Abstract
The Drosophila Suppressor of Hairy wing [Su(Hw)] insulator protein has an essential role in the development of the female germline. Here we investigate the function of Su(Hw) in the ovary. We show that Su(Hw) is universally expressed in somatic cells, while germ cell expression is dynamic. Robust levels accumulate in post-mitotic germ cells, where Su(Hw) localization is limited to chromosomes within nurse cells, the specialized cells that support oocyte growth. Although loss of Su(Hw) causes global defects in nurse cell chromosome structure, we demonstrate that these architectural changes are not responsible for the block in oogenesis. Connections between the fertility and insulator functions of Su(Hw) were investigated through studies of the two gypsy insulator proteins, Modifier of (mdg4)67.2 (Mod67.2) and Centrosomal Protein of 190 kD (CP190). Accumulation of these proteins is distinct from Su(Hw), with Mod67.2 and CP190 showing uniform expression in all cells during early stages of oogenesis that diminishes in later stages. Although Mod67.2 and CP190 extensively co-localize with Su(Hw) on nurse cell chromosomes, neither protein is required for nurse cell chromosome development or oocyte production. These data indicate that while the gypsy insulator function requires both Mod67.2 and CP190, these proteins are not essential for oogenesis. These studies represent the first molecular investigations of Su(Hw) function in the germline, which uncover distinct requirements for Su(Hw) insulator and ovary functions.
Keywords: Su(Hw), insulator, Drosophila, oogenesis, CP190, Mod(mdg4)
Introduction
Insulators are a class of DNA regulatory elements that constrain the action of enhancers and silencers (Gurudatta and Corces, 2009; Raab and Kamakaka, 2010; Wallace and Felsenfeld, 2007). Two functional properties have been used to identify insulators (Kellum and Schedl, 1991, 1992; Udvardy et al., 1985). First, insulators block enhancer and silencer action in a position-dependent manner. Enhancer and silencer function is disrupted only when insulators are positioned between these cis-regulatory elements and a promoter, a property referred to as blocking activity. Second, insulators protect promoters from positive and negative chromosomal position effects associated with ectopic placement of genes within genomes, a property referred to as barrier activity. Elements with one or both properties have been identified in most eukaryotic genomes, suggesting that insulators are important for maintaining transcriptional integrity (Geyer and Clark, 2002; Kuhn and Geyer, 2003).
The Drosophila gypsy insulator possesses both blocking and barrier functions (Dorsett et al., 1989; Geyer and Corces, 1992; Roseman et al., 1995a; Roseman et al., 1993; Roseman et al., 1995b). This insulator contains twelve binding motifs for the twelve zinc finger protein Suppressor of Hairy-wing [Su(Hw)] (Parkhurst et al., 1988; Spana and Corces, 1990). Enhancer blocking of the gypsy insulator requires Su(Hw) recruitment of two Broad-complex, Tramtrack and Bric-a-brac/ Poxvirus and Zinc Finger (BTB/POZ) domain proteins, Modifier of (mdg4) 67.2 (Mod67.2) (Georgiev and Gerasimova, 1989) and Centrosomal Protein of 190 kD (CP190) (Pai et al., 2004), while the barrier function requires recruitment of Enhancer of y2 [E(y)2, the fly homologue of yeast Sus1, newly named ENY2] (Kopytova et al., 2010; Kurshakova et al., 2007). Using these protein, the gypsy insulator has a versatile capacity for defining regulatory interchanges throughout the Drosophila genome.
Existing su(Hw) alleles have been identified based on screens scoring for reversal of gypsy-induced mutant phenotypes (Harrison et al., 1993; Klug et al., 1968; Parkhurst et al., 1988). Analyses of these mutants demonstrated that loss of Su(Hw) also causes female sterility. Mutant ovaries are arrested in mid-oogenesis, with evidence that the developing oocyte undergoes apoptosis. Prior to oocyte loss, defects occur within the nuclei of nurse cells (NC), specialized cells that supply RNAs and proteins to the developing oocyte through interconnected bridges (King, 1970; Spradling, 1993). In su(Hw) mutant NC nuclei, NC chromosomes remain condensed, and morphological development of the nucleolus is disrupted. This has led to the hypothesis that loss of Su(Hw) causes defects in nucleolar function, resulting in insufficient oocyte growth and activation of the mid-oogenesis checkpoint (Klug et al., 1968; Klug et al., 1970).
We studied ovarian phenotypes in females carrying multiple su(Hw) mutant alleles to understand the role of Su(Hw) in oogenesis. Our analyses revealed that global changes in NC nuclear architecture are not responsible for the block in oogenesis. These data suggest that Su(Hw) dependent sterility may be due to local changes in chromatin structure that alter NC function. Additional immunohistochemical and genetic analyses addressed the connection between the Su(Hw) ovarian and insulator functions. In these studies, the developmental expression patterns of Su(Hw), CP190 and Mod67.2 were defined, as well as the ovary phenotypes associated with females carrying null or nearly null mutations of each gene. Our studies uncovered differences between Su(Hw) and the other gypsy insulator proteins, both in the pattern of localization during oogenesis and the mutant phenotypes caused by protein loss. Our data demonstrate that neither CP190 nor Mod67.2 is essential for oogenesis, suggesting that the functional requirements for Su(Hw) in the germline and at the gypsy insulator are different.
Materials and Methods
Drosophila stocks and culture conditions
Flies were raised at 25°C, 70% humidity on standard corn meal/agar medium. Multiple su(Hw) mutants were used in these studies (Figure 1A). These include 1) su(Hw)2 that is caused by insertion of a jockey element within the first intron (Harrison et al., 1993; Parkhurst et al., 1988), 2) su(Hw)Pb, called su(Hw)e04061 in Flybase, that is caused by an insertion of a white marked piggy-bac transposon at the 5’ end of the second exon, 3) su(Hw)E8 that is caused by a point mutation that disrupts formation of zinc finger 7 (Harrison et al., 1993; Parkhurst et al., 1988), 4) su(Hw)v that carries a deletion encompassing the promoters of the su(Hw) and the neighboring essential RpII15 gene (Harrison et al., 1992), 5) su(Hw)f that is caused by a point mutation that disrupts formation of zinc finger 10 (Harrison et al., 1993; Kuhn-Parnell et al., 2008). Western analysis shows that females carrying the su(Hw)2 and su(Hw)v alleles produce no Su(Hw) protein, while su(Hw)Pb produces a nearly undetectable level of protein (Figure 1B), estimated to be ~35 fold lower than wild type based on analyses of su(Hw) RNA levels. Further, females carrying the su(Hw)E8 and su(Hw)f alleles produce near wild type levels of full-length proteins (Figure 1B), consistent with analyses of su(Hw) RNA levels in these mutants. Sterility associated with su(Hw)v females was by transgenes containing RPII15 and the su(Hw) promoter fused to the su(Hw) cDNA (Kim et al., 1996), suggesting that sterility is due to loss of Su(Hw). The mod(mdg4) mutant studied was mod(mdg4)u1, which specifically causes loss of the 67.2 kD gypsy insulator isoform. This allele carries an insertion of a Stalker retrotransposon into an exon encoding the C-terminus of Mod67.2, resulting in a deletion of the last 145 amino acids (Gause et al., 2001; Georgiev and Gerasimova, 1989). The Cp190 mutants studied were 1) the Cp190H4-1allele that carries a premature stop codon that results in a carboxyl-terminal deletion of the last 331 amino acids (Pai et al., 2004), 2) Cp190P11 that carries a deletion spanning from the 5’ end of Set to the 3’ end of MRG15 that removes Cp190 and four other genes (17), and 3) the Cp1901 null allele that carries a premature stop codon that results in a carboxyl-terminal deletion of the last 964 amino acids (51). Polytene chromosome squashes from ovaries were completed using females carrying the ovarian tumor (otu) mutation (y w sn3 otu11) (Koryakov et al., 2004).
Figure 1. Molecular properties of su(Hw) alleles.
A) Diagram of the su(Hw) genomic region, including the su(Hw) and RpII15 genes. The su(Hw) coding region is mapped onto the gene structure and includes two acidic domains (AD), the Mod67.2 interaction domain (MID) and the locations of the twelve zinc-fingers (black boxes). The su(Hw)v allele carries a deletion of the su(Hw) and RpII15 promoters. The positions of the insertions in su(Hw)2 (jockey) and su(Hw)Pb(Piggy-bac) are shown by inverted triangles, the su(Hw)E8 and su(Hw)f alleles contain point mutations in coding regions for zinc finger 7 and zinc finger 10, respectively. B) Shown is a western blot of protein extracts obtained from wild type (Canton S, CS) and su(Hw) mutant ovaries probed with Su(Hw) and alpha-Tubulin antibodies, with the latter used as a loading control.
Western analyses
For western blot analysis of ovary extracts, ovaries were dissected from 10 adult females, extracts were separated on a 6% polyacrylamide gel, transferred to a nitrocellulose membrane, and incubated with primary antibody overnight at 4°C. Guinea pig anti-Su(Hw) antibody was used at 1:500. Blots were incubated with secondary antibody, HRP-conjugated donkey anti-guinea pig IgG (Jackson ImmunoResearch, 706-035-148) for 2 hr at room temperature and detected using Immun-Star HRP Chemiluminescent Kit (Bio-Rad). To control for amounts of protein loaded, blots were incubated with the mouse anti-alpha-tubulin IgG primary antibody (Sigma, T5168) and detected with the HRP-conjugated rabbit anti-mouse IgG secondary antibody (Sigma, A9044).
Immunohistochemical analyses
Ovaries were dissected from two to four day old females in phosphate buffered saline solution (PBS). Ovaries were fixed in 3% EM-grade paraformaldehyde and washed in PBT (PBS + 0.3% TritonX-100). Ovaries were teased apart, blocked overnight in PBT + 5% normal goat serum or 5% bovine serum albumin at 4°C, and incubated overnight with primary antibodies at 4°C. Next, samples were washed in PBT and incubated with secondary antibodies for two hours. Following this incubation, ovaries were washed in PBT and DAPI stained (0.1μg/ml in PBT). Following an additional PBT wash, ovaries were mounted in Vectashield H-1000 (Vector Laboratories). Whole mount salivary glands from third instar larvae were dissected in PBS and prepared using the staining protocol outlined above. Slides were examined using a Bio-Rad Multi-Photon or Zeiss 710 confocal microscope. Images were processed using ImageJ.
