Abstract
Recent morphologic, immunohistochemical and molecular genetic studies have led to the development of a new paradigm for the pathogenesis and origin of epithelial ovarian cancer (EOC) based on a dualistic model of carcinogenesis that divides EOC into two broad categories designated type I and type II. Type I tumors are comprised of low-grade serous, low-grade endometrioid, clear cell and mucinous carcinomas and Brenner tumors. They are generally indolent, present in stage I (tumor confined to the ovary) and are characterized by specific mutations, including KRAS, BRAF, ERBB2, CTNNB1, PTEN PIK3CA, ARID1A, and PPPR1A, which target specific cell signaling pathways. Type I tumors rarely harbor TP53 and are relatively stable genetically. Type II tumors are comprised of high-grade serous, high-grade endometrioid, malignant mixed mesodermal tumors (carcinosarcomas) and undifferentiated carcinomas. They are aggressive, present in advanced stage, and have a very high frequency of TP53 mutations but rarely harbor the mutations detected in type I tumors. In addition, type II tumors have molecular alterations that perturb expression of BRCA either by mutation of the gene or by promotor methylation. A hallmark of these tumors is that they are genetically highly unstable. Recent studies strongly suggest that fallopian tube epithelium (benign or malignant) that implants on the ovary is the source of low-grade and high-grade serous carcinoma rather than the ovarian surface epithelium as previously believed. Similarly, it is widely accepted that endometriosis is the precursor of endometrioid and clear cell carcinomas and as endometriosis is thought to develop from retrograde menstruation these tumors can also be regarded as involving the ovary secondarily. The origin of mucinous and transitional cell (Brenner) tumors is still not well established, although recent data suggest a possible origin from transitional epithelial nests located in paraovarian locations at the tubo-peritoneal junction. Thus, it now appears that type I and type II ovarian tumors develop independently along different molecular pathways, and that both types develop outside the ovary and involve it secondarily. If this concept is confirmed it leads to the conclusion that the only true primary ovarian neoplasms are gonadal stromal and germ cell tumors analogous to testicular tumors. This new paradigm of ovarian carcinogenesis has important clinical implications. By shifting the early events of ovarian carcinogenesis to the fallopian tube and endometrium instead of the ovary, prevention approaches, for example, salpingectomy with ovarian conservation, may play an important role in reducing the burden of ovarian cancer while preserving hormonal function and fertility.
Keywords: Ovarian cancer, borderline tumors, molecular pathogenesis, origin, p53 mutations, prevention
Paradigms, as defined by Kuhn in his seminal work “The Structure of Scientific Revolutions”, are the best ways of explaining progress in science (1). Kuhn believed that textbooks, which describe progress, as a cumulative, incremental process leading to a growing corpus of scientific knowledge present an unrealistic and biased view. Instead, he felt that a more accurate depiction could be gleaned by looking at what scientists do most of the time, which he termed normal science, and normal science is governed by paradigms. A paradigm generates a consensus among scientists working in a particular field about how work in that field should be done. It also identifies puzzles, assures scientists that each puzzle has a solution, and provides standards for evaluating solutions. Normal science involves showing how nature can be fitted into the categories provided by the paradigm. When puzzles arise that repeatedly resist solutions a crisis of confidence occurs. During a crisis the paradigm is subjected to testing and might be rejected. If that occurs a new paradigm replaces the previous one and a scientific revolution has occurred.
The operative paradigm of ovarian carcinogenesis is that epithelial ovarian cancer (EOC) is composed of several different types, but since the vast majority is high-grade serous carcinoma (HGSC) differences between the types are obscured and therefore, EOC is regarded as a single disease. Moreover, since carcinomas in the female pelvis tend to involve the ovary, often as the dominant mass, they have all been regarded as ovarian in origin. This paradigm is based on taxonomy, specifically morphologic classifications, which took shape in the 1930s and 40s (2-4) matured in the 1950s and 60s (5) and culminated with the WHO Classification in 1973 (6). The histologic classifications created a structure that provided the basis for performing clinicopathologic studies but apart from these studies, the tools necessary to study pathogenesis were not available and therefore our understanding of ovarian carcinogenesis was limited.
Arguably, research within this paradigm has failed to identify the precursor of EOC (7-19) and as a result current management is empirical. Despite advances in radical surgery and cytotoxic chemotherapy, overall survival has not changed in over 50 years. In the last two decades attention has focused on early detection but unfortunately, this strategy has also failed to provide a survival benefit. Accordingly, there are “persistent puzzles” which have resisted solutions and hence a “crisis of confidence” exists.
The introduction of molecular biology and the development of new methods of tissue sampling are now ushering in a paradigm shift, which can be considered “revolutionary”. The concepts that are emerging and shaping this new paradigm are novel and highly provocative. Some of them will be confirmed and others will be modified or discarded, as scientists in the process of performing “normal science” within the framework of the new paradigm attempt to clarify and resolve issues that have frustrated progess in reducing the burden of this disease.
Molecular Pathogenesis of Epithelial Ovarian Carcinoma
The introduction of the “borderline (low malignant potential)” category was an important step in refining the morphologic classification of EOC by identifying a group of tumors, defined as lacking destructive invasive growth that had a significantly better outcome than the invasive carcinomas. Since it was rare to find a borderline tumor coexisting with an invasive carcinoma it was generally believed that they were unrelated. In 1996 a relationship between serous borderline tumor (SBT) and invasive serous carcinoma was described based on the subdivision of SBT into two groups. One group designated “atypical proliferative serous tumor (APST)” behaved in a benign fashion and a second, smaller group, designated “micropapillary serous carcinoma (MPSC)” also termed “noninvasive low-grade serous carcinoma” behaved like a low-grade malignant tumor (20). Moreover, this latter subset was closely associated with invasive low-grade serous carcinoma (LGSC) and the investigators proposed that MPSC was the immediate precursor of LGSC. The key element leading to this conclusion was the recognition that LGSC was a distinct entity that differed from HGSC in several ways (see below). Prior to this, serous carcinoma was graded well, moderately and poorly differentiated with the implication that serous carcinoma was a spectrum of disease in which well differentiated carcinoma (LGSC) progressed to poorly differentiated carcinoma (HGSC). Following this morphologic study linking APST to MPSC and LGSC, a series of molecular genetic studies was performed, which culminated in the proposal of a dualistic model to explain the pathogenesis of EOC (21).
Dualistic Model of Carcinogenesis
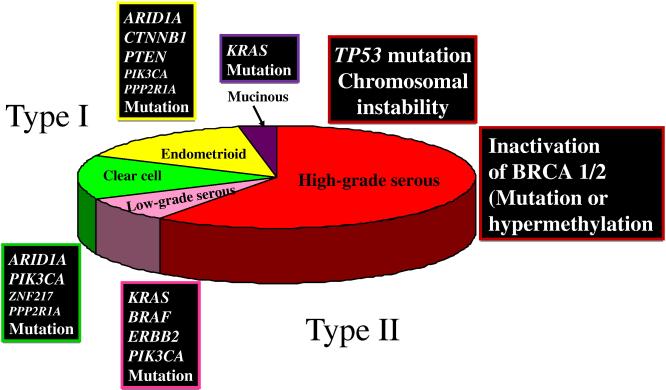
Briefly, the dualistic model accommodates and confirms the heterogeneous nature of EOC and places the major histologic types into two groups (type I and type II) based on their distinctive clinicopathologic and molecular genetic features. It also links specific histologic types with their putative precursor lesions. Thus, type I tumors are comprised of low-grade serous carcinomas, low-grade endometrioid, clear cell and mucinous carcinomas which develop in a stepwise fashion from well-established precursor lesions, such as borderline tumors and endometriosis. They typically present as large masses that are confined to one ovary (stage Ia), are indolent and have a good prognosis. The type I tumors are relatively genetically stable and typically display a variety of somatic sequence mutations that include KRAS, BRAF, PTEN, PIK3CA CTNNB1 (the gene encoding beta catenin), ARID1A and PPP2R1A but very rarely TP53 (21-23). In contrast, type II tumors are comprised of HGSC (usual type of serous carcinoma), high-grade endometrioid carcinoma, malignant mixed mesodermal tumors (carcinosarcomas) and undifferentiated carcinomas, which present in advanced stage (stages II-IV) in over 75% of cases; they grow rapidly and are highly aggressive. Type II tumors, of which HGSC is the prototypic type, are chromosomally highly unstable and harbor TP53 mutations in >95% of cases (24); they rarely display the mutations found in the type I tumors. BRCA inactivation, either by mutation or inactivation of expression of BRCA and its downstream genes via promoter methylation occurs in up to 40-50% of HGSC (25). BRCA inactivation has not been reported in the type I tumors.
