1 liter of expression culture produced 0.7 mg of the purified complex with ∼95% purity, which was qualitatively compared with the band intensity of BSA with 95% purity (Sigma-Aldrich) (supplemental Fig. S4).
The NP gene from the Nanchang strain was cloned to pET28a (Novagen), expressing NP protein fused to the His6 tag at its N-terminal end and purified as previously described (24). The RNA binding activity of the purified NP was examined by a filter binding assay (data not shown).
Template Preparation
5′ vRNA (5′-AGUAGAAACAAGGCC-3′) was purchased from Dharmacon. The 30-nucleotide (nt) template that encodes the first 30 nucleotides from the 3′ end of the Nanchang PA gene and a nonspecific 11-nt RNA that is used as a loading control was synthesized by Integrated DNA Technologies. The long template (137-nt) was prepared by in vitro transcription as described previously (24).
ApG-primed RNA Transcription
The ApG-primed transcription assay was performed as described elsewhere (24, 26). The reactions were performed in 10 μl of reaction volume containing transcription buffer, 0.25 unit RNasin μl−1, 500 μm NTPs, 0.16 μm [α-32P]NTPs (3,000 Ci mmol−1), 1.6 μm each 5′ and 3′ end vRNA (30 or 137-nt), 0.3 mm ApG (Biosynthesis), and purified polymerase. Transcription was allowed to occur for 1 h at 30, 32, 34, 37, and 42 °C, and the reactions were terminated by the addition of 10 μl of stop buffer (10 mm EDTA, pH 8.0, 90% formamide). Under this condition, the incorporation of the radioactive substrate was linear to the enzyme concentration and time. The transcription products were separated by 20% PAGE in 7 m urea and detected by autoradiography using a PhosphorImager (Molecular Dynamics). Densitometry was performed using Quantity One (Bio-Rad).
Steady-state Kinetics
For steady-state analysis, the reactions were performed as above at substrate concentrations of 500, 250, 100, 25, and 10 μm at 34, 37, and 42 °C. The data were obtained from nonlinear regression fit of the Michaelis-Menten equation. For the reactions with NP, sufficient NP was added to achieve 100% coating of the 137-nt template (1 NP molecule/24 nucleotides of the template). The reactions were repeated at least three times for statistic analysis.
Cap-initiated Transcription Using Globin Primer
The globin mRNA-primed (cap-dependent) transcription assay was performed similarly. Instead of 0.3 mm ApG, 10 ng/μl rabbit-globin mRNA (Sigma) was used as a cap donor. The reactions were performed for 1 h at 34, 37, and 42 °C. For the long template (137-nt), reactions were also performed in the presence of NP. Under this condition, the incorporation of the radioactive substrate was linear to the enzyme concentration and time. The fully extended product is longer than the original 30-nt template because of the extra nucleotides added by the capped primer (cap + 30). The reactions were done at least in triplicate.
RESULTS
Effect of the Human E627K PB2 Mutation on Temperature-dependent RNA Polymerase Enzyme Activity
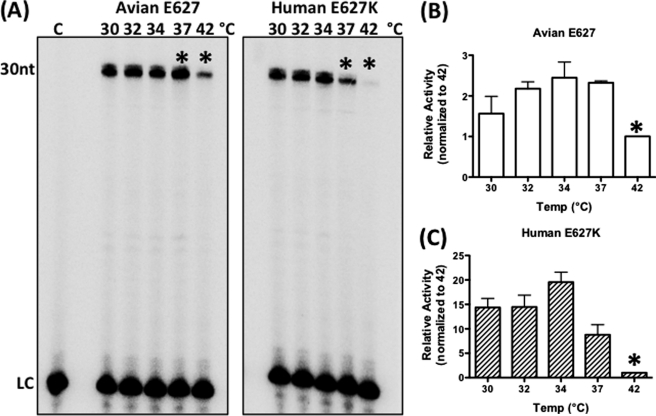
First, we tested whether the human E627K mutation influences the biochemical enzyme activity of an avian H3N2 RNA polymerase complex at various physiologically relevant temperatures. For this test we employed the H3N2 Nanchang strain, which is a well characterized avian strain cloned directly from birds and has never been exposed to humans (21). This avian influenza strain replicates efficiently at high temperatures in avian cells and contains E at the 627 position in PB2 (27). In addition, we introduced the human E627K mutation to the PB2 subunit of the avian Nanchang Pol complex. These two Nanchang Pol complexes with either the avian Glu-627 or human E627K residue in their PB2 subunit were expressed using a baculovirus system as described in our previous study (24). Next, we performed an RNA synthesis assay using ApG primer with these two Pol complexes at various temperatures with 500 μm NTPs including a small molar ratio of radioactive NTPs (0.16 μm [α-32P]NTPs). The template used in this test is a 30-nt single-stranded RNA encoding the 3′ end sequence of the Nanchang PA gene, and the reaction generates a 30-nt-long product (Fig. 1A). Importantly, the increase in band intensity of the 30-nt fully extended product is proportional to the enzyme concentration under this experimental condition (24). As shown in Fig. 1A, by comparing the amount of the 30-nt-long reaction product, the avian Glu-627 PB2 Pol displays highest activity at 37 °C with significant activity even at 42 °C (see the lanes with asterisks). In contrast, the human E627K-containing Nanchang Pol complex shows its highest activity at 34 °C, with almost no activity at 42 °C. In addition, the E627K Pol complex shows a drastic decrease in activity even at 37 °C, when compared with 34 °C.