Multiple primary antibodies were used for immunohistochemical analyses of ovaries. Polyclonal guinea pig (Covance) and goat (Elmira Biologicals) anti-Su(Hw) antibodies were generated against a full-length C-terminal 6xHis tagged protein and purified using a Su(Hw) affinity column (Actigel, Sterogene) that was generated from the bacterially expressed protein. Guinea pig anti-Su(Hw) was used at 1:500, Goat anti-Su(Hw) was used at 1:200. The dilutions of other antibodies include 1) chicken anti-Mod67.2 at 1:2,000 (Parnell et al., 2006), 2) rabbit anti-CP190 (a generous gift from M. Moritz) at 1:1,000, 3) rabbit anti-Stand still (Stil; a generous gift from D. Pauli) at 1:4,000, 4) mouse anti-Spectrin (Iowa DSHB, 3A9) at 1:10, 5) mouse anti-Fibrillarin (Abcam, ab4566) at 1:500, 6) rabbit anti-caspase-3 (Asp 175-cleaved, Cell Signaling) at 1:100 dilution, 7) guinea pig anti-Traffic Jam (a generous gift from D. Godt) at 1:10,000, and 8) mouse anti-LaminDm0 at 1:500 (Iowa DSHB, ADL67.10). All Alexa Fluor secondary antibodies (Invitrogen, Molecular Probes) were used at a 1:1,000 dilution, including AF488 goat anti-guinea pig IgG (A11041), AF568 goat anti-guinea pig IgG (A11075), AF488 goat anti-chicken (A11039), AF568 goat anti-chicken (A11041), AF488 goat anti-rabbit (A11008), AF568 goat anti-rabbit (A11011), AF488 goat anti-mouse (A11001), AF568 goat anti-mouse (A11004), AF488 donkey anti-sheep (A11015), AF488 donkey anti-sheep (A21098), AF488 donkey anti-goat (A11055).
Ovarian polytene chromosomes were prepared by dissection of otu11 mutant ovaries in 0.7% NaCl/ 0.5% NP-40, fixed for 2 min in 0.7% NaCl/ 3.7% formaldehyde/ 1% NP-40 and squashed in 45% acetic acid/ 2% formaldehyde. Following squashing, slides were frozen in liquid nitrogen and cover slips removed. These preparations were kept in ice-cold PBS buffer. Chromosomes were incubated with antibodies (rabbit anti-Su(Hw) 1:1,000 in PBS/3% BSA (Parnell et al., 2006), guinea pig anti-Su(Hw) 1:250 in PBS/3% BSA, chicken anti-Mod67.2 1:2,000 in PBS/3% BSA and sheep anti-CP190 1:250 in PBS/3% BSA) at 4°C overnight, followed by incubation with secondary antibodies (goat anti-chicken, goat anti-guinea pig and donkey anti-sheep; all were Alexa Fluor 488 or 568; 1:1,000 in PBS) for 2 h at 37°C. Subsequently, all preparations were stained with DAPI and analyzed on a fluorescent microscope.
Analyses of rRNA processing
Total RNA was isolated from su(Hw) wild type and mutant ovaries and 5 μg were analyzed by northern blotting (Soshnev et al., 2008). Intermediates in rRNA processing were detected using radioactively labeled oligonucleotide probes, as described by Giordano et al. (Giordano et al., 1999) with the following modifications: probes II and III contain an additional 3’-TG to increase efficiency of labeling. Oligonucleotide probes were labeled using T4 Polynucleotide Kinase (NEB, M0201S).
Analyses of fertility phenotypes associated with loss of Su(Hw), Mod67.2 and CP190
Fertility phenotypes of su(Hw), mod(mdg4) and Cp190 mutants included the level of apoptosis observed, as well as the number and size of eggs produced. The level of apoptosis was determined in ovaries isolated from 2-3 day old females of each genotype that were DAPI stained. An ovariole was evaluated only if it contained egg chambers progressing at least to stage 8. The percent apoptosis was defined as the number of ovarioles with egg chamber degeneration divided by the total number of ovarioles scored. To analyze fecundity, 20 to 25 two to five day old females were mated to Canton S males and allowed to lay on orange juice plates with wet yeast for 2 hours. Egg numbers produced during this period were counted. To measure egg length, eggs were placed next to a ruler and a picture was taken. Eggs were measured lengthwise using ImageJ.
Chromatin immunoprecipitation (ChIP) and quantitative real time PCR analyses
Fifty pairs of ovaries were dissected from newly eclosed Drosophila females and incubated in PBS with 1.8% formaldehyde for 10 min at room temperature to cross-link chromatin. Samples were sonicated with the Fisher sonic dismembrator 100 flat microtip, using eight pulses of 30 seconds at 6W, leaving 90 seconds between pulses. Chromatin was sheared to average size of 200-300 bp as determined by agarose gel electrophoresis. Immunoprecipitation was performed with affinity-purified Su(Hw) and CP190 antibodies. ChIP was analyzed by quantitative real time PCR (QPCR) with SYBR green and primers amplifying SBSs, named by the cytological position in the chromosome. Primers are available upon request. These regions were selected based on the ModENCODE dataset (Negre et al., 2010). The average size of the amplified DNA samples was 100-200 bp, centered on the Su(Hw) consensus-binding site. Percent input was calculated by ChIP/input cycle threshold change ratios, using the formula %input = 2Ct(input)-Ct(IP) * 1/DF * 100, where DF is dilution factor between IP and input samples. All analyses were performed in three biological replicates. Average values and standard deviation were determined using Microsoft Excel. For QPCR analyses of five-blob gene expression, fifty ovary pairs were isolated from newly eclosed Drosophila females and processed as described (Soshnev et al., 2008), with the modification that expression of Rpl32 was used as an internal control. Primers are available upon request.
Results
The Su(Hw) insulator protein is required for oogenesis (Harrison et al., 1993; Klug et al., 1968; Parkhurst et al., 1988). The ovary is comprised of fifteen to twenty ovarioles that contain an assembly line of increasingly mature egg chambers (King, 1970; Spradling, 1993; Verheyen and Cooley, 1994). Egg production begins at the anterior tip of the ovariole in the germarium, which is divided into regions depending upon the developmental state of the germ cells (Figure 2A). Region 1 contains mitotically active cells. Here, the germline stem cells divide asymmetrically to give rise to daughter cystoblasts and stem cells. Cystoblasts undergo four mitotic divisions with incomplete cytokinesis to generate a sixteen-cell cyst. Differentiation of cells within the cyst produces one oocyte and fifteen nurse cells (NC). Region 2 contains cysts with oocytes undergoing meiosis. Region 3 contains the first egg chamber, which is formed by migration of a monolayer of somatic follicle cells around the interconnected oocyte and NCs. As egg chambers leave the germarium, NCs enter a variant cell cycle, called the endocycle, in which multiple rounds of DNA replication occur without intervening mitoses. In egg chambers undergoing early endocycles, the replicated homologues pair to form polytene chromosomes. During stages 4 to 6 of egg chamber development, NC chromosomes undergo striking structural changes (Figure 2B, Figure 3). At the end of stage 4, the polytene chromosomes condense to generate NC nuclei with five DAPI stained “blobs” that correspond to the five large euchromatic chromosome arms. Progression from stage 5 to stage 6 is accompanied by NC chromosome decondensation and dispersal (Dej and Spradling, 1999).
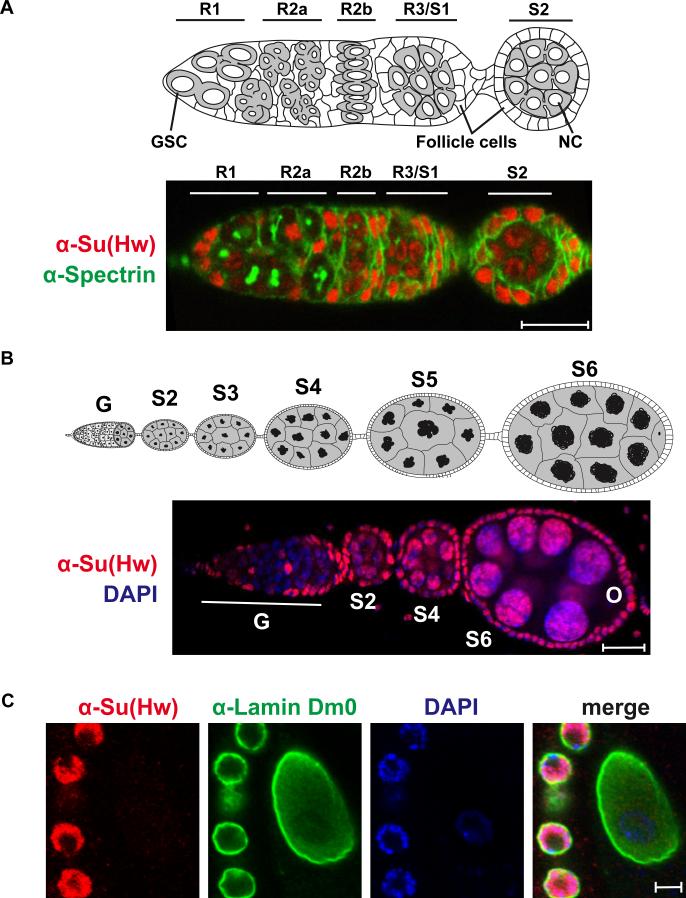
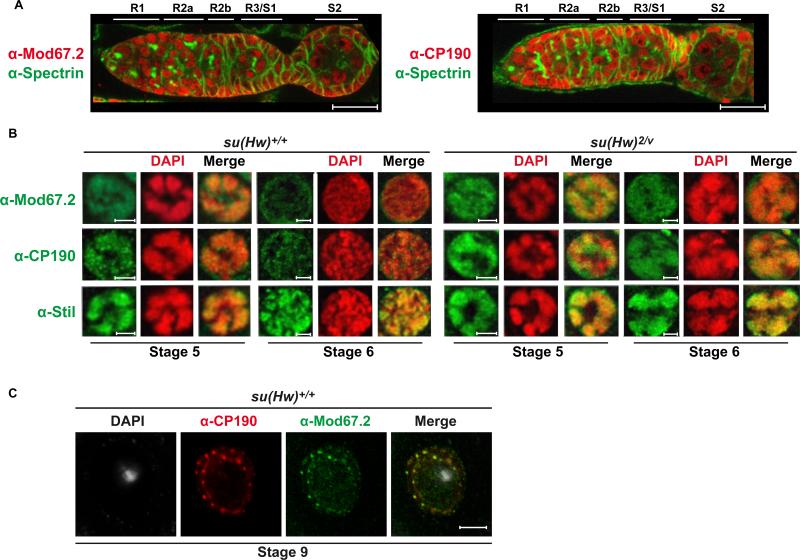
Figure 2. Su(Hw) localizes to somatic and post-mitotic NC nuclei in the ovary.
A) Top: Diagram of the germarium, marking regions 1 (R1), R2a, R2b, R3/egg chamber stage 1 (S1). The first egg chamber that emerges from the germarium is a stage 2 (S2) chamber. Germline stem cells (GSC) are located at the anterior end of the germarium. Egg chambers include germ cells and somatic follicle cells. NCs within the stage 2 egg chamber are labeled. Bottom: A representative image of the germarium from a su(Hw)+/+ (Canton S) female stained with anti-Spectrin (green) and anti-Su(Hw) (red) antibodies. Accumulation of Su(Hw) in R1/ R2a is limited to somatic cells. Cells labeled in R1/R2a are somatic cells. B) Top: Diagram of an ovariole that contains the germarium (G) and developing egg chambers up to stage 6 (S6) of development. Below: Shown is a representative image of an ovariole from su(Hw)+/+ (Canton S) stained with anti-Su(Hw) antibodies (red) and the DNA stain DAPI (blue). The germarium (G), egg chamber stages (S2 to S6) and oocyte (O) are indicated. Scale bars are 20 microns in length. C. Shown is a representative image of a wild type (Canton S) stage 9 oocyte nucleus (center) surrounded by follicle cells (right) that was stained with antibodies against Su(Hw) (red), laminDm0 (green) and DAPI (blue).