Serous Tumors
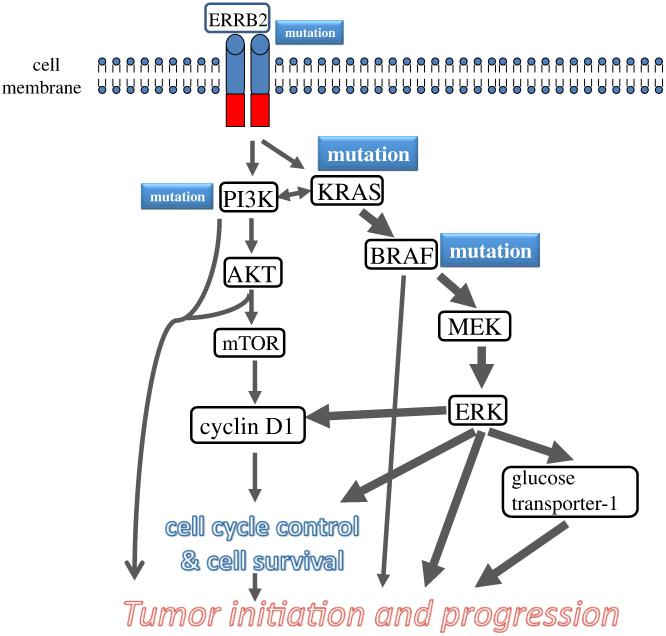
The relationship of APST and MPSC to LGSC based on morphologic studies was supported by mutational analysis, gene expression studies and methylation profiling demonstrating that these three tumor types shared molecular alterations that differed dramatically from HGSC (25-30). Initial molecular genetic studie focused on individual genes (Fig 1) but more recent studies have highlighted the importance of molecular signaling pathways (Fig 2). For example, the MAPK signaling pathway is important for the cellular response to a variety of growth and differentiation factors and activating mutations in KRAS or one of its downstream effectors, BRAF, (mutations of KRAS and BRAF are mutually exclusive) results in constitutive activation of MAPK-mediated signaling in more than half of APSTs, MPSCs and LGSCs (31-34). In addition, a 12-bp insertion mutation of ERBB2 (encoding HER2/neu), which activates an upstream regulator of K-Ras, has been detected in 9% of these tumors. Interestingly, tumors with ERBB2 mutations lack KRAS and BRAF mutations (35,36). Accordingly, 60-70% of APSTs, MPSCs and LGSCs express active MAPK (37); they rarely harbor TP53 mutations. Recent studies have further clarified the molecular pathogenesis of APST, MPSC and LGSC. First, KRAS and BRAF mutations have not been detected in serous cystadenomas, the putative precursor of SBTs, but laser capture microdissection studies have detected these mutations in the adenoma epithelium and APST epithelium in serous cystadenomas containing small APSTs suggesting that these mutations occur early in the development of APST (38). In an attempt to elucidate the relationship of APST to LGSC a recent study compared the gene expression profiles of APST, MPSC and LGSC and found that MPSC is closer molecularly to invasive LGSC than to APST (26) and that the genes involved in MAPK signaling showed higher expression in MPSC than in APST. In addition, a previous study reporting that MPSC harbors a pattern of chromosomal imbalance distinct from that of APST (39) confirms the proposal that LGSC develops in a stepwise fashion from cystadeno(fibro)ma to APST and MPSC, supporting the biological role of the KRAS-BRAF-MEK-MAPK pathway in the development of LGSC. By globally profiling the epigenetic landscape, we have recently reported that the methylation profiles in low-grade serous carcinoma are closer to APST and serous cystadenoma than high-grade serous carcinoma (30). This finding lends further support to the dualistic model of ovarian serous carcinogenesis.
Fig. 1.
Prevalence of histologic types of epithelial ovarian cancer and their associated molecular genetic changes.
Fig. 2.
Schematic illustration of pathway alterations involved in the development of low-grade serous carcinoma. The cardinal molecular genetic changes include somatic mutations in KRAS, BRFA and occasionally ERRB2 (encoding Her2/Neu) and PIK3CA. The mutated gene products constitutively activate the signaling pathways that regulate cellular proliferation and survival and promote tumor initiation and progression through several mechanisms including up regulation of glucose transporter-1. The size of the boxes containing specific genes reflects the relative frequency of the mutation and the thickness of the arrows indicates the relative contribution of the pathway alterations to tumor development.
In contrast to LGSC, HGSC harbors TP53 mutations in >95% of cases (25), but rarely contains KRAS or BRAF mutations. Aside from TP53 mutations no other mutations are consistently found in sporadic (nonfamilial) HGSCs including mutations of BRCA1 and BRCA2, which characterize familial HGSC (The Cancer Genome Atlas, unpublished). On the other hand, inactivation of the BRCA1/2 genes by other mechanisms, such as hypermethylation of the BRCA1 promoter, occurs relatively frequently and as a result inactivation of BRCA1/2 by mutation or other mechanisms occurs in 40-50% of sporadic HGSCs (26). The most striking molecular feature of HGSC is diffuse and high levels of DNA copy number gains or losses, which include CCNE1 (cyclin E1), NOTCH3, AKT2, RSF1, and PIK3CA loci (40). Despite their distinct molecular signatures, LGSC and even an APST is sometimes clonally associated with a synchronous HGSC, suggesting that such progression rarely does occur (41).
Clear Cell and Endometrioid Tumors
After serous carcinoma, endometrioid and clear cell carcinomas are the most frequent types of EOC accounting for approximately 15-20% of EOC in Western countries. The molecular genetic alterations that underlie the development of these tumors are now beginning to emerge. Based on genome-wide mutational analysis, the most common molecular genetic changes in clear cell carcinoma are a somatic inactivating mutation of ARID1A (22,23), a tumor suppressor gene detected in about 50% of cases, an activating mutation of PIK3CA in about 50% of tumors (42) and deletion of PTEN, a tumor suppressor gene involved in the PI3K/PTEN signaling pathway, in about 20%(43), supporting the role of an aberrant PI3K/PTEN pathway in the development of clear cell carcinoma. In addition, SNP array analysis has identified frequent amplification of the ZNF217 (zinc finger protein 217) locus and deletion of the CDKN2A/2B locus in clear cell carcinomas, suggesting that the pathways involving these two genes are also important in their development.
Like clear cell carcinoma, mutations that deregulate PI3K/PTEN signaling are common in low-grade endometrioid carcinoma and, in fact, mutation of the tumor suppressor gene PTEN, which occurs rarely in other types of EOC, has been reported in approximately 20% of ovarian low-grade endometrioid carcinomas (44,45). Another mechanism by which activation of PI3K signaling occurs is through activating mutations of PIK3CA, which has been detected in 20% of low-grade endometrioid carcinomas (42). The Wnt/b-catenin signaling pathway, which is involved in the regulation of several important cellular processes including proliferation, motility and survival, is deregulated in up to 40% of ovarian endometrioid carcinomas, usually on the basis of activating mutations of CTNNB1, the gene that encodes β-catenin (46). Notably, mutation of CTNNB1 has been associated with squamous differentiation, low tumor grade and a favorable outcome, features that characterize low-grade endometrioid carcinoma (47-50).
Although low-grade endometrioid carcinomas are easily recognized, the distinction of high-grade endometrioid carcinoma from HGSC can be very difficult. Some pathologists even question the existence of high-grade endometrioid carcinoma regarding it as a variant of HGSC whereas others classify high-grade endometrioid carcinoma as “mixed high-grade endometrioid carcinoma and HGSC” or as “HGSC with features of endometrioid carcinoma”. It is therefore of interest that in a study of ovarian endometrioid carcinomas of all grades, low-grade endometrioid carcinomas were characterized by mutations that deregulate the canonical Wnt/β-catenin and PI3K/PTEN signaling pathways and lacked TP53 mutations whereas high-grade endometrioid carcinomas lacked Wnt/β catenin or PI3K/PTEN signaling pathway defects and frequently harbored TP53 mutations (47). A few high-grade endometrioid carcinomas did, in addition, to TP53 mutation, display molecular changes found in the low-grade endometrioid carcinomas suggesting that some low-grade endometrioid carcinoma may progress to high-grade carcinoma. The findings parallel those seen in the serous tumors, namely that generally low- and high-grade tumors develop independently but that rarely progression of a low-grade to a high-grade tumor occurs. The similar high frequency of TP53 mutations in the high-grade endometrioid carcinoma as in HGSC suggests that both develop in a similar fashion, via TP53 mutation, and that most high-grade endometrioid carcinoma is closely related or is a variant of HGSC.
