Abstract
Summary
Prostate cancer is characterized by its dependence on androgen receptor and frequent activation of PI3K signaling. We find that AR transcriptional output is decreased in human and murine tumors with PTEN deletion and that PI3K pathway inhibition activates AR signaling by relieving feedback inhibition of HER kinases. Similarly, AR inhibition activates AKT signaling by reducing levels of the AKT phosphatase PHLPP. Thus, these two oncogenic pathways cross-regulate each other by reciprocal feedback. Inhibition of one activates the other, thereby maintaining tumor cell survival. However, combined pharmacologic inhibition of PI3K and AR signaling caused near complete prostate cancer regressions in a Pten-deficient murine prostate cancer model and in human prostate cancer xenografts, indicating that both pathways coordinately support survival.
Significance
The two most frequently activated signaling pathways in prostate cancer are driven by AR and PI3K. Inhibitors of the PI3K pathway are in early clinical trials and AR inhibitors confer clinical responses in most patients. However, these inhibitors rarely induce tumor regression in preclinical models. Here we show that these pathways regulate each other by reciprocal negative feedback, such that inhibition of one activates the other. Therefore, tumor cells can adapt and survive when either single pathway is inhibited pharmacologically. Our demonstration of profound tumor regressions with combined pathway inhibition in preclinical prostate tumor models provides rationale for combination therapy in patients.
Introduction
Prostate cancer is the most common malignancy diagnosed in males and the second most common cause of male cancer deaths (American Cancer Society 2010 Facts and Figures). Despite advances made in the early detection and treatment of localized prostate cancer, the American Cancer Society estimates that 32,050 men will have died from metastatic disease in 2010. Androgen deprivation therapy remains the standard treatment of metastatic prostate cancer; however, progression to castrate resistance disease occurs in the majority of patients (Rini and Small, 2002; Singh et al., 2010). Following the emergence of castrate resistant prostate cancer, docetaxel chemotherapy has been shown to be therapeutically efficacious; however, the median increase in survival was only 4 months (Petrylak et al., 2004). Thus there is a significant need for improvements in therapy for prostate cancer.
The PI3K pathway plays a central role in tumorigenesis across a variety of malignancies (Courtney et al., 2010; Liu et al., 2009). Prostate cancers are associated with genetic alterations involving the PI3K and AR pathways, both of which mediate survival signals in prostate cancer. Roughly 40 percent of primary and 70 percent of metastatic prostate cancers have genomic alterations in the PI3K signaling pathway, mostly through loss of PTEN (El Sheikh et al., 2008; Reid et al., 2010; Taylor et al., 2010). Preclinical studies of mice with conditional, prostate-specific Pten deletion and of cell lines with stable silencing of Pten by RNA interference have established that loss of PTEN promotes resistance to castration (Gao et al., 2006; Jiao et al., 2007). However, this effect of PTEN loss is not absolute because certain prostate cancer xenograft models with PTEN loss (LNCaP, LAPC9) remain at least partially sensitive to castration (Craft et al., 1999). Furthermore, the high clinical response rate to castration therapy indicates that at least some PTEN-deficient tumors retain some degree of sensitivity.
The critical role of PTEN in regulating flux through the PI3K signaling pathway raises the possibility that PI3K pathway inhibitors might be effective in PTEN-deficient prostate cancer. Indeed, genetic loss of either mTOR or AKT1 is sufficient to significantly reduce the initiation of prostate cancer in the conditional Pten model (Chen et al., 2006; Guertin et al., 2009; Nardella et al., 2009). The mTORC1 inhibitor rapamycin has been shown to revert early PIN lesions in young mAKT mice; however, results in Pten prostate conditional null mouse models have been modest (Majumder et al., 2004; Zhang et al., 2009). Furthermore, clinical trials of rapamycin analogs in castration-resistant prostate cancer have failed to demonstrate clinical activity (George et al 2008 ASCO abstract). One potential liability of mTORC1 inhibition is disruption of a negative feedback loop, resulting in hyper-activation of AKT and MAPK that can promote cell survival independent of mTORC1, thereby limiting therapeutic efficacy (Carracedo et al., 2008; O’Reilly et al., 2006; Sarbassov et al., 2005).
The availability of a number of PI3K pathway inhibitors in clinical development targeting various critical components of the pathway (PI3K, AKT, mTORC1/2) allows this issue to be readdressed (Serra et al., 2008). The goal of our study was to evaluate the therapeutic efficacy of PI3K pathway inhibition in pre-clinical models of prostate cancer and to define the molecular mechanism of PI3K and AR feedback regulation. Through this work we propose combination therapy based on targeting compensatory survival pathways associated with relief of feedback inhibition observed following PI3K or AR inhibition.
Results
Inhibition of the PI3K pathway causes growth arrest but not significant tumor regression in Pten negative prostate cancers
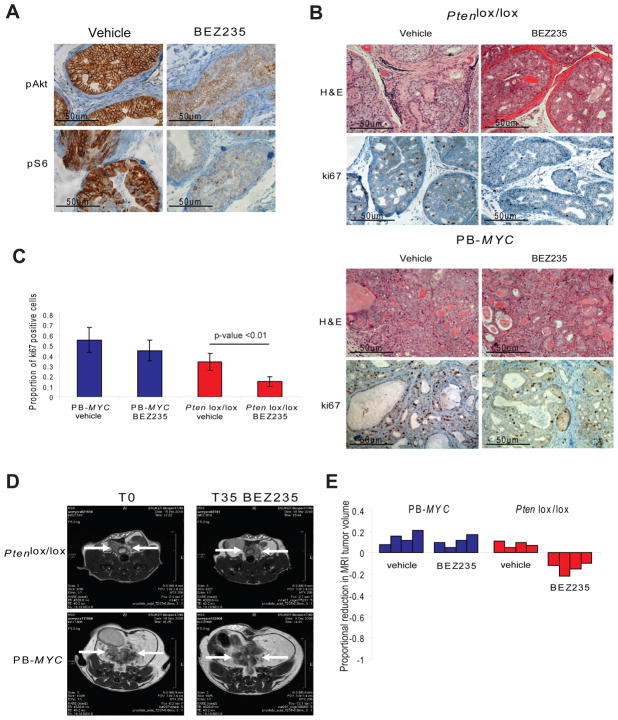
We evaluated the therapeutic efficacy of PI3K pathway inhibition in mice with established prostate cancers caused by either conditional deletion of Pten (Ptenlox/lox PB-Cre, hereafter called Ptenlox/lox) or transgenic expression of MYC (PB-MYC) using BEZ235, a dual PI3K and mTORC1/2 inhibitor (Serra et al., 2008). PB-MYC mice were chosen because MYC amplification or overexpression is also commonly found in human tumors. This model likely represents a subset of human prostate cancer distinct from that driven by PTEN loss. PI3K/mTOR inhibition was confirmed in the Ptenlox/lox mice using pAKT and pS6 and in the PB-MYC mice using pS6 (Figure 1A, Figure S1A). Cell proliferation as measured by Ki67 staining was significantly reduced in the Ptenlox/lox mice (proportion of positive cells: 0.34 versus 0.15, for vehicle control and BEZ235, respectively, p<0.01) but not in PB-MYC mice (Figure 1B, 1C). However, there was minimal reduction in prostate cancer tumor volume as measured by MRI (mean volume reduction of 14.75%) and no obvious effect on tumor histology (Figure 1B, 1D, 1E). PB-MYC prostate cancers showed no radiographic or histologic response (Figure 1B, 1C, 1D, 1E). In summary, BEZ235 has modest, primarily cytostatic, activity in Ptenlox/lox mice but no activity in PB-MYC mice, consistent with earlier studies in vitro studies in breast cancer cell lines (Brachmann et al., 2009).
