Abstract
Fanconi anemia (FA) is a rare genetic disease characterized by congenital abnormalities, bone marrow failure and heightened cancer susceptibility. The FA proteins are known to function in the cellular defense against DNA interstrand crosslinks (ICLs), a process that remains poorly understood. A recent spate of discoveries has led to the identification of one new FA gene, FANCP/SLX4, and two strong candidate FA genes, FAN1 and RAD51C. In this perspective we describe the discovery of FANCP/SLX4 and discuss how these new findings collectively refine our understanding of DNA ICL repair.
Key words: Fanconi anemia, DNA repair, ubiquitin, FANCP/SLX4, DNA interstrand crosslink repair
Introduction
Fanconi anemia is an autosomal recessive and X-linked genetic disorder characterized by congenital abnormalities, progressive bone marrow failure and pronounced cancer susceptibility. This rare disease, with an estimated incidence of 1 in 200,000 to 400,000 live births, was first described in 1927 by the Swiss pediatrician Guido Fanconi. While working at the Kinderspital in Zurich, Fanconi encountered a family of five siblings, three of whom perished from an early-onset condition resembling pernicious anemia. The affected siblings displayed additional clinical features including microcephaly, vitiligo, as well as hypoplasia of the testes.1 Through the pioneering work of Fanconi and others, this heterogeneous constellation of congenital anomalies and pediatric hematopoietic dysfunction became formally recognized as Fanconi anemia (FA) in 1931. Almost sixty years later the first FA gene was identified: in 1992 Manuel Buchwald and colleagues at the Hospital for Sick Children in Toronto cloned the FANCC gene (biallelic mutations in which underlie FA complementation group C or FA-C for short).2 This was followed by discoveries of the FANCA, FANCE, FANCF and FANCG genes.3–6 The pace of FA gene discovery has accelerated in the 21st century with an additional eight genes, FANCD2 (2001), FANCD1/BRCA2 (2002), FANCL (2003), FANCB (2004), FANCJ/BRIP1 (2005), FANCM (2005), FANCI (2007) and FANCN/PALB2 (2007), being identified in the first decade alone.7 Several exciting recent reports describing the identification of a bona fide new FA gene, FANCP/SLX4, and two very strong candidate FA genes, FAN1 and RAD51C, suggest that the pace of FA gene discovery is set to continue.
The FA-BRCA DNA Damage Response Network
Prior to these recent discoveries, the FA-BRCA DNA damage response network was known to be comprised of thirteen bona fide disease-linked proteins, as well as numerous non-FA proteins, that function cooperatively in ICL repair.7,8 The hallmark of FA patient cells, as well as BRCA1/2-deficient cells, is hypersensitivity to the clastogenic effects of DNA crosslinking agents, examples of which include mitomycin C (MMC) and cisplatin.9 Indeed, the diagnostic test for FA is based directly upon this fact, and involves exposing FA patient cells to DNA crosslinking agents and scoring the percentage of metaphases exhibiting radial chromosome formations.10 The following model for the FA-BRCA pathway has evolved over the past decade: following exposure to DNA damaging agents and during unperturbed S-phase of the cell cycle, the FANC proteins A, B, C, E, F, G, L and M, the Fanconi anemia associated proteins (FAAP) 100 and 24, and the E2 ubiquitin-conjugating enzyme UBE2T, assemble into an active E2/E3 ubiquitin holoenzyme complex in chromatin.11–18 This ‘upstream core’ FA complex catalyzes the monoubiquitination of the paralogous FANCD2 and FANCI proteins on K561 and K523, respectively.9,19,20 Importantly, patient-derived mutations in the FA core complex genes FANCA, B, C, E, F, G and L, lead to a complete failure to monoubiquitinate both FANCD2 and FANCI.9,19,20 Monoubiquitination of the FANCD2 and FANCI proteins results in their targeting to discrete chromatin-associated foci, where they co-localize with numerous well known DNA repair proteins, including BRCA1, RAD51 and phosphorylated H2AX (γH2AX).9,21,22 The monoubiquitination of FANCD2/I is reversible: the USP1 ubiquitin hydrolase, in association with the UAF1 protein, deubiquitinates both FANCD2 and FANCI, presumably deactivating these proteins once their function in DNA repair is complete.23,24