Figure 3. Mutations in the su(Hw) block oogenesis and produce apoptotic egg chambers.
Representative images of DAPI stained ovarioles from wild type (su(Hw)+/+), su(Hw)2/v and su(Hw)f/v ovaries, oriented such that the germarium is at the left. Egg chamber stages (S4 to S8) and oocyte (O) are indicated. Magnified below each ovariole are representative NC nuclei from S4 to S7 egg chambers. Note that the late stage egg chamber in the su(Hw)2/v ovariole displays condensed DAPI foci, indicating apoptosis. Ovariole and nurse cell scale bars are 20 and 5 microns, respectively.
As a first step in understanding the role of Su(Hw) in oogenesis, we determined the temporal and spatial localization of Su(Hw) in the developing ovary. Immunohistochemical analyses were completed on ovaries dissected from wild type females using antibodies against Su(Hw) and Spectrin, a germline cytoskeletal marker used to discriminate distinct regions of the germarium (Lin et al., 1994). These studies indicate that Su(Hw) is present in all somatic cells within the germarium (Figure 2), and supported by additional immunohistochemical analyses using antibodies against Su(Hw) and Traffic Jam (TJ), a transcription factor present in somatic cells that contact germ cells in the germarium (Li et al., 2003) (Supplemental Figure 1). Accumulation of Su(Hw) in germ cells was cell type specific. Su(Hw) is present at low levels in germline stem cells and absent in mitotically active germ cells in region 2. As egg chambers are formed in region 3 of the germarium, Su(Hw) accumulation increases, with protein localized to NCs chromosomes (Figure 2B), but excluded from the oocyte nucleus (Figure 2C). Su(Hw) remains associated with NC chromosomes to late stages of oogenesis (Figure 2B, data not shown). These findings demonstrate that Su(Hw) accumulation in germ cells is limited to NC nuclei.
The ovarian phenotype in su(Hw) null mutants
Previous phenotypic analysis was completed using two different su(Hw) null backgrounds, su(Hw)2 and su(Hw)v (Harrison et al., 1993; Kim et al., 1996; Klug et al., 1968). These studies found that su(Hw) mutant ovaries contained egg chambers that first manifest a mutant phenotype at stage 6, wherein the five-blob NC chromosome structure was retained. These aberrant egg chambers were found to degenerate around stage 8. One of these studies reported further defects that included altered NC numbers in many egg chambers (Harrison et al., 1993). To gain a better understanding of the su(Hw) mutant ovarian phenotype, we dissected ovaries from females carrying five null or near null su(Hw) heteroallelic mutant combinations, including su(Hw)2/v, su(Hw)E8/v, su(Hw)E8/2, su(Hw)Pb/v, su(Hw)Pb/2 (Figure 1). These females were all sterile, with ovaries displaying a similar mutant phenotype (Figure 3). As expected, differences in egg chamber development between su(Hw) wild type and mutant backgrounds occurred at stage 6, where NC chromosomes in su(Hw) mutants failed to decondense and disperse. Our studies showed, however, that these defects were largely resolved by stage 7. Even so, all stage 8 to 9 egg chambers had DAPI bright nuclear foci and stained with antibodies against activated caspase, indicating apoptosis of egg chambers (Figure 3; data not shown). Only one su(Hw) heteroallelic combination, su(Hw)Pb/2, produced egg chambers with increased NC numbers (data not shown), implying that these changes are not a common phenotype associated with Su(Hw) loss. These data confirm that Su(Hw) is required for the proper execution of the developmentally programmed structural changes of NC chromosomes, with loss of Su(Hw) resulting in activation of the mid-oogenesis checkpoint (McCall, 2004; Nezis et al., 2000).
Loss of Su(Hw) does not alter expression of genes required for NC chromosome development
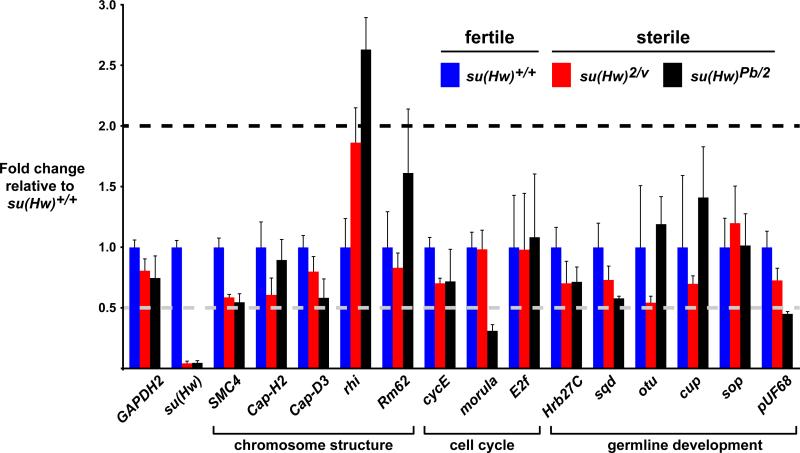
Several female sterile mutants have been identified that display global defects in NC chromosome development similar to those found in su(Hw) mutants (Buszczak and Spradling, 2006; Cramton and Laski, 1994; Hartl et al., 2008; Keyes and Spradling, 1997; King et al., 1981; Klug et al., 1968; Lilly and Spradling, 1996; Reed and Orr-Weaver, 1997; Royzman et al., 2002; Volpe et al., 2001), which we refer to as five-blob mutants. These genes encode proteins with diverse regulatory functions, including structural chromosomal proteins (SMC4, Cap-H2, Cap-D3, rhino, Rm62), cell cycle regulators (cycE, morula, E2f), and germline growth and development (Hrb27C, sqd, otu, cup, sop, pUF68). We examined whether any of the genes falling into the five-blob class were mis-regulated in su(Hw) mutants. In these experiments, RNAs were isolated from ovaries dissected from females from a wild type and two su(Hw) null mutant genetic backgrounds. Levels of RNA produced by each five-blob gene were determined using quantitative real time PCR. We reasoned that if defects in regulation of a five-blob gene were responsible for the su(Hw) mutant phenotype, then RNA levels should decrease in both mutant backgrounds, because the characterized alleles that caused five-blob phenotypes were loss of function. These studies revealed that only one gene changed expression greater than two-fold in both su(Hw) null backgrounds, rhino (rhi, Figure 4). However, expression of rhi increased, not decreased. Taken together, these data suggest that down regulation of known five-blob genes is not responsible for altered NC chromosome development in su(Hw) mutants.
Figure 4. Loss of Su(Hw) does not alter expression of five-blob genes in the ovary.
Levels of expression of genes required for NC chromosome development, referred to as five-blob genes, were determined using quantitative real time PCR. The fold change in expression of wild type to su(Hw)+/+ was set to 1 (blue). Two su(Hw) sterile backgrounds were tested, su(Hw)2/v (red) and su(Hw)Pb/2 (black). The functional class of the five-blob gene is noted. Error bars indicate standard deviation (n=3).
Analysis of su(Hw)f/v ovaries reveals defects in NC chromosome maturation
The su(Hw)f allele is exceptional, as this mutation reverses effects of the gypsy insulator without causing female sterility (Harrison et al., 1993). This allele encodes a full-length protein with a defective zinc finger 10 that has reduced chromosome association (Kuhn-Parnell et al., 2008). We expected that ovaries isolated from su(Hw)f females would have a wild type phenotype because these females are fertile. In contrast to this prediction, DAPI stained su(Hw)f/v ovaries showed abnormal egg chamber development and NC chromosomes remained in the five-blob state throughout oogenesis (Figure 3). Similarly, the five-blob state was also retained in ovaries dissected from su(Hw)f/2 females (data not shown). These data demonstrate that global changes in NC nuclear architecture are not responsible for sterility of su(Hw) mutant females.
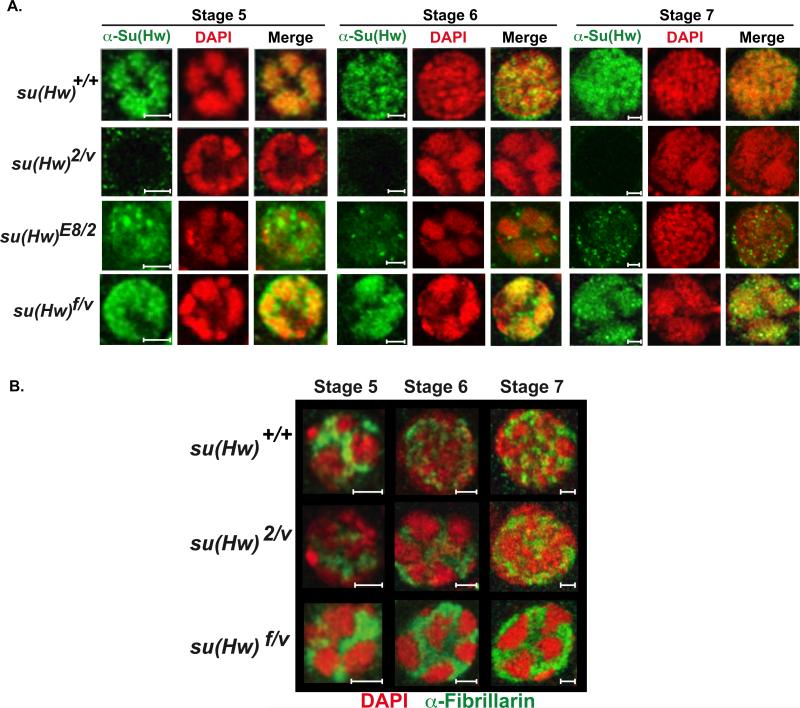
Immunohistochemical analyses showed that Su(Hw)f accumulates in spaces between the condensed NC chromosomes (Figure 5A). Su(Hw)f forms bright staining foci. We find that these foci largely co-stain with antibodies against Mod67.2, with more limited association with CP190 (Supplemental Figure 2). These findings suggest that Su(Hw)f foci may be similar to insulator bodies (Bushey, 2008).
Figure 5. Mutations in su(Hw) alter nuclear architecture within NC nuclei.
A) Magnified images of representative NC nuclei of stage 5 to 7 egg chambers from su(Hw)+/+ (Canton S), su(Hw)2/v, su(Hw)E8/2 and su(Hw)f/v egg chambers. Nuclei were stained with DAPI (red) and antibodies against Su(Hw) (green). B) Merged images of nurse cell nuclei representing stages 5 to 7 egg chambers from su(Hw)+/+ (Canton S), su(Hw)2/v and su(Hw)f/v ovaries stained with antibodies against the nucleolar marker Fibrillarin (green) and DAPI (red). Scale bars are 5 microns.