FIGURE 1.
Temperature-dependent ApG primer-initiated RNA synthesis profiles of avian Glu-627 and human E627K PB2 influenza A virus RNA polymerase complex proteins purified from baculovirus expression system. A, ApG primer was extended by Glu-627 and E627K Pol proteins purified from insect cells at five different physiologically relevant temperatures (30, 32, 34, 37, and 42 °C) as described under “Experimental Procedures.” The 30-nt reaction products are radioactively labeled by the incorporation of [α-32P]NTPs during RNA synthesis and analyzed by urea-denaturing gels. An equal amount of 5′ end 32P-labeled 11-nt primer was added in each reaction and used as a loading control (LC) to avoid loading errors. The asterisks are used for comparison only. Lane C, no polymerase control. B and C, the 30-nt product band intensity at each temperature with avian Glu-627 and human E627K Pol complexes was normalized by the band intensity at 42 °C (asterisk), and the normalized relative enzyme activities were plotted. At least three independent reactions were conducted for the analysis. The data represent mean values; the error bars denote standard deviations.
Next, to compare the temperature-dependent enzymatic behavior of the two polymerase proteins, we quantitated the intensity of the full extended products and plotted the values as shown in Fig. 1 (B and C). The amount of extended products was first normalized for loading error by a nonspecific 5′ end labeled 11-nt loading control RNA (see LC in Fig. 1A) that was added to each reaction at an equal amount. The data were arbitrarily normalized to activity at 42 °C (see asterisk in Fig. 1, B and C), and the fold differences at varying temperatures were determined. As shown in Fig. 1, the Nanchang Pol complex harboring the human E627K mutation (Fig. 1C) showed optimal activity at 30–34 °C, whereas the avian Pol sustained its activity up to 42 °C. Overall, the Nanchang Pol complex containing the human E627K PB2 mutation showed a 20-fold difference in activity between 34 and 42 °C, compared with the avian Glu-627 Nanchang Pol complex, which differed by only 2-fold across this temperature range. These data suggest that the human E627K PB2 mutation alone can alter the temperature-dependent polymerase activity profile of the avian Nanchang Pol complex in the absence of any mammalian or avian host or cellular factors. However, although the data described in Fig. 1 clearly demonstrates a difference in the temperature-dependent activity profile between these two polymerases, further kinetic analysis is necessary for more quantitative and mechanistic comparisons of enzyme catalysis.
Steady-state Kinetic Analysis of the Nanchang Pol Complex Containing the Avian Glu-627 or Human E627K PB2 Subunit
Next, we investigated the impact of the human E627K PB2 mutation on the enzyme kinetics of the avian Nanchang Pol complex. For this, we determined steady-state kinetic parameters of these Pol complex proteins, Km and apparent Kcat, which indicate enzymatic potentials for substrate binding and enzyme catalysis, respectively. Importantly, these steady-state kinetic parameters allowed us to compare the nature of the temperature dependence between the two Pol proteins shown in Fig. 1. Here, the steady-state analysis was performed at three relevant temperatures, 34, 37, and 42 °C by ApG-initiated polymerase reaction with the 30-nt template used in Fig. 1. The reactions were performed with varying concentrations of NTPs using known concentrations of the two Pol proteins, which were normalized using the PB2 concentration of each purified complex.
Visual inspection of the intensity of the full-length product levels in Fig. 2A reveals that the avian Glu-627 Pol complex maintained significant polymerase activity with all NTP concentrations tested and also at high temperatures, consistent with the data presented in Fig. 1. Also, at 42 °C, there was detectable activity down to an NTP concentration of 10 μm. The intensity of the fully extended products was estimated by densitometry and normalized to the loading control as discussed in Fig. 1, and the relative reaction rates at varying NTP concentrations were plotted. The Km values were then determined by steady-state Michaelis-Menten analysis. Next, we normalized our enzyme activity data in terms of the amount of input polymerase complex (as measured by the PB2 concentration in the Pol complexes) and the amount of fully extended product produced (as measured by the product band intensity) and determined the apparent Kcat values (product band intensity/mol of polymerase/min) as described (28).
FIGURE 2.