Morphologic studies over the past two to three decades have repeatedly shown an association of endometrioid and clear cell carcinoma with endometriosis and early molecular genetic studies demonstrated LOH in the same chromosomal regions in endometrioid carcinoma and adjacent endometriosis (51) confirming a clonal relationship between endometriosis and endometrioid carcinoma. In addition, a recent study reported mutation of ARID1A in atypical endometriosis adjacent to clear cell carcinoma but not in distant sites of endometriosis further linking endometriosis to clear cell carcinoma and thereby providing further evidence that endometriosis is the likely precursor of endometrioid and clear cell carcinoma (23). Although both clear cell and endometrioid carcinomas are derived from endometriosis and share some molecular genetic features, such as mutation of ARID1A and deletion of PTEN, they clearly adopt different molecular programs for their development, as is evident by their distinctly different morphologic phenotype and clinical behavior. For example, canonical Wnt signaling pathway defects and microsatellite instability, which occur frequently in low-grade endometrioid carcinoma have only rarely been detected in clear cell carcinoma (46). Also it has been recently demonstrated that unlike all the other types of EOC, clear cell carcinoma has significantly longer telomeres and this finding correlates with poor outcome (52).
Finally, morphologic studies have linked the endocervical-type mucinous borderline tumor, also referred to as “atypical proliferative seromucinous tumor”, to endometriosis in about a third of cases (53). We are unaware of any molecular genetic studies of these neoplasms, however, we have recently detected ARID1A mutations in two of these neoplasms suggesting that they are more closely related to endometrioid than to serous or mucinous tumors (unpublished data) further confirming the role of endometriosis as a precursor for a variety of “endometrioid-related” ovarian neoplasms.
Mucinous Tumors
These tumors have been the least studied histologic types probably due to their relative rarity (approximately 3% of EOC). KRAS mutations occur in up to 75% of primary mucinous carcinomas and using KRAS as a molecular marker, laser capture microdissection studies have shown the identical KRAS mutation in mucinous carcinomas and adjacent mucinous cystadenomas and borderline tumors (32,54,55) supporting the morphological continuum and tumor progression in ovarian mucinous neoplasms.
In summary, each of the major histologic types of EOC is associated with a different set of cell signaling pathways abnormalities, which for the type I tumors are shared with their respective precursor lesions (borderline tumors and endometriosis) supporting their stepwise progression (Fig 2). In contrast, the type II tumors, aside from a very high frequency of TP53 mutations and molecular alterations of BRCA1/2, are characterized by marked genetic instability and lack other mutations. The identification and characterization of their precursor lesions have only recently been recognized (see below).
Origin of Epithelial Ovarian Carcinoma
Serous Tumors
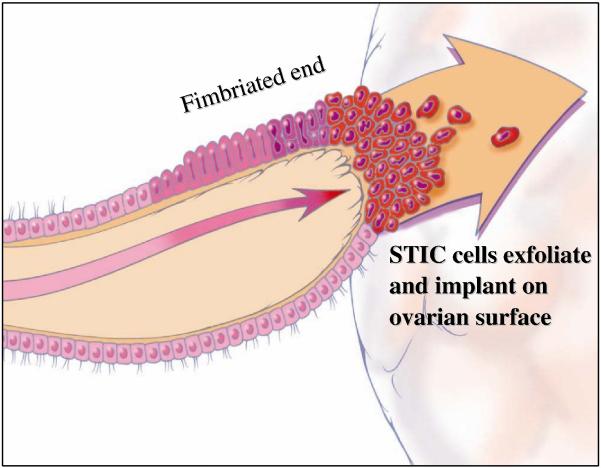
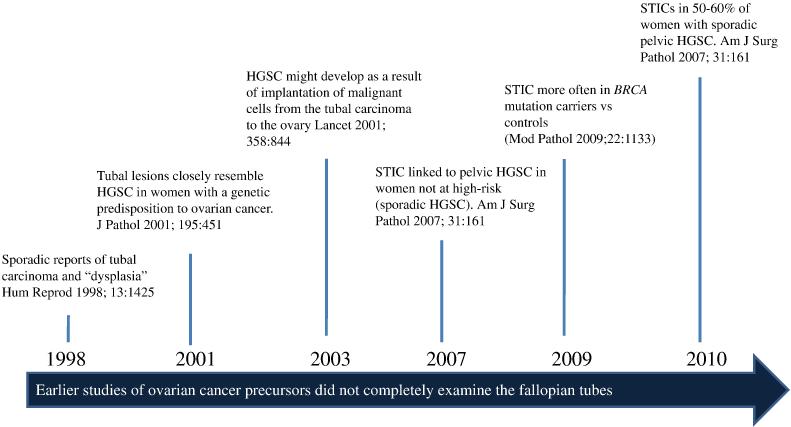
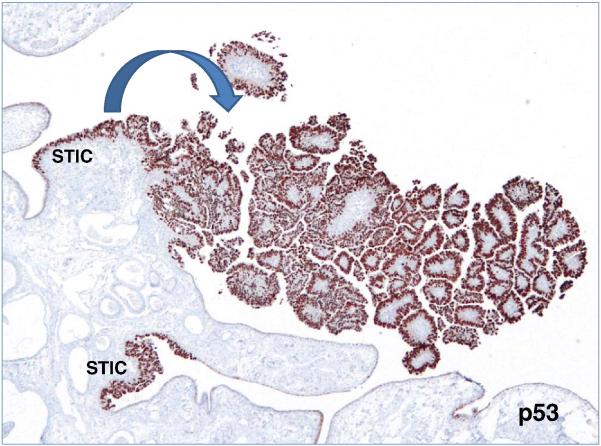
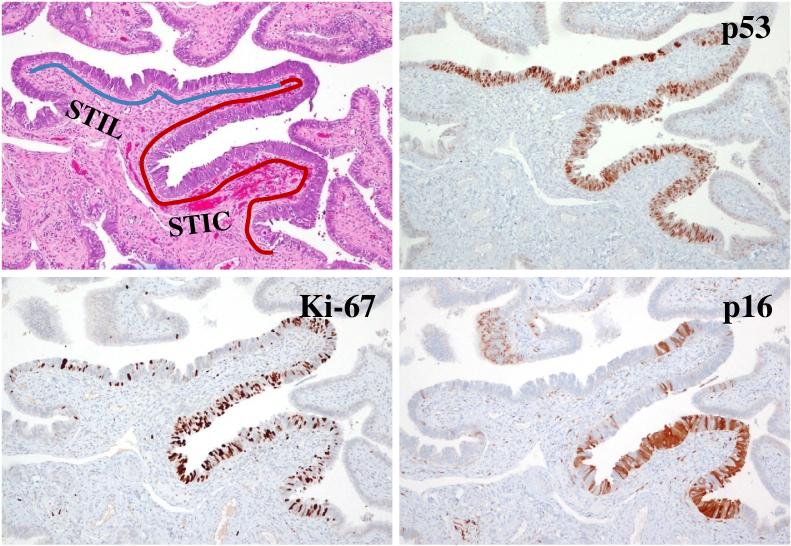
The conventional view of the origin of serous tumor has been that they were derived from the ovarian surface epithelium or cortical inclusion cysts. Therefore, there was surprise and skepticism when a group of Dutch investigators in 2001 first described tubal intraepithelial carcinomas (TICs), later designated “serous tubal intraepithelial carcinomas (STICs)” and occult invasive HGSCs in the fallopian tube that closely resembled ovarian HGSC, in women with a genetic predisposition to ovarian cancer (56). Similar lesions were not found in the ovaries of the same women. In hindsight, the failure to identify the tubal carcinomas in the past was because it was assumed that precursors of ovarian carcinoma would logically be in the ovaries, and therefore the fallopian tubes were not carefully examined (7-19). It was subsequently proposed that implantation of malignant cells from the tubal carcinoma to the ovary develop into a tumor mass that gives the impression that the tumor originated in the ovary (57,58) (Fig. 3). Additional studies in which fallopian tubes were carefully examined confirmed that STICs and small, early invasive tubal carcinomas occurred not only in women with a genetic predisposition for the development of ovarian cancer but also in 50-60% of women without known BRCA mutations (sporadic ovarian cancer) (Fig 4) (59-67). Moreover, these carcinomas were almost always detected in the fimbria (Fig 5) and it has been proposed that earliest neoplastic change begins in secretory-type cells (63,66). Further evidence supporting the proposal that STICs are precursors was the identification of STICs in women without ovarian cancer as well as the presence of identical TP53 mutations in STICs and concomitant ovarian HGSCs, indicating a clonal relationship between them (66,68). A gene profiling study showing that the gene expression profile of HGSC is more closely related to fallopian tube epithelium than to ovarian surface epithelium (69) and immunohistochemical studies showing that HGSC expresses PAX8, a müllerian marker, but not calretinin, a mesothelial marker (ovarian surface epithelium has a mesothelial not a müllerian morphologic phenotype) lends further support to the proposal that the tubal lesions are precursors of HGSC and not the ovarian surface epithelium (70). Further confirmation of the link between STICs and HGSC is the demonstration that both STICs and concomitant ovarian HGSCs, besides co-expressing p53, also co-express p16, FAS, Rsf-1, and cyclin E1 (71) (Fig 6). In addition, a recent study showed that STICs, like other precancerous lesions, have relatively short telomeres (72).