Figure 1. Pre-clinical trial of PI3K pathway inhibition in GEM models of prostate cancer.
(A) Target inhibition was confirmed with BEZ235 treatment (45 mg/kg/day) by analyzing the expression of pAkt and pS6 in the Ptenlox/lox mice (microscopy at 400x). (B) Histologic and immunohistochemical staining for ki67 in PB-MYC and Ptenlox/lox mice treated with vehicle control or BEZ235 (microscopy at 200x). (C) Quantification of ki67 staining (mean of 3 HPF +/− SD) for PB-MYC and Ptenlox/lox mice treated with vehicle and BEZ235. (D) MRI images of representative PB-MYC and Ptenlox/lox mice at initiation and completion of study evaluating PI3K pathway inhibition with BEZ235. (E) Waterfall plot depicting proportional change in tumor response for PB-MYC (n=4) and Ptenlox/lox (n=4) mice treated with vehicle and BEZ235. See also Figure S1.
Inhibition of the PI3K pathway activates the AR pathway in PTEN negative prostate cancers
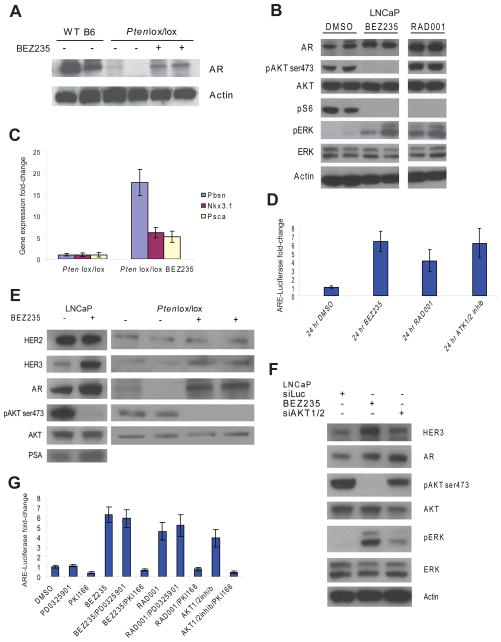
Given the critical role of AR in prostate cancer initiation and progression, we hypothesized that sustained AR activity might explain the persistent survival of Pten null prostate cells in Ptenlox/lox mice treated with BEZ235. To our surprise, we found that Ptenlox/lox mice had reduced AR protein levels compared to their Pten wild-type littermates. Treatment of Ptenlox/lox mice with BEZ235 partially rescued AR protein levels, indicating that increased PI3K/mTOR activity likely explains the decrease in AR levels (Figure 2A). Similar effects of PI3K/mTOR inhibition (BEZ235) or mTORC1 (RAD001) inhibition on AR protein levels were observed in the PTEN-deficient human prostate cancer cell line LNCaP (Figure 2B). As expected from earlier studies with rapamycin (Carracedo et al., 2008), p-ERK levels were increased following treatment with either BEZ235 or RAD001 (Figure 2B). Thus, PI3K pathway inhibition in PTEN-deficient prostate cancer resulted in the activation of two critical cell survival pathways (AR and MAPK).
Figure 2. Inhibition of the PI3K pathway restores, in part, androgen responsive signaling in PTEN loss prostate cancers.
(A) AR protein levels by Western blot analysis in wild-type, Ptenlox/lox, and Ptenlox/lox mice treated with BEZ235. Ventral prostates from individual 8-week old mice were analyzed. (B) Western blot analysis of AR, pAKT, pS6, pERK in the LNCaP cell line treated for 24 hours with DMSO, BEZ235 (500nM), and RAD001 (100nM). (C) Expression analysis (qRT-PCR) of the androgen regulated genes Pbsn, Nkx3.1, and Psca in wild-type, Ptenlox/lox, and Ptenlox/lox mice treated with BEZ235, normalized to Hprt and wild-type intact mice. Ventral prostates from individual 8-week old mice were analyzed and mean fold-change +/− SD is reported. (D) Luciferase mean fold-change +/− SD measurements in the LNCaP AR-ARE-Luciferase cell line treated for 24 hours with DMSO, BEZ235 (500nM), RAD001 (100nM), and AKT1/2 inhibitor (1uM) normalized to DMSO. (E) Western blot analysis of HER2, HER3, AR, and PSA in the LNCaP cell line treated with DMSO and BEZ235 (500nM) for 24 hours and Ptenlox/lox mice treated with BEZ235. (F) Western blot analysis for HER3, AR, pAKT, pERK in LNCaP cells treated with BEZ235 or siRNA AKT1/2 for 24 hours. (G) Luciferase mean fold-change +/− SD measurements in the LNCaP AR-ARE-Luciferase cell line treated for 24 hours with DMSO, BEZ235 (500nM), RAD001 (100nM), with or without PKI166 (5uM) or PD0325901 (1uM) normalized to DMSO.
We next evaluated whether the increase in AR protein levels seen with PI3K pathway inhibition resulted in increased AR target gene activity. Indeed, mRNA levels of three canonical AR target genes, Pbsn, Nkx3.1 and Psca, were increased by short-term treatment of Ptenlox/lox mice with BEZ235 (Figure 2C). Similarly, the activity of an androgen responsive reporter gene was increased in LNCaP cells exposed to BEZ235 or RAD001, consistent with other reports using rapamycin (Figure 2D) (Lin et al., 2003; Wang et al., 2008). Increased androgen responsive reporter gene activity was also observed following treatment with an allosteric, highly specific, inhibitor of AKT1 and AKT2, providing further evidence that these pharmacologic effects are due to PI3K pathway blockade (Figure 2D). Collectively, the data from these PTEN deficient murine and human models indicate that PI3K pathway inhibitors can activate AR target gene expression.