Several FA proteins function ‘downstream’ of FANCD2/I monoubiquitination: namely FANCD1/BRCA2, FANCJ/BRIP1 and FANCN/PALB2. The FANCN/PALB2 and FANCD1/BRCA2 proteins stably associate in chromatin and cooperatively regulate the activation of the RAD51 protein.25–27 RAD51 is the major eukaryotic homologous recombination (HR) protein. During the process of HR, RAD51 forms a nucleoprotein filament on single-stranded DNA (ssDNA) and catalyzes invasion of a homologous intact DNA template [forming a D-loop (displacement loop) structure in the process] to prime repair DNA synthesis.28 FANCD1/BRCA2 and FANCN/PALB2 promote RAD51 nucleoprotein filament formation and strand invasion.27,29 Accordingly, cells from FA-D1 and FA-N patients exhibit severely reduced MMC-inducible RAD51 nuclear foci formation.25,26,30 In certain sub-pathways of HR, D-loop formation is followed by the formation of X-shaped heteroduplex DNA structures known as Holliday junctions (HJs), named after the geneticist Robin Holliday who in 1964 proposed a mechanism for DNA strand exchange during fungal meiotic recombination.31 Of particular importance for this article, the resolution of these recombination intermediates necessitates the coordinated activity of HJ resolvases and a host of structure-specific endonucleases.32
The FANCJ/BACH1/BRIP1 (for BRCA1-Associated C-terminal Helicase or BRCA1-Interacting Protein 1) protein was originally identified as a BRCA1 BRCT (for BReast Cancer C-terminal repeat) domain-interacting protein.33 FANCJ/BRIP1 is a 5′–3′ DNA helicase that can unwind D-loop structures, yet is incapable of unwinding HJs in vitro.33–35 Importantly, the FANCD1/BRCA2, FANCN/PALB2 and FANCJ/BRIP1 proteins are all required for the HR-mediated repair of an I-SceI-induced DNA double-strand break (DSB).27,35,36 Collectively, these findings strongly suggest that one major function of the FA-BRCA DNA damage response network is to promote error-free, conservative HR DNA repair.
The Discovery of FANCP/SLX4
Genome-wide screens in the budding yeast Saccharomyces cerevisiae had previously uncovered an important role for the Slx4p protein in the cellular response to DNA ICLs.37,38 Similarly, the Drosophila melanogaster Slx4p homolog, MUS312, was also shown to be required for ICL repair.39 Consistent with these findings, in 2009 three independent groups demonstrated that depletion of the human homolog of Slx4p, SLX4 (also known as BTBD12) sensitizes cells to the cytotoxic effects of ICLs.40–42 In light of these findings, the groups of Johan de Winter and Agata Smogorzewska at Vrije Universiteit Medical Center and the Rockefeller University, respectively, sequenced the SLX4 gene in several FA patients not previously assigned to any of the thirteen known FA complementation groups.43,44 Biallelic SLX4 mutations were uncovered in a total of six individuals from four unrelated kindreds of distinct geographical origin. The clinical phenotypes of these six individuals, while heterogeneous, were typical of that of classic FA and included congenital abnormalities, pediatric hematopoietic dysfunction, and in the case of one individual, susceptibility to squamous cell carcinoma of the tongue.43–45 As is characteristic of FA patient cells, either fibroblasts or lymphoblasts, or both, from all six affected individuals displayed hypersensitivity to the cytotoxic and clastogenic effects of ICLs. Interestingly, two of the six patient cell lines also displayed marked sensitivity to the topoisomerase type I poison camptothecin, a unique phenotype of cells from FA complementation groups D1, M and N, as well as provisional FA complementation group O (RAD51C-/-, see below).46,47 The cellular ICL hypersensitivity of these patient cells was rescued, albeit partially, by expression of either the wild-type human SLX4 protein,43 or a truncated murine Slx4 protein.44 Thus, biallelic mutations in the SLX4 gene underlie FA complementation group P, and FANCP becomes synonymous with SLX4.
In the same issue of Nature Genetics as that reporting the findings of the de Winter and Smogorzewska groups, Crossan and colleagues at the Medical Research Council Laboratory of Molecular Biology in Cambridge describe the phenotype of Btbd12/Slx4 knockout mice.48 Slx4-deficient mice are born at sub-Mendelian ratios, display growth retardation and developmental defects, have greatly reduced fertility, and are prone to hematological dysfunction. For example, bone marrow progenitors from Slx4-deficient mice are markedly compromised in their ability to differentiate into both lymphoid and myeloid lineages, compared with those from their wild-type littermates. Thus, Slx4-deficient mice recapitulate many of the hallmark clinical features of FA. At the cellular level, Slx4-/- mouse embryonic fibroblasts (MEFs) exhibit premature replicative senescence as a consequence of increased spontaneous chromosome instability, which is exacerbated upon treatment with MMC. Furthermore, similar to FA patient cells, Slx4-/- MEFs are hypersensitive to the cytotoxic effects of MMC.48 Importantly, all three groups demonstrated that FANCD2 (and almost certainly FANCI) monoubiquitination and nuclear foci formation are unaffected by biallelic SLX4 mutation, establishing that the FANCP/SLX4 protein functions downstream of this central posttranslational modification in the FA-BRCA DNA damage response network.