Fig. 3.
Spread of serous ubal intraepitelial carcinoma (STIC) from the fimbria to the ovarian surface. Adapted and reprinted with permission from American J Surg Pathol 2010;34:433-443. Reference 70.
Fig. 4.
Tubal origin of ovarian high-grade serous carcinoma (HGSC). A brief history. HGSC= high-grade serous carcinoma, STIC= serous tubal intraepithelial carcinoma.
Fig. 5.
Fimbria with two serous tubal intraepithelial carcinomas (STICs) and an associated papillary invasive high-grade serous carcinoma highlighted by p53 immunostain.
Fig. 6.
A serous tubal intraepithelial lesion (STIL) immediately adjacent to a serous tubal intraepithelial carcinoma (STIC).
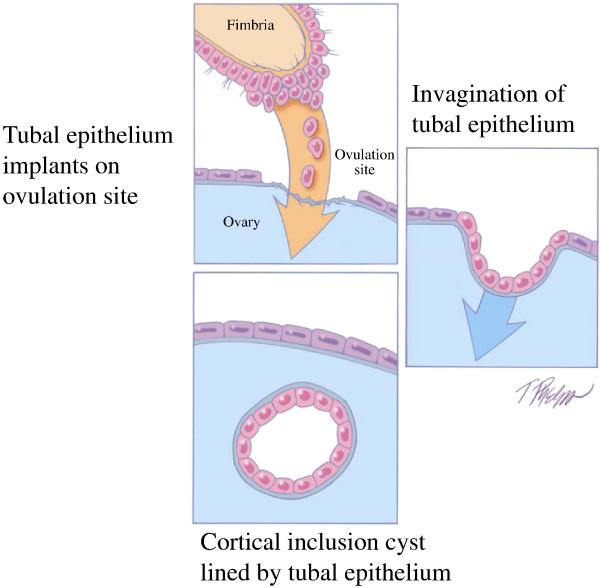
As previously noted, in studies of ovarian and primary peritoneal HGSC in which the fallopian tubes were completely sectioned using the SEE-FIM protocol (63), STICs were identified in 50-60% of cases (66,67). This raises the question as to the source of the remaining ovarian carcinomas that lack evidence of tubal involvement. One possibility is that small STICs were missed despite complete sampling of the tubes and, in fact, it has been shown that leveling the fallopian tube blocks can detect additional STICs not found in the original sections (67). Another possibility is that the carcinoma developed from ovarian cortical inclusion cysts. Although it is generally stated that these cysts develop by invagination of ovarian surface epithelium, there is reason to believe that during ovulation, as the fimbria come into close contact with the ovary, tubal epithelial cells implant on the disrupted ovarian surface to form a cortical inclusion cyst (70) (Fig 7). Parenthetically, tubal epithelial cells are easily dislodged for culture in the laboratory by flushing the fallopian tube (56, Shih, I-M, unpublished data). In addition, ovulation itself with the release of follicular fluid, which has been shown to contain reactive oxygen species (free radicals), and possibly associated changes in the microenvironment, such as inflammation, may play a role in early ovarian carcinogenesis. This is consistent with epidemiologic evidence linking decreased ovulation (either as a result of oral contraceptive usage or multiple pregnancies) with a decreased risk of ovarian cancer (73,74). Therefore, some HGSCs may develop from ovarian cortical inclusion cysts (75) but these cysts could be derived, not from the ovarian surface epithelium but from implanted fimbrial tubal epithelium (70) (Fig 8). Also as the fallopian tubes are now being more carefully studied additional abnormalities in the fallopian tube epithelium have been discovered. These include cytologic abnormalities that fall short of STICs which we have tentatively designated “serous tubal intraepithelial lesions (STILs)” (Fig 6) and termed by others “tubal intraepithelial lesions in transition (TILT)” (76). In addition, short stretches of normal appearing fallopian tube epithelium that strongly expresses p53, and in which TP53 mutations have occasionally been identified, have been termed “p53 signatures” (68). Although these lesions may represent the very early events in serous carcinogenesis, it is not clear, at this time, whether STILs and p53 signatures are precursor lesions or that they are benign “reactive” changes that over express p53 and have no biological relevance to neoplasia. It is conceivable that some are precursors and others are not; clearly this is an area that requires further investigation.
Fig. 7.
Development of a cortical inclusion cyst from tubal epithelium. Adapted and reprinted with permission from American J Surg Pathol 2010;34:433-443. Reference 70.
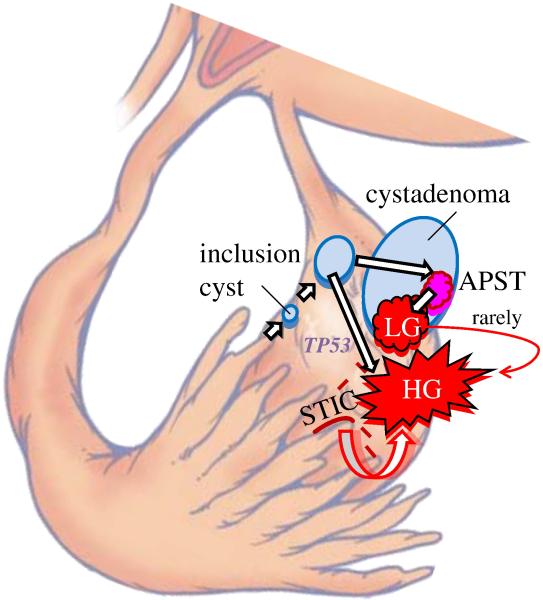
Fig. 8.
Development of low-grade [type I pathway with KRAS or BRAF mutation) and high-grade serous carcinoma [type II pathway with TP53 mutation] from tubal epithelium by way of a cortical inclusion cyst and cystadenoma or an intraepithelial carcinoma (STIC) implanting directly on the ovary developing into a high-grade serous carcinoma. Reprinted with permission from American J Surg Pathol 2010;34:433-443. Reference 70.
It appears that LGSC may also be derived from fallopian tube epithelium. Careful examination of fallopian tubes in women with APSTs discloses what we have recently described as “papillary tubal hyperplasia”. This lesion is characterized by small, papillary clusters of bland-appearing tubal epithelium (both secretory and ciliated cells) that are often associated with psammoma bodies. Varying numbers of these clusters can be detected in the tubal lumen and appear to bud from the tubal epithelium (unpublished data). We speculate that these detached clusters of tubal epithelium pass through the tube and implant on the ovary where they can develop into APSTs or implant on the pelvic or abdominal peritoneum to produce noninvasive implants.