HER family receptor tyrosine kinases and the insulin-like growth factor receptor (IGFR) are feedback inhibited by the PI3K pathway and reactivated in breast tumor cell lines and xenograft models exposed to AKT inhibitors (Chandarlapaty et al., 2011). Consistent with these data, others have found that inhibition of PI3K pathway in breast cancer cell lines results in up-regulation of HER3 (Yao et al., 2009). We therefore considered that similar effects may be seen in PTEN-deficient prostate cancers. Indeed, the level of HER3 was increased in LNCaP cells and in prostates of Ptenlox/lox mice after exposure to BEZ235 (Figure 2E). To address potential off target effects associated with pharmacologic inhibition of PI3K pathway kinases, we targeted AKT1/2 using siRNA. AKT1/2 knock-down led to up-regulation of both HER3 and AR, as well as pERK similar to that observed with BEZ235, albeit to more modest levels (Figure 2F). We and others previously demonstrated that HER2/HER3 promotes AR activity and stability by an AKT-independent mechanism by enhancing both AR stability and transcriptional activity, similar to the effects observed here with BEZ235 (Mahajan et al., 2007; Mellinghoff et al., 2004; Yeh et al., 1999). Therefore, we postulated that the increase in HER3 expression induced by PI3K pathway inhibition might explain the increase in AR transcriptional output,. Consistent with this hypothesis, co-treatment with the HER family kinase inhibitor PKI166 (EGFR and HER2 inhibitor) abolished the up-regulation of AR activity observed with either BEZ235, RAD001 or AKT1/2 inhibitor (Figure 2G).
To address the potential role of ERK activation in the upregulation of AR activity, we examined the effects of MEK inhibition using the MEK inhibitor PD0325901. In contrast to the stimulatory effects of BEZ235 and RAD001 on AR signaling, PD0325901 did not augment AR signaling. Furthermore, PD0325901 did not reverse AR activation induced by BEZ235 or RAD001, despite the fact that ERK is activated by both drugs (Figure 2G). Thus, inhibition of the PI3K pathway up-regulates AR target gene expression in a HER kinase-dependent manner independent of MEK.
PTEN loss is associated with repression of androgen responsive genes
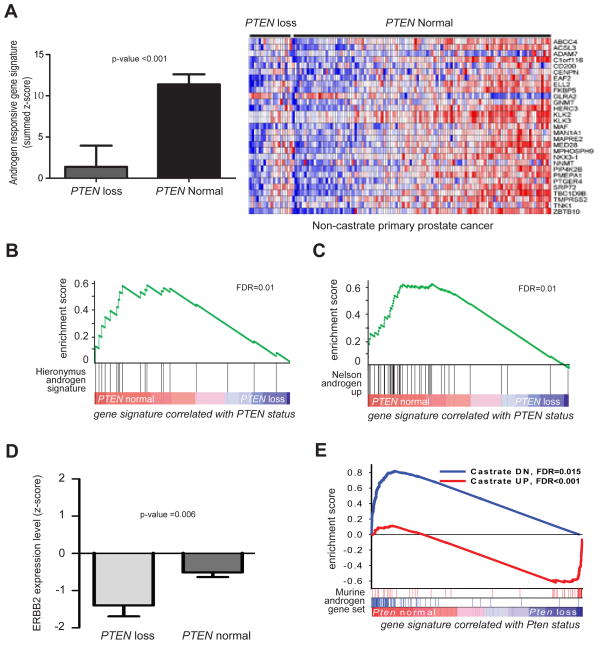
Having demonstrated that inhibition of the PI3K pathway results in increased AR activity in two prostate cancer models, we explored the relevance of this finding in human prostate cancer specimens. Because clinical trials of PI3K pathway inhibitors in prostate cancer are still in early stages, we asked the reciprocal question of whether PI3K activation caused by PTEN loss impairs AR activity in primary human prostate tumors. One-hundred and six tumors from a previously reported MSKCC dataset were designated PTEN loss or PTEN normal based on PTEN copy number and PTEN mRNA expression level. These PTEN status assignments were validated by gene set enrichment analysis (GSEA) showing concordance with a transcriptome-based signature of PTEN loss developed independently from breast cancer specimens (Figure S2A) (Saal et al., 2007). We then analyzed AR pathway activation by PTEN status using a previously reported mRNA signature of AR target genes (Hieronymus et al., 2006). AR activity was significantly repressed in PTEN loss prostate tumors (t-test on summed z-score, p-value <0.001, Figure 3A). Consistent with this finding, GSEA of gene sets differentially regulated in PTEN loss and PTEN normal prostate tumors revealed that the same androgen regulated gene set was significantly repressed in the PTEN loss cancers (FDR=0.01, Fig 3B) (Hieronymus et al., 2006). This association was also observed with two other independently derived AR target gene sets (Nelson FDR=0.01, Figure 3C, and Creighton FDR<0.001, data not shown) (Creighton, 2008; Nelson et al., 2002).
Figure 3. PTEN loss prostate cancer is associated with reduced androgen responsive gene signaling.
(A) Mean summed z-score +/− SD for an androgen responsive gene signature in human PTEN loss and PTEN normal prostate cancer showing PTEN loss tumors have a reduced androgen responsive gene signature with corresponding heat map. Significance was determined by the Student’s t-test. GSEA showing the (B) Hieronymus and (C) Nelson androgen responsive gene signatures revealed that androgen responsive gene signaling is reduced in PTEN loss human prostate cancers. (D) Mean ERBB2 expression levels +/− SD in PTEN loss and PTEN normal prostate cancer. Significance was determined by the Student’s t-test. (E) Gene signature enrichment analysis (GSEA) of a murine androgen responsive gene set in Pten loss prostate cancers compared to an intact wild-type mice. See also Figure S2.
Our observation that PI3K inhibition leads to increased HER3 levels in Ptenlox/lox mice and in LNCaP cells raises the possibility that human tumors with PTEN loss might have decreased HER2/3 activity. We did not observe significant differences in HER3 mRNA levels, but HER2 (ERBB2) expression was significantly (p=0.006) reduced in PTEN loss prostate cancers (Figure 3D). Furthermore, HER2 expression was significantly (p<0.003) correlated with AR target gene signature output (Figure S2B).