We studied the nuclear localization of Su(Hw)E8 in the ovary, to determine whether foci commonly form when chromosome association of Su(Hw) is compromised. Su(Hw)E8 carries a defective zinc finger 7 and does not bind DNA in vitro or in vivo (Harrison et al., 1993; Kuhn-Parnell et al., 2008). Immunohistochemical analyses of dissected su(Hw)E8/v ovaries showed that Su(Hw)E8 had a diffuse nuclear distribution and formed many Su(Hw) foci that contained Mod67.2 and CP190 (Figure 5A, Supplemental Figure 2), implying that foci formation is a consequence of lost chromosome association. To determine whether these mutant Su(Hw) proteins form foci in somatic tissues, we examined the nuclear distribution of the Su(Hw), Mod67.2 and CP190 in whole mount salivary glands isolated from su(Hw)f/v and su(Hw)E8/v larvae. Confocal imaging demonstrated the presence of nuclear foci containing all three proteins, again with more foci present in salivary glands containing Su(Hw)E8 (Supplemental Figure 2). Taken together, these data correlate formation of Su(Hw) foci with altered association of Su(Hw) to chromosomes, supporting proposals that insulator bodies are composed of protein, and do not contain DNA (Golovnin et al., 2008).
Retention of the five-blob chromosome state does not affect rRNA production
We were surprised that NC chromosomes maintained the five-blob state throughout oogenesis in su(Hw)f/v ovaries (Figure 3). Previous studies suggested that retention of the five-blob chromosome state caused defects in ribosome biogenesis that diminished oocyte growth and activated the mid-oogenesis checkpoint (Klug et al., 1970). We examined the structure of the nucleolus in su(Hw)f/v ovaries through immunohistochemical analyses of fibrillarin, a nucleolar protein required for pre-rRNA processing (Tollervey et al., 1993; Wu et al., 1998). In wild type stage 5 NC nuclei, fibrillarin is restricted to the space between the condensed chromosomes, while in stage 6 nuclei a reticular network is formed between the dispersed chromosomes (Figure 5B; (Klug et al., 1970)). In su(Hw)f/v ovaries, fibrillarin staining remained compact throughout oogenesis (Figure 5B). To address whether these defects in nucleolar structure affected ribosome biogenesis, we conducted several studies. First, we determined whether intermediates in pre-RNA processing accumulated in su(Hw)f/v mutants, as reduction in ribosome biogenesis leads to increases in processing intermediates (Ross et al., 2007). Total RNA was isolated from wild type and su(Hw)f/v ovaries and northern analysis was undertaken, using radiolabeled oligonucleotide probes that specifically recognized pre-rRNA processing intermediates (Giordano et al., 1999; Long and Dawid, 1980). These studies showed that levels of pre-rRNA intermediates in su(Hw)f/v RNA were indistinguishable from those found in wild type RNA (Supplemental Figure 3). Second, we quantified the level of apoptosis in su(Hw)f/v ovaries and found that only ~13% of all ovarioles had mid-stage apoptosis. This percentage of apoptosis is greatly reduced relative to su(Hw)2/v females (100%), but increased relative to wild type females (1.5%, Table 1). Third, we measured the length of eggs produced by wild type and su(Hw)f/v females to evaluate oocyte growth. These studies showed that egg sizes in both groups were the same, with each producing similar numbers of offspring (Table 1). Taken together, these observations suggest that defects in nucleolar structure in su(Hw)f/v ovaries do not strongly affect ribosome biogenesis or oocyte production, implying that the absence of structural reorganization of NC chromosomes is not responsible for sterility of su(Hw) mutant females.
Table 1.
Characterization of su(Hw), mod(mdg4), and Cp190 mutant phenotypes.
| Genotype | % apoptosis (# ovarioles) | eggs/day/female (# females tested) | embryo length (# measured) | progeny/day/female (total # progeny) |
|---|---|---|---|---|
| su(Hw)+/+ (Canton S) | 1.5 (63) | 74.0 (240) | 0.5 mm (50) | 16.9 (1354) |
| y2 w ct6 f1; su(Hw)2/v | 100 (50) | NA1 | NA1 | NA1 |
| y2 w ct6 f1; su(Hw)f/v | 13.1 (145) | 83.2 (240) | 0.5 mm (50) | 12.9 (1032) |
| y2 v ct6; mod(mdg4)u1/u1 | 7.7 (91) | 71.0 (240) | 0.5 mm (50) | 12.9 (1028) |
| Cp190H4-1/Cp190P11 | 6.0 (83) | 74.8 (69) | 0.5 mm (50) | ND2 |
| Cp190H4-1/Cp1901 | 4.7 (85) | 7.6 (63) | 0.5 mm (8) | ND2 |
| Cp190H4-1/Cp190H4-1 | 100 (35) | NA1 | NA1 | NA1 |
| Cp190H4-1, mod(mdg4)u1 ( 3 R145/R93) | 3.3 (92) | 63.0 (38) | 0.5 mm (50) | NA1 |
| Cp190H4-1, mod(mdg4)u1 (R165/R93) | 3.0 (100) | 69.1 (37) | 0.5 mm (50) | NA1 |
| Cp190H4-1, mod(mdg4)u1 (R195/R93) | 3.5 (86) | 71.9 (37) | 0.5 mm (50) | NA1 |
NA = Not Applicable: these genetic backgrounds either fail to produce eggs or produce eggs bu embryos do not hatch.
ND = Not Determined.
R = recombinant chromosome.
Su(Hw)f binds many SBSs in the ovary
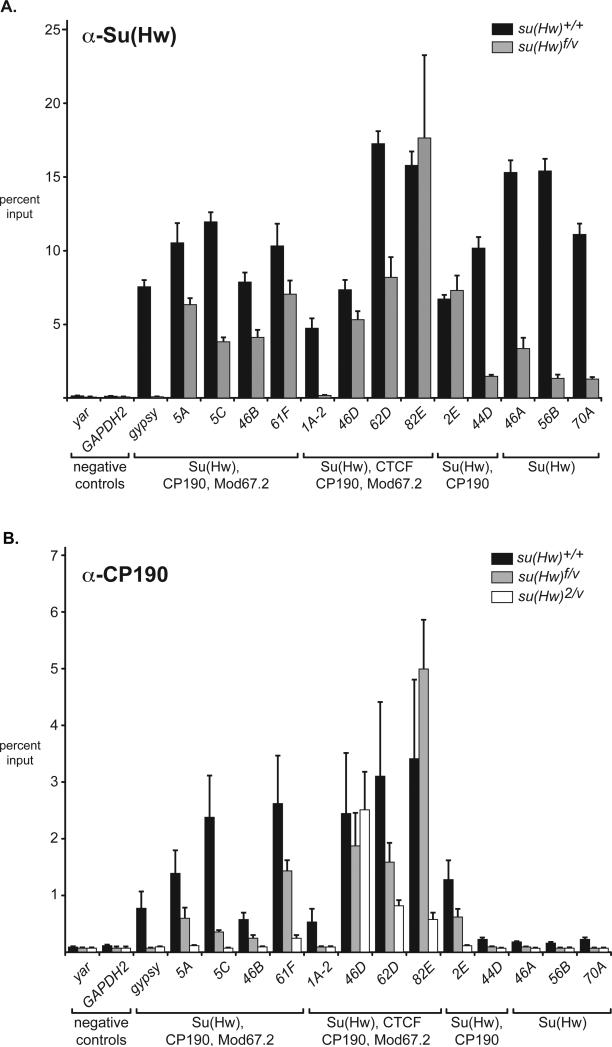
Binding of Su(Hw)f to NC chromosomes is decreased (Figure 5). To determine the extent of this altered chromosome association, we carried out chromatin immunoprecipitation (ChIP). Chromatin was isolated from ovaries dissected from females less than six hours old, because ovaries in newly eclosed females contain egg chambers younger than stage 6. As such, these ovaries contain a nearly equal contribution of chromatin from germ cells and somatic cells. We studied the gypsy insulator and fourteen non-gypsy SBSs that each contained at least one gypsy insulator-like consensus sequence (Adryan et al., 2007). These SBSs were chosen as strongly enriched Su(Hw) binding regions in genome-wide studies in somatic cells. We predicted that each SBS would bind Su(Hw) in the ovary, because studies to date indicate that Su(Hw) binding has limited tissue specificity (Adryan et al., 2007; Bushey et al., 2009; Negre et al., 2010; Parnell et al., 2006). The fourteen SBSs fell into four classes, based on association with other insulator proteins (Negre et al., 2010). These included 1) gypsy and gypsy-like SBSs that bind Su(Hw), CP190 and Mod67.2, 2) SBSs that bind dCTCF, CP190 and Mod67.2, 3) SBSs that bind Su(Hw) and CP190 only, and 4) SBSs that bind Su(Hw) only.
ChIP studies demonstrated that gypsy and all SBSs bound Su(Hw) (Figure 6A). Parallel experiments were completed using chromatin isolated from newly eclosed su(Hw)f/v ovaries. Real time quantitative PCR analysis demonstrated that Su(Hw)f bound twelve of the fourteen tested regions, with loss of binding to the gypsy insulator and 1A-2. Of the twelve bound SBSs, eight were enriched to a similar degree as in wild type ovaries, while four (44D, 46A, 56B, 70A) showed binding that was reduced but still greater than the negative controls (yar, GAPDH2). These data indicate that Su(Hw)f associates with a majority of SBSs, but with reduced occupancy. The class of Su(Hw) only SBSs had the lowest retention, suggesting that the presence of other insulator proteins facilitates association of Su(Hw)f to chromosomes. Yet, this relationship was not absolute, as 1A-2 failed to bind Su(Hw)f, even though other insulator proteins bind this region. These data suggest that multiple factors contribute to Su(Hw)f binding.
Figure 6. Properties of the ovary SBSs are similar to those in somatic cells.
A) Su(Hw) binding in the ovary. Shown is ChIP data obtained using Su(Hw) antibodies on chromatin isolated from newly eclosed ovaries isolated from wild type (su(Hw)+/+) and su(Hw)f/v females. Noted below each SBS are the insulator protein partners present as determined in (Negre et al., 2010). Error bars indicate standard deviation (n=3). B) CP190 binding in the ovary. Shown ChIP data obtained using CP190 antibodies on chromatin isolated from newly eclosed ovaries isolated from su(Hw) wild type and mutant females. Axes are labeled as in A. Error bars indicate standard deviation (n=3).
