Abstract
Change blindness (CB), the inability to detect changes in visual scenes, may increase with age and early Alzheimer’s disease (AD). To test this hypothesis, participants were asked to localize changes in natural scenes. Dependent measures were response time (RT), hit rate, false positives (FP), and true sensitivity (d′). Increased age correlated with increased sensitivity and RT; AD predicted even slower RT. Accuracy and RT were negatively correlated. Differences in FP were nonsignificant. CB correlated with impaired attention, working memory, and executive function. Advanced age and AD were associated with increased CB, perhaps due to declining memory and attention. CB could affect real-world tasks, like automobile driving.
Keywords: Automobile driving, Change detection, Cognitive aging, Inattentional blindness, Natural scenes, Visual search, Working memory
INTRODUCTION
In his productive and influential career as a neuropsychologist, Dr Arthur L. Benton combined neurological, experimental, and clinical psychometric approaches to investigate brain–behavior relationships. One particular area of interest for Dr Benton was the study of spatial and nonspatial visual perceptual abilities.
In his paper on neuropsychological assessment (Benton, 1994), Benton states that the primary purpose of neuropsychological assessment is to gather data that enable one to make inferences about the structure and functional characteristics of an individual’s brain. By eliciting behavior in structured stimulus–response situations, particularly standardized tests, we can compare neuropsychological function within and between individuals with diverse neurological and psychological conditions.
The objectivity and standardization of neuropsychological tests have contributed greatly to our understanding of typical cognitive function, cognitive decline, and the intricacies of neurological disorders. Through experimental rigor, sound methodology, and oftentimes creative statistical analysis (e.g., see The Fiction of the “Gerstmann syndrome,” Benton, 1961), Benton played a significant role in laying the foundation of neuropsychological assessment and research.
In fact, one of his major accomplishments was the development of widely used, standardized neuropsychological assessment tools, several of which bear his name. And while it appears that “no cognitive domain was left unturned,” one primary area of emphasis was visuo-spatial perception and recognition, including the development of the Benton Visual Retention Test (BVRT) and the Judgment of Line Orientation (JLO) test. The BVRT is a measure of visuospatial perception and memory and requires subjects to reproduce geometric designs after viewing them briefly. The JLO removes the memory component and is primarily a measure of visuospatial perception. Both measures are representative of skills that contribute to our ability to successfully navigate through the world.
Dr Benton recognized the particular difficulties faced when attempting to infer cerebral integrity from behavioral observations in old age and dementia. He noted that dementia may have diverse interactive effects with aging and that these effects are difficult to distinguish from the variable effects of “normal aging” (Benton & Sivan, 1984). In a study assessing spatial and nonspatial visuo-perceptual abilities in “normal aging” and dementia, Eslinger and Benton (1983) found moderate decline in judgment of line orientation and facial discrimination in normal older adults and evidence of severe declines in these abilities in demented participants. They concluded that assessment of these abilities may be useful in the detection of abnormal cognitive decline.
In addition to the BVRT, JLO, and several other neuropsychological measures, the current study utilizes computer technology and complex real-world scenes in order to build upon Benton’s work by evaluating visuo-spatial perception in older adults with and without dementia.
Background
To interact effectively with the visible world, it is necessary to not only be able to store visual perceptual information in memory, but also to be able to perceive both stability and changes across views of a scene, such as those that occur when eye movements are made. Because people with normal vision typically can rely on the ability to rapidly and accurately detect environmental changes, they are able to accomplish feats such as keeping track of a person in a crowd, crossing the street in traffic, or driving safely in traffic even at highway speeds. Despite normal vision, we sometimes fail to notice even very conspicuous changes. In one striking example, normal younger observers failed to notice a gorilla walking though a small group of people playing catch (Simons & Chabris, 1999). This failure to notice some changes has been called “change blindness” (CB) and is thought to be caused by limited attentional capacity and memory failures. Not all objects can be attended to and remembered in a complex scene, often allowing a changing object to go unnoticed. This study investigates whether CB increases because of aging and cognitive decline in early Alzheimer’s disease (AD).
To notice change, we must focus attention on an image and store relevant information in visual short-term memory (VSTM) for comparison against image updates (Rensink, O’Regan, & Clark, 1997; Simons, 2000). Failure to attend to or remember the objects in a scene may prevent a comparison between current and recent images, thus producing CB. Neurodegenerative processes due to aging, mild cognitive impairment (MCI), and AD can impair visual attention and control of VSTM (Rizzo, Akutsu, & Dawson, 2001; Rizzo, Anderson, Dawson, & Nawrot, 2000a, 2000b; Ball, Vance, Edwards, & Wadley, 2004; Jackson & Owsley, 2003; Vecera & Rizzo, 2004). In aging, MCI, and AD, neuroanatomical substrates for these cognitive impairments may be neuronal loss within visual cortex (Wang, Zhou, Ma, & Leventhal, 2005) and other brain regions. Findings using functional magnetic resonance imaging (fMRI) suggest that frontal and parietal areas, as well as the pulvinar and cerebellum, deploy attention to loci of a change in visual scenes for further processing of stimuli (Pessoa & Ungerleider, 2004). Single neurons in human medial temporal lobe are also active during change detection (Reddy, Quiroga, Wilken, Koch, & Fried, 2006).
These impairments could increase the susceptibility to CB and, in turn, reduce the ability to respond effectively in visually dynamic, real-world tasks such as automobile driving (Caird, Edwards, Creaser, & Horrey, 2005; Hoffman, McDowd, Atchley, & Dubinsky, 2005). To address this issue, we tested the hypothesis that individuals with advancing age and AD are less able to detect change in traffic-related visual scenes.
METHOD
Participants
A total of 81 individuals participated in this study of vision, cognition, and CB. This included 13 participants with early AD (mean age 74.8 years, SD 6.4, range 63–84) and 68 cognitively healthy individuals, including 26 older comparison participants (mean age 72.9 years, SD 5.1, range 63–84), 11 participants ages 50–59 years (mean age 55.6 years, SD 3.2), 9 participants ages 40–49 years (mean age 44.7 years, SD 3.1), 9 participants ages 30–39 years (mean age 35.8 years, SD 2.8), and 13 participants ages 20–29 years (mean age 23.7 years, SD 2.3).