The Structure and Function of FANCP/SLX4
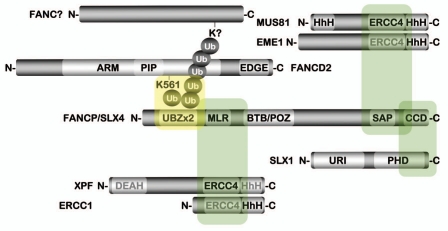
FANCP/SLX4 is an 1,834 amino acid ∼200 kDa multidomain protein (Fig. 1). FANCP/SLX4 is comprised of two N-terminal C2HC zinc finger domains, related to the UBZ4 family of ubiquitin-binding domains (UBDs);49 a MEI9XPF-Interaction-Like Region referred to as the MLR; a Broad-Complex, Tramtrack and Bric a brac/POxvirus and Zinc finger (BTB/POZ) protein-protein interaction domain; a SAF-A/B, Acinus and PIAS (SAP) domain; and a highly conserved C-terminal domain (CCD) containing a helix-turn-helix motif. Proteomic analysis of SLX4 immune complexes from HEK293 cells revealed that, similar to fungal Slx4, human SLX4 interacts with the structure-specific endonucleases XPF-ERCC1, MUS81-EME1 and SLX1, and stimulates their enzymatic activities.41,42 XPF-ERCC1 and MUS81-EME1 are members of the XPF/MUS81 family of structure specific endonucleases. In vitro, XPF-ERCC1 preferentially cleaves splayed-arm, bubble and stem-loop structures, while MUS81-EME1 preferentially cleaves 3′ flap and replication fork structures as well as nicked HJs.32 SLX1 is a structurally distinct endonuclease that contains an N-terminal UvrC-intron-endonuclease (URI) domain and a C-terminal PHD-type zinc finger, often found in proteins with chromatin-localized functions.50,51 SLX1, in association with SLX4, is capable of efficiently processing HJ structures.40–42 XPF-ERCC1, MUS81-EME1 and SLX1 interact directly with SLX4 via the MLR, SAP and CCD domains, respectively (Fig. 1).40,42 In addition, SLX4 was shown to physically interact with the telomere-binding protein TRF2 and its partner TERF2IP/RAP1, the PLK1 protein kinase, as well as the mismatch repair (MMR) heterodimer MSH2-MSH3.42 Thus, FANCP/SLX4 appears to be uniquely poised to coordinate multiple DNA repair activities.
Figure 1.
FANCP/SLX4 functions as a molecular platform for several structure specific endonucleases. Depiction of several known or suspected FANCP/SLX4 protein interactions. Domains typewritten in gray text are evolutionarily diverged and are no longer active. Shaded green boxes indicate the regions of FANCP/SLX4 necessary for interaction with the corresponding heterodimer. The shaded yellow box depicts a speculative interaction between monoubiquitin on K561 of the FANCD2 protein, or a K63-linked ubiquitin chain on an unknown protein,43 and the UBZ ubiquitin-binding domain of FANCP/SLX4. ARM, Armadillo/beta-catenin-like repeat; BTB/POZ, broad-complex, tramtrack and bric a brac/Poxvirus and zinc finger; CCD, conserved C-terminal domain; DEAH, aspartic acid-glutamic acid-alanine-histidine helicase motif; EDGE, glutamic acid-aspartic acid-glycine-glutamic acid motif; ERCC4, excision repair cross complementation group 4 nuclease domain; HhH, helix-hairpin-helix domain; PHD, plant homeodomain; PIP, PCNA-interacting protein motif; MLR, MEI9XPF-interaction-like region; SAP, SAF-A/B, acinus and PIAS domain; UBZ, ubiquitin-binding zinc finger domain; URI, UvrC-intron-endonuclease domain. Figure adapted from reference 40.