Steady-state kinetic analysis of the avian Glu-627 PB2 influenza A virus RNA polymerase complex protein at varying temperatures. A, ApG-initiated RNA synthesis was conducted with the avian Pol complex protein at 34, 37, and 42 °C with varying concentrations of NTP substrates (10, 25, 100, 250, and 500 μm). The reactions were conducted as described in the legend to Fig. 1, and the products were analyzed by 20% denaturing gels. Lane C, no polymerase control. LC, loading control. B–D, the reaction rates at each of the temperature and NTP concentration settings were normalized with the maximum rate (apparent Kcat = 1/mol min) at 42 °C at the highest NTP concentration of 500 μm, and the calculated relative reaction rates were plotted for the determination of Km values by the Michaelis-Menten equation. The PB2 concentration in the avian Glu-627 Pol complex was used for determining the apparent Kcat values. At least three independent reactions were conducted for the analysis. The data represent the mean values; the error bars denote standard deviations.
Next, we conducted the same analysis with the E627K Pol complex. As shown in Fig. 3, unlike the avian Pol complex, the E627K Pol complex displayed a significant decrease of the full-length product at high temperatures, even at high NTP concentrations, which is consistent with the data shown in Fig. 1. We also determined Km and apparent Kcat values for two Pol complexes (Table 1). Importantly, our Km and apparent Kcat values are comparable with ones previously published (29). Comparisons of temperature-dependent changes in the kinetic values of the two complexes are presented in Fig. 4.
FIGURE 3.
Steady-state kinetic analysis of ApG-initiated RNA synthesis by human E627K PB2 influenza A virus RNA polymerase complex at varying temperatures. A, ApG-initiated RNA synthesis was conducted with the human Pol complex at 34, 37, and 42 °C with varying concentrations of NTP substrates (10, 25, 100, 250, and 500 μm). The reactions were conducted as described in the legend to Fig. 2, and the products were analyzed by 20% denaturing gels. LC, loading control. B–D, the reaction rates at each of the temperature and NTP concentration settings were normalized with the maximum rate (apparent Kcat = 1/mol min) at 42 °C at the highest NTP concentration of 500 μm, and the calculated relative reaction rates were plotted for the determination of Km values by the Michaelis-Menten equation. The PB2 concentration of the human E627K Pol complex was used for determining the apparent Kcat values. At least three independent reactions were conducted for the analysis. The data represent mean values; the error bars denote standard deviations.
TABLE 1.
Steady-state parameters in ApG-primed transcription assay using a 30-nt RNA template
| Avian E627 | Human E627K | |
|---|---|---|
| 34 °C | ||
| Km, μm | 32.0 ± 13 | 33.0 ± 4.3 |
| Apparent Kcata | 1.8 ± 0.2 | 8.1 ± 0.2 |
| Apparent Kcat/Km | 0.06 | 0.25 |
| 37 °C | ||
| Km, μm | 25.6 ± 7.8 | 83.9 ± 26.9 |
| Apparent Kcat | 1.6 ± 0.1 | 4.9 ± 0.3 |
| Apparent Kcat/Km | 0.06 | 0.06 |
| 42 °C | ||
| Km, μm | 37.1 ± 6.9 | 60.7 ± 8.7 |
| Apparent Kcat | 1.1 ± 0.1 | 1.1 ± 0.04 |
| Apparent Kcat/Km | 0.02 | 0.01 |
The fold differences between the determined values are summarized in Fig. 4.
a Apparent Kcat was calculated as product band intensity/min/mol of enzyme used. Km is expressed in μm.
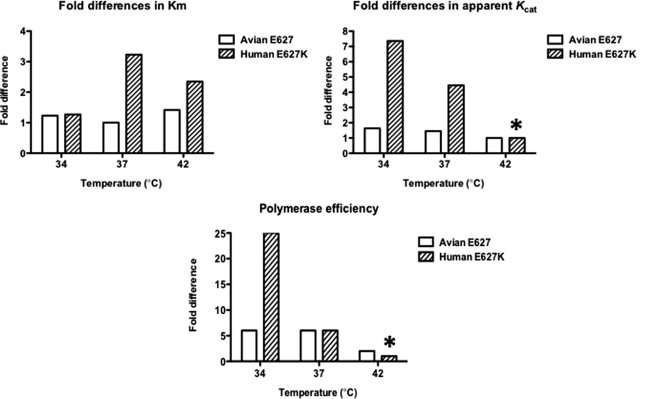
FIGURE 4.
Comparison of steady-state kinetic values of avian Glu-627 and human E627K Pol complex proteins summarized in Table 1. The fold differences between the Km values (top left panel), apparent Kcat values (top right panel), and polymerase efficiency (apparent Kcat/Km) (bottom panel) of avian and human Pol complex proteins at the three temperatures (Table 1) were calculated and compared.