Participants with AD were recruited from a registry in the University of Iowa Department of Neurology. Their diagnosis of probable AD relied on the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria (McKhann et al., 1984). All had symptoms of memory impairment and related cognitive complaints that negatively affected activities of daily living, but they were all still living at home and able to attend to personal needs (e.g., eating and dressing). Their scores on the Mini-Mental State Examination (MMSE; 27.15, SD 2.58) and performance on a battery of standardized neuropsychological tests (Table 1) reflected early cognitive decline (Salmon et al., 2002). Computed tomography and magnetic resonance imaging scans excluded destructive brain lesions due to cerebrovascular and neoplastic disease. No participant had visual field loss based on confrontation perimetry and visual field sensitivity assessed by Humphrey Frequency Doubling Technology perimetry (Anderson & Johnson, 2002). Exclusion criteria included acute medical illness, alcoholism and other forms of drug abuse, stroke, and depression. Informed consent was obtained in accord with institutional and federal guidelines for human participant safety and confidentiality.
TABLE 1.
Participant demographics
| Alzheimer’s group | Older normal group | p-value | |
|---|---|---|---|
| Age, years | 74.9 (6.4) | 72.9 (5.1) | .2037 |
| Years of education | 15.0 (3.0) | 15.2 (2.29) | .7982 |
| Near visual acuity (LogMAR) | 0.09 (0.12) | 0.04 (0.08) | .1231 |
| Far visual acuity (LogMAR) | 0.006 (0.11) | 0.018 (0.13) | .8603 |
| Contrast sensitivity (Pelli–Robson) | 1.67 (0.15) | 1.72 (0.16) | .0906 |
| JLO | 25.0 (4.05) | 25.1 (4.38) | .9467 |
| CFT-COPY | 29.5 (6.29) | 30.7 (4.40) | .6756 |
| CFT-RECALL | 9.77 (4.88) | 16.4 (5.49) | .0018 |
| BLOCKS | 28.4 (12.6) | 37.4 (12.1) | .1189 |
| BVRT Errors | 8.77 (4.27) | 4.91 (2.56) | .0039 |
| AVLT 30-min recall | 3.08 (2.87) | 9.46 (3.94) | .0002 |
| COWA | 30.5 (7.20) | 38.4 (10.7) | .0360 |
| COGSTAT | 301.7 (60.0) | 391.3 (49.6) | .0003 |
| UFOVTOT | 1,088.7 (317.9) | 654.1 (154.0) | .0001 |
| TMT (B–A) | 110.4 (67.9) | 42.9 (35.6) | .0004 |
Note. Standard deviations in parentheses.
LogMAR = logarithm of the minimum angle of resolution. JLO = Judgment of Line Orientation test. CFT-COPY = Rey–Osterrieth Complex Figure Test Copy version. CFT-RECALL = Rey–Osterrieth Complex Figure Test Recall version. BLOCKS = Block Design subtest from the Wechsler Adult Intelligence Scale–Revised. BVRT = Benton Visual Retention Test. AVLT = Rey Auditory Verbal Learning Test. COWA = Controlled Oral Word Association. COGSTAT = composite measure of cognitive impairment. UFOV-TOT = sum of Subtests 1–4 of the Useful Field of View task. TMT (B–A) = Trail Making Test Subtest B minus Subtest A.
Cognitive and visual tests
In addition to the CB task, all AD and older normal participants were tested on a battery of cognitive and visual tasks (Table 1). Judgment of Line Orientation (JLO) assessed visuospatial perception. Visuoconstructional ability was measured using the Rey–Osterrieth Complex Figure Test Copy version (CFT-COPY) and Block Design subtest (BLOCKS) from the Wechsler Adult Intelligence Scale–Revised (WAIS–R). The CFT-RECALL version and the Benton Visual Retention Test (BVRT) tested visual memory. The Rey Auditory Verbal Learning Test (AVLT) provided an index of anterograde verbal memory. The Trail Making Test (TMT) Subtest B minus Subtest A, TMT (B–A), provided an index of set-shifting (an executive function) independent of motor speed. Controlled Oral Word Association (COWA) is also a measure of executive functions (maintaining task). These tasks are described in detail elsewhere (Lezak, 1995). We calculated a composite measure of cognitive impairment (COGSTAT) by summing T scores (mean = 50, SD = 10) derived from raw (uncorrected) data from each of the eight tests from the neuropsychological assessment battery, as in our previous work (Rizzo, McGehee, Dawson, & Anderson, 2001).
The Useful Field of View (UFOV) task (Visual Attention Analyzer Model 3000, Visual Awareness Inc.), a predictor for crashes in drivers with age-related cognitive decline (Owsley, Ball, Sloane, Roenker, & Bruni, 1991; Rizzo et al., 2001), depends on speed of visual processing, divided attention, and selective attention. We used the sum of Subtests 1–4 (in ms) of the UFOV task (UFOV-TOT) in our analyses. Contrast sensitivity was assessed using a standard technique (Pelli, Robson, & Wilkins, 1988). The best corrected visual acuity was measured using the ETDRS chart (Ferris, Kassoff, Bresnick, & Bailey, 1982) and was expressed as a LogMAR (logarithm of the minimum angle of resolution) score, with zero equivalent to 20/20 vision.
Apparatus
A personal computer controlled the presentation and timing of the CB experiment. Stimulus images were displayed on a 21-inch (50-cm) color monitor positioned approximately 2 feet (60 cm) from the participant. The experiment was conducted in a dimly lit room to reduce the reflection from the removable touch-screen overlying the monitor.
Stimuli
The CB task used 21 pairs of color images (Figure 1). Each pair consisted of an original image and a modified original (i.e., an image with a well-defined change to a single object). The images were created from photographs taken from inside a motor vehicle and depicted various driving circumstances. All photographs were resized to 800 × 600 pixels. The modifications made to the original image did not introduce any anomalies—that is, both images were physically plausible, appeared natural, and did not violate logical traffic patterns.
Figure 1.