Clear Cell and Endometrioid Tumors
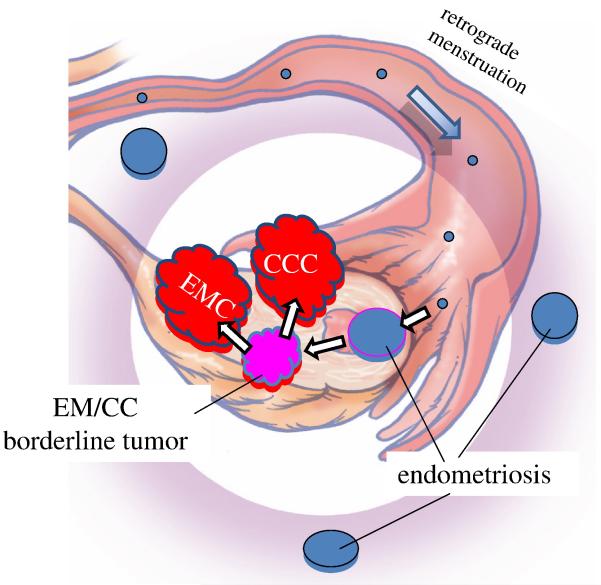
As previously noted it is well established by morphologic and, more recently, by molecular genetic studies that low-grade endometrioid and clear cell carcinomas develop from endometriotic cysts (endometriomas) and are frequently associated with implants of endometriosis elsewhere in the pelvis (77). Although the precise origin of endometriosis has not been completely established, specifically, whether it develops in situ in the peritoneum through a process of metaplasia or from retrograde menstrual flow, the preponderance of data favor the latter mechanism (78). Admittedly, the former theory is more difficult to prove experimentally. Thus, if retrograde menstruation accounts for most cases of endometriosis, it is logical to assume that endometrioid and clear cell tumors develop from endometrial tissue that implanted on the ovary and therefore the ovary is involved secondarily (79) (Fig. 9). Of further interest has been the observation that the eutopic endometrium in women with endometriosis exhibits intrinsic molecular abnormalities, including activation of oncogenic pathways (78). Presumably, these changes permit the endometrial tissue to implant, survive, and invade ovarian and peritoneal tissue. This hypothesis, by which endometrioid and clear cell carcinoma develop from endometrial tissue implanted on the ovary, is supported by epidemiologic evidence showing that the protective effect for tubal ligation is seen only for endometrioid and clear cell carcinoma as tubal ligation would interrupt passage of endometrial tissue from entering the peritoneal cavity but would not interfere with tubal cells from the fimbria implanting on the ovary and developing into HGSC (80).
Fig. 9.
Development of low-grade endometrioid and clear cell carcinoma from endometriosis by retrograde menstruation. Reprinted with permission from American J Surg Pathol 2010;34:433-443. Reference 70.
Mucinous Tumors
Studies over the last decade have shown that the majority of the gastrointestinal-type tumors involving the ovary are secondary (81,82) and that, in fact, primary mucinous carcinomas of the ovary are one of the least common types of EOC comprising about 3% of EOC. Malignant Brenner tumors are the least common type of EOC. The origin of these mucinous tumors and Brenner tumors tumors is puzzling, as unlike serous, endometrioid, and clear cell tumors, they do not display a müllerian phenotype. Although it has been argued that mucinous tumors bear some relationship to the endocervix, the mucinous epithelium that characterizes them more closely resembles gastrointestinal mucosa. It seems unlikely that they develop from cortical inclusion cysts, as mucinous metaplasia involving cortical inclusion cysts is a very rare finding. On the other hand, the association of Brenner tumors and mucinous tumors has been recognized for many years. In a provocative study of mucinous cystadenomas and Brenner tumors, it was reported that after extensive sectioning, mucinous cystadenomas contained foci of Brenner tumor in 18% of cases (83). Interestingly, mucinous tumors were frequently associated with Walthard cell nests that are composed of benign transitional-type epithelium, frequently found in paraovarian and paratubal locations. This raises the possibility that mucinous tumors and Brenner tumors have the same histogenesis arising from these microscopic transitional cell nests at the tubal-peritoneal junction, which would be consistent with their non-müllerian appearance (84). The study reported that Brenner tumors are small (median size 0.5 cm), whereas mucinous cystadenomas are large (median size 9 cm). The investigators then went on to speculate that as a small Brenner tumor grows, the mucinous component becomes dominant resulting in the development of a mucinous cystadenoma that, as it enlarges, compresses and eventually obliterates the adjacent ovary and Brenner tumor giving the appearance that it arose in the ovary. The findings in this study are intriguing but must be regarded as preliminary. Additional morphologic and molecular genetic studies are necessary to determine whether this concept is valid. Another subset of gastrointestinal-type mucinous tumors can arise from ovarian mature cystic teratomas (85).
In summary, the data support the view that serous tumors develop from the fallopian tube, that endometrioid, and clear cell tumors arise from endometrial tissue passing through the fallopian tube resulting in endometriosis and mucinous, and Brenner tumors develop from transitional-type epithelium located at the tubal-peritoneal junction (84). The concept that EOC originates outside the ovary and involves it secondarily has emerged only recently, because in the past, the default diagnosis of carcinomas involving the pelvis and abdomen was that these were ovarian. A carcinoma is classified as tubal in origin only when the bulk of the tumor involves the fallopian tube rather than the ovary, and there is evidence of an intraepithelial (in situ) tubal carcinoma (86). Similarly, HGSC that extensively involves the peritoneum, omentum, and other abdominal organs, is classified as primary ovarian, if there is as little as 5mm of tumor involving the ovaries. Although the recent data suggesting that EOC arises in extraovarian sites and involves the ovaries secondarily are compelling, serous neoplasms (low- and high-grade) involve the ovaries and other pelvic and abdominal organs, such as the omentum and mesentery, much more extensively than the fallopian tubes. Similarly, although endometrioid and clear cell carcinomas develop from endometriosis that frequently involve multiple sites in the pelvis, these neoplasms are almost always confined to the ovaries. It is likely that the propensity for growth in the ovary is mulifactorial, but the precise reasons for this are unknown.
The New Paradigm and its Clinical Implications
The aforementioned molecular genetic and morphologic studies have provided new insight into the pathogenesis and origin of EOC and in so doing have ushered in a new paradigm. These studies provide compelling evidence that contrary to what was previously believed, EOC is not primarily ovarian in origin but rather secondary leading to the conclusion that the only true primary ovarian neoplasms are gonadal stromal and germ cell tumors analogous to testicular tumors. This is not merely of academic interest because it also has profound clinical implications. Given the distinctly different morphologic, molecular genetic and clinical features of this diverse group of tumors classified as EOC it is important to evaluate the triad of early detection, treatment and prevention according to whether tumors are type I or type II and then to consider the various histologic types that constitute the type I group individually as there is considerable diversity in their pathogenesis which will have a direct impact on how they are managed.
Early Detection
The dualistic model highlights the heterogeneity of ovarian carcinoma and points out that one screening test will not be effective in detecting all the different types of ovarian carcinomas. Type I tumors (low-grade serous, low-grade endometrioid, clear cell, and mucinous) are slow growing and attain a large size while still confined to the ovary. They are easily detected by pelvic examination and/or transvaginal ultrasound. Moreover, they constitute only 25% of ovarian cancers and account for approximately 10% of ovarian cancer deaths (87). Therefore, it can be argued that the development of a biomarker screening test is not urgently needed for type I tumors. More importantly, the recognition that type II tumors [high-grade serous and undifferentiated carcinomas, and malignant mixed mesodermal tumors (carcinosarcomas)] represent 75% of all ovarian carcinomas, are responsible for 90% of ovarian cancer deaths (87) and originate outside the ovary underscores the importance of diagnosing these tumors early in their evolution. Unfortunately, screening approaches designed to detect them while confined to the ovary have been unsuccessful (87), and are not likely to succeed, because these tumors are almost never confined to the ovary at diagnosis. This has been clearly demonstrated by a study of nearly 400 carefully staged patients from the Washington Center Hospital in Washington DC, which is a primary care hospital that found less than 1.1% of HGSCs were confined to the ovary at diagnosis (82) and a report from the British Columbia Tumor Registry which found only 0.5% of HGSCs limited to the ovary at diagnosis (89). The futility of detecting early-stage ovarian cancer was recently underscored in a large multi-institutional prospective study [Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial] in which, despite intensive annual screening of nearly 35,000 women with CA 125 and transvaginal ultrasound, 70% of the women presented with advanced stage disease, which was no different from unscreened populations (88). For type II tumors, the goal in screening should be the detection of low volume disease even if outside the ovary rather than stage I disease (tumor confined to the ovary). This can only be accomplished by developing a panel of sensitive and specific biomarkers that are expressed early in ovarian carcinogenesis.