Because other genomic alterations may impact the interpretation of the human tumor studies, we examined AR activity in primary prostate tissue harvested from 8 week Ptenlox/lox mice (with or with Pten deletion) before the onset of prostate cancer. To define a murine AR gene signature, we first compared transcriptomes of prostates from wild-type mice to those from littermates isolated 3 days post-castration (Table S1). In parallel, we compared transcriptome data from prostates isolated from intact Pten+/+ (wild-type) and Pten−/− mice (without castration). GSEA revealed that genes up- or down-regulated in response to castration in wild-type mice were significantly enriched in intact Pten−/− prostates compared to intact Pten+/+ prostates, indicating that Pten loss is associated with reduced AR activity (Figure 3E). Examination of individual genes revealed that a substantial number of the genes up- or downregulated by castration in intact mice are already up- or downregulated in intact Pten−/− mice (Figure S2C, Table S2). Together with the human prostate tumor data and the BEZ235 treatment studies, these findings establish that the increase in PI3K activation associated with PTEN loss impairs AR signaling.
Inhibition of AR promotes PI3K activity in PTEN loss prostate cancer
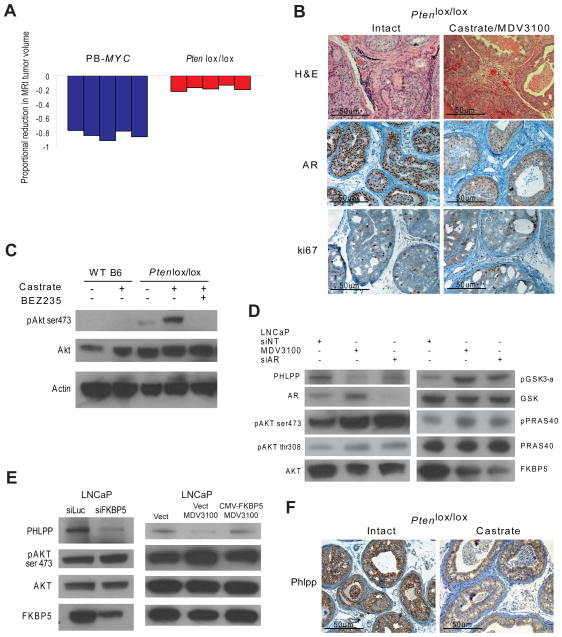
Previous studies in mouse models and cell lines have implicated PTEN loss as a potential cause of castration resistance (Gao et al., 2006; Zhang et al., 2009). Our finding that PI3K activation is associated with reduced AR output suggest a potential explanation, e.g. these tumors are less dependent on AR. However, it is possible that AR function, albeit low, remains intact due to low circulating androgens that remain after castration. To investigate the potential role of persistent AR signaling in this context, we evaluated the effect of combined androgen blockade (surgical castration plus the next generation AR inhibitor MDV3100 that impairs AR nuclear translocation and DNA binding) (Tran et al., 2009) in the Pten−/− model. After 7 days of treatment, mRNA levels of the androgen regulated genes Pbsn, Nkx3.1, and Psca were decreased 25–50 fold (Figure S3A) and AR protein levels were primarily cytoplasmic (Figure 4B), confirming substantial inhibition of AR pathway output in tumors isolated from treated mice. Despite this magnitude of pathway inhibition, tumors showed only modest regression (mean reduction in tumor volume of 19.2%) without obvious histologic changes (Figure 4A, 4B, Figure S3B). In addition, there was minimal effect on proliferation as measured by Ki67 staining (proliferative indices of 0.34 and 0.28 pre and post-treatment) (Figure 4B, Figure S3D). In contrast, the same treatment regimen in PB-MYC mice resulted in profound reductions in tumor volume (mean of 81.42%), near complete pathologic responses and virtually absent Ki67 staining (reduction in proliferative index from 0.554 to 0.012, p-value <0.001) (Figure 4A, Figure S3B, S3C, S3D). We conclude that even combined AR blockade remains ineffective in Pten−/− mice.
Figure 4. Pre-clinical trial of combined androgen blockade in GEM models of prostate cancer.
(A) Waterfall plot depicting proportional change in tumor response for PB-MYC (n=5) and Ptenlox/lox (n=5) mice treated with castration and MDV3100 (30 mg/kg/day). (B) Histologic and immunohistochemical staining for AR and ki67 and Ptenlox/lox mice treated with vehicle control or castration and MDV3100 (microscopy at 200x). (C) Western blot analysis of prostates from wild-type and Ptenlox/lox mice treated with surgical castration and/or BEZ235 for 7-days. Ventral prostates from individual 8-week old mice were analyzed. (D) Western blot analysis for PHLPP, FKBP5, pAKT in LNCaP treated with MDV3100 (10uM) or siRNA AR for 24 hours. (E) Western blot analysis for PHLPP, FKBP5, pAKT in LNCaP transfected with siRNA FKBP5. Western blot analysis for PHLPP, FKBP5, pAKT in LNCaP transfected with vector control, treated with MDV3100 (10uM) or transfected with CMV-FKBP5 and treated with MDV3100. (F) Phlpp immunohistochemistry of intact and castrate Ptenlox/lox mice. See also Figure S3.
Although it is formally possible that the 50-fold impairment in AR output was simply not enough to impair survival of PTEN deficient prostate cells, another explanation could be persistent survival signaling through AKT. Remarkably, AKT phosphorylation at Ser473 was increased in prostates of Ptenlox/lox mice following castration. This increase was likely PI3K pathway dependent since it was inhibited by concurrent treatment with BEZ235 (Figure 4C). Similar results, including increased phosphorylation of downstream AKT targets such as GSK-alpha and PRAS40, were observed in PTEN negative LNCaP cells treated with MDV3100 (Figure 4D). We also observed increased levels of pAKT in the AR positive cell line LAPC4 following treatment with MDV3100 (Figure S3E). The effects of MDV3100 on AKT activation are likely specific to AR inhibition since siRNA knockdown of AR gave similar results (Figure 4D) and no change in pAKT levels was observed in AR-negative PC3 cells (Figure S3F).