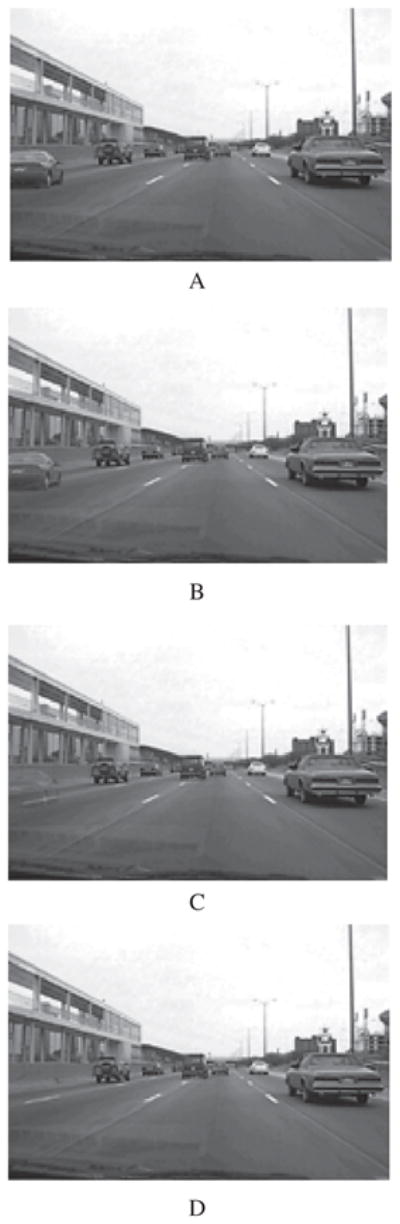
Example of one complete image change. In this case, a vehicle appears and disappears in the leftmost lane. To view a color version of this figure, please see the online issue of the Journal.
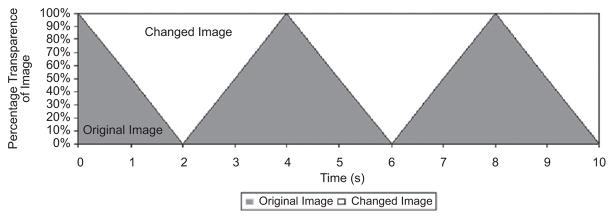
The 21 pairs of images (original plus modified original) were presented with one discrete changing element. The original image progressively faded into the modified image, so that the participant was continuously viewing the same global scene, except for the changing “feature” that was gradually blending in and out (Simons, Franconeri, & Reimer, 2000). This blending, or melting, appeared continuous and was unobtrusive to the viewer (i.e., there was no flashing element). Each complete change in the feature (i.e., one appearance or disappearance of the feature) required 2 s. A maximum time limit for each scene was set at 10 s, which allowed time for a maximum of five complete cycles of the change to occur (Figure 2).
Figure 2.
Cycle of image pairs in change trial.
The 21 original (unmodified) images were also used in catch trials where no change occurred in the presentation. These trials were included in order to investigate the occurrence of false positive (FP) responses or random guessing. The static images were also presented for a maximum duration of 10 s.
Procedure
Practice and training
Participants were not allowed to begin the experiment until they successfully completed practice and training sessions. A first session ensured that participants could accurately touch 1-inch diameter white dots against a black background. Dots were presented one at a time, in random order, in the center of the monitor and in each of its four corners. A second session presented five CB task images on the same monitor: Three trials presented images that changed (“change trials”), and two trials contained no change (“catch trials”). Changing trials in the practice session were selected based on high salience. Speed and duration of the training trials were the same as those in the subsequent CB experiment.
CB task
The experimental session was self-paced, and participants initiated each trial by touching a clearly demarcated box in the center of the screen. Each participant viewed 42 randomly ordered trials; half were change trials, and half were catch trials. The change trials and catch trials were interspersed in a random sequence that was unpredictable to the participants. Participants were instructed that one aspect of an image might appear, disappear, change location, or change color. They were also told that an image might not change, but were not informed of the frequencies of the two alternatives.
Participants were instructed to touch the location on the monitor where they perceived a change in each trial. A standard script read aloud to the participant instructed: “Your task is to touch the area of change as quickly and accurately as possible. If you do not see a change, press the space bar to indicate that nothing in the image changed.”
Any touch within the perimeter of a rectangle just large enough to encompass the changed element was scored as correct. Touches outside the region of the change were scored as incorrect. If no change occurred, participants were instructed to indicate this by pressing the spacebar of the computer keyboard instead of touching the screen. Trials with extremely fast response times (less than 250 ms) were excluded, to eliminate spurious trials in which participants unintentionally touched the screen twice while starting a new trial.
Dependent measures in the CB experiment were (a) hit rate (HR), the percentage of true positive responses on change trials; (b) false positive (FP) rate, the percentage of catch trials in which a participant reported a change; (c) response time (RT) in ms for change trials; and (d) d′, the true sensitivity, computed from the difference between the z-scores for percentage of hits (maximum score 99%) and the z-score for percentage of false positives (minimum score 1%).