Treatment
Treatment of type I and type II tumors, like early detection, must be individualized. Type I tumors are generally low-grade, slow growing and localized to the ovary at diagnosis spreading late in their evolution. Accordingly, when confined to the ovary, salpingo oophorectomy probably suffices. On the other hand, when these tumors have spread beyond the ovary, chemotherapeutic agents that are effective against the more rapidly proliferating type II tumors are not as effective for the slow growing type I tumors. Therefore, new approaches for advanced- stage type I tumors are needed. Since deregulation of signaling pathways as a result of somatic mutation of genes is responsible for driving progression in type I tumors, these genes could provide potential targets for therapeutic intervention. For example, in many type I carcinomas, there is constitutive activation of the MAPK signaling pathway because of mutations in ERBB2, KRAS or BRAF, the upstream regulators of MAPK. It is therefore conceivable that MAPK kinase inhibitors could prolong disease-free interval and improve overall survival in patients with such advanced stage type I tumors, when combined with conventional therapeutic modalities.
In contrast to the type I tumors, treatment for type II tumors should be initiated on the basis of detection of sensitive and specific biomarkers before the appearance of morphologically recognizable disease, when therapy will likely be more effective. A related and unresolved question is what should be the management of a patient in whom a STIC is diagnosed but who has no other evidence of disease. The finding of positive pelvic washings in patients with only a STIC indicates that these microscopic lesions can shed malignant cells (59). This clinical dilemma highlights the importance of an accurate diagnosis of a STIC. As this is a recently described entity and pathologists have limited experience with it this can be extremely challenging. A recent study showed that even among expert gynecologic pathologists the reproducibility of a diagnosis of STIC was only moderate (90). We have developed an algorithm that utilizes p53 and Ki-67 immunostaining in conjunction with morphology to make a diagnosis. With this algorithm we were able to achieve high reproducibility (kappa of 0.74) (91) (see http://www.ovariancancerprevention.org).
The frequent inactivation of the DNA repair pathways involving BRCA 1/2 offers a new approach to treatment by taking advantage of BRCA pathway inactivation to induce cell death using small molecule inhibitors that suppress other DNA repair pathways. In fact, the feasibility and efficacy of this approach have recently been demonstrated in preclinical and clinical studies of ovarian cancer patients with poly(ADP-ribose) polymerase (PARP) inhibitors such as olaprib and AG014699. It is therefore likely that PARP inhibitors will be effective in treating sporadic and hereditary ovarian type II carcinomas as a monotherapy or in combination with other cytotoxic reagents (92-95).
Prevention
The mounting evidence that ovarian cancer does not develop in the ovary, and the lack of success of ovarian cancer screening provide a strong argument for directing efforts at prevention. It has been well established in epidemiologic studies that reducing the number of ovulations during a woman’s life has a significant protective effect. Thus, the risk of EOC is reduced by as much as 50% for women using oral contraceptives for 5 or more years (74) and parity compared with nulliparity confers approximately a 50% decrease in risk (96). These data provide strong evidence that ovulation plays an important role in ovarian carcinogenesis and as previously described implantation of tubal epithelium from the fimbria on denuded ovarian surface epithelium at the site of ovulation may be the culprit. Accordingly, the entire approach to prophylaxis, not only for women at high risk of developing ovarian cancer but also for the general female population, needs to be reevaluated in the light of the evolving new paradigm of ovarian carcinogenesis. The traditional approach for reducing risk for women with a family history of ovarian traditional approach for reducing risk for women with a family history of ovarian carcinoma or who are found to have BRCA1/2 mutations has been hysterectomy and bilateral salpingo-oophorectomy. The ovarian tumors that develop are almost always HGSC and there has been no convincing evidence that these women are at a higher risk of developing uterine serous carcinoma. Therefore, if it can be unequivocally shown that the HGSC in these women develop almost exclusively in the fimbria, then salpingectomy or fimbriectomy alone would be sufficient to reduce the risk of ovarian cancer while providing the additional benefit of conserving ovarian function and preserving fertility. This approach would have to be evaluated in a randomized clinical trial comparing it to the standard treatment of bilateral salpingo oophorectomy.
For women who are not considered to be at high risk but who undergo a hysterectomy for benign uterine disease, many gynecologists have argued that bilateral oophorectomy should be carried out to reduce the risk of developing ovarian cancer. However, in a recent prospective study of nearly 30,000 women in the Nurses’ Health Study, it was shown that compared with ovarian conservation, bilateral oophorectomy at the time of hysterectomy was associated with an increased risk of death from all causes as well as being associated with at increased risk of nonfatal coronary heart disease (97). Accordingly, for women undergoing a hysterectomy for benign uterine disease, removal of only the fallopian tubes with sparing of the ovaries would improve quality of life and overall survival while still reducing the risk of ovarian carcinoma. Such an approach has important public health implications, as approximately 300,000 women in the United States undergo elective oophorectomy each year (97). Finally, for young women who are not at high-risk but who are seeking a more permanent form of contraception, fimbriectomy or salpingectomy instead of tubal ligation (tubal ligation leaves the fimbria intact and STICs are almost always confined to the fimbria) could be performed thereby reducing their risk of developing EOC.
Conclusions
Recent morphologic, immunohistochemical and molecular genetic studies have led to the development of a new paradigm for the pathogenesis and origin of EOC. The paradigm is based on a dualistic model of carcinogenesis that divides EOC into two broad categories designated type I and type II. Type I tumors are generally indolent, present in stage I (tumor confined to the ovary) and develop from borderline tumors and endometriosis. They are characterized by specific mutations, including KRAS, BRAF, ERBB2, CTNNB1, PTEN PIK3CA, ARID1A, and PPPR1A but rarely TP53 and are relatively stable genetically.
Type II tumors are aggressive, present in advanced stage, and develop from intraepithelial carcinomas in the fallopian tube. They have a very high frequency of TP53 mutations but rarely harbor the mutations detected in type I tumors. In addition, type II tumors have molecular alterations that perturb expression of BRCA either by mutation of the gene or by promotor methylation. They are also genetically highly unstable. Recent studies indicate that both type I and type II tumors develop from extraovarian tissue that implants on the ovary. In addition, the precursor lesions of type I and type II tumors are being characterized and have been linked to their respective carcinomas. Thus, the fallopian tube appears to be the source of LGSC and HGSC. We believe that the low-grade serous tumors develop from a recently described lesion designated “papillary tubal hyperplasia” and the high-grade carcinomas from an intraepithelial carcinoma designated “serous intraepithelial tubal carcinoma (STIC)”. Another possible mechanism for the development of HGSC is dislodgement of normal tubal epithelium from the fimbria, which implants on the site of rupture where ovulation occurred resulting in the formation of an inclusion cyst that may then undergo malignant transformation. Endometrioid and clear cell carcinomas may also originate from nonovarian, müllerian-type tissue, as it is widely accepted that these tumors develop from endometriosis that is thought to develop as a result of retrograde menstruation. The origin of mucinous and transitional cell (Brenner) tumors is still not well established, although recent data suggest a possible origin from transitional epithelial nests located in paraovarian locations at the tubo-peritoneal junction. Thus, there is mounting evidence that type I and type II ovarian tumors develop independently along different molecular pathways, and that both types develop outside the ovary and involve it secondarily. If this concept is confirmed it leads to the conclusion that the only true primary ovarian neoplasms are gonadal stromal and germ cell tumors analogous to testicular tumors. This new paradigm has profound clinical implications. By shifting the early events of ovarian carcinogenesis to the fallopian tube and endometrium instead of the ovary, prevention approaches, for example salpingectomy with ovarian conservation, which was never seriously considered in the past, may play an important role in reducing the burden of this disease while at the same time preserving hormonal function and fertility.