Importantly, the hypomorphic SLX4 mutations uncovered in the FA-P patients did not appear to overtly affect the ability of the mutant SLX4 protein to physically interact with XPF-ERCC1, MUS81-EME1 and SLX1. These findings suggest that the cellular defects observed may be due to low levels of SLX4 in general, and are not likely to be a consequence of a defect in the interaction with one specific nuclease.43,44 However, subcellular fractionation experiments by Stoepker and colleagues revealed a specific defect in the chromatin localization of XPF-ERCC1 in cells from FA-P patient EUFA1354, while MUS81-EME1 and SLX1 chromatin localization was unaffected. These findings were corroborated by immunofluoresence microscopy experiments demonstrating a striking defect in ERCC1 nuclear foci formation in EUFA1354 cells.44 Defective basal and DNA damage-inducible Ercc1 chromatin association was also observed in the Slx4-/- MEFs.48 Furthermore, Slx4 with a C-terminal truncation (defective in the Slx1 interaction) complemented the MMC hypersensitivity of Slx4-/- MEFs, in contrast to Slx4 with an N-terminal truncation (defective in the Xpf-Ercc1 interaction).48 Collectively, these findings strongly suggest that at least one cellular function of SLX4 is to promote the chromatin localization of XPF-ERCC1 and that this function, in particular, could define its role in the FA-BRCA DNA damage response network.
FANCP/SLX4 and ICL Repair
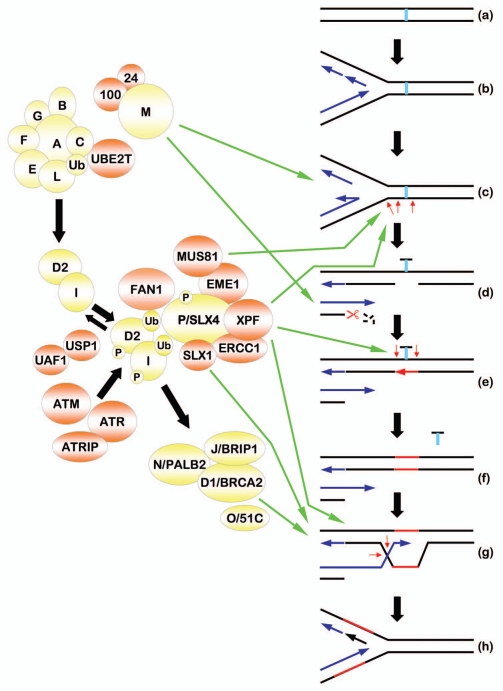
Here we review our current understanding of cellular ICL repair and consider how FANCP/SLX4 might function in this process: the covalent linkage of DNA nucleobases represents a direct physical block to both DNA replication and RNA transcription processes. Extensive studies in mammalian cells, D. melanogaster, S. cerevisiae and E. coli have established that proteins with defined roles in HR, MMR, NER and translesion DNA synthesis (TLS) also function cooperatively in ICL repair. ICL repair appears to be instigated primarily upon stalling of the DNA replication fork machinery at the covalently linked nucleobases (Fig. 2a and b).52 Several studies indicate that the MUS81-EME1 heterodimer is the first structure-specific endonuclease to catalyze nick incision on the template strand, leading to the generation of a one-ended DSB with a 3′ ssDNA overhang (Fig. 2c).53 Indeed, ICL-induced DSB formation is severely compromised in the absence of MUS81.54,55 While FANCP/SLX4 interacts directly with MUS81-EME1, FANCP/SLX4 does not seem to be required for ICL-induced DSB formation as robust and persistent levels of γH2AX nuclear foci are observed in FANCP/SLX4-deficient cells.41,44 The FANCM-FAAP24 heterodimer, another member of the XPF/MUS81 family of structure-specific endonucleases, may play several important roles in these early stages of ICL repair. First, FANCM-FAAP24 can promote replication fork reversal and remodeling, and could perhaps facilitate MUS81-EME1 endonuclease activity (Fig. 2c).56 Second, FANCM-FAAP24 is required for the recruitment of the ssDNA binding protein RPA to ICL-induced ssDNA, and for the induction of the ATR-mediated ICL-induced checkpoint response.57 Many proteins in the FA-BRCA DNA damage response network, including FANCP/SLX4, are phosphorylated by the ATR or ATM DNA damage response kinases.20,58,59 Third, FANCM-FAAP24 promotes the recruitment of the FA core complex to chromatin and the monoubiquitination of FANCD2/I.17,60,61 However, Fancd2 monoubiquitination is observed in a Fancm-/- mouse model generated by targeted deletion of exon 2.62 Furthermore, chicken DT40 ΔΔFANCC cells are considerably more sensitive to ICLs than DT40 ΔFANCM cells.61 These results suggest that FANCM may participate in only a subset of ICL repair events.