RESULTS
Effects of age
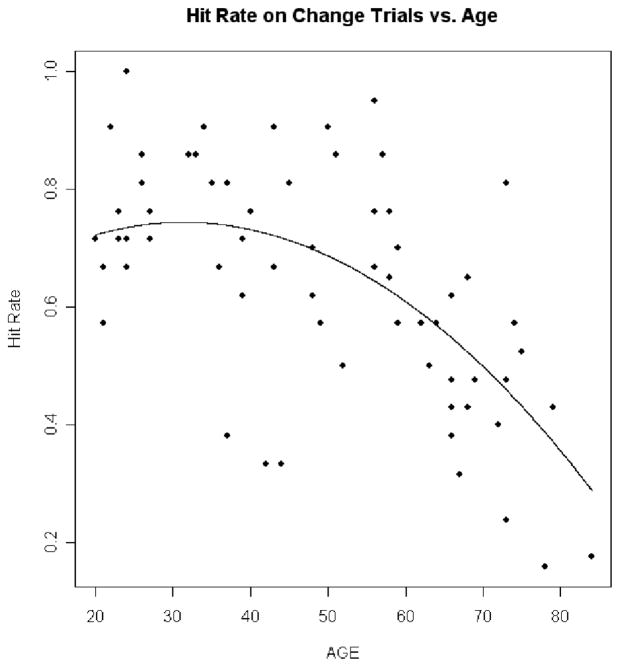
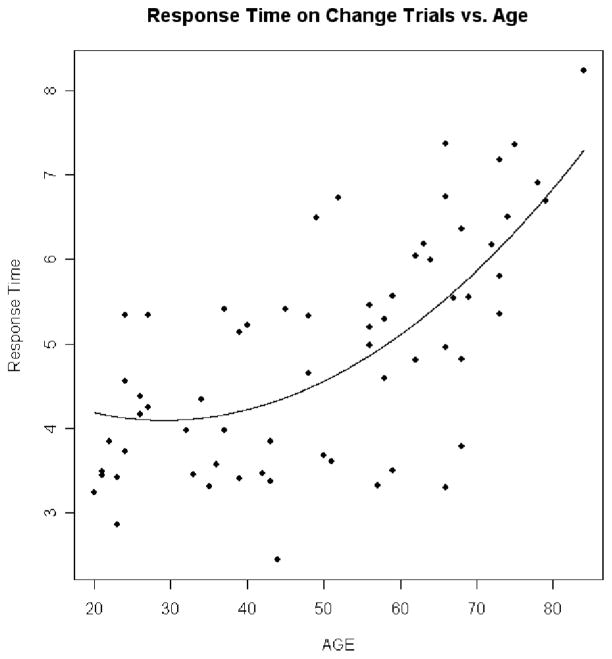
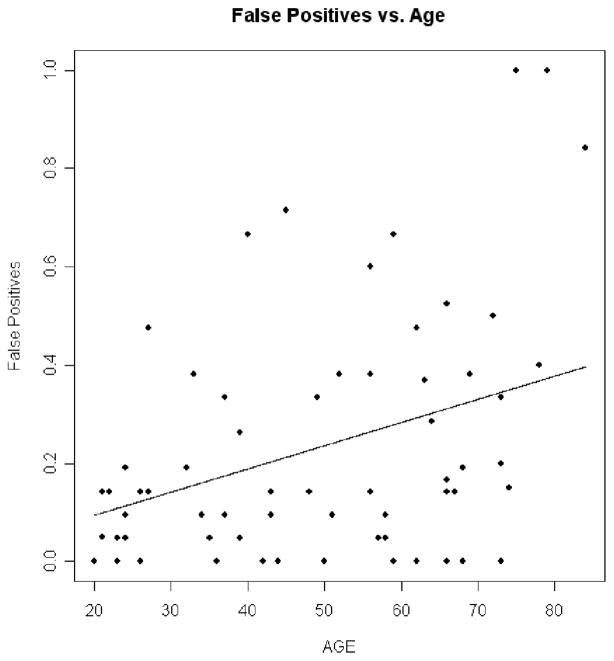
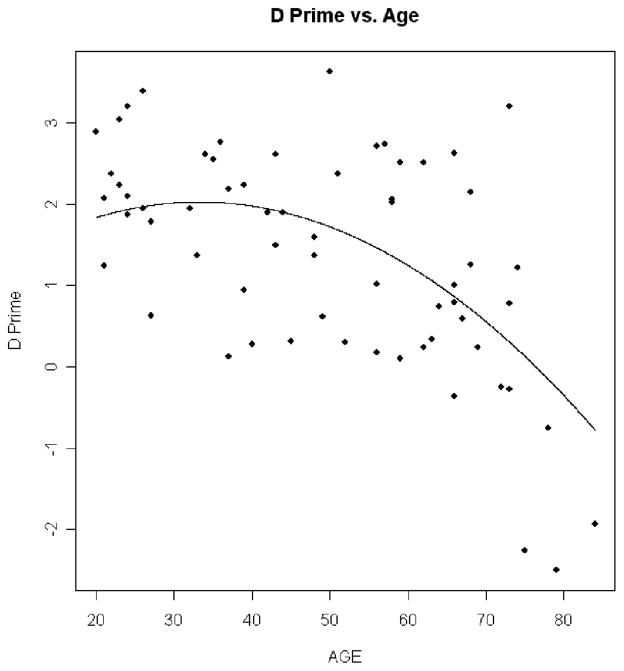
Linear regression analyses in the 68 participants in the cognitively healthy control group addressed the effects of age on the dependent measures in the CB experiment, with adjustments for contrast sensitivity, near visual acuity, and far visual acuity. Results in the CB experiment showed that as age increased hit rate decreased (p = .006 for quadratic trend; Figure 3), RT increased (p < .0001 for linear trend; Figure 4), false positives did not significantly increase (p = .351 for linear trend; Figure 5), and d′ decreased (p = .028 for quadratic trend; Figure 6).
Figure 3.
Hit rate on change trials versus age. This plot shows how hit rate decreases with age. The dots are the individual hit rate percentages for the normal controls plotted against age. The line is the prediction line from the regression equation.
Figure 4.
Response time on change trials versus age. This plot shows how response time increases with age. The dots are the individual response times for the normal controls plotted against age. The line is the prediction line from the regression equation.
Figure 5.
False positives (FP) versus age. The dots are the individual false positive percentages for the normal controls plotted against age. The line is the prediction line from the regression equation. The linear trend depicted was not significant.
Figure 6.
d′ versus age. This plot shows how d′ decreases with age. The dots are the individual d′ values for the normal controls plotted against age. The line is the prediction line from the regression equation.
Age-related threshold effects
Inspection of Figures 3, 4, and 6 suggests that CB begins to accelerate beyond a certain age. Linear regression models were fit to statistically assess this age threshold. The cognitively healthy control participants were divided into two groups using different ages as cutoff values, and slope estimate was calculated within each group. The maximum value of the difference of slope estimates between the two groups indicated where the age threshold occurs. To ensure that each group had a sufficient number of participants for valid slope estimates, the group sizes were constrained so that each group had at least 30% of the total number of control participants. These procedures showed that the age thresholds at which CB accelerated were 68 years old for RT, 54 years old for HR, and 68 years old for d′. In comparison, the threshold at which total UFOV score decline accelerated was 64 years old.
Results in aging and AD
Table 2 shows the HR, RT, FP rate, and d′ scores in the CB experiment in the older control group and in the AD group. Linear regression models were fit to estimate the differences between the AD and normal control groups on the dependent measures. For each dependent measure, a model was fit without adjusting for possible confounding variables, and models were also fit that adjusted for age and contrast sensitivity individually. Significant differences in RT persisted after adjusting for age and contrast sensitivity. The marginally worse performances in the other dependent measures were not significant.
TABLE 2.