Many questions relating to the pathogenesis of EOC remain unanswered but the inability to reconcile all of these issues at this time does not invalidate or negate the utility of the new paradigm. As Kuhn remarked “To be accepted as a paradigm, a theory must seem better than its competitors, but it need not, and in fact never does, explain all the facts with which it can be confronted.”(1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuhn TS. The Structure of Scientific Revolutions. 3rd ed. University of Chicago Press; 1996. enlarged. [Google Scholar]
- 2.Geist SH. Ovarian Tumors. Paul B. Hoeber, Inc; New York: 1942. [Google Scholar]
- 3.Frank RT. Gynecological and Obstetrical Pathology. D Appleton and Co; New York: 1931. [Google Scholar]
- 4.Gemma B. Atlas of Ovarian Tumors. Grune & Stratton; New York: 1943. [Google Scholar]
- 5.Hertig AT, Gore H. Atlas of Tumor Pathology, Section IX-Fascicle 3,Tumors of the Female Sex Organs Part 3 Tumors of the Ovary and Fallopian Tube. Armed Forces Institute of Pathology; Washington DC: 1961. [Google Scholar]
- 6.Serov SF, Scully RE, Sobin LH. International Histological Classification of Tumours No. 9. Histological Typing of Ovarian Tunours. World Health Organization; Geneva: 1973. [Google Scholar]
- 7.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 8.Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases. Cancer. 1994;73:1859–1864. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Cai KQ, Wu H, Klein-Szanto AJ, Xu XX. Acquisition of a second mutation of the Tp53 alleles immediately precedes epithelial morphological transformation in ovarian tumorigenicity. Gynecol Oncol. 2009;114:18–25. doi: 10.1016/j.ygyno.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deligdisch L. Ovarian dysplasia: a review. Int J Gynecol Cancer. 1997;7:89–94. [Google Scholar]
- 11.Deligdisch L, Gil J, Kerner H, Wu HS, Beck D, Gershoni-Baruch R. Ovarian dysplasia in prophylactic oophorectomy specimens: cytogenetic and morphometric correlations. Cancer. 1999;86:1544–1550. [PubMed] [Google Scholar]
- 12.Salazar H, Godwin AK, Daly MB, Laub PB, Hogan WM, Rosenblum N, Boente MP, Lynch HT, Hamilton TC. Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst. 1996;88:1810–1820. doi: 10.1093/jnci/88.24.1810. [DOI] [PubMed] [Google Scholar]
- 13.Werness BA, Afify AM, Bielat KL, Eltabbakh GH, Piver MS, Paterson JM. Altered surface and cyst epithelium of ovaries removed prophylactically from women with a family history of ovarian cancer. Hum Pathol. 1999;30:151–157. doi: 10.1016/s0046-8177(99)90269-1. [DOI] [PubMed] [Google Scholar]
- 14.Sherman ME, Lee JS, Burks RT, Struewing JP, Kurman RJ, Hartge P. Histopathologic features of ovaries at increased risk for carcinoma. A case-control analysis. Int J Gynecol Pathol. 1999;18:151–157. doi: 10.1097/00004347-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Stratton JF, Buckley CH, Lowe D, Ponder BA. Comparison of prophylactic oophorectomy specimens from carriers and noncarriers of a BRCA1 or BRCA2 gene mutation. United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. J Natl Cancer Inst. 1999;91:626–628. doi: 10.1093/jnci/91.7.626. [DOI] [PubMed] [Google Scholar]
- 16.Seidman JD, Wang BG. Evaluation of normal-sized ovaries associated with primary peritoneal serous carcinoma for possible precursors of ovarian serous carcinoma. Gynecol Oncol. 2007;106:201–206. doi: 10.1016/j.ygyno.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Yang DH, Smith ER, Cohen C, Wu H, Patriotis C, Godwin AK, Hamilton TC, Xu XX. Molecular events associated with dysplastic morphologic transformation and initiation of ovarian tumorigenicity. Cancer. 2002;94:2380–2392. doi: 10.1002/cncr.10497. [DOI] [PubMed] [Google Scholar]
- 18.Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 2000;89:383–390. doi: 10.1002/1097-0142(20000715)89:2<383::aid-cncr25>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Chene G, Penault-Llorca F, Le Bouedec G, Mishellany F, Dauplat MM, Jaffeux P, Aublet-Cuvelier B, Pouly JL, Dechelotte P, Dauplat J. Ovarian epithelial dysplasia and prophylactic oophorectomy for genetic risk. Int J Gynecol Cancer. 2009;19:65–72. doi: 10.1111/IGC.0b013e3181990127. [DOI] [PubMed] [Google Scholar]
- 20.Burks RT, Sherman ME, Kurman RJ. Micropapillary serous carcinoma of the ovary. A distinctive low-grade carcinoma related to serous borderline tumors. Am J Surg Pathol. 1996;20:1319–1330. doi: 10.1097/00000478-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Shih I-M, Kurman RJ. Ovarian tumorigenesis- a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S, Wang TL, Shih IM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Jr., Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010 doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, et al. N Eng J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio A, Bowtell D, Brenton JD. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senturk E, Cohen S, Dottino PR, Martignetti JA. A critical re-appraisal of BRCA1 methylation studies in ovarian cancer. Gynecol Oncol. 2010;119:376–383. doi: 10.1016/j.ygyno.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 26.May T, Virtanen C, Sharma M, Milea A, Begley H, Rosen B, Murphy KJ, Brown TJ, Shaw PA. Low malignant potential tumors with micropapillary features are molecularly similar to low grade serous carcinoma of the ovary. Gynecol Oncol. 2010;117:9–17. doi: 10.1016/j.ygyno.2010.01.006. l. 2010. [DOI] [PubMed] [Google Scholar]
- 27.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, Bauknecht T, Park TW, Jonat W, Jacobsen A, Sehouli J, Luttges J, Krajewski M, Krajewski S, Reed JC, Arnold N, Hampton GM. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65. doi: 10.1038/sj.onc.1208298. l. [DOI] [PubMed] [Google Scholar]
- 28.Bonome T, Lee JY, Park DC, Radonovich M, Pise-Masison C, Brady J, Gardner GJ, Hao K, Wong WH, Barrett JC, Lu KH, Sood AK, Gershenson DM, Mok SC, Birrer MJ. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005;65:10602–12. doi: 10.1158/0008-5472.CAN-05-2240. l. [DOI] [PubMed] [Google Scholar]
- 29.Gilks CB, Vanderhyden BC, Zhu S, van de Rijn M, Longacre TA. Distinction between serous tumors of low malignant potential and serous carcinomas based on global mRNA expression profiling. Gynecol Oncol. 2005;96:684–94. doi: 10.1016/j.ygyno.2004.11.039. l. [DOI] [PubMed] [Google Scholar]
- 30.Dehari R, Kurman RJ, Logani S, Shih IM. The Development of High-grade Serous Carcinoma From Atypical Proliferative (Borderline) Serous Tumors and Low-grade Micropapillary Serous Carcinoma: A Morphologic and Molecular Genetic Analysis. Am J Surg Pathol. 2007;31:1007–1012. doi: 10.1097/PAS.0b013e31802cbbe9. [DOI] [PubMed] [Google Scholar]
- 31.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih IeM. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 32.Mok SC, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, Berkowitz RS, Tsao SW. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–92. [PubMed] [Google Scholar]
- 33.Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Fleuren G Jan, Houmadi R, Diss T, Warren B, Al Adnani M, De Goeij AP, Krausz T, Flanagan AM. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202:336–40. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 34.Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: A comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–7. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, Carpenter G, Gazdar AF, Muthuswamy SK, Arteaga CL. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y, Velculescu VE, Wang TL, Shih IeM. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther. 2006;5:779–85. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CY, Bristow R, Cha MS, Wang BG, Ho CL, Kurman RJ, Wang TL, Shih IeM. Characterization of active mitogen-activated protein kinase in ovarian serous carcinomas. Clin Cancer Res. 2004;10:6432–6. doi: 10.1158/1078-0432.CCR-04-0893. [DOI] [PubMed] [Google Scholar]
- 38.Ho CL, Kurman RJ, Dehari R, Wang TL, Shih IeM. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004;64:6915–8. doi: 10.1158/0008-5472.CAN-04-2067. [DOI] [PubMed] [Google Scholar]
- 39.Staebler A, Heselmeyer-Haddad K, Bell K, Riopel M, Perlman E, Ried T, Kurman RJ. Micropapillary serous carcinoma of the ovary has distinct patterns of chromosomal imbalances by comparative genomic hybridization compared with atypical proliferative serous tumors and serous carcinomas. Hum Pathol. 2002;33:47–59. doi: 10.1053/hupa.2002.30212. l. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama K, Nakayama N, Jinawath N, Salani R, Kurman RJ, Shih IeM, Wang TL. Amplicon profiles in ovarian serous carcinomas. Int J Cancer. 2007;120:2613–7. doi: 10.1002/ijc.22609. [DOI] [PubMed] [Google Scholar]
- 41.Shih Ie M, Chen L, Wang CC, Gu J, Davidson B, Cope L, Kurman RJ, Xuan J, Wang TL. Distinct DNA methylation profiles in ovarian sreous neoplasms and their implications in ovarian carcinogenesis. Am J Obstet Gynecol. 2010;584:e1–e22. doi: 10.1016/j.ajog.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 43.Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- 44.Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58:2095–7. [PubMed] [Google Scholar]