The immunophilin FKBP5 is a chaperone for the AKT phosphatase PHLPP and its expression in prostate cancer is androgen dependent (Gao et al., 2005; Pei et al., 2009). We hypothesized that AR inhibition would result in reduced FKBP5 expression and, consequently, lower PHLPP protein levels, and this could cause increased phosphorylation of AKT. Indeed, FKBP5 and PHLPP protein levels were both reduced in LNCaP cells treated with MDV3100 or siRNA AR, and this was accompanied by an increase in phosphoAKT (Figure 4D). siRNA knockdown of PHLPP in the LNCaP cell line resulted in increased levels of pAKT as expected and importantly, knockdown of FKPB5 resulted in decreased levels of PHLPP and upregulation of pAKT, phenocopying the effects of MDV3100 (Figure 4E, Figure S3G). Furthermore, constitutive expression of FKBP5 (CMV-FKBP5) resulted in stable levels of PHLPP and blocked the up-regulation of pAKT in the presence of MDV3100 (Figure 4E). Protein levels of PHLPP were also lower in Ptenlox/lox mice following castration (Figure 4F). These data suggest that AR negatively regulates AKT activity through stabilization of PHLPP. Consequently, AR inhibition destabilizes PHLPP and results in unchecked AKT activation, especially in the setting of PTEN loss.
Combined PI3K and AR pathway inhibition gives profound tumor regressions
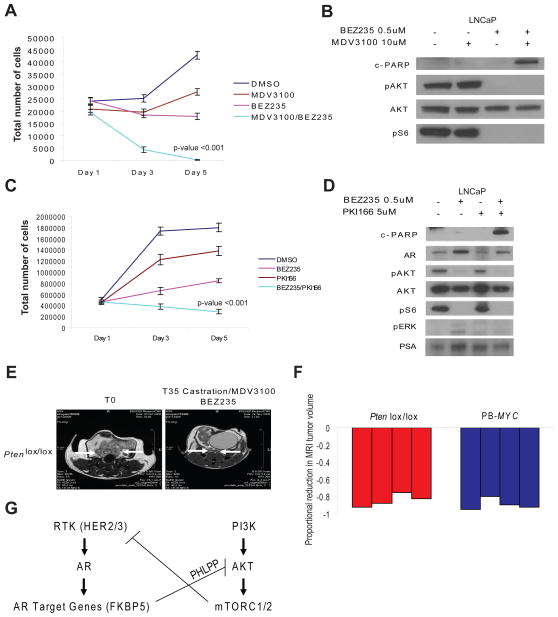
Taken together, the effects of PI3K inhibitors on the AR pathway and AR inhibitors on the PI3K pathway in PTEN deficient prostate cells demonstrate that perturbations in the activity of one pathway impact signaling through the other pathway. We therefore evaluated the effect of combined PI3K and AR pathway inhibition in PTEN-deficient LNCaP cells and in the conditional Pten−/− prostate cancer model. BEZ235 and MDV3100 each displayed modest single agent antiproliferative activity in LNCaP cells (primarily cytostatic), but neither treatment promoted apoptotic cell death (Figure 5A, 5B). However, the combination of BEZ235 with MDV3100 led to a profound decrease in cell number and an increase in cleaved PARP, a marker of apoptosis (Figure 5A, 5B). To determine if similar effects might be observed by inhibiting mTORC1 or MEK, we compared the effects of RAD001 or PD0325901 to BEZ235, alone and in various combinations, including with MDV3100 (Figure S4A, S4B). The greatest antiproliferative effect was observed with combined treatment with BEZ235 and MDV3100, indicating that PI3K and/or mTORC1/2 and AR, but not mTORC1 or MEK, appear to be the most critical targets in this model. Based on our discovery that inhibition of the PI3K pathway promotes AR activity in a HER2/3 dependent manner, we reasoned that that a HER2/3 inhibitor might be similarly efficacious (replacing MDV3100) in combination with BEZ235. Indeed, combined treatment with BEZ235 and PKI166 was as effective as BEZ235 plus MDV3100 (Figure 5C, 5D). Furthermore, inhibition of HER2/3 abolished the upregulation of AR protein levels and transcriptional activity observed with PI3K pathway inhibition (Figure 5D), as measured by PSA expression.
Figure 5. Therapeutic efficacy of combined androgen blockade and PI3K pathway inhibition in pre-clinical models of prostate cancer.
(A) LNCaP cell proliferation assay reported as mean cell count +/− SD following treatment with DMSO, MDV3100 (10uM), BEZ235 (500nM) and combinations. (B) Western blot analysis of LNCaP cells treated with DMSO, MDV3100, BEZ235, and BEZ235/MDV3100 for cleaved-PARP, pAKT, AKT, and pS6. (C) LNCaP cell proliferation assay reported as mean cell count +/− SD following treatment with DMSO, MDV3100 (10uM), PKI166 (5uM) and combinations. (B) Western blot analysis of LNCaP cells treated with DMSO, PKI166, BEZ235, and BEZ235/PKI166 for cleaved-PARP, pAKT, pERK, pS6, and PSA. (E) MRI images of representative Ptenlox/lox mice at initiation and completion of study evaluating combined AR and PI3K pathway inhibition with castration, MDV3100 (30 mg/kg/day), and BEZ235 (45 mg/kg/day). (F) Waterfall plot depicting proportional change in tumor response for Ptenlox/lox (n=4) and PB-MYC (n=4) mice treated with combined AR and PI3K pathway inhibition. (G) Model of PI3K pathway and AR crosstalk demonstrating activation of the PI3K pathway in the setting of PTEN loss leads to up-stream feed-back inhibition on receptor tyrosine kinases (HER2/3) leading to repressed AR activity. Inhibition of the PI3K pathway stimulates up-stream HER2/3 resulting in activation of AR, while blockade of AR reduces FKBP5 levels impairing PHLPP function leading to up-regulation of pAKT. See also Figure S4.
To test the impact of combined PI3K/AR therapy in tumor models, Ptenlox/lox mice with established prostate tumors were treated with BEZ235 + MDV3100 and castration. Combined PI3K and AR pathway inhibition led to dramatic reductions in tumor volume (mean tumor volume reduction of 84.25%) with near complete pathologic responses and no evidence of residual cell proliferation detectable by Ki67 staining (Figure 5E, 5F, Figure S4C). Combined PI3K/AR therapy also induced regressions in LNCaP xenografts (24% mean reduction in tumor volume) whereas average tumor volume in mice treated with vehicle or single pathway therapy (BEZ235 or castration) increased (28%, 30%, and 31%, respectively) (Figure S4D). Addition of BEZ235 to castration plus MDV3100 in PB-MYC mice showed no measurable benefit, but the substantial response to combined androgen blockade alone in this model makes it difficult to detect any effect of combined PI3K/AR therapy (Figure 5F).