Summary statistics and group differences between AD and older controls for dependent measures
| AD
|
Older controls
|
Unadjusted
|
Age-adjusted
|
CS-adjusted
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mdn | Mean | SD | Mdn | Diff. | SE | p | Diff. | SE | p | Diff. | SE | p | |
| Response time on change trials (in s) | 7.42 | 1.03 | 7.88 | 5.72 | 1.58 | 6.08 | 1.70 | 0.49 | .0012 | 1.54 | 0.47 | .0024 | 1.53 | 0.46 | .0021 |
| Hit rate of true positives (%) | 33.5 | 21.5 | 25.0 | 41.2 | 16.4 | 42.9 | −7.72 | 6.18 | .2194 | −4.36 | 5.39 | .4237 | −6.96 | 5.9 | .2485 |
| False positives (%) | 11.8 | 22.6 | 4.8 | 4.50 | 6.60 | 0.0 | 7.28 | 4.73 | .1324 | 6.17 | 4.74 | .2009 | 6.66 | 4.9 | .1849 |
| d′ | 1.14 | 1.31 | 1.61 | 1.64 | 0.81 | 1.62 | −0.49 | 0.34 | .1543 | −0.33 | 0.31 | .2938 | −0.43 | 0.35 | .2188 |
Note. AD = Alzheimer’s disease. CS = contrast sensitivity. p-values and the estimates of group differences and standard errors are computed using linear regression.
Speed–accuracy (s–a) tradeoffs
Speed–accuracy (s–a) tradeoffs occur when participants perform a task more quickly at the expense of accuracy (Pachella, 1974). This may affect the interpretation of between-group differences in accuracy in CB tasks (Mitroff, Simons, & Levin, 2004; Schofield, Bishop, & Allen, 2006). To assess s–a tradeoffs in this study, we plotted RT and HR data for each participant and calculated Spearman correlation coefficients between RT and HR in each group of participants. A positive correlation (i.e., an increase in HR as RT increases) would provide evidence for an s–a tradeoff. However, HR correlated negatively with RT in AD (rs = −.76, p = .002) and in all controls (rs = −.40, p < .001). Furthermore, there was no significant correlation in the older comparison group (rs = −.03, p = .889). These findings do not support s–a tradeoff as an explanation for the slowed detection of change (increased CB) in the AD group. It could be suggested that a positive correlation between HR and RT indicates inefficient search strategies; however, this seems implausible given that this relationship held even in young controls.
Relationship between CB and cognitive decline in aging and AD
Across both the AD and older comparison groups (n = 39), d′ (true sensitivity to change) on the CB task correlated with the total score of the UFOV task (Spearman r = −.50, p = .001). In other words, greater UFOV loss (higher scores), which reflects decreased processing speed and attention, is associated with greater CB, the inability to perceive change. The level to which d′ correlated with the BVRT errors (rs = −.32, p = .059) suggests dependence of CB on short-term visual memory. There was a correlation of d′ with TMT (B–A) (rs = −.38, p = .018) and with COWA (rs = .32, p = .052), which indicates that CB increases with decline in executive functions, including the ability to track competing streams of information. d′ also correlated significantly with JLO (rs = .39, p = .018). d′ increased positively with COGSTAT (rs = .41, p = .020), suggesting that failure to detect change on the CB task increases with overall cognitive decline. RT on the CB task showed similar relationships to cognitive measures including UFOVTOT (rs = .55, p < .001), TMT (B–A) (rs = .44, p = .006), and COGSTAT (rs = −.32, p = .072), as d′ did.
Stronger correlations within AD alone helped drive correlations across the combined groups. Within AD these included correlation of d′ with BLOCKS (rs = .66, p = .019), JLO (rs = .73, p = .011), BVRT errors (rs = −.67, p = .018), UFOV-TOT (rs = −.75, p = .004), and COGSTAT (rs = .81, p = .003) and a marginally significant correlation between RT and CFT-COPY (rs = −.54, p = .068). Within the older control group, d′ showed trends toward significant correlations with cognitive tests including COWA (rs = .36, p = .070), TMT (B–A) (rs = −.38, p = .054), and UFOV total (rs = −.34, p = .089), whereas RT showed a trend toward a significant association with CFT-COPY (rs = .38, p = .072).
DISCUSSION
The results of this study show that cognitively healthy normal older individuals as well as individuals with early AD show diminished ability to respond to visual change while performing a change detection task. This decrement in performance is more evident in early AD, suggesting that the natural aging process decreases an individual’s ability to perceive visual change, and that transition to early AD diminishes this even further. FP rates did not increase with age and were similar in the AD and older comparison group. This indicates that they had no greater bias to respond (“I saw a change”) in catch trials in which no elements changed (noise in the absence of signal; Macmillan & Creelman, 1991; Swets & Pickett, 1982). Across both the cognitively healthy and AD groups, CB correlated with poor performance on cognitive tests of attention, short-term visual memory, and executive function.
Analyses of age-related threshold effects showed that increases in response times for change detection progressively decline across the life span. The progressive slowing precedes accelerated decline in change detection accuracy during the seventh decade. This pattern suggests that aging individuals normally develop alternative or compensatory strategies for visual search or control of attention that allow relatively good detection until the compensation mechanisms also fail about two decades later and no longer allow accurate detection of change.
Perceptual mechanisms
Aging produces a variety of visual processing impairments (see review by Jackson & Owsley, 2003), and increased CB with aging is likely to reflect a decline in both static processes and dynamic (temporal) processes concerned with movement and change. Increased CB in aging and AD can be due to decreased signal strength (from reduced sampling of visual images because of neuronal loss in the visual system) or to decreased ability to discriminate between signals plus noise and noise alone (arising from sources in the environment and degenerating nervous system).
Regarding temporal processes needed to detect change, aging can impair processing of low-contrast moving contours (Sekuler, Hutman, & Owsley, 1980), optical flow (Atchley & Andersen, 1998), heading (Warren, Blackwell, & Morris, 1998), coherent motion amid background noise, and speed (Snowden & Kavanagh, 2006). Although this study cannot identify the loci of reduced temporal processing that impede change detection, growing evidence suggests that these declines reflect degraded information handling in cortical areas. Old primates show delayed intracortical and intercortical transfer of information throughout visual area V2 and parts of visual area V1 (Wang et al., 2005). These temporal impairments coincide with degraded intracortical inhibition reduction of gamma aminobutyric acid (GABA) that may be reversible with pharmacologic interventions (Leventhal, Wang, Pu, Zhou, & Ma, 2003). Changes in GABA also affect frontal lobe areas associated with visual working memory (Sawaguchi & Iba, 2001).