As shown in Fig. 4A, no significant change was observed in the Km values at the three different temperatures for the avian Glu-627 Pol complex, whereas the E627K Pol showed a 2–3-fold increase in Km values at 37 and 42 °C, as compared with 34 °C. Importantly, both the E627K and avian Glu-627 Pol complexes showed almost identical Km values at 34 °C, indicating that the E627K mutation does not alter the Km value of the avian Pol complex at 34 °C (the temperature at which human viruses preferentially replicate). In contrast, as shown in Fig. 4B, whereas the apparent Kcat values of avian Pol complex were similar at the three temperatures tested, the apparent Kcat values of the human E627K Pol complex decreased by 8-fold at 42 °C, compared with 34 °C (see asterisk in Fig. 4B). This suggests that the human E627K PB2 mutation influences the temperature-dependent profile of the avian Pol by restricting enzyme catalysis (apparent Kcat) rather than NTP interaction (Km). More importantly, when we compared the apparent Kcat values of the avian and human complexes at 34 °C, the human E627K Pol complex showed a 4.5 times higher apparent Kcat value, suggesting that the E627K mutation enhances enzyme catalysis of the avian Pol complex at lower temperatures (i.e. 34 °C). Finally, when we compared the overall polymerase efficiency (apparent Kcat/Km) of the two proteins (Fig. 4C), the avian Glu-627 Pol complex displayed no difference at 37 °C and only a 3-fold decrease in polymerase efficiency at 42 °C, compared with 34 °C. In contrast, the human E627K showed 4- and 25-fold decreases in efficiencies at 37 and 42 °C, respectively, compared with that at 34 °C. In summary, this steady-state kinetic analysis suggests that the E627K mutation alone can alter the temperature-dependent enzymatic profile of the avian Pol complex in the absence of putative host factors. In addition, the efficient RNA synthesis capability of the human E627K Pol complex at low temperatures (Fig. 4C) is mainly due to elevation of apparent Kcat rather than a decrease in Km values.
Temperature-dependent Cap-initiated RNA Synthesis of the Avian Glu-627 and Human E627K-containing Influenza Pol Complexes
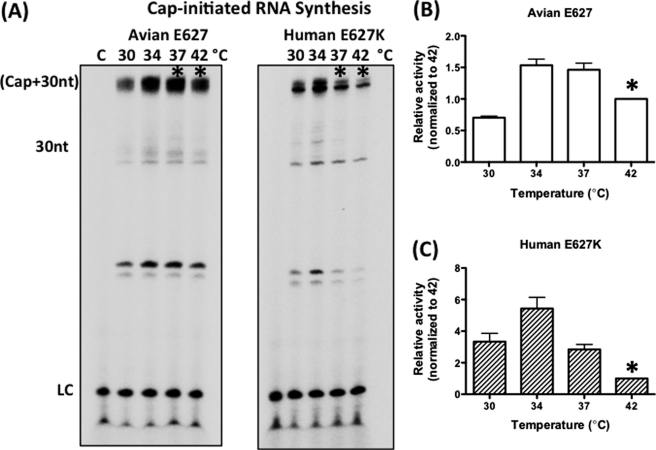
Initial enzymatic conversion of (−) strand RNA segments to viral mRNAs by Pol complex, called transcription, is initiated by using 5′ capped cellular mRNAs, which are captured by PB2 subunit and undergo endonucleolytic cleavage at their 5′ end by the PA subunit generating primers. Thus, next, we tested whether the human E627K mutation also alters the temperature-dependent transcription activity of the Pol complex using a RNA synthesis reaction with capped primers instead of ApG. For this test, we conducted the globin mRNA-initiated RNA polymerization assay with H3N2 Pol complex containing the avian Glu-627 PB2 or human E627K PB2 mutation with the same 30-nt template used in the ApG-initiated reaction (Fig. 1) at varying temperatures. As shown in Fig. 5, the avian Glu-627 Pol complex maintained significant polymerase activity even at high temperatures, whereas the human E627K Pol complex quickly lost activity at these high temperatures (37 and 42 °C) (see asterisk in Fig. 5). These data confirm that the E627K PB2 mutation also induces temperature-sensitive reduction of Pol activity during transcription using a globin primer, which was also observed in the ApG-initiated reaction. In addition, to generalize this finding, we also employed a longer RNA template (137-nt) encoding the 3′ end 137-nt sequence of the Nanchang PA gene. The temperature-dependent activity profile of the two proteins with this long template (supplemental Fig. S1) was identical to that found with the 30-nt template. These data further support that the temperature-sensitive effect of the human E627K PB2 mutation may mechanistically affect either both ApG- and cap-dependent initiation steps or only a post-initiation step of RNA synthesis, such as elongation without affecting cap binding.
FIGURE 5.