Mod67.2 and CP190 have distinct properties from Su(Hw) in the ovary
The expression patterns of Mod67.2 and CP190 were determined to investigate connections between the Su(Hw) ovary and insulator functions. Ovaries were dissected from wild type females, stained with DAPI and incubated with antibodies against each protein. As a control, ovaries were stained with antibodies against Stand still (Stil), a germline transcription factor that is associated with NC chromosomes throughout oogenesis (Sahut-Barnola and Pauli, 1999). These studies reveal that in the germarium, Mod67.2 and CP190 show the same accumulation pattern, which largely coincides with Su(Hw). We find that Mod67.2 and CP190 accumulate in all somatic and germ cells in the germarium (Figure 7A), including the mitotically active germ cells that lack Su(Hw) (Figure 2A, Supplemental Figure 1). Once egg chambers are formed, Mod67.2 and CP190 localize to NC chromosomes. This association is maintained until stage 6, at which time Mod67.2 and CP190 levels on NC are reduced, even though Su(Hw) levels are unchanged (compare Mod67.2 and CP190 staining to Stil, Figure 7B). These studies reveal that the major gypsy insulator proteins extensively co-localize during the period when Su(Hw) mutant phenotypes are first observed.
Figure 7. Mod67.2 and CP190 display both Su(Hw)-dependent and -independent localization.
A) Representative images of germaria from su(Hw)+/+ (Canton S) females stained with anti-Spectrin (green) and anti-Mod67.2 (red) or anti-CP190 (red) antibodies. Note Mod67.2 and CP190 staining in R1 and R2a, regions that lack Su(Hw). Scale bars are 20 microns. B) NC nuclei representing stages 5 to 6 egg chambers from su(Hw)+/+ (Canton S) and su(Hw)2/v ovaries. Nuclei were stained with antibodies against Mod67.2, CP190 or Stil (positive control) (green) and DAPI (red). Merged images are shown to display colocalization of each signal. Scale bars are 5 microns. C). A representative image of a wild type stage 8/9 oocyte nucleus (Canton S) is shown. This nucleus was stained with antibodies against CP190 (red), Mod67.2 (green) and DAPI (white), with the merged image of CP190 and Mod67.2 staining on the right.
Germline accumulation of Mod67.2 and CP190 is not limited to NC nuclei. In fact, localization to the oocyte nucleus occurs in the germarium and is maintained throughout oogenesis (Figure 7). Before stage 6 of egg chamber development, Mod67.2 and CP190 distribution is mostly uniform within the oocyte nucleus. At stage 6, these proteins begin to co-localize in foci, a distribution that is maintained throughout oogenesis (Figure 7C). Interestingly, this reorganization of Mod67.2 and CP190 coincides with loss of the synaptonemal complex, suggesting that these changes are linked to differences in oocyte chromosome structure. Further studies are needed to understand the significance of the changes in Mod67.2 and CP190 distribution. That Mod67.2 and CP190 but not Su(Hw) localize to the oocyte nucleus indicates that gypsy insulator proteins have distinct properties in germ cells.
Three studies were conducted to investigate whether the three gypsy insulator proteins bound to overlapping chromosomal regions. First, the genome-wide distribution of Su(Hw), Mod67.2 and CP190 was examined using the giant NC chromosomes in the ovarian tumor (otu11 ) mutant (Koryakov et al., 2004). Incubation of otu11 ovaries with antibodies against these three proteins showed extensive colocalization on euchromatic chromosome arms (Supplemental Figure 4). Second, we determined whether localization of Mod67.2 and CP190 depended on Su(Hw). Ovaries were dissected from su(Hw)2/v females and incubated with antibodies against Mod67.2 and CP190. These experiments showed that in the absence of Su(Hw), increased levels of Mod67.2 and CP190 were present in the inter-chromosomal spaces (Figure 7B). Even so, both proteins show overlap with DAPI staining, suggesting that both retain some chromosome association. Retention of CP190 was expected because this protein interacts with other DNA binding proteins, such as BEAF and dCTCF (Bartkuhn et al., 2009; Bushey et al., 2009). Retention of Mod67.2 is consistent with genome-wide studies that demonstrate that Mod67.2 association does not always overlap with SBSs (Negre et al., 2010). Third, we used ChIP to determine whether CP190 binding depended on Su(Hw). In these studies, CP190 antibodies were incubated with chromatin isolated from newly eclosed ovaries dissected from wild type and su(Hw) mutant females. CP190 enrichment at the fourteen SBSs was tested. Of these sites, eleven were expected to bind CP190, from studies completed in somatic cells (Negre et al., 2010). Quantitative real time PCR analyses demonstrated that ten of these SBSs had significant CP190 association in a wild type su(Hw) background; the exceptional site being 1A-2 (Figure 6B). Of the ten ovary-bound CP190 SBSs, six lost CP190 binding in the su(Hw)2/v null background. Interestingly, the three SBSs that retained CP190 binding in the absence of Su(Hw) bind dCTCF in the soma (Negre et al., 2010), consistent with the prediction that dCTCF also recruits CP190 (Bushey et al., 2009; Mohan et al., 2007). Further, we determined whether CP190 bound the ten SBSs in chromatin obtained from su(Hw)f/v chromatin. Reduced CP190 occupancy was observed at these sites, possibly because Su(Hw)f occupancy is lower (Figure 5, Figure 6a). These data indicate that Su(Hw) recruits CP190 to many genomic sites in the ovary and that mutation of zinc finger 10 does not disrupt this function.
The extensive co-localization of Su(Hw) with other gypsy insulator proteins on NC chromosomes suggests that the ovary function of Su(Hw) might be related to gypsy insulator function. To test this possibility, we examined the phenotypes associated with ovaries dissected from mod(mdg4) and Cp190 mutants. The mod(mdg4) mutant phenotype was studied in ovaries dissected from mod(mdg4)u1 females, because this mutation causes complete loss of the insulator specific Mod67.2 isoform (Gause et al., 2001; Georgiev and Gerasimova, 1989). Multiple nearly null mutant backgrounds were studied that included the Cp190H4-1 allele. The Cp190H4-1 allele encodes a truncated protein that retains enough CP190 function to permit development of adults, although these adults are unhealthy and die within a few days of eclosion (Butcher et al., 2004; Pai et al., 2004).
Previous studies have shown that mod(mdg4)u1 females are fertile (Gerasimova et al., 1995). Even so, it was unclear whether aspects of the su(Hw) mutant phenotype were present, such as a failure to decondense and disperse nurse cell chromosomes. Ovaries were dissected from one to two-day-old mod(mdg4)u1 females, stained with DAPI and imaged by confocal microscopy. These studies showed normal oogenesis, including an appropriate timing of structural changes in NC chromosomes (Figure 8). Further, we quantified levels of apoptosis in mod(mdg4)u1 ovaries, as well as fertility and fecundity of the mutant females. These studies revealed that loss of Mod67.2 slightly increased egg chamber apoptosis relative to the wild type controls (7.7% relative to 1.5%, Table 1), with no effect on the other parameters.
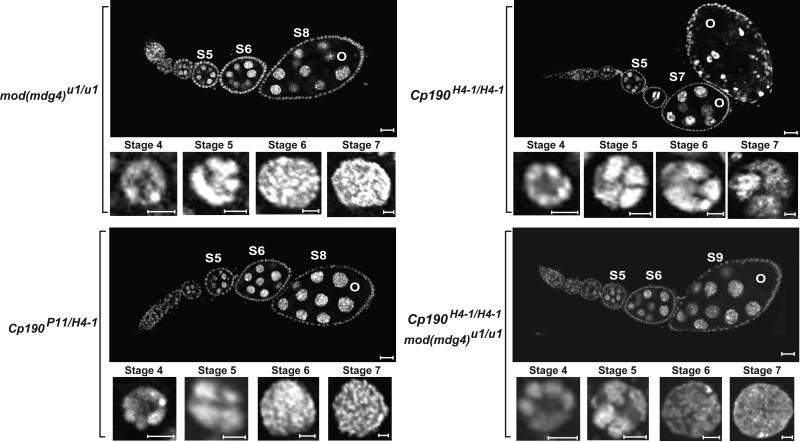
Figure 8. Mutations in the mod(mdg4) and Cp190 genes have minor effects on oogenesis.
Images of DAPI stained ovarioles isolated from mod(mdg4) mutant females and Cp190 mutant females carrying three different heteroallelic combinations. Genotypes of the females are indicated on the left. For each genotype, an ovariole and magnified NC nuclei are shown. Ovariole and nurse cell scale bars are 20 and 5 microns in length, respectively.
We studied effects of CP190 on oogenesis in Cp190H4-1, Cp190 H4-1/1, Cp190H4-1/P11 females. We found that the ovary phenotype depended upon genetic background tested (Figure 8, data not shown). Ovaries dissected from homozygous Cp190H4-1 females were defective in NC chromosome structure and egg chamber development, with all egg chambers showing apoptosis (Table 1). In addition, many egg chambers showed aberrant NC numbers, with both increased and decreased numbers of NCs found. However, ovaries dissected from heteroallelic Cp190 mutant backgrounds (Cp190H4-1/P11 and Cp190H4-1/1 ) had ovarioles with normal egg chamber progression, with only a slight elevation in the level of apoptosis (Figure 8, Table 1). In fact, Cp190H4-1/P11 females produced near wild type numbers of eggs. Based on these data, we conclude that the severe phenotype observed in Cp190H4-1 homozygotes may result from effects of a second site mutation that influence oogenesis. This possibility is supported by our subsequent studies of mod(mdg4), Cp190 double mutants (see below). That near complete loss of functional CP190 in Cp190 H4-1/1 and Cp190H4-1/P11 females permits near wild type oogenesis suggests that this protein is not essential for oogenesis.
Given the extensive partnership between these insulator proteins and Su(Hw), we were surprised that only minor defects occurred when Mod67.2 and CP190 are compromised. We considered that the apparent lack of requirement for these proteins might reflect redundant roles in oogenesis. Both proteins contain an N-terminal BTB domain that may support recruitment of a common protein partner to a SBS, such that loss of one protein would not interfere with partner association. To address redundant functions, we isolated four recombinant chromosomes carrying the Cp190H4-1 and mod(mdg4)u1 alleles and studied ovary phenotypes in females carrying heterozygous combinations of different recombinants (Table 1). Again, oogenesis proceeded normally in ovaries both lacking Mod67.2 and containing greatly reduced CP190 function (Figure 8, Table 1). In fact, in all cases, levels of apoptosis were only slightly elevated relative to the wild type control (~3.3% versus ~1.5%). These data imply that Mod67.2 and CP190 do not play redundant roles in oogenesis, indicating that neither protein is essential in these processes.
Discussion
Su(Hw) is essential for oogenesis, but little is known about the function of this protein in the ovary. Here, we completed the first detailed molecular analysis of properties of Su(Hw). We found that during oogenesis somatic cells uniformly express Su(Hw) (Figure 2; Supplemental Figure 1). In contrast, germ cell accumulation is temporally and spatially regulated. Robust accumulation of Su(Hw) begins upon formation of egg chambers, where Su(Hw) localization is restricted to NC nuclei and is absent from the oocyte nucleus, a distribution that is maintained throughout oogenesis. Other nuclear factors do not show dynamic expression in the germarium (Figure 7, see below), suggesting that regulated accumulation of Su(Hw) may be important for its role in oogenesis.