One explanation for greater difficulties with change detection in aging and AD is that these participants may tend to respond faster at the expense of decreased accuracy, engaging in a “speed–accuracy trade-off” (Pachella, 1974). However, this explanation is unwarranted in this study because the participants who took less time to respond appeared to find the CB task easier and performed more accurately. Furthermore, the results of this study indicate that slowed change detection in AD cannot be explained solely in terms of aging, reduced visual acuity, or contrast sensitivity. Participants with AD took significantly longer to detect change than comparison participants, even after adjusting for vision and age.
Cognition and change blindness (CB)
Increases in CB on the change detection task in aging and AD were related to decreases in performance on cognitive tests that depend on attention, memory, and executive functions. These findings fit with current theories, in which executive control over attention permits consolidation of information temporarily stored in visual working memory (Chun & Jiang, 2003; Dosher & Sperling, 1998; Irwin & Andrews, 1996; Luck & Vecera, 2002; Luck & Vogel, 1997).
Without focused attention, we may be unaware of changes to objects or scenes made during a saccade, flicker, blink, movie cut (O’Regan, Rensink, & Clark, 1999; Rensink et al., 1997; Rensink, O’Regan, & Clark, 2000; Simons & Levin, 1997), or even a gradual change (David, Laloyaux, Devue, & Cleeremans, 2006; Simons et al., 2000). Items stored in VSTM may fade if focused attention is withdrawn from them (“inattentional amnesia”). Because VSTM capacity is limited, interruptions of processing may result if an item is being stored in VSTM when another item arrives, leading to perceptual errors such as the “attentional blink” (Chun & Potter, 1995). Individuals with brain lesions and neurological conditions affecting neural systems for working memory and attention are particularly likely to err in detecting visual changes (Rizzo et al., 2001). They are liable to be “looking but not seeing” despite low information load (Rizzo, Anderson, Dawson, & Nawrot, 2000a, 2000b; Rizzo & Kellison, 2004; Rizzo, McGehee, Cumming, Dawson, & Laird, 1997); such errors resemble those made by air traffic controllers during extended viewing of radar images.
Both aging and AD have been shown to reduce visual attention and VSTM capacity (Kellison, Rizzo, & Vecera, 2004; Vecera & Rizzo, 2004). Visual-attention impairments hinder an individual’s ability to search the entire visual field, reducing the features of the visual array that can be perceived and encoded into visual working memory (Kellison, Rizzo, Vecera, & Dawson, 2003). Should a location or feature in the visual field be attended and encoded into memory, a working-memory impairment increases the likelihood that this stored image will decay before it can be compared to a new image in order to detect change.
Optimal detection of change depends on tracking and controlling cognitive resources that may decline in aging and AD. This includes executive systems that control the focus of attention and depends on a network of structures that include prefrontal regions (Cabeza et al., 2003; Nyberg et al., 2003). Shallice (1982) and Shallice and Burgess (1996) theorized that frontal executive areas play a role in a supervisory attention system for contention scheduling that coordinates the sharing of processes or resources used by several cognitive operations. Along similar lines, Baddeley (1986) invoked “central executive” and working-memory “slave” systems for verbal working memory and for visuospatial memory. The central executive system controls these slave systems and coordinates their concurrent operation when a person must track simultaneous streams of information, as during automobile driving.
Real-world considerations and new directions
Part of the motivation for using pictures of natural scenes in CB experiments is that much of human behavior is situational in context and depends on the level of naturalistic detail presented. The idea that cognition should be studied in relation to natural information is embodied by Gibson’s ecological approach to visual perception (Gibson, 1979), which anticipated the concept of situated-when-embodied cognition expressed by Clark (1997) and by Hutchins (1995).
Like other studies on the effects of incremental change on perception of natural scenes, such as a living room (Hollingworth & Henderson, 2004), this study tested CB using a task that used gradually changing images of traffic-related scenes. Aging affects the ability to perform simultaneous visual tasks, such as obeying traffic signals and signs while driving behind a vehicle that is intermittently braking (McDowd & Shaw, 2000). Because the visual array of a driver constantly changes, a potentially hazardous situation can evolve slowly, catch a driver unaware, and climax seconds later in a crash. A driver who is susceptible to CB in the real world may fail to (a) maintain situation awareness, (b) interpret feedback when vehicle actions fail to match driver expectations, and (c) take decisive corrective actions before it is too late. Failure to detect such changes increases when information load is high, as at road intersections with high traffic and visual clutter (Batchelder, Rizzo, Vanderleest, & Vecera, 2003; Caird, Edwards, & Creaser, 2001). Driver errors occur when attention is focused away from a critical roadway event in which vehicles, traffic signals, and signs are seen but not acted upon, or are missed altogether (Treat, 1980). Sometimes eye gaze is captured by irrelevant distractors (Kramer, Cassavaugh, Irwin, Peterson, & Hahn, 2001; Theeuwes, 1991), such as “mudsplashes” on a windshield, that disrupt a driver from seeing a critical object or event (O’Regan et al., 1999) like an incurring vehicle, a child chasing a ball, or an emergency vehicle parked by the roadside (Rizzo et al., 2005).
Participants with mild AD and older controls who showed reduced change detection also showed greater levels of impairment on the UFOV task, which has provided sensitive and specific predictions of crash risk in longitudinal studies of cognitively impaired older drivers (Ball, Owsley, Sloane, Roenker, & Bruni, 1993; Edwards et al., 2005; Owsley et al., 1991). Similar research could address whether measurements of CB predict crash records of older drivers with mental decline due to aging, AD, or forms of “mild cognitive impairment” (MCI) that may precede dementia (Grundman, Petersen, & Ferris, 2004; Petersen, 2004).
CONCLUSION
With a career spanning nearly seven decades, Dr Benton witnessed and cultivated many advancements in clinical neuropsychology. While early, important objectives of the field included lesion localization, Benton, along with others (e.g., Karl Lashley), appreciated the importance of interlobular and interhemispheric contributions to normal cognitive function. In other words, a focal lesion in the posterior right hemisphere can result in visuo-spatial deficits, but other combinations of neurological changes or abnormalities can also result in the same visuo-spatial deficits. This paper underscores the idea that global changes, whether age related or the pathological process of AD, can affect a relatively “localized” domain of cognitive function.