Discussion
AR pathway inhibition has long been the treatment of choice for men with metastatic prostate cancer. While much attention has been devoted to mechanisms of acquired resistance, there has been little investigation of the considerable variability in primary response among patients. Here we show, by mRNA transcriptome analyses, that activation of the PI3K pathway is associated with repressed androgen signaling in mouse and human prostate cancers and that this may, in part, be responsible for the castrate resistant phenotype observed with these prostate tumors. Importantly, we demonstrate that this resistance is reversible because inhibition of the PI3K pathway restores AR signaling in PTEN deficient prostate cells. At least one mechanism appears to be through relief of negative feedback to HER kinases. Similarly, blockade of AR relieves feedback inhibition of AKT by the phosphatase PHLPP. This reciprocal feedback regulation of the PI3K and AR pathways provides a compelling explanation for the poor efficacy of single pathway therapy in PTEN null cancers and the substantially better effects of combined PI3K/AR pathway inhibition (Figure 5G).
Prior work has implicated PTEN loss as a potential cause of castration resistance in mice and in humans (Gao et al., 2006; Zhang et al., 2009). Zhang and colleagues reported that Pten prostate conditional null mice treated with surgical castration have a delay in tumor growth and minimal tumor regression (Zhang et al., 2009). Although no human studies have formally addressed this question, there is evidence from presurgical treatment studies that tumors with PTEN loss are relatively refractory to bicalutamide (Ham et al., 2009; Schulman et al., 2000). Despite the evidence that PTEN loss can promote castration resistance, there is little insight into the mechanism. Some reports have suggested that PTEN loss activates AR, through PI3K-mediated stabilization of AR protein levels or AKT-mediated phosphorylation and transcriptional activation of AR. Conversely, other studies have demonstrated that PI3K activation promotes degradation of AR and inhibits AR transcriptional activity (Lin et al., 2004; Lin et al., 2003; Wang et al., 2008). Our transcriptome studies make a strong case for the latter model. In addition, our finding that reduced expression of the AR target gene FKBP5 results in an increase in AKT activation (via PHLPP degradation) in PTEN null cancers further explains the survival advantage of these tumor cells in the setting of castration.
This work has immediate implications for the design of clinical trials evaluating PI3K pathway inhibitors in prostate cancer. Our preclinical data predict that single agent PI3K pathway inhibitor therapy will most likely result in disease stabilization rather that tumor regression, particularly in PTEN null tumors which represent ~40 percent of primary cancers and ~70 percent of metastases (Taylor et al., 2010). Additionally, given that the primary serum marker used to monitor disease progression (prostate-specific antigen or PSA) is androgen regulated, patients treated with PI3K pathway inhibitors may experience a rise in PSA level if their tumors are PTEN deficient. Our data argue that combined therapy with an AR pathway inhibitor is required for maximal efficacy in PTEN null cancers. In patients with hormone-naïve disease this could be achieved using currently available antiandrogen therapy, but patients with castration resistant prostate cancer are likely to require next generation AR pathway inhibitors such as abiraterone or MDV3100.
Because BEZ235 inhibits both PI3K and mTORC1/2, our data do not delineate which target is most critical for the observed effects of combination therapy. Others reported beneficial effects of combined AR and mTORC1 (rapalog) inhibition in a similar Ptenlox/lox model, but the magnitude of tumor response was less substantial since mice had significant amounts of residual tumor tissue at the time of sacrifice (Zhang et al., 2009). In addition, these investigators monitored tumor volume by ultrasound, which makes it difficult to distinguish between shrinkage caused by true tumor regression versus a reduction in the cystic dilation that accompanies Pten−/− prostate tumors. Kinkade et al also reported benefit from combining rapamycin with a MEK inhibitor in Nkx3.1−/−; Pten+/− mice, but this experiment differs in that Pten+/− mice have a less aggressive cancer phenotype than the Ptenlox/lox model (Kinkade et al., 2008). Side-by-side experiments using identical endpoints in the same model are required to properly compare these regimens. In the meantime, our in vitro studies establish that dual PI3K/mTORC1/2 inhibition (BEZ235) is superior to mTORC1 inhibition (RAD001) when combined with AR blockade and that MEK inhibition (PD0325901) is relatively ineffective. Because BEZ235 inhibits mTORC1/2 more potently than PI3K, it is possible that the superiority of BEZ235 over RAD001 is solely through TORC1/2 blockade (Serra et al., 2008). This question can be addressed using selective TORC1/2 inhibitors (Chresta et al., 2010; Thoreen et al., 2009).
Our finding that HER2/3 activation (and subsequent AR activation) is associated with PI3K pathway inhibition also has important clinical implications since a HER2 kinase inhibitor such as lapatinib could, in theory, replace the requirement for an antiandrogen in combination with a PI3K pathway inhibitor. Our studies with the preclinical HER2 inhibitor PKI-166 establish this principle in vitro. Single agent trials with HER2 inhibitors (trastuzamab, pertuzumab, lapatinib) in men with castration resistant prostate cancer have been largely negative (Solit and Rosen, 2007), but our data suggest that combination of these inhibitors with PI3K pathway inhibitors is required to elicit activity.
In summary, our results demonstrate that inhibition of the PI3K pathway in PTEN negative prostate cancer results in feedback signaling to the receptor tyrosine kinase HER2/HER3 leading to activation of AR. Conversely, blockade of AR results in activation of AKT through reduced levels of FKBP5 impairing the stability of PHLPP. This bidirectional crosstalk between two critical survival pathways in prostate cancer provides the molecular rationale for simultaneously targeting both pathways. The success of clinical trials evaluating PI3K pathway inhibitors in prostate cancer could be optimized by enrolling patients with documented activation of the PI3K pathway and treating in combination with appropriate AR pathway inhibition.
Experimental Procedures
Mouse models of prostate cancer
Animal studies were carried out under protocol 06-07-012 approved by the MSKCC Institutional Animal Care and Use Committee. Institutional guidelines for the proper, humane use of animals in research were followed. The GEM models of human prostate cancer (PB-MYC and Ptenlox/lox PB-Cre) have been described previously (Ellwood-Yen et al., 2003; Trotman et al., 2003). Genotyping was conducted through our core facility using previously published primer sets and protocols. PB-MYC (ages 12 mo and 18 mo) and Ptenlox/lox (age 6 mo) were imaged by our MRI small animal imaging core prior to and at the completion of treatment (see experimental methods) (Chen et al., 2005). Surgical castration was performed under anesthesia with isoflurane. Mice were monitored post-operatively for recovery from anesthesia and checked daily for 2 days post-operatively. Surgical skin clips were removed on post-operative day 5. Mice undergoing treatment were administered control vehicle or therapeutic doses of the appropriate agents by oral gavage on a Monday through Friday schedule for a total of 35 days. Any mouse suffering distress or greater than 15% weight loss during treatment was euthanized by CO2 asphyxiation. MRI tumor volumes were reported for each mouse at time point 0 (T0) at initiation of study and time point 35 days (T35) at completion of study. Changes in tumor volumes between T0 and T35 were calculated for individual mice and reported in waterfall plots. At the completion of study mice were euthanized by CO2 asphyxiation and tissue was procured for histology, mRNA analysis, protein analysis and tissue banking.