The global defects in NC chromosome structure are not responsible for su(Hw) sterility
Phenotypic defects caused by loss of Su(Hw) were investigated through studies of ovaries obtained from females carrying different heteroallelic combinations of su(Hw) mutant alleles. These investigations demonstrate that NC chromosome development is delayed (Figure 3), but not blocked as previously reported (Klug et al., 1968). Structural defects in NC chromosomes appear to be independent of known genes involved in NC chromosome development, because transcription of these genes was largely maintained in su(Hw) null backgrounds (Figure 4). Finally, our studies addressed the long-standing hypothesis that the sterility in su(Hw) mutant females is caused by retention of the five-blob chromosome state that affects ribosome biogenesis, oocyte growth, and activates apoptosis (Klug et al., 1970). We demonstrate that rRNA processing occurs normally in su(Hw) mutants (Supplemental Figure 3), suggesting that ribosome biogenesis is not impaired. Further, we discovered that NC chromosomes never disperse in ovaries obtained from fertile su(Hw)f/v females, even though oocyte growth, rRNA processing and fecundity are wild type (Figure 3, Table 1). These observations establish that decondensation and dispersal of NC chromosomes is not the cause of sterility in su(Hw) mutants.
The uncoupling of the NC structural defects and Su(Hw) dependent fertility raises the question of which cells require Su(Hw) function for completion of oogenesis. At present, we do not know whether the essential function resides in NCs or the surrounding follicle cells, as these latter cells provide signals to the germline needed for egg chamber development. Additional investigations are required to resolve this issue.
Functional requirements for the insulator and fertility functions of Su(Hw) are different
Mod67.2 and CP190 are BTB-domain proteins that are required for enhancer blocking by the gypsy insulator (Georgiev and Gerasimova, 1989; Gerasimova et al., 1995; Gerasimova et al., 2007; Pai et al., 2004). We investigated the role of these proteins in oogenesis, to gain insights into the connections between the insulator and fertility functions of Su(Hw). We found that during oogenesis Mod67.2 and CP190 show parallel accumulation, with these BTB-domain proteins found in all somatic and germ cells, including cells that lack Su(Hw) such as the oocyte nucleus. In early egg chambers, Mod67.2 and CP190 extensively co-localize with Su(Hw) (Figure 5, Figure 7), while older egg chambers display diminished levels. NC chromosome association of Mod67.2 and CP190 is largely dependent on Su(Hw), although both proteins retain NC chromosome binding in su(Hw) mutants. Our findings are consistent with genome-wide studies of protein binding in somatic cells that show that chromosome association of Mod67.2 and CP190 does not always overlap with SBSs (Negre et al., 2010).
Extensive co-localization of Su(Hw), Mod67.2 and CP190 is present in stages of oogenesis where the su(Hw) mutant phenotype becomes evident. Even so, null or nearly null mod(mdg4) and Cp190 single and double mutant females lay eggs of normal size (Table 1). No evidence was observed for defects in NC chromosome development or increased apoptosis (Table 1), implying that Mod67.2 and CP190 are not required for oogenesis. These observations imply that the fertility and insulator functions of Su(Hw) are different. Such findings may be explained if lower levels of BTB domain proteins are needed for oogenesis than are needed to establish an insulator. We note that while we used a mod(mdg4) allele that fails to produce any of the Mod67.2 isoform, the Cp190 heteroallelic combinations studied were hypomorphic, because null alleles are pharate lethal. As such, Cp190 mutant ovaries may have enough CP190 activity to support Su(Hw) functions. However, CP190 null embryos have been generated from germline clones, implying that oogenesis is not blocked when CP190 is absent from germ cells (Chodagam et al., 2005). These data, coupled with our studies, indicate that Mod67.2 and CP190 are not essential for oogenesis. We predict that Su(Hw) has Mod67.2 and CP190 independent functions. Support for this postulate comes from genome-wide studies that demonstrate that ~50% of SBSs do not bind Mod67.2 or CP190 (Negre et al., 2010).
The function of Su(Hw) in oogenesis
The different requirements for Su(Hw) in insulation and fertility raise the question of whether the essential role of Su(Hw) in oogenesis involves formation of chromatin insulators. We predict that if insulator function is involved, then novel interaction partners may be required for Su(Hw) to demarcate chromatin domains. Alternatively, Su(Hw) function may extend beyond that of an insulator protein, a possibility that is supported by recent genome wide studies of SBSs (Adryan et al., 2007; Golovnin et al., 2003; Parnell et al., 2006; Parnell et al., 2003; Ramos et al., 2006). The vast majority of non-gypsy SBSs contain a single motif, in contrast to the twelve Su(Hw) binding motifs found in the gypsy insulator. This observation is striking considering that enhancer blocking by the gypsy insulator requires at least four tightly spaced SBSs (Hagstrom et al., 1996; Scott et al., 1999; Spana and Corces, 1990). Direct tests of the insulator activity of individual SBSs in transgene assays have shown that ~40% block enhancer action (Golovnin et al., 2003; Negre et al., 2011; Negre et al., 2010; Parnell et al., 2006; Parnell et al., 2003; Ramos et al., 2006), suggesting that not all SBSs are insulators. If the formation of chromatin insulators by Su(Hw) is not required for fertility, then how does this protein contribute to nuclear functions during oogenesis? It is possible that Su(Hw) has the capacity to directly to modulate transcription of target genes. For example, studies of the function of one SBS revealed that this SBS was required for activation of transcription of the adjacent gene (Soshnev et al., 2008). Further, a repressor activity is suggested by genome-wide studies that correlate Su(Hw) localization with repressive chromatin and gene silencing (Filion et al., 2010; Roy et al., 2010). Interestingly, diverse regulatory functions have been documented for the major vertebrate insulator protein, CCCTC binding factor (CTCF). While CTCF is best known as an insulator protein (Ohlsson et al., 2010; Phillips and Corces, 2009; Zlatanova and Caiafa, 2009), early studies of CTCF documented direct involvement in transcriptional activation and repression (Burcin et al., 1997; Lobanenkov et al., 1990). More recent genetic studies in transgenic mice provide additional support for direct regulation of gene expression (Heath et al., 2008; Ribeiro de Almeida et al., 2009; Soshnikova et al., 2010; Wan et al., 2008). These observations suggest that Su(Hw) may be similar to CTCF, functioning as a multi-faceted transcriptional regulator.
Supplementary Material
Supplemental Figure 1. Somatic cells of the germarium express Su(Hw). A representative image of a wild type (su(Hw)+/+) germarium stained with DAPI (blue) and antibodies against Su(Hw) (green) and Traffic Jam (red). Merged images are shown to display colocalization of each signal. Scale bars are 20 microns.
Supplemental Figure 2. Su(Hw) zinc finger mutants form foci that co-localize with Mod67.2 and CP190 in somatic and germ cells. A) Representative magnified images of stage 6 egg chamber NC nuclei from su(Hw)f/v and su(Hw)E8/2 ovaries stained with DAPI and antibodies against Su(Hw), Mod67.2 or CP190. Scale bars are 5 microns. B) Representative images of whole mount salivary gland nuclei from su(Hw)+/+ (Canton S), su(Hw)f/v and su(Hw)E8/v third instar larvae stained with DAPI (red) and antibodies against Su(Hw) (green). Scale bars are 10 microns.
Supplemental Figure 3. Processing of rRNA is normal in su(Hw) mutant ovaries. A) Diagram of the ribosomal DNA (rDNA) locus in Drosophila. Mature 2S, 5S, 18S, 28Sa and 28Sb rRNAs are produced via cleavages at six different sites (labeled 1 to 6). Three different probes (I, II, III) detect the processing intermediates a, b and d. (B) Northern analysis of total RNA isolated from Canton S (+/+), su(Hw)f/v (f/v) and su(Hw)2/v (2/v) ovaries. The sizes of standards are shown on the left. The identity of each rRNA intermediate detected is labeled on the far right. (C) Methylene blue staining of membranes used in northern analyses showing mature 18S, 28Sa and 28Sb rRNAs.
Supplemental Figure 4. Su(Hw), Mod67.2 and CP190 co-localize on nurse cell polytene chromosomes. Nurse cell polytene chromosomes obtained from otu11, su(Hw)+/+ females were stained with DAPI (blue) and antibodies against Su(Hw) (green), Mod67.2 or CP190 (red). Merged images are shown for both Su(Hw) (green)/Mod67.2 (red) and Su(Hw) (green)/CP190 (red).
Highlights.
Highlights are mandatory for this journal. They consist of a short collection of bullet points that convey the core findings of the article and should be submitted in a separate file in the online submission system. Highlights will be displayed in online search result lists, the contents list and in the online article, but will not (yet) appear in the article PDF file or print.
• Include 3 to 5 highlights.
• Max. 85 characters per highlight including spaces.
Only the core results of the paper should be covered.
The insulator and fertility functions of Suppressor of Hairy-wing are different.
Sterility of su(Hw) mutants is not due to altered ribosome biogenesis.
Architectural changes in nurse cell chromosomes are not required for oogenesis.
Mod67.2 and CP190 are not essential for Drosophila oogenesis.