Future research can further evaluate specific display characteristics (e.g., color, shape, size, content, context, location) that render some scene changes more conspicuous than others to older observers and determine how change detection depends on total information content and interference among display components. Also, must observers experience and identify change before they localize it? Can they detect changes that they fail to consciously perceive (Fernandez-Duque, Grossi, Thornton, & Neville, 2003; a form of knowledge without awareness that relies on preattentive mechanisms) or are attention-driven conscious comparisons needed (Mitroff, Simons, & Franconeri, 2002)? Do these automatic (preattentive) and controlled (effortful attentive) processes decline differentially with aging and cognitive impairment? How do long-term knowledge, experience, and training in particular tasks and settings facilitate change detection over shorter (tactical) time frames (Simons & Rensink, 2004)? How do these processes differ between cognitively healthy and impaired individuals, and how could patient awareness and performance be improved using alerting systems or “augmented cognition” displays (Durlach, 2004) that cue observers to loci of critical change? Dr Benton might like to know.
Acknowledgments
We thank Digital Artefacts, LLC and Joan Severson for their assistance in developing the experimental software and Art Kramer, University of Illinois at Urbana-Champaign, for the stimulus images. This work was supported by National Institute on Aging grants NIA AG 17177 and NIA AG 15071.
References
- Anderson AJ, Johnson CA. Mechanisms isolated by frequency-doubling technology perimetry. Investigative Ophthalmology & Visual Science. 2002;43:398–401. [PubMed] [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and Aging. 1998;13:297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Baddeley A, editor. Working memory. New York: Oxford University Press; 1986. [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology & Visual Science. 1993;34:3110–3123. [PubMed] [Google Scholar]
- Ball K, Vance D, Edwards J, Wadley VG. Aging and the brain. In: Rizzo M, Eslinger PJ, editors. Principles and practice of behavioral neurology and neuropsychology. Philadelphia: Saunders; 2004. pp. 795–809. [Google Scholar]
- Batchelder S, Rizzo M, Vanderleest R, Vecera S. Traffic scene related change blindness in older driver. Paper presented at the 2nd International Driving Symposium on Human Factors in Driver Assessment, Training and Vehicle Design; Park City, UT, USA. 2003. Jul, [Google Scholar]
- Benton AL. The fiction of the “Gerstmann syndrome. Journal of Neurology, Neurosurgery & Psychiatry. 1961;24:176–181. doi: 10.1136/jnnp.24.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL. Neuropsychological assessment. Annual Review of Psychology. 1994;45:1–23. doi: 10.1146/annurev.ps.45.020194.000245. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB. Problems and conceptual issues in neuropsychological research in aging and dementia. Journal of Clinical Neuropsychology. 1984;6:57–63. doi: 10.1080/01688638408401196. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: A cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Caird JK, Edwards CJ, Creaser J. The effect of time constraints on older and younger driver decisions to turn at intersections using a modified change blindness paradigm. Presented at the 1st International Driving Symposium on Human Factors in Driver Assessment, Training and Vehicle Design; Aspen, CO, USA. 2001. Aug, [Google Scholar]
- Caird JK, Edwards CJ, Creaser JI, Horrey WJ. Older driver failures of attention at intersections: Using change blindness methods to assess turn decision accuracy. Human Factors. 2005;47:235–249. doi: 10.1518/0018720054679542. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Implicit, long-term spatial contextual memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:224–234. doi: 10.1037/0278-7393.29.2.224. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Clark A. Being there: Putting brain, body, and the world together again. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- David E, Laloyaux C, Devue C, Cleeremans A. Change blindness to gradual changes in facial expressions. Psychologica Belgica. 2006;46:253–268. [Google Scholar]
- Dosher BA, Sperling G. A century of human information-processing theory: Vision, attention, and memory. In: Hochberg J, editor. Perception and cognition at century’s end: Handbook of perception and cognition. 2. San Diego, CA: Academic Press; 1998. pp. 199–252. [Google Scholar]
- Durlach PJ. Change blindness and its implications for complex monitoring and control systems design and operator training. Human-Computer Interaction. 2004;19:423–451. [Google Scholar]
- Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. Reliability and validity of Useful Field of View test scores as administered by personal computer. Journal of Clinical and Experimental Neuropsychology. 2005;27:529–543. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Benton AL. Visuoperceptual performances in aging and dementia: Clinical and theoretical implications. Journal of Clinical Neuropsychology. 1983;5:213–220. doi: 10.1080/01688638308401170. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Grossi G, Thornton IM, Neville HJ. Representation of change: Separate electrophysiological markers of attention, awareness, and implicit processing. Journal of Cognitive Neuroscience. 2003;15:491–507. doi: 10.1162/089892903321662895. [DOI] [PubMed] [Google Scholar]
- Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology. 1982;94:91–96. [PubMed] [Google Scholar]
- Gibson JJ. The ecological approach to visual perception. Boston, MA: Houghton Mifflin; 1979. [Google Scholar]
- Grundman M, Petersen RC, Ferris SH. Alzheimer’s disease cooperative study. Archives of Neurology. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Hoffman L, McDowd JM, Atchley P, Dubinsky R. The role of visual attention in predicting driving impairment in older adults. Psychology and Aging. 2005;20:610–622. doi: 10.1037/0882-7974.20.4.610. [DOI] [PubMed] [Google Scholar]
- Hollingworth A, Henderson JM. Sustained change blindness to incremental scene rotation: A dissociation between explicit change detection and visual memory. Perception & Psychophysics. 2004;66:800–807. doi: 10.3758/bf03194974. [DOI] [PubMed] [Google Scholar]
- Hutchins E. Cognition in the wild. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Irwin DE, Andrews RV. Integration and accumulation of information across saccadic eye movements. In: Inui T, McClelland JL, editors. Attention and performance XVI: Information integration in perception and communication. Cambridge, MA: MIT Press; 1996. pp. 125–155. [Google Scholar]
- Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurologic Clinics. 2003;21:709–728. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- Kellison IL, Rizzo M, Vecera SP. Visual short-term memory deficits in Alzheimer’s disease. Journal of Vision. 2004;4:770. [Google Scholar]
- Kellison I, Rizzo M, Vecera S, Dawson J. Blindness to context-relevant visual change in advancing age. Paper presented at the Society for Neurosciences; San Francisco, CA, USA. 2003. Nov, [Google Scholar]