For xenograft experiments, 1×106 LNCaP cells were injected into the bilateral flanks of SCID mice. When mice tumors were approximately 500 mm3 mice were randomized to the treatment groups. Tumor volume was measured bi-weekly for a total of 2 weeks and the animals were sacrificed according to our protocol. All animal experiments conform to the relevant regulatory standards and were approved by our IACUC committee under our approved animal protocol.
Targeted pathway inhibitors
The AR inhibitor MDV3100 was synthesized by the MSKCC chemistry core and used in vitro at a concentration of 10uM and in vivo with a dose of 30 mg/kg/day administered once daily by oral gavage on a Monday through Friday schedule. The PI3K pathway inhibitors NVP-BEZ235 (PI3K and mTORC1/2 inhibitor) and RAD001 (mTORC1 inhibitor) were provided by Novartis under a Materials Transfer Agreement. The concentration of BEZ235 and RAD001 used for in vitro experiments was 500nM and 100nM, respectively. For in vivo experiments the dose of BEZ235 used was 45 mg/kg/day administered once daily by oral gavage on a Monday through Friday schedule. The HER2 kinase inhibitor PKI166 was provided by Novartis and used for in vitro experiments at a concentration of 5uM. PD0325901 (MEK inhibitor) was synthesized by the MSKCC Chemistry core and used for in vitro studies at a concentration of 1uM. AKT1/2 inhibitor (AKT VIII) was purchased from Calbiochem and used in vitro at a concentration of 1uM.
Mouse mRNA expression analysis
Prostate tissues frozen for total RNA isolation were homogenized in TRIzol Reagent (Invitrogen #15596-026), followed by phase separation, washing, precipitation and resuspension of RNA in RNAse free water according to manufacturer’s protocols. The RNA was further purified using the RNeasy kit (Qiagen) according to manufacturer’s protocols, followed by quantification and normalization using A260/A280. cDNA synthesis from 1 μg RNA was carried out using the TaqMan Reverse Transcription Reagents (Applied Biosystems) with random hexamers according to the manufacturer’s protocol. Triplicate samples for quantitative PCR were run in the Realplex MasterCycler (Eppendorf) using the Power SYBR Green PCR Mastermix (Applied Biosystems). Each reaction contained 1 μL of cDNA in a total volume of 20 μL. ΔCt for each gene was determined after normalization to Hprt and Δ ΔCt was calculated relative to the designated reference sample. Gene expression values were then expressed as a fold change, calculated by 2− Δ ΔCt. See experimental methods for primer sequences.
Mouse Microarray expression profiling
Microarray gene expression profiling was performed on RNA prepared from the prostates of wild-type and Ptenlox/lox Pb-Cre intact and castrate mice. Eight week old wild-type (n=7) and Pten prostate conditional null (n=7) mice in the C57B6 background were used. Three mice of each genotype were castrated. Three days after castration, mice were euthanized and RNA was isolated from prostates then profiled on the Illumina MouseRef-8 v2 bead arrays. Raw data was imported into Partek Genomics Suite v6.5 where data was Log2 transformed and quartile normalized. The raw and normalized microarray data has been deposited into the NIH NCBI Gene Expression Omnibus (GEO), GSE24691. See experimental methods for detailed methods for generation of murine androgen responsive gene signature and GSEA analysis.
In vitro Experiments
In vitro experiments were conducted using the LNCaP and PC3 cell lines obtained from American Type Culture Collection and cell lines generated in our lab LAPC4 and LNCaP AR-ARE-Luciferase, which expresses exogenous AR and Luciferase expression under control of an androgen regulated promoter (Tran et al., 2009). Proliferation assays were conducted by plating 1×105 cells per well of a 12 well cell culture plate and treating with vehicle (DMSO) control or AR/PI3K inhibitors at the aforementioned concentrations. Viable cells were counted using a hemocytometer using trypan blue exclusion on days 1, 3, and 5. Cell lysates for western blot analysis were prepared using standard RIPA buffer. Luciferase assays were conducted using the Promega ONE-Glo luciferase assay system and measured using a luminometer plate reader. All in vitro experiments were conducted in triplicate and standard deviations were reported. Significance was determined by the Student’s t-test. The FKBP5, PHLPP, AKT1, AKT2, and AR siRNA smart-pool was obtained from Dharmacon. Control siRNA luciferase was used for all experiments. The CMV-FKPB5 plasmid was purchased from Origene.
Immunohistochemical and Western blotting Antibodies
The antibodies used for western blot analysis and immunohistochemistry were pAKT Ser473 (Cell Signaling Technology, 1:1000 dilution), pAKT Thr308 (Cell Signaling Technology 1:500 dilution), AKT (Cell Signaling Technology, 1:1000 dilution ), pS6 Ser240/244 (Cell Signaling Technology, 1:1000 dilution), pERK Thr202/Tyr204 (Cell Signaling Technology, 1:1000 dilution), ERK (Cell Signaling Technology, 1:1000 dilution), pPRAS40 Thr246 (Cell Signaling Technology, 1:1000 dilution), PRAS40 (Cell Signaling Technology, 1:1000 dilution), pGSK3-a Ser21 (Cell Signaling Technology, 1:1000 dilution), GSK (Cell Signaling Technology, 1:1000 dilution), PARP (Cell Signaling Technology, 1:1000 dilution), AR N-20 (Santa Cruz, 1:500 dilution), c-MYC (Epitomics, 1:500 dilution), PHLPP (Bethyl Laboratories 1:500 dilution), and FKBP5 (Bethyl Laboratories 1:500 dilution), HER2 (Cell Signaling Technology, 1:1000 dilution), HER3 (Cell Signaling Technology, 1:1000 dilution), and Actin (Cell Signaling Technology, 1:1000 dilution). All immunohistochemical analyses were conducted by the MSKCC Molecular Cytology core.