Temperature-dependent capped primer-initiated RNA synthesis profiles of the avian Glu-627 and human E627K PB2 influenza A virus RNA polymerase complexes. A, globin mRNA was used as a primer and extended by purified Glu-627 and E627K Pol proteins at four different physiologically relevant temperatures (30, 34, 37, and 42 °C) as described under “Experimental Procedures.” The reaction products (Cap+30nt) are radioactively labeled by the incorporation of [α-32P]NTPs during RNA synthesis and analyzed by urea-denaturing gels. An equal amount of 5′ end 32P-labeled 11-nt primer was added in each reaction and used as a loading control (LC) for avoiding loading errors. The asterisks are used for comparison only. Lane C, no polymerase control. B and C, the product band intensity (Cap+30nt) at each temperature with avian Glu-627 and human E627K Pol complexes was normalized by the band intensity at 42 °C (asterisks), and the normalized relative enzyme activities were plotted. At least three independent reactions were conducted for the analysis. The data represent mean values; the error bars denote standard deviations.
Effect of NP on the Temperature Sensitivity of the Human E627K PB2 Mutation
Viral NP is a RNA-binding protein that physically interacts with single-stranded viral RNAs forming coiled structures (30). Importantly, NP is known to affect the polymerase activity by directly interacting with the influenza Pol complex (31). Lastly, we tested whether the difference in temperature-dependent activity observed in the ApG-initiated RNA synthesis between the avian Glu-627 and human E627K Pol complex is also observed in the presence of NP. For this assay, we employed 1) NP protein purified from Escherichia coli as described in our previous study (24) and 2) a 137-nt-long RNA template encoding the 5′ end sequence of Nanchang PA gene. An average of one NP molecule binds to 24 nucleotides of RNA template, and NP sufficient for 100% coating of the 137-nt template was used in this analysis. Under this condition, we conducted steady-state ApG-initiated transcription assays with the avian Glu-627 (supplemental Fig. S2) and human E627K Pol complexes (supplemental Fig. S3). The calculated kinetic parameters from these experiments are summarized in Table 2. The data show that, at 34 °C, the human E627K mutation was able to induce a 3-fold increase in the polymerase efficiency (apparent Kcat/Km), compared with the avian Pol at the same temperature. In addition, although the avian Pol complex sustained polymerase efficiency even at 42 °C, a 9-fold reduction was observed with the human E627K Pol at 42 °C, compared with 34 °C. Because there was little difference in Km values between these two Pol complexes and between the three temperatures tested here, the effect of the E627K mutation on temperature-dependent polymerase efficiency is due to the effect on apparent Kcat values, which is consistent with the observation with the ApG-initiated reactions in the absence of NP. Thus, these data show that NP does not alter the effect of the E627K mutation on the temperature-dependent activity of the Pol complex.
TABLE 2.
Steady-state parameters in ApG-primed transcription assay using a 137-nt template with NP
| Avian E627 | Human E627K | |
|---|---|---|
| 34 °C | ||
| Km, μm | 20.9 ± 7.1 | 19.8 ± 3.0 |
| Apparent Kcat | 1.4 ± 0.1 (1×)a | 3.5 ± 0.08 (2.5×)a |
| Apparent Kcat/Km | 0.06 (1×)b | 0.18 (3×)b (9×)c |
| 37 °C | ||
| Km, μm | 20.9 ± 7.1 | 42.7 ± 5.9 |
| Apparent Kcat | 1.4 ± 0.1 | 2.7 ± 0.06 |
| Apparent Kcat/Km | 0.06 (1×)b | 0.06 (1×)b (3×)c |
| 42 °C | ||
| Km, μm | 30.2 ± 11.1 | 69.5 ± 19.4 |
| Apparent Kcat | 1.3 ± 0.1 | 1.5 ± 0.08 |
| Apparent Kcat/Km | 0.04 (0.67×)b | 0.02 (0.3×)b (1×)c |
a Fold differences in apparent Kcat values of avian and human polymerases at 34 °C (1× = apparent Kcat of avian polymerase).
b Fold differences in apparent Kcat/Km values of avian and human polymerases at the three temperatures were marked (1× = apparent Kcat/Km value of avian E627 polymerase at 34 °C).
c Fold differences in apparent Kcat/Km values of human E627K polymerase at the three temperatures (1× = apparent Kcat/μm value at 42 °C).
DISCUSSION
Influenza A virus is capable of efficient transmission among different host species including birds and mammals, and this unique evolutionary potential of influenza A viruses is derived from their assortment capability between heterozygous viral gene segments (32, 33). This host adaptability can be a result of a series of specific viral mutations found in the various influenza gene segments. Among those host-specific mutations (34, 35), the E627K mutation of influenza polymerase PB2 subunit is particularly interesting, because this suggests that influenza viruses use polymerase mutations for their natural evolution in addition to mutations in the viral surface proteins that recognize target cell receptors. In fact, in other viruses, most viral polymerase mutations emerge mainly when their polymerases are targeted by potent pharmacological agents (i.e. HIV reverse transcriptase mutations against reverse transcriptase inhibitors (36, 37) and Hanta virus-resistant mutations against ribavirin (38, 39)). Indeed, these known polymerase mutations render viral resistance against the applied inhibitors. Thus, it is very intriguing to understand how influenza A viruses utilize the residue 627 PB2 polymerase mutation for host-specific replication adaptation.