- Kramer AF, Cassavaugh ND, Irwin DE, Peterson MS, Hahn S. Influence of single and multiple onset distractors on visual search for singleton targets. Perception & Psychophysics. 2001;63:952–968. doi: 10.3758/bf03194515. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- Luck SJ, Vecera SP. Attention: From tasks to mechanisms. In: Pashler HE, Yantis S, Stevens SS, editors. Stevens’ handbook of experimental psychology: Vol. 1. Sensation and perception. 3. New York: Wiley; 2002. pp. 235–286. [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. New York: Cambridge University Press; 1991. [Google Scholar]
- McDowd JM, Shaw RJ. Attention and aging: A functional perspective. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2000. pp. 221–292. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mitroff SR, Simons DJ, Franconeri SL. The siren song of implicit change detection. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:798–815. [PubMed] [Google Scholar]
- Mitroff SR, Simons DJ, Levin DT. Nothing compares two views: Change blindness can occur despite preserved access to the changed information. Perception & Psychophysics. 2004;66:1268–1281. doi: 10.3758/bf03194997. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, et al. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- O’Regan JK, Rensink RA, Clark JJ. Change-blindness as a result of “mudsplashes”. Nature. 1999;398:34. doi: 10.1038/17953. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, Sloane ME, Roenker DL, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychology and Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- Pachella RG. The interpretation of reaction time in information processing research. In: Kantowitz BH, editor. Human information processing: Tutorials in performance and cognition. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1974. pp. 41–81. [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science. 1988;2:187–199. [Google Scholar]
- Pessoa L, Ungerleider L. Neural correlates of change detection and change blindness in a working memory task. Cerebral Cortex. 2004;14:511–520. doi: 10.1093/cercor/bhh013. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Reddy L, Quiroga RQ, Wilken P, Koch C, Fried I. Single-neuron correlate of change detection and change blindness in the human medial temporal lobe. Current Biology. 2006;16:2066–2072. doi: 10.1016/j.cub.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Rensink RA, O’Regan JK, Clark JJ. To see or not to see: The need for attention to perceive changes in scenes. Psychological Sciences. 1997;8:368–373. [Google Scholar]
- Rensink RA, O’Regan JK, Clark JJ. On the failure to detect changes in scenes across brief interruptions. Visual Cognition. 2000;7:127–145. [Google Scholar]
- Rizzo M, Akutsu H, Dawson J. Increased attentional blink after focal cerebral lesions. Neurology. 2001;57:795–800. doi: 10.1212/wnl.57.5.795. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Anderson SW, Dawson J, Nawrot M. Vision and cognition in Alzheimer’s disease. Neuropsychologia. 2000a;38:1157–1169. doi: 10.1016/s0028-3932(00)00023-3. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Anderson SW, Dawson J, Nawrot M. Visual function and cognitive deficits with cerebral lesions. Investigative Ophthalmology & Visual Science. 2000b;41:S434. [Google Scholar]
- Rizzo M, Kellison IL. Eyes, brains, and autos. Archives of Ophthalmology. 2004;122:641–647. doi: 10.1001/archopht.122.4.641. [DOI] [PubMed] [Google Scholar]
- Rizzo M, McGehee D, Cumming T, Dawson J, Laird K. Simulated crashes at intersections in drivers with Alzheimer’s disease. Annals of Neurology. 1997;42:394. [Google Scholar]
- Rizzo M, McGehee DV, Dawson JD, Anderson SW. Simulated car crashes at intersections in drivers with Alzheimer disease. Alzheimer Disease and Associated Disorders. 2001;15:10–20. doi: 10.1097/00002093-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Shi Q, Dawson J, Anderson SW, Kellison I, Petras TA. Stops for cops: Impaired response implementation in older drivers with cognitive decline. Journal of the Transportation Research Board. 2005;1922:1–8. [Google Scholar]
- Salmon DP, Thomas RG, Pay MM, Booth CR, Hofstetter CR, Thal LJ, et al. Alzheimer’s disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59:1022–1028. doi: 10.1212/wnl.59.7.1022. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Iba M. Prefrontal cortical representation of visuospatial working memory in monkeys examined by local inactivation with muscimol. Journal of Neurophysiology. 2001;86:2041–2053. doi: 10.1152/jn.2001.86.4.2041. [DOI] [PubMed] [Google Scholar]
- Schofield AJ, Bishop NJ, Allen J. Oscillatory motion induces change blindness. Acta Psychologica. 2006;122:109. doi: 10.1016/j.actpsy.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Hutman LP, Owsley CJ. Human aging and spatial vision. Science. 1980;209:1255–1256. doi: 10.1126/science.7403884. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society B: Biological Sciences. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behavior. Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:1405–1412. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Current approaches to change blindness. Visual Cognition. 2000;7:1–15. [Google Scholar]
- Simons DJ, Chabris CF. Gorillas in our midst: Sustained inattentional blindness for dynamic events. Perception. 1999;28:1059–1074. doi: 10.1068/p281059. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Franconeri SL, Reimer RL. Change blindness in the absence of a visual disruption. Perception. 2000;29:1143–1154. doi: 10.1068/p3104. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Levin DT. Change blindness. Trends in Cognitive Sciences. 1997;1:261–267. doi: 10.1016/S1364-6613(97)01080-2. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Rensink RA. Change blindness: Past, present, and future. Trends in Cognitive Sciences. 2004;9:16–20. doi: 10.1016/j.tics.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: Minimum motion, motion coherence, and speed discrimination thresholds. Perception. 2006;35:9–24. doi: 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- Swets JA, Pickett RM. The evaluation of diagnostic systems: Methods from signal detection theory. New York: Academic Press; 1982. [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention: The effect of visual onsets and offsets. Perception & Psychophysics. 1991;49:83–90. doi: 10.3758/bf03211619. [DOI] [PubMed] [Google Scholar]
- Treat JR. A study of precrash factors involved in traffic accidents. HRSI Research Review. 1980;10:1–35. [Google Scholar]
- Vecera S, Rizzo M. Visual attention and visual short-term memory in Alzheimer’s disease. In: Cronin-Golomb A, Hof PR, editors. Vision in Alzheimer’s disease. New York: Karger Press; 2004. pp. 248–270. [Google Scholar]
- Wang Y, Zhou Y, Ma Y, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cerebral Cortex. 2005;15:403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Warren WH, Blackwell AW, Morris MW. Age differences in perceiving the direction of self-motion from optical flow. Journal of Gerontology. 1989;44:147–153. doi: 10.1093/geronj/44.5.p147. [DOI] [PubMed] [Google Scholar]