Figure 2.
A speculative model of DNA interstrand crosslink repair. Please see the main text for details. Proteins encoded by bona fide Fanconi anemia (FA) genes are depicted in yellow. Non-FA proteins are depicted in orange. Green arrows depict stages of DNA interstrand crosslink (ICL) repair where the indicated proteins may, or are known to, function. Small red arrows depict sites of endonucleolytic cleavage. The red scissors indicates exonucleolytic DNA strand resection. The ICL is depicted in light blue. De novo DNA synthesis is depicted in navy blue, while newly patched DNA (repair synthesis) is depicted in red. P, phosphate group; Ub, ubiquitin.
The next step of ICL repair, namely unhooking of the ICL, is perhaps the most contentious. ICL unhooking requires structural recognition of the ICL, unwinding of the DNA double helix adjacent to the ICL, incision 3′ and 5′ of the ICL, and displacement of the ICL from the double helix. The NER machinery is thought to play a major role in ICL unhooking, exemplified by the ICL sensitivity of numerous mammalian NER mutants.63 During NER, the catalytic activity for strand unwinding is provided by TFIIH, which is comprised of the ERCC3/XPB and ERCC2/XPD DNA helicases, as well as several other factors.63 As XPB- and XPD-deficient cells exhibit ICL sensitivity, TFIIH may play a similar catalytic function during ICL repair.63,64 Other candidate helicases for this ICL unhooking step include FANCJ/BRIP1 and BLM (mutated in Bloom syndrome). Indeed, recent studies demonstrate that FANCJ/BRIP1 and BLM can act synergistically to unwind a DNA duplex containing a strand discontinuity.65
During NER, XPF-ERCC1 creates a nick incision 5′ of the DNA lesion (most often a UV-C irradiation-inducible cyclobutane pyrimidine dimer).32 XPF- and ERCC1-deficient cells exhibit pronounced ICL hypersensitivity, and by analogy with its function in NER, XPF-ERCC1 is thought to be responsible for nick incision 5′ of the ICL (Fig. 2c or e). However, the precise enzymatic function(s) of the XPF-ERCC1 heterodimer in ICL repair remains a matter of considerable debate.32,63,66 The observation that the chromatin localization of XPF-ERCC1 is defective in FANCP/SLX4-deficient cells,44,48 yet ICL-inducible RAD51 nuclear foci formation appears to be unaffected, suggests that XPF-ERCC1 could play a role downstream of DSB formation and RAD51-mediated DNA strand invasion (Fig. 2g). An alternative interpretation of this data is that, in the absence of XPF-ERCC1 and despite the continued presence of the ICL, RAD51-mediated strand invasion proceeds at a non- or micro-homologous DNA sequence, leading to deletion and/or chromosomal rearrangement. Another candidate unhooking nuclease is the recently identified FAN1 protein (for FANCD2-associated nuclease 1).67–69 FAN1 harbors a VRR_nuc domain and exhibits 5′ flap endonuclease as well as 5′–3′ exonuclease activities.67–69 FAN1 is recruited to chromatin via a noncovalent interaction between its UBZ domain and monoubiquitinated K561 of FANCD2.67–69 Like XPF-ERCC1,70 and unlike MUS81-EME1,55 depletion of FAN1 does not affect ICL-induced DSB formation,68 suggesting that FAN1 may also function downstream of the ICL unhooking step (Fig. 2g).