Acknowledgements
We thank for Daniel Pauli, Michelle Moritz and Dorothea Godt for generously supplying Stand still, CP190 and Traffic Jam antibodies, respectively. We thank Victor Corces for the Cp190 mutant alleles. We thank Chantal Allamargot and other staff in the Carver College of Medicine Microscopy Facility for assistance in immunohistochemical experiments. We thank the Geyer laboratory for their critical reading of this manuscript. This work was supported by the National Institute of Health Grant GM42539 to P.K.G., National Institute of Health (T32073610) training position to R.M.B., and the Russian Foundation for Basic Research grant 09-04-00437, grant 6.4 of the program of Presidium RAS “Molecular and cellular biology”, Interdisciplinary integration project of SB RAS N 37, Government contract ROSNAUKA 02.740.11.0099 for funding to D.E.K. and I.F.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adryan B, Woerfel G, Birch-Machin I, Gao S, Quick M, Meadows L, Russell S, White R. Genomic mapping of Suppressor of Hairy-wing binding sites in Drosophila. Genome Biol. 2007;8:R167. doi: 10.1186/gb-2007-8-8-r167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkuhn M, Straub T, Herold M, Herrmann M, Rathke C, Saumweber H, Gilfillan GD, Becker PB, Renkawitz R. Active promoters and insulators are marked by the centrosomal protein 190. Embo J. 2009;28:877–888. doi: 10.1038/emboj.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcin M, Arnold R, Lutz M, Kaiser B, Runge D, Lottspeich F, Filippova GN, Lobanenkov VV, Renkawitz R. Negative protein 1, which is required for function of the chicken lysozyme gene silencer in conjunction with hormone receptors, is identical to the multivalent zinc finger repressor CTCF. Mol Cell Biol. 1997;17:1281–1288. doi: 10.1128/mcb.17.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey AM, Dorman ER, Corces VG. Chromatin insulators: regulatory mechanisms and epigenetic inheritance. Mol Cell. 2008;32:1–9. doi: 10.1016/j.molcel.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey AM, Ramos E, Corces VG. Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev. 2009;23:1338–1350. doi: 10.1101/gad.1798209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Spradling AC. The Drosophila P68 RNA helicase regulates transcriptional deactivation by promoting RNA release from chromatin. Genes Dev. 2006;20:977–989. doi: 10.1101/gad.1396306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RD, Chodagam S, Basto R, Wakefield JG, Henderson DS, Raff JW, Whitfield WG. The Drosophila centrosome-associated protein CP190 is essential for viability but not for cell division. J Cell Sci. 2004;117:1191–1199. doi: 10.1242/jcs.00979. [DOI] [PubMed] [Google Scholar]
- Chodagam S, Royou A, Whitfield W, Karess R, Raff JW. The centrosomal protein CP190 regulates myosin function during early Drosophila development. Curr Biol. 2005;15:1308–1313. doi: 10.1016/j.cub.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Cramton SE, Laski FA. string of pearls encodes Drosophila ribosomal protein S2, has Minute-like characteristics, and is required during oogenesis. Genetics. 1994;137:1039–1048. doi: 10.1093/genetics/137.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dej KJ, Spradling AC. The endocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development. 1999;126:293–303. doi: 10.1242/dev.126.2.293. [DOI] [PubMed] [Google Scholar]
- Dorsett D, Viglianti GA, Rutledge BJ, Meselson M. Alteration of hsp82 gene expression by the gypsy transposon and suppressor genes in Drosophila melanogaster. Genes & Development. 1989;3:454–468. doi: 10.1101/gad.3.4.454. [DOI] [PubMed] [Google Scholar]
- Filion GJ, van Bemmel JG, Braunschweig U, Talhout W, Kind J, Ward LD, Brugman W, de Castro IJ, Kerkhoven RM, Bussemaker HJ, van Steensel B. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell. 2010;143:212–224. doi: 10.1016/j.cell.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause M, Morcillo P, Dorsett D. Insulation of enhancer-promoter communication by a gypsy transposon insert in the Drosophila cut gene: cooperation between suppressor of hairy-wing and modifier of mdg4 proteins. Mol Cell Biol. 2001;21:4807–4817. doi: 10.1128/MCB.21.14.4807-4817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev PG, Gerasimova TI. Novel genes influencing the expression of the yellow locus and mdg4 (gypsy) in Drosophila melanogaster. Mol Gen Genet. 1989;220:121–126. doi: 10.1007/BF00260865. [DOI] [PubMed] [Google Scholar]
- Gerasimova TI, Gdula DA, Gerasimov DV, Simonova O, Corces VG. A Drosophila protein that imparts directionality on a chromatin insulator is an enhancer of position-effect variegation. Cell. 1995;82:587–597. doi: 10.1016/0092-8674(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Gerasimova TI, Lei EP, Bushey AM, Corces VG. Coordinated Control of dCTCF and gypsy Chromatin Insulators in Drosophila. Mol Cell. 2007;28:761–772. doi: 10.1016/j.molcel.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer PK, Clark I. Protecting against promiscuity: the regulatory role of insulators. Cell Mol Life Sci. 2002;59:2112–2127. doi: 10.1007/s000180200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer PK, Corces VG. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes & Development. 1992;6:1865–1873. doi: 10.1101/gad.6.10.1865. [DOI] [PubMed] [Google Scholar]
- Giordano E, Peluso I, Senger S, Furia M. minifly, a Drosophila gene required for ribosome biogenesis. J Cell Biol. 1999;144:1123–1133. doi: 10.1083/jcb.144.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovnin A, Birukova I, Romanova O, Silicheva M, Parshikov A, Savitskaya E, Pirrotta V, Georgiev P. An endogenous Su(Hw) insulator separates the yellow gene from the Achaete-scute gene complex in Drosophila. Development. 2003;130:3249–3258. doi: 10.1242/dev.00543. [DOI] [PubMed] [Google Scholar]
- Golovnin A, Melnikova L, Volkov I, Kostuchenko M, Galkin AV, Georgiev P. ‘Insulator bodies’ are aggregates of proteins but not of insulators. EMBO Rep. 2008;9:440–445. doi: 10.1038/embor.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurudatta BV, Corces VG. Chromatin insulators: lessons from the fly. Brief Funct Genomic Proteomic. 2009;8:276–282. doi: 10.1093/bfgp/elp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom K, Muller M, Schedl P. Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes & Development. 1996;10:3202–3215. doi: 10.1101/gad.10.24.3202. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Gdula DA, Coyne RS, Corces VG. A leucine zipper domain of the suppressor of Hairy-wing protein mediates its repressive effect on enhancer function. Genes & Development. 1993;7:1966–1978. doi: 10.1101/gad.7.10.1966. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Mortin MA, Corces VG. The RNA polymerase II 15-kilodalton subunit is essential for viability in Drosophila melanogaster. Molecular & Cellular Biology. 1992;12:928–935. doi: 10.1128/mcb.12.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl TA, Smith HF, Bosco G. Chromosome alignment and transvection are antagonized by condensin II. Science. 2008;322:1384–1387. doi: 10.1126/science.1164216. [DOI] [PubMed] [Google Scholar]
- Heath H, Ribeiro de Almeida C, Sleutels F, Dingjan G, van de Nobelen S, Jonkers I, Ling KW, Gribnau J, Renkawitz R, Grosveld F, Hendriks RW, Galjart N. CTCF regulates cell cycle progression of alphabeta T cells in the thymus. EMBO J. 2008;27:2839–2850. doi: 10.1038/emboj.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64:941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- Kellum R, Schedl P. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Molecular & Cellular Biology. 1992;12:2424–2431. doi: 10.1128/mcb.12.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes LN, Spradling AC. The Drosophila gene fs(2)cup interacts with otu to define a cytoplasmic pathway required for the structure and function of germ-line chromosomes. Development. 1997;124:1419–1431. doi: 10.1242/dev.124.7.1419. [DOI] [PubMed] [Google Scholar]
- Kim J, Shen B, Rosen C, Dorsett D. The DNA-binding and enhancer-blocking domains of the Drosophila suppressor of Hairy-wing protein. Molecular & Cellular Biology. 1996;16:3381–3392. doi: 10.1128/mcb.16.7.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RC. Ovarian Development in Drosophila melanogaster. Academic Press; New York: 1970. [Google Scholar]
- King RC, Riley SF, Cassidy JD, White PE, Paik YK. Giant polytene chromosomes from the ovaries of a Drosophila mutant. Science. 1981;212:441–443. doi: 10.1126/science.6782674. [DOI] [PubMed] [Google Scholar]
- Klug WS, Bodenstein D, King RC. Oogenesis in the suppressor of hairy-wing mutant of Drosophila melanogaster. I. Phenotypic characterization and transplantation experiments. J Exp Zool. 1968;167:151–156. doi: 10.1002/jez.1401670203. [DOI] [PubMed] [Google Scholar]
- Klug WS, King RC, Wattiaux JM. Oogenesis in the suppressor of hairy-wing mutant of Drosophila melanogaster. II. Nucleolar morphology and in vitro studies of RNA protein synthesis. J Exp Zool. 1970;174:125–140. doi: 10.1002/jez.1401740203. [DOI] [PubMed] [Google Scholar]
- Kopytova DV, Orlova AV, Krasnov AN, Gurskiy DY, Nikolenko JV, Nabirochkina EN, Shidlovskii YV, Georgieva SG. Multifunctional factor ENY2 is associated with the THO complex and promotes its recruitment onto nascent mRNA. Genes Dev. 2010;24:86–96. doi: 10.1101/gad.550010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koryakov DE, Mal'ceva NI, King RC, Zhimulev IF. Polytene chromosomes from ovarian nurse cells of Drosophila melanogaster otu mutants. Methods Mol Biol. 2004;247:139–161. doi: 10.1385/1-59259-665-7:139. [DOI] [PubMed] [Google Scholar]
- Kuhn EJ, Geyer PK. Genomic insulators: connecting properties to mechanism. Curr Opin Cell Biol. 2003;15:259–265. doi: 10.1016/s0955-0674(03)00039-5. [DOI] [PubMed] [Google Scholar]
- Kuhn-Parnell EJ, Helou C, Marion DJ, Gilmore BL, Parnell TJ, Wold MS, Geyer PK. Investigation of the properties of non-gypsy suppressor of hairy-wing-binding sites. Genetics. 2008;179:1263–1273. doi: 10.1534/genetics.108.087254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurshakova M, Maksimenko O, Golovnin A, Pulina M, Georgieva S, Georgiev P, Krasnov A. Evolutionarily conserved E(y)2/Sus1 protein is essential for the barrier activity of Su(Hw)-dependent insulators in Drosophila. Mol Cell. 2007;27:332–338. doi: 10.1016/j.molcel.2007.05.035. [DOI] [PubMed] [Google Scholar]
- Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- Lilly MA, Spradling AC. The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 1996;10:2514–2526. doi: 10.1101/gad.10.19.2514. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Lobanenkov VV, Nicolas RH, Adler VV, Paterson H, Klenova EM, Polotskaja AV, Goodwin GH. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5'-flanking sequence of the chicken c-myc gene. Oncogene. 1990;5:1743–1753. [PubMed] [Google Scholar]
- Long EO, Dawid IB. Alternative pathways in the processing of ribosomal RNA precursor in Drosophila melanogaster. J Mol Biol. 1980;138:873–878. doi: 10.1016/0022-2836(80)90070-4. [DOI] [PubMed] [Google Scholar]