The major hypothesis for the evolutionary role of the human E627K mutation, which has been extensively pursued, is that the mutation allows avian viruses to either escape from putative human-specific host factors that inhibit the PB2 functions or allows for interactions with human-specific factors that are essential for viral replication in human cells. This hypothesis was conceptually reinforced by the fact that residue 627 of PB2 lies on the surface of the molecule (19, 40). Although this hypothesis is clearly logical, the predicted host factors have yet to be identified.
In this study, we explored an alternative, although not mutually exclusive, hypothesis that the E627K PB2 mutation may directly alter the enzymatic activity of influenza Pol complex in the absence of putative host factors. One key basis for this alternative hypothesis was obtained from the distinct optimal replication temperatures of avian and human influenza strains: avian viruses optimally replicate at high temperatures (37–40 °C), which are observed in the in vivo target organ of avian viruses (the GI tract), whereas human-adapted viruses preferentially replicate at the low temperature (32–34 °C) observed in the human respiratory tract (1). Indeed, the temperature-dependent influenza replication and viral host specificity are closely linked. In addition, even within a host species, temperature-dependent viral growth also contributes to pathogenesis as genetically demonstrated by studies on both nonpathogenic cold-adapted influenza viruses and their revertants that can replicate at high temperature and become pathogenic (41). More importantly, viral cold adaptation and reversions have been associated with mutations in the polymerase genes (41).
Here, we tested our hypothesis by employing biochemical RNA synthesis reactions with purified influenza Pol complex proteins at varying incubation temperatures between 30 and 42 °C. Most importantly, to test our hypothesis, it is crucial to use the true avian strain that was directly isolated from birds and has never evolved in humans. Currently available cloned and well characterized avian strains are mainly H5N1 isolates; most of them were isolated from tissue samples of infected humans and often cultured in human cells for a while to obtain viral clones. Thus, these clones are not ideal to test our hypothesis. In contrast, H3N2 Nanchang strain was directly isolated from birds and had never been exposed to humans. First, to eliminate human-specific host factors bound to the PB2 subunit, we purified influenza H3N2 Pol complex proteins from the baculovirus expression system that we recently established (24). Second, we performed biochemical RNA synthesis reactions with identical purified Pol complexes that differed only at a single amino acid within the PB2 subunit (avian Glu-627 residue or human E627K). Third, to generalize biochemical observations, the reactions were conducted 1) with multiple templates, 2) with ApG- and Cap-initiating primers, and 3) in the presence and absence of the avian influenza NP protein, which was overexpressed and purified from E. coli as described in our previous study (24).
Indeed, both primer extension reactions and steady-state kinetic analysis clearly demonstrate that the E627K mutation can directly alter the temperature-dependent enzymatic behavior of the purified influenza Pol complex under all of the conditions analyzed (i.e. with templates of varying length, in reactions with different polymerization initiation modes, and in the presence of absence of the viral NP protein). More specifically, the avian Glu-627 Pol complex protein maintains significant polymerase activity even at 42 °C, whereas the E627K mutation induces a loss of polymerase activity of the Pol complex at high temperature. The steady-state kinetic analysis, which measures the NTP binding profile (Km) and chemical catalysis (apparent Kcat) of the polymerase, demonstrates that the capability of the avian Glu-627 PB2 Pol complex to remain catalytically active at high temperatures is mechanistically because of similar apparent Kcat values that did not change at high temperatures. In contrast, the human E627K mutation reduces apparent Kcat at high temperatures. Importantly, no significant alteration in the Km values of either Glu-627 or E627K influenza Pol complexes was observed regardless of the temperature. Thus, the kinetic and biochemical data presented in this study clearly demonstrate that the Glu-627 residue plays a key role in the temperature-dependent RNA synthesis of influenza Pol complex, likely by modulating the overall enzymatic catalytic potential of the Pol complex, rather than by altering NTP binding. Furthermore, upon examining the calculated apparent Kcat/Km values (Table 1), the E627K mutation actually increases the polymerase efficiency of the avian polymerase at low temperature (34 °C). This is an important finding, because it suggests that one of the roles of the human E627K mutation could be to elevate viral replication capability at the low temperatures found in the human respiratory tract.
How, then, does the Glu-627 residue control the enzymatic catalysis (apparent Kcat) step of the influenza Pol complex at varying temperatures? One simple explanation is that the Glu-627 residue may affect the structural integrity of the heterotrimeric polymerase complex. Possibly, the E627K mutation destabilizes the polymerase complex at high temperatures and thereby reduces overall enzymatic activity at high temperatures. This possibility can be further tested by investigating the stability of the complex of the three Pol subunits in solutions at various temperatures. An alternative scenario is that the avian Glu-627 residue could exert a direct, temperature-dependent effect on the apparent Kcat of the polymerase.