- 45.Catasus L, Bussaglia E, Rodrguez I, Gallardo A, Pons C, Irving JA, Prat J. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360–8. doi: 10.1016/j.humpath.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Cho KR, Shih I-E. Ovarian Cancer. Ann Rev Pathol Mech Dis. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DM, Katabuchi H, Williams BO, Fearon ER, Cho KR. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/B-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–33. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, Swisher EM. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007 doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Gamallo C, Palacios J, Moreno G, de Mora J Calvo, Suarez A, Armas A. beta-catenin expression pattern in stage I and II ovarian carcinomas: relationship with beta-catenin gene Mutations, Clinicopathological features, and clinical outcome. Amer J Path. 1999;155:527–36. doi: 10.1016/s0002-9440(10)65148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saegusa M, Okayasu I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001;194:59–67. doi: 10.1002/path.856. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X, Morland SJ, Hitchcock A, Thomas EJ, Campbell IG. Allelo typing of endometriosis with adjacent ocarian carcinomna reveals evidence of a common lineage. Cancer Research. 1998;58:1707–1712. [PubMed] [Google Scholar]
- 52.Kuhn E, Meeker A, Visvanathan K, Gross AL, Wang T-L, Kurman RJ, Shih I-M. Telomere length in different histologic types of epithelial ovarian carcinoma with emphasis on clear cell carcinoma. In press. [DOI] [PMC free article] [PubMed]
- 53.Rutgers JL, Scully RE. Ovarian mullerian mucinous papillary cystadenomas of borderline malignancy. A clinicopathologic analysis. Cancer. 1988;61:340–48. doi: 10.1002/1097-0142(19880115)61:2<340::aid-cncr2820610225>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 54.Ichikawa Y, Nishida M, Suzuki H, Yoshida S, Tsunoda H, Kubo T, Uchida K, Miwa M. Mutation of K-ras protooncogene is associated with histological subtypes in human mucinous ovarian tumors. Cancer Res. 1994;54:33–5. [PubMed] [Google Scholar]
- 55.Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Lin O, Soslow R, Venkatraman E, Boyd J. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90:378–81. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 56.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 57.Piek JM, van Diest PJ, Zweemer RP, Kenemans P, Verheijen RH. Tubal ligation and risk of ovarian cancer. Lancet. 2001;358:844. doi: 10.1016/S0140-6736(01)05992-X. [DOI] [PubMed] [Google Scholar]
- 58.Piek JM, Verheijen RH, Kenemans P, Massuger LF, Bulten H, van Diest PJ. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol. 2003;90:491. doi: 10.1016/s0090-8258(03)00365-2. [DOI] [PubMed] [Google Scholar]
- 59.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 60.Carcangiu ML, Radice P, Manoukian S, Spatti G, Gobbo M, Pensotti V, Crucianelli R, Pasini B. Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. Int J Gynecol Pathol. 2004;23:35–40. doi: 10.1097/01.pgp.0000101082.35393.84. [DOI] [PubMed] [Google Scholar]
- 61.Paley PJ, Swisher EM, Garcia RL, Agoff SN, Greer BE, Peters KL, Goff BA. Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol. 2001;80:176–180. doi: 10.1006/gyno.2000.6071. [DOI] [PubMed] [Google Scholar]
- 62.Finch A, Shaw P, Rosen B, Murphy J, Narod SA, Colgan TJ. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 63.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 64.Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–1289. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Shaw PA, Rouzbahman M, Pizer ES, Pintilie M, Begley H. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;22:1133–1138. doi: 10.1038/modpathol.2009.89. [DOI] [PubMed] [Google Scholar]
- 66.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 67.Przybycin CG, Kurman RJ, Ronnett BM, Shih IM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 68.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–3569. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 69.Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L, Fishman D, Berchuck A, Whitaker R, Gershenson DM, Mills GB, Bast RC, Jr., Lu KH. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–612670. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 70.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sedhav A Smith, Kurman RJ, Kuhn E, Shih I-M. Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP(, ctclin E and fatty acid synthase. Mod Pathol. 2010;23:844–85572. doi: 10.1038/modpathol.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhn E, Meeker A, Wang TL, Sehdev AS, Kurman RJ, Shih Ie M. Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. Am J Surg Pathol. 2010;34:829–836. doi: 10.1097/PAS.0b013e3181dcede7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beral V, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369:1703–1710. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- 74.Risch HA, Weiss NS, Lyon JL, Daling JR, Liff JM. Events of reproductive life and the incidence of epithelial ovarian cancer. Am J Epidemiol. 1983;117:128–139. doi: 10.1093/oxfordjournals.aje.a113523. [DOI] [PubMed] [Google Scholar]
- 75.Pothuri B, Leitao MM, Levine DA, Viale A, Olshen AB, Arroyo C, Bogomolniy F, Olvera N, Lin O, Soslow RA, Robson ME, Offit K, Barakat RR, Boyd J. Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One. 2010;5:e10358. doi: 10.1371/journal.pone.0010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 77.Veras E, Mao TL, Ayhan A, et al. Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases. Am J Surg Pathol. 2009;33:844–853. doi: 10.1097/PAS.0b013e31819c4271. [DOI] [PubMed] [Google Scholar]
- 78.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 79.Martin DC. Cancer and endometriosis: do we need to be concerned? Semin Reprod Endocrinol. 1997;15:319–324. doi: 10.1055/s-2008-1068762. [DOI] [PubMed] [Google Scholar]
- 80.Rosenblatt KA, Thomas DB. Reduced risk of ovarian cancer in women with a tubal ligation or hysterectomy. The World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Cancer Epidemiol Biomarkers Prev. 1996;5:933–935. [PubMed] [Google Scholar]
- 81.Riopel MA, Ronnett BM, Kurman RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, and metastatic carcinomas. Am J Surg Pathol. 1999;23:617–635. doi: 10.1097/00000478-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Seidman JD, Cho KR, Ronnett BM, Kurman RJ. Surface epithelial tumors of the ovary. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. 6th Springer-Verlag; New York: 2011. pp. 679–784. [Google Scholar]
- 83.Seidman JD, Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: a study of 120 tumors. Arch Pathol Lab Med. 2008;132:1753–1760. doi: 10.5858/132.11.1753. [DOI] [PubMed] [Google Scholar]
- 84.Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tubeperitoneal junction: A potential site of carcinogenesis. Int J Gynecol Pathol. 2010;30:4–11. doi: 10.1097/PGP.0b013e3181f29d2a. [DOI] [PubMed] [Google Scholar]
- 85.Vang R, Gown AM, Zhao C, Barry TS, Isacson C, Richardson MS, Ronnett BM. Ovarian mucinous tumors associated with mature cystic teratomas. Morphologic and immunohistochemical analysis identifies a subset of potential teratomatous origin that shares features of lower gastrointestinal tract mucinous tumors more commonly encountered in the ovary. Am J Surg Pathol. 2007;31:854–869. doi: 10.1097/PAS.0b013e31802efb45. [DOI] [PubMed] [Google Scholar]
- 86.Sedlis A. Primary carcinoma of the fallopian tube. Obstet Gynecol Surv. 1961;16:209–226. doi: 10.1097/00006254-196104000-00022. [DOI] [PubMed] [Google Scholar]
- 87.Guth U, Huang DJ, Bauer G, et al. Metastatic patterns at autopsy in patients with ovarian carcinoma. Cancer. 2007;110:1272–1280. doi: 10.1002/cncr.22919. [DOI] [PubMed] [Google Scholar]
- 88.Partridge E, Kreimer AR, Greenlee RT, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775–782. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salvador S, Gilks B, Kobel M, et al. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer. 2009;19:58. doi: 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 90.Carlson JW, Jarboe EA, Kindelberger D, Nucci MR, Hirsch MS, Crum CP. Serous tubal intraepithelial carcinoma: diagnostic reproducibility and its implications. Int J Gynecol Pathol. 2010;29:310–314. doi: 10.1097/PGP.0b013e3181c713a8. [DOI] [PubMed] [Google Scholar]
- 91.Visvanathan K, et al. Submitted for publication.
- 92.Drew Y, Mulligan EA, Vong WT, Thomas HD, Kahn S, Kyle S, Mukhopadhyay A, Los G, Hostomsky Z, Plummer ER, Edmondson RJ, Curtin NJ. Therapeutic Potential of Poly(ADP-ribose) Polymerase Inhibitor AG014699 in Human Cancers With Mutated or Methylated BRCA1 or BRCA2. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- 93.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 94.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 95.Konstantinopoulos PA, Spentzos D, Karlan BY, Taniguchi T, Fountzilas E, Francoeur N, Levine DA, Cannistra SA. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28:3555–3561. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–437. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 97.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]