- McCall K. Eggs over easy: cell death in the Drosophila ovary. Dev Biol. 2004;274:3–14. doi: 10.1016/j.ydbio.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Mohan M, Bartkuhn M, Herold M, Philippen A, Heinl N, Bardenhagen I, Leers J, White RA, Renkawitz-Pohl R, Saumweber H, Renkawitz R. The Drosophila insulator proteins CTCF and CP190 link enhancer blocking to body patterning. Embo J. 2007;26:4203–4214. doi: 10.1038/sj.emboj.7601851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre N, Brown CD, Ma L, Bristow CA, Miller SW, Wagner U, Kheradpour P, Eaton ML, Loriaux P, Sealfon R, Li Z, Ishii H, Spokony RF, Chen J, Hwang L, Cheng C, Auburn RP, Davis MB, Domanus M, Shah PK, Morrison CA, Zieba J, Suchy S, Senderowicz L, Victorsen A, Bild NA, Grundstad AJ, Hanley D, MacAlpine DM, Mannervik M, Venken K, Bellen H, White R, Gerstein M, Russell S, Grossman RL, Ren B, Posakony JW, Kellis M, White KP. A cis-regulatory map of the Drosophila genome. Nature. 2011;471:527–531. doi: 10.1038/nature09990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre N, Brown CD, Shah PK, Kheradpour P, Morrison CA, Henikoff JG, Feng X, Ahmad K, Russell S, White RA, Stein L, Henikoff S, Kellis M, White KP. A comprehensive map of insulator elements for the Drosophila genome. PLoS Genet. 2010;6:e1000814. doi: 10.1371/journal.pgen.1000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP, Stravopodis DJ, Papassideri I, Robert-Nicoud M, Margaritis LH. Stage-specific apoptotic patterns during Drosophila oogenesis. Eur J Cell Biol. 2000;79:610–620. doi: 10.1078/0171-9335-00088. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Lobanenkov V, Klenova E. Does CTCF mediate between nuclear organization and gene expression? Bioessays. 2010;32:37–50. doi: 10.1002/bies.200900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai CY, Lei EP, Ghosh D, Corces VG. The centrosomal protein CP190 is a component of the gypsy chromatin insulator. Mol Cell. 2004;16:737–748. doi: 10.1016/j.molcel.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Parkhurst SM, Harrison DA, Remington MP, Spana C, Kelley RL, Coyne RS, Corces VG. The Drosophila su(Hw) gene, which controls the phenotypic effect of the gypsy transposable element, encodes a putative DNA-binding protein. Genes & Development. 1988;2:1205–1215. doi: 10.1101/gad.2.10.1205. [DOI] [PubMed] [Google Scholar]
- Parnell TJ, Kuhn EJ, Gilmore BL, Helou C, Wold MS, Geyer PK. Identification of genomic sites that bind the Drosophila suppressor of Hairy-wing insulator protein. Mol Cell Biol. 2006;26:5983–5993. doi: 10.1128/MCB.00698-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell TJ, Viering MM, Skjesol A, Helou C, Kuhn EJ, Geyer PK. An endogenous suppressor of hairy-wing insulator separates regulatory domains in Drosophila. Proc Natl Acad Sci U S A. 2003;100:13436–13441. doi: 10.1073/pnas.2333111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab JR, Kamakaka RT. Insulators and promoters: closer than we think. Nat Rev Genet. 2010;11:439–446. doi: 10.1038/nrg2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos E, Ghosh D, Baxter E, Corces V. Genomic organization of gypsy-like chromatin insulators in Drosophila melanogaster. Genetics. 2006 doi: 10.1534/genetics.105.054742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BH, Orr-Weaver TL. The Drosophila gene morula inhibits mitotic functions in the endo cell cycle and the mitotic cell cycle. Development. 1997;124:3543–3553. doi: 10.1242/dev.124.18.3543. [DOI] [PubMed] [Google Scholar]
- Ribeiro de Almeida C, Heath H, Krpic S, Dingjan GM, van Hamburg JP, Bergen I, van de Nobelen S, Sleutels F, Grosveld F, Galjart N, Hendriks RW. Critical role for the transcription regulator CCCTC-binding factor in the control of Th2 cytokine expression. J Immunol. 2009;182:999–1010. doi: 10.4049/jimmunol.182.2.999. [DOI] [PubMed] [Google Scholar]
- Roseman RR, Johnson EA, Rodesch CK, Bjerke M, Nagoshi RN, Geyer PK. A P element containing suppressor of hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics. 1995a;141:1061–1074. doi: 10.1093/genetics/141.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman RR, Pirrotta V, Geyer PK. The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO Journal. 1993;12:435–442. doi: 10.1002/j.1460-2075.1993.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman RR, Swan JM, Geyer PK. A Drosophila insulator protein facilitates dosage compensation of the X chromosome mini-white gene located at autosomal insertion sites. Development. 1995b;121:3573–3582. doi: 10.1242/dev.121.11.3573. [DOI] [PubMed] [Google Scholar]
- Ross CL, Patel RR, Mendelson TC, Ware VC. Functional conservation between structurally diverse ribosomal proteins from Drosophila melanogaster and Saccharomyces cerevisiae: fly L23a can substitute for yeast L25 in ribosome assembly and function. Nucleic Acids Res. 2007;35:4503–4514. doi: 10.1093/nar/gkm428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Ernst J, Kharchenko PV, Kheradpour P, Negre N, Eaton ML, Landolin JM, Bristow CA, Ma L, Lin MF, Washietl S, Arshinoff BI, Ay F, Meyer PE, Robine N, Washington NL, Di Stefano L, Berezikov E, Brown CD, Candeias R, Carlson JW, Carr A, Jungreis I, Marbach D, Sealfon R, Tolstorukov MY, Will S, Alekseyenko AA, Artieri C, Booth BW, Brooks AN, Dai Q, Davis CA, Duff MO, Feng X, Gorchakov AA, Gu T, Henikoff JG, Kapranov P, Li R, MacAlpine HK, Malone J, Minoda A, Nordman J, Okamura K, Perry M, Powell SK, Riddle NC, Sakai A, Samsonova A, Sandler JE, Schwartz YB, Sher N, Spokony R, Sturgill D, van Baren M, Wan KH, Yang L, Yu C, Feingold E, Good P, Guyer M, Lowdon R, Ahmad K, Andrews J, Berger B, Brenner SE, Brent MR, Cherbas L, Elgin SC, Gingeras TR, Grossman R, Hoskins RA, Kaufman TC, Kent W, Kuroda MI, Orr-Weaver T, Perrimon N, Pirrotta V, Posakony JW, Ren B, Russell S, Cherbas P, Graveley BR, Lewis S, Micklem G, Oliver B, Park PJ, Celniker SE, Henikoff S, Karpen GH, Lai EC, MacAlpine DM, Stein LD, White KP, Kellis M. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science. 2010;330:1787–1797. doi: 10.1126/science.1198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royzman I, Hayashi-Hagihara A, Dej KJ, Bosco G, Lee JY, Orr-Weaver TL. The E2F cell cycle regulator is required for Drosophila nurse cell DNA replication and apoptosis. Mech Dev. 2002;119:225–237. doi: 10.1016/s0925-4773(02)00388-x. [DOI] [PubMed] [Google Scholar]
- Sahut-Barnola I, Pauli D. The Drosophila gene stand still encodes a germline chromatin-associated protein that controls the transcription of the ovarian tumor gene. Development. 1999;126:1917–1926. doi: 10.1242/dev.126.9.1917. [DOI] [PubMed] [Google Scholar]
- Scott KC, Taubman AD, Geyer PK. Enhancer blocking by the Drosophila gypsy insulator depends upon insulator anatomy and enhancer strength. Genetics. 1999;153:787–798. doi: 10.1093/genetics/153.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshnev AA, Li X, Wehling MD, Geyer PK. Context differences reveal insulator and activator functions of a Su(Hw) binding region. PLoS Genet. 2008;4:e1000159. doi: 10.1371/journal.pgen.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshnikova N, Montavon T, Leleu M, Galjart N, Duboule D. Functional analysis of CTCF during mammalian limb development. Dev Cell. 2010;19:819–830. doi: 10.1016/j.devcel.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Spana C, Corces VG. DNA bending is a determinant of binding specificity for a Drosophila zinc finger protein. Genes & Development. 1990;4:1505–1515. doi: 10.1101/gad.4.9.1505. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. 1993.
- Tollervey D, Lehtonen H, Jansen R, Kern H, Hurt EC. Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell. 1993;72:443–457. doi: 10.1016/0092-8674(93)90120-f. [DOI] [PubMed] [Google Scholar]
- Udvardy A, Maine E, Schedl P. The 87A7 chromomere. Identification of novel chromatin structures flanking the heat shock locus that may define the boundaries of higher order domains. Journal of Molecular Biology. 1985;185:341–358. doi: 10.1016/0022-2836(85)90408-5. [DOI] [PubMed] [Google Scholar]
- Verheyen E, Cooley L. Looking at oogenesis. Methods Cell Biol. 1994;44:545–561. [PubMed] [Google Scholar]
- Volpe AM, Horowitz H, Grafer CM, Jackson SM, Berg CA. Drosophila rhino encodes a female-specific chromo-domain protein that affects chromosome structure and egg polarity. Genetics. 2001;159:1117–1134. doi: 10.1093/genetics/159.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev. 2007;17:400–407. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan LB, Pan H, Hannenhalli S, Cheng Y, Ma J, Fedoriw A, Lobanenkov V, Latham KE, Schultz RM, Bartolomei MS. Maternal depletion of CTCF reveals multiple functions during oocyte and preimplantation embryo development. Development. 2008;135:2729–2738. doi: 10.1242/dev.024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Brockenbrough JS, Metcalfe AC, Chen S, Aris JP. Nop5p is a small nucleolar ribonucleoprotein component required for pre-18 S rRNA processing in yeast. J Biol Chem. 1998;273:16453–16463. doi: 10.1074/jbc.273.26.16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova J, Caiafa P. CCCTC-binding factor: to loop or to bridge. Cell Mol Life Sci. 2009;66:1647–1660. doi: 10.1007/s00018-009-8647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Somatic cells of the germarium express Su(Hw). A representative image of a wild type (su(Hw)+/+) germarium stained with DAPI (blue) and antibodies against Su(Hw) (green) and Traffic Jam (red). Merged images are shown to display colocalization of each signal. Scale bars are 20 microns.
Supplemental Figure 2. Su(Hw) zinc finger mutants form foci that co-localize with Mod67.2 and CP190 in somatic and germ cells. A) Representative magnified images of stage 6 egg chamber NC nuclei from su(Hw)f/v and su(Hw)E8/2 ovaries stained with DAPI and antibodies against Su(Hw), Mod67.2 or CP190. Scale bars are 5 microns. B) Representative images of whole mount salivary gland nuclei from su(Hw)+/+ (Canton S), su(Hw)f/v and su(Hw)E8/v third instar larvae stained with DAPI (red) and antibodies against Su(Hw) (green). Scale bars are 10 microns.
Supplemental Figure 3. Processing of rRNA is normal in su(Hw) mutant ovaries. A) Diagram of the ribosomal DNA (rDNA) locus in Drosophila. Mature 2S, 5S, 18S, 28Sa and 28Sb rRNAs are produced via cleavages at six different sites (labeled 1 to 6). Three different probes (I, II, III) detect the processing intermediates a, b and d. (B) Northern analysis of total RNA isolated from Canton S (+/+), su(Hw)f/v (f/v) and su(Hw)2/v (2/v) ovaries. The sizes of standards are shown on the left. The identity of each rRNA intermediate detected is labeled on the far right. (C) Methylene blue staining of membranes used in northern analyses showing mature 18S, 28Sa and 28Sb rRNAs.
Supplemental Figure 4. Su(Hw), Mod67.2 and CP190 co-localize on nurse cell polytene chromosomes. Nurse cell polytene chromosomes obtained from otu11, su(Hw)+/+ females were stained with DAPI (blue) and antibodies against Su(Hw) (green), Mod67.2 or CP190 (red). Merged images are shown for both Su(Hw) (green)/Mod67.2 (red) and Su(Hw) (green)/CP190 (red).