Previous kinetic analyses of other DNA polymerases (42) have shown that nucleotide binding to the active site of polymerases is followed by three subsequent steps: 1) conformational change, 2) chemical catalysis for phosphodiester bond formation between the 3′ end OH of the primer and the α-phosphate of the incoming nucleotide, and finally 3) product (PPi) release. Product release, which removes the pyrophosphate (PPi) from the active site after the formation of a new phosphodiester bond, is rate-limiting during the steady-state nucleic acid synthesis of many polymerases (42). Presumably, the avian Glu-627 residue accelerates the rate-limiting PPi product release from the active site at high temperature, maintaining efficient steady-state polymerase activity at high temperatures. In contrast, the human E627K mutation loses this capability simply because influenza replication in human does not encounter the high temperature-specific rate-limiting steps.
Our polymerase assay is an RNA synthesis reaction incorporating multiple nucleotides. Thus, it is important to consider the translocation step where the polymerase moves to the next template nucleotide to initiate the incorporation of the next new nucleotide substrate involved in the primer extension. It is possible that the 627 residue may exert a temperature-dependent effect on the translocation step, which could then become rate-limiting. Future experiments will be needed to address this hypothesis.
It is also intriguing that the E627K mutation improves the enzymatic activity at low temperatures, compared with the avian Pol complex. This effect will also require future analysis. In conclusion, this study supports the possibility that the Glu-627 mutation can directly alter enzyme kinetic behaviors of influenza A virus RNA polymerase, which may be further amplified in the presence of the host factors that currently remain to be discovered.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Contract HHSN266200700008C granted to the New York Influenza Center of Excellence.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- Pol
- polymerase
- NP
- nucleoprotein
- TAP
- tandem affinity purification
- nt
- nucleotide.
REFERENCES
- 1. Murphy B. R., Hinshaw V. S., Sly D. L., London W. T., Hosier N. T., Wood F. T., Webster R. G., Chanock R. M. (1982) Infect. Immun. 37, 1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scholtissek C., Rohde W., Von Hoyningen V., Rott R. (1978) Virology 87, 13–20 [DOI] [PubMed] [Google Scholar]
- 3. Alford R. H., Kasel J. A., Gerone P. J., Knight V. (1966) Proc. Soc. Exp. Biol. Med. 122, 800–804 [DOI] [PubMed] [Google Scholar]
- 4. Webster R. G., Yakhno M., Hinshaw V. S., Bean W. J., Murti K. G. (1978) Virology 84, 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Connor R. J., Kawaoka Y., Webster R. G., Paulson J. C. (1994) Virology 205, 17–23 [DOI] [PubMed] [Google Scholar]
- 6. Ito T., Suzuki Y., Suzuki T., Takada A., Horimoto T., Wells K., Kida H., Otsuki K., Kiso M., Ishida H., Kawaoka Y. (2000) J. Virol. 74, 9300–9305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kobasa D., Kodihalli S., Luo M., Castrucci M. R., Donatelli I., Suzuki Y., Suzuki T., Kawaoka Y. (1999) J. Virol. 73, 6743–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naffakh N., Massin P., Escriou N., Crescenzo-Chaigne B., van der Werf S. (2000) J. Gen. Virol 81, 1283–1291 [DOI] [PubMed] [Google Scholar]
- 9. Snyder M. H., Buckler-White A. J., London W. T., Tierney E. L., Murphy B. R. (1987) J. Virol. 61, 2857–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subbarao E. K., London W., Murphy B. R. (1993) J. Virol. 67, 1761–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massin P., van der Werf S., Naffakh N. (2001) J. Virol. 75, 5398–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hatta M., Gao P., Halfmann P., Kawaoka Y. (2001) Science 293, 1840–1842 [DOI] [PubMed] [Google Scholar]
- 13. Salomon R., Franks J., Govorkova E. A., Ilyushina N. A., Yen H. L., Hulse-Post D. J., Humberd J., Trichet M., Rehg J. E., Webby R. J., Webster R. G., Hoffmann E. (2006) J. Exp. Med. 203, 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Labadie K., Dos Santos Afonso E., Rameix-Welti M. A., van der Werf S., Naffakh N. (2007) Virology 362, 271–282 [DOI] [PubMed] [Google Scholar]