Human CGH and mRNA profiling
Our human prostate cancer data set has been previously published (Taylor et al., 2010). All patients provided informed consent and samples were procured and the study was conducted under Memorial Sloan-Kettering Cancer Center Institutional Review Board approval. Briefly, copy number data was generated on Agilent 244K aCGH arrays and mRNA expression data was obtained on Affymetrix Human Exon 1.0 ST arrays. The complete genomics dataset and analytic methods is reported separately and is available at: http://cbio.mskcc.org/cancergenomics-dataportal/. PTEN status was determined using primary hormone naïve tumors that had both mRNA expression data and copy number data available (n = 106). Tumors were classified as showing genomic PTEN loss (n = 19) if they showed PTEN copy number loss (single copy loss or greater by aCGH) and/or decreased PTEN mRNA level (z<−2). The remaining tumors were classified as PTEN normal (n = 87). Expression of the Hieronymus androgen-responsive gene set was scored by summing the expression z-scores per tumor within our human prostate cancer cohort. GSEA was carried out with the gene-level expression from primary hormone naïve tumor set described above stratified by genomic PTEN status, using Student’s t-test on the collapsed probe sets after normalization. Enrichment of two specific androgen responsive gene sets as well as the MSigDB curated gene set collection (c2 version 5) were tested (1000 permutations by gene set, GenePattern).
Supplementary Material
Highlights.
Inhibition of the PI3K pathway promotes AR activity
Androgen blockade activates AKT signaling
Combined PI3K and AR inhibition is superior to single agent therapy in PTEN loss prostate cancer
Acknowledgments
The authors would like to thank M. Isaka and the Genetically Engineered Mouse Facility for maintaining our mouse colonies. We would like to thank P.P. Pandolfi for providing the Ptenlox/lox PB-Cre GEM model. A special thanks to all members of the Sawyers lab and the Rosen lab for providing informative discussion. This work was funded in part through: NIH Prostate SPORE P50-CA92629; NIH Small-Animal Imaging Research Program (SAIRP) R24 CA83084; NIH Center Grant P30 CA08748. B. S. C. has a Prostate Cancer Foundation Young Investigator Award and STARR Cancer Consortium Award. C. L. S. is a Howard Hughes Medical Institute Investigator. B.S.C. and C.L.S. designed the study; B.S.C., J.W., and C.C. performed the mouse experiments. B.S.C., C.C., and V.A. performed the in vitro experiments. H.H. and Y.C. performed the expression array analyses in human and mouse prostate cancers, respectively. S.C. and N.R. provided expert guidance in defining the mechanism of PI3K feed-back inhibition on AR. J.K. and C.L. conducted the MRI animal imaging. P.T.S. and H.S. provided valuable discussion with regards to clinical correlates. B.S.C. and C.L.S. prepared the manuscript. All authors approved of the final manuscript. C.L.S. is a coinventor of MDV3100 and owns stock in Medivation.
Footnotes
Accession number
NIH NCBI Gene Expression Omnibus (GEO), GSE24691:
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=fxeffccomigcsvs&acc=GSE24691.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, Wang S, Garcia-Echeverria C, Maira SM. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. The Journal of clinical investigation. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Xu PZ, Peng XD, Chen WS, Guzman G, Yang X, Di Cristofano A, Pandolfi PP, Hay N. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes & development. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer research. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, Said J, Reiter RE, Sawyers CL. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer research. 1999;59:5030–5036. [PubMed] [Google Scholar]
- Creighton CJ. Multiple oncogenic pathway signatures show coordinate expression patterns in human prostate tumors. PloS one. 2008;3:e1816. doi: 10.1371/journal.pone.0001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sheikh SS, Romanska HM, Abel P, Domin J, Lalani el N. Predictive value of PTEN and AR coexpression of sustained responsiveness to hormonal therapy in prostate cancer--a pilot study. Neoplasia (New York, NY. 2008;10:949–953. doi: 10.1593/neo.08582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C. Emergence of androgen independence at early stages of prostate cancer progression in Nkx3.1; Pten mice. Cancer research. 2006;66:7929–7933. doi: 10.1158/0008-5472.CAN-06-1637. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Molecular cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, Mullholland DJ, Magnuson MA, Wu H, Sabatini DM. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham WS, Cho NH, Kim WT, Ju HJ, Lee JS, Choi YD. Pathological effects of prostate cancer correlate with neuroendocrine differentiation and PTEN expression after bicalutamide monotherapy. The Journal of urology. 2009;182:1378–1384. doi: 10.1016/j.juro.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, Nieto M, Du J, Stegmaier K, Raj SM, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, Wu H. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer research. 2007;67:6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. The Journal of clinical investigation. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Molecular endocrinology (Baltimore, Md. 2004;18:2409–2423. doi: 10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]
- Lin HK, Hu YC, Yang L, Altuwaijri S, Chen YT, Kang HY, Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. The Journal of biological chemistry. 2003;278:50902–50907. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature reviews. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, Earp HS, Whang YE. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nature medicine. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M, et al. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Science signaling. 2009;2:ra2. doi: 10.1126/scisignal.2000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, Hood L, Lin B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- Reid AH, Attard G, Ambroisine L, Fisher G, Kovacs G, Brewer D, Clark J, Flohr P, Edwards S, Berney DM, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. British journal of cancer. 2010;102:678–684. doi: 10.1038/sj.bjc.6605554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Small EJ. Hormone-refractory Prostate Cancer. Current treatment options in oncology. 2002;3:437–446. doi: 10.1007/s11864-002-0008-1. [DOI] [PubMed] [Google Scholar]
- Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, Koujak S, Ferrando AA, Malmstrom P, Memeo L, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4-Year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. European urology. 2000;38:706–713. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer research. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- Singh P, Yam M, Russell PJ, Khatri A. Molecular and traditional chemotherapy: a united front against prostate cancer. Cancer letters. 2010;293:1–14. doi: 10.1016/j.canlet.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Solit DB, Rosen N. Targeting HER2 in prostate cancer: where to next? J Clin Oncol. 2007;25:241–243. doi: 10.1200/JCO.2006.08.8187. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. The Journal of biological chemistry. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science (New York, NY. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, et al. Pten dose dictates cancer progression in the prostate. PLoS biology. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mikhailova M, Bose S, Pan CX, deVere White RW, Ghosh PM. Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene. 2008;27:7106–7117. doi: 10.1038/onc.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao E, Zhou W, Lee-Hoeflich ST, Truong T, Haverty PM, Eastham-Anderson J, Lewin-Koh N, Gunter B, Belvin M, Murray LJ, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–4156. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhu J, Efferson CL, Ware C, Tammam J, Angagaw M, Laskey J, Bettano KA, Kasibhatla S, Reilly JF, et al. Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a Pten-deficient mouse model of prostate cancer. Cancer research. 2009;69:7466–7472. doi: 10.1158/0008-5472.CAN-08-4385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.