Covalently linked nucleobases may also be recognized and initially processed by components of the MMR machinery. Numerous studies have established important physical connections between the FA and MMR proteins. For example, FAN1 and FANCJ/BRIP1 are components of the MLH1 interactome,69,71 while MSH2 and MSH3 were recently identified in FANCP/SLX4 immune complexes.42 From a functional perspective, Peng and colleagues demonstrated that the interaction between FANCJ/BRIP1 and MLH1 is required for correction of the ICL hypersensitivity of FA-J cells.72 Furthermore, MutLα (MLH1-PMS2) is capable of incising a nicked mismatch-containing DNA heteroduplex,73 and MSH2-deficient cells are sensitive to the cytotoxic effects of ICLs.74 It will be important to clearly define the molecular interplay between FAN1, FANCJ/BRIP1, FANCP/SLX4 and the MMR proteins during ICL repair. Nevertheless, once unhooking is completed, the resulting strand discontinuity/gap is filled by homologs of the yeast RAD6 epistasis group in TLS (Fig. 2e and f). Accordingly, cells with defective TLS display marked sensitivity to ICL-inducing agents.38,75 The MMR machinery could also function post-TLS to remove any mismatches arising as a consequence of low fidelity TLS DNA polymerases. Recent experiments with ICL-bearing plasmids and Xenopus cell-free extracts suggest an important role for FANCD2 monoubiquitination in both the early endonucleolytic and TLS steps of ICL repair.76 Similar to that recently observed for FAN1, perhaps monoubiquitinated FANCD2 and FANCI recruit additional UBD-harboring ligands to perform these functions.67–69 Following gap filling by TLS, homologs of the yeast RAD52 epistasis group initiate HR through the displacement of RPA from the 3′ ssDNA overhang and the promotion of RAD51 nucleoprotein filament formation and strand invasion of the complementary duplex (Fig. 2g). As mentioned earlier, FANCD1/BRCA2 and FANCN/PALB2 promote RAD51 nucleoprotein filament formation and strand invasion.27,29 Recently, homozygous hypomorphic mutations in the RAD51 paralogous gene RAD51C were uncovered in three siblings with clinical manifestations resembling FA.47 Owing to the absence of hematological deficiencies or cancer in the one surviving affected child, a formal diagnosis of FA has yet to be assigned. Nonetheless, RAD51C is provisionally referred to as FANCO.47 Importantly, similar to FANCD1/BRCA2 and FANCN/PALB2, FANCO/RAD51C acts downstream of FANCD2/I monoubiquitination and promotes ICL-inducible RAD51 nuclear foci formation, consistent with previous studies.47,77,78 Following strand invasion, a D-loop forms and the invading 3′ ssDNA primes de novo DNA synthesis (Fig. 2g). Once strand extension is complete, the newly synthesized DNA will disengage and reanneal with its original contiguous DNA strand, restoring the DNA replication fork (Fig. 2h). Recombination intermediates, including D-loops and HJs, that arise during these latter stages of HR will require the coordinated activity of HJ resolvases and structure-specific endonucleases for their resolution. It is highly likely that FANCP/SLX4 plays a major role in coordinating several of these enzymatic activities. Precisely defining these enzymatic activities and identifying their specific recombination intermediate substrates in vivo poses a considerable challenge.
Concluding Remarks
Over the course of the past two decades, we have witnessed remarkable advancements in our understanding of the genetics and molecular etiology of FA. While the first six identified FA proteins (A, C, D2, E, F and G) harbored no recognizable enzymatic domains, many proteins with putative or proven enzymatic function have now entered the fray. The identification of biallelic mutations in the SLX4 gene in FA-P patients potentially adds three new structure-specific endonucleases to the FA DNA damage response network: MUS81-EME1, SLX1-SLX4 and XPF-ERCC1. Future studies will seek to uncover the in vivo substrates, enzymatic activities and regulation of these structure-specific endonucleases, in particular as they relate to ICL repair. These studies will surely continue to improve our understanding of the molecular basis of this devastating disease and offer hope for much needed therapeutic advancements.
References
- 1.Lobitz S, Velleuer E. Guido Fanconi (1892–1979): a jack of all trades. Nat Rev Cancer. 2006;6:893–898. doi: 10.1038/nrc2009. [DOI] [PubMed] [Google Scholar]
- 2.Strathdee CA, Gavish H, Shannon WR, Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992;356:763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- 3.de Winter JP, Leveille F, van Berkel CGM, Rooimans MA, van der Weel L, Steltenpool J, et al. Isolation of a cDNA representing the Fanconi Anemia Complementation Group E gene. Am J Hum Genet. 2000;67:1306–1308. doi: 10.1016/s0002-9297(07)62959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Winter JP, Rooimans MA, van der Weel L, Van Berkel CM, Alon N, Bosnoyan-Collins L, et al. The Fanconi Anemia Complementation Gene FANCF encodes a novel protein with homology to ROM. Nat Genet. 2000;24:15–16. doi: 10.1038/71626. [DOI] [PubMed] [Google Scholar]
- 5.de Winter JP, Waisfisz Q, Rooimans MA, van Berkel CGM, Bosnoyan-Collins L, Alon N, et al. The Fanconi anaemia group G gene is identical with human XRCC9. Nat Genet. 1998;20:281–283. doi: 10.1038/3093. [DOI] [PubMed] [Google Scholar]
- 6.Lo Ten Foe JR, Rooimans MA, Bosnoyan-Collins L, et al. Expression cloning of a cDNA for the major Fanconi anemia gene, FAA. Nat Genet. 1996;14:320–323. doi: 10.1038/ng1196-320. [DOI] [PubMed] [Google Scholar]
- 7.Moldovan GL, D'Andrea AD. How the fanconi anemia pathway guards the genome. Annu Rev Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rego MA, Kolling FW, Howlett NG. The Fanconi anemia protein interaction network: Casting a wide net. Mutat Res. 2009;668:27–41. doi: 10.1016/j.mrfmmm.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 10.Auerbach AD. Fanconi anemia diagnosis and the diepoxybutane (DEB) test. Exp Hematol. 1993;21:731–733. [PubMed] [Google Scholar]
- 11.Alpi A, Langevin F, Mosedale G, Machida YJ, Dutta A, Patel KJ. UBE2T, the Fanconi anemia core complex, and FANCD2 are recruited independently to chromatin: A basis for the regulation of FANCD2 monoubiquitination. Mol Cell Biol. 2007;27:8421–8430. doi: 10.1128/MCB.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciccia A, Ling C, Coulthard R, Yan Z, Xue Y, Meetei AR, et al. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol Cell. 2007;25:331–343. doi: 10.1016/j.molcel.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Ling C, Ishiai M, Ali AM, Medhurst AL, Neveling K, Kalb R, et al. FAAP100 is essential for activation of the Fanconi anemia-associated DNA damage response pathway. EMBO J. 2007;26:2104–2114. doi: 10.1038/sj.emboj.7601666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, et al. A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol Cell Biol. 2003;23:3417–3426. doi: 10.1128/MCB.23.10.3417-3426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 16.Meetei AR, Levitus M, Xue Y, Medhurst AL, Zwaan M, Ling C, et al. X-linked inheritance of Fanconi anemia complementation group B. Nat Genet. 2004;36:1219–1224. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- 17.Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, et al. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat Genet. 2005;37:958–963. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joenje H, Patel KJ. The emerging genetic and molecular basis of fanconi anaemia. Nat Rev Genet. 2001;2:446–459. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 19.Sims AE, Spiteri E, Sims RJ, 3rd, Arita AG, Lach FP, Landers T, et al. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nat Struct Mol Biol. 2007;14:564–567. doi: 10.1038/nsmb1252. [DOI] [PubMed] [Google Scholar]
- 20.Smogorzewska A, Matsuoka S, Vinciguerra P, McDonald ER, 3rd, Hurov KE, Luo J, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogliolo M, Lyakhovich A, Callen E, Castella M, Cappelli E, Ramirez MJ, et al. Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. EMBO J. 2007;26:1340–1351. doi: 10.1038/sj.emboj.7601574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D'Andrea AD. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100:2414–2420. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- 23.Cohn MA, Kowal P, Yang K, Haas W, Huang TT, Gygi SP, et al. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol Cell. 2007;28:786–797. doi: 10.1016/j.molcel.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Nijman SM, Huang TT, Dirac AM, Brummelkamp TR, Kerkhoven RM, D'Andrea AD, et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell. 2005;17:331–339. doi: 10.1016/j.molcel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2007;39:162–164. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 26.Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, et al. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet. 2007;39:159–161. doi: 10.1038/ng1942. [DOI] [PubMed] [Google Scholar]
- 27.Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719–729. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Boulton SJ. Cellular functions of the BRCA tumour-suppressor proteins. Biochem Soc Trans. 2006;34:633–645. doi: 10.1042/BST0340633. [DOI] [PubMed] [Google Scholar]
- 29.Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell. 2001;7:273–282. doi: 10.1016/s1097-2765(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 30.Godthelp BC, Wiegant WW, Waisfisz Q, Medhurst AL, Arwert F, Joenje H, et al. Inducibility of nuclear Rad51 foci after DNA damage distinguishes all Fanconi anemia complementation groups from D1/BRCA2. Mutat Res. 2005;594:39–48. doi: 10.1016/j.mrfmmm.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Holliday R. A mechanism for gene conversion in fungi. Genetical Research. 1964;5:282–304. doi: 10.1017/S0016672308009476. [DOI] [PubMed] [Google Scholar]
- 32.Ciccia A, McDonald N, West SC. Structural and functional relationships of the XPF/MUS81 family of proteins. Annu Rev Biochem. 2008;77:259–287. doi: 10.1146/annurev.biochem.77.070306.102408. [DOI] [PubMed] [Google Scholar]
- 33.Cantor SB, Bell DW, Ganesan S, Kass EM, Drapkin R, Grossman S, et al. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–160. doi: 10.1016/s0092-8674(01)00304-x. [DOI] [PubMed] [Google Scholar]
- 34.Gupta R, Sharma S, Sommers JA, Jin Z, Cantor SB, Brosh RM., Jr. Analysis of the DNA substrate specificity of the human BACH1 helicase associated with breast cancer. J Biol Chem. 2005;280:25450–25460. doi: 10.1074/jbc.M501995200. [DOI] [PubMed] [Google Scholar]
- 35.Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S, et al. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell. 2005;8:255–265. doi: 10.1016/j.ccr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 37.Lee W, St Onge RP, Proctor M, Flaherty P, Jordan MI, Arkin AP, et al. Genome-wide requirements for resistance to functionally distinct DNA-damaging agents. PLoS Genet. 2005;1:24. doi: 10.1371/journal.pgen.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu HI, Brown JA, Dorie MJ, Lazzeroni L, Brown JM. Genome-wide identification of genes conferring resistance to the anticancer agents cisplatin, oxaliplatin and mitomycin C. Cancer Res. 2004;64:3940–3948. doi: 10.1158/0008-5472.CAN-03-3113. [DOI] [PubMed] [Google Scholar]
- 39.Yildiz O, Majumder S, Kramer B, Sekelsky JJ. Drosophila MUS312 interacts with the nucleotide excision repair endonuclease MEI-9 to generate meiotic crossovers. Mol Cell. 2002;10:1503–1509. doi: 10.1016/s1097-2765(02)00782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fekairi S, Scaglione S, Chahwan C, Taylor ER, Tissier A, Coulon S, et al. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138:78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munoz IM, Hain K, Declais AC, Gardiner M, Toh GW, Sanchez-Pulido L, et al. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol Cell. 2009;35:116–127. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Svendsen JM, Smogorzewska A, Sowa ME, O'Connell BC, Gygi SP, Elledge SJ, et al. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138:63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y, Lach FP, Desetty R, Hanenberg H, Auerbach AD, Smogorzewska A. Mutations of the SLX4 gene in Fanconi anemia. Nat Genet. 2011;43:142–146. doi: 10.1038/ng.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoepker C, Hain K, Schuster B, Hilhorst-Hofstee Y, Rooimans MA, Steltenpool J, et al. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat Genet. 2011;43:138–141. doi: 10.1038/ng.751. [DOI] [PubMed] [Google Scholar]
- 45.FARF I, author. Fanconi Anemia: Standards for Clinical Care. Eugene, Oregon: Fanconi Anemia Research Fund, Inc.; 2003. [Google Scholar]
- 46.Singh TR, Bakker ST, Agarwal S, Jansen M, Grassman E, Godthelp BC, et al. Impaired FANCD2 monoubiquitination and hypersensitivity to camptothecin uniquely characterize Fanconi anemia complementation group M. Blood. 2009;114:174–180. doi: 10.1182/blood-2009-02-207811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaz F, Hanenberg H, Schuster B, Barker K, Wiek C, Erven V, et al. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet. 2010;42:406–409. doi: 10.1038/ng.570. [DOI] [PubMed] [Google Scholar]
- 48.Crossan GP, van der Weyden L, Rosado IV, Langevin F, Gaillard PH, McIntyre RE, et al. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat Genet. 2011;43:147–152. doi: 10.1038/ng.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofmann K. Ubiquitin-binding domains and their role in the DNA damage response. DNA Repair (Amst) 2009;8:544–556. doi: 10.1016/j.dnarep.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Fricke WM, Brill SJ. Slx1-Slx4 is a second structure-specific endonuclease functionally redundant with Sgs1-Top3. Genes Dev. 2003;17:1768–1778. doi: 10.1101/gad.1105203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2001;157:103–118. doi: 10.1093/genetics/157.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akkari YM, Bateman RL, Reifsteck CA, Olson SB, Grompe M. DNA replication is required To elicit cellular responses to psoralen-induced DNA interstrand cross-links. Mol Cell Biol. 2000;20:8283–8289. doi: 10.1128/mcb.20.21.8283-8289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osman F, Whitby MC. Exploring the roles of Mus81-Eme1/Mms4 at perturbed replication forks. DNA Repair (Amst) 2007;6:1004–1017. doi: 10.1016/j.dnarep.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 54.Hanada K, Budzowska M, Davies SL, van Drunen E, Onizawa H, Beverloo HB, et al. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat Struct Mol Biol. 2007;14:1096–1104. doi: 10.1038/nsmb1313. [DOI] [PubMed] [Google Scholar]
- 55.Hanada K, Budzowska M, Modesti M, Maas A, Wyman C, Essers J, et al. The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. EMBO J. 2006;25:4921–4932. doi: 10.