- 15. Gabriel G., Herwig A., Klenk H. D. (2008) PLoS Pathog. 4, e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mayer D., Molawi K., Martínez-Sobrido L., Ghanem A., Thomas S., Baginsky S., Grossmann J., García-Sastre A., Schwemmle M. (2007) J. Proteome Res. 6, 672–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehle A., Doudna J. A. (2008) Cell Host Microbe 4, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuzuhara T., Kise D., Yoshida H., Horita T., Murazaki Y., Nishimura A., Echigo N., Utsunomiya H., Tsuge H. (2009) J. Biol. Chem. 284, 6855–6860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarendeau F., Crepin T., Guilligay D., Ruigrok R. W., Cusack S., Hart D. J. (2008) PLoS Pathog 4, e1000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada S., Hatta M., Staker B. L., Watanabe S., Imai M., Shinya K., Sakai-Tagawa Y., Ito M., Ozawa M., Watanabe T., Sakabe S., Li C., Kim J. H., Myler P. J., Phan I., Raymond A., Smith E., Stacy R., Nidom C. A., Lank S. M., Wiseman R. W., Bimber B. N., O'Connor D. H., Neumann G., Stewart L. J., Kawaoka Y. PLoS Pathog 6, e1001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu M., He S., Walker D., Zhou N., Perez D. R., Mo B., Li F., Huang X., Webster R. G., Webby R. J. (2003) Virology 305, 267–275 [DOI] [PubMed] [Google Scholar]
- 22. Bradel-Tretheway B. G., Kelley Z., Chakraborty-Sett S., Takimoto T., Kim B., Dewhurst S. (2008) J. Gen Virol 89, 2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bussey K. A., Desmet E. A., Mattiacio J. L., Hamilton A., Bradel-Tretheway B., Bussey H. E., Kim B., Dewhurst S., Takimoto T. (2011) J. Virol. 85, 7020–7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aggarwal S., Bradel-Tretheway B., Takimoto T., Dewhurst S., Kim B. (2010) PLoS One 5, e10372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Puig O., Caspary F., Rigaut G., Rutz B., Bouveret E., Bragado-Nilsson E., Wilm M., Séraphin B. (2001) Methods 24, 218–229 [DOI] [PubMed] [Google Scholar]
- 26. Fodor E., Crow M., Mingay L. J., Deng T., Sharps J., Fechter P., Brownlee G. G. (2002) J. Virol. 76, 8989–9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bussey K. A., Bousse T. L., Desmet E. A., Kim B., Takimoto T. (2010) J. Virol. 84, 4395–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wainberg M. A., Drosopoulos W. C., Salomon H., Hsu M., Borkow G., Parniak M., Gu Z., Song Q., Manne J., Islam S., Castriota G., Prasad V. R. (1996) Science 271, 1282–1285 [DOI] [PubMed] [Google Scholar]
- 29. Zhang S., Weng L., Geng L., Wang J., Zhou J., Deubel V., Buchy P., Toyoda T. (2010) Biochem. Biophys. Res. Commun. 391, 570–574 [DOI] [PubMed] [Google Scholar]
- 30. Ng A. K., Wang J. H., Shaw P. C. (2009) Sci. China C. Life Sci. 52, 439–449 [DOI] [PubMed] [Google Scholar]
- 31. Mena I., Jambrina E., Albo C., Perales B., Ortín J., Arrese M., Vallejo D., Portela A. (1999) J. Virol. 73, 1186–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Desselberger U., Nakajima K., Alfino P., Pedersen F. S., Haseltine W. A., Hannoun C., Palese P. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 3341–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Webster R. G., Bean W. J., Gorman O. T., Chambers T. M., Kawaoka Y. (1992) Microbiol. Rev. 56, 152–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vines A., Wells K., Matrosovich M., Castrucci M. R., Ito T., Kawaoka Y. (1998) J. Virol. 72, 7626–7631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shinya K., Watanabe S., Ito T., Kasai N., Kawaoka Y. (2007) J. Gen. Virol. 88, 547–553 [DOI] [PubMed] [Google Scholar]
- 36. Menéndez-Arias L. (2008) Virus Res. 134, 124–146 [DOI] [PubMed] [Google Scholar]
- 37. Boyer P. L., Sarafianos S. G., Arnold E., Hughes S. H. (2002) J. Virol. 76, 9143–9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun Y., Chung D. H., Chu Y. K., Jonsson C. B., Parker W. B. (2007) Antimicrob. Agents Chemother. 51, 84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parker W. B. (2005) Virus Res. 107, 165–171 [DOI] [PubMed] [Google Scholar]
- 40. Yamada S., Hatta M., Staker B. L., Watanabe S., Imai M., Shinya K., Sakai-Tagawa Y., Ito M., Ozawa M., Watanabe T., Sakabe S., Li C., Kim J. H., Myler P. J., Phan I., Raymond A., Smith E., Stacy R., Nidom C. A., Lank S. M., Wiseman R. W., Bimber B. N., O'Connor D. H., Neumann G., Stewart L. J., Kawaoka Y. (2010) PLoS Pathogens 6, e1001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Treanor J., Perkins M., Battaglia R., Murphy B. R. (1994) J. Virol. 68, 7684–7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rothwell P. J., Waksman G. (2005) Adv. Protein Chem. 71, 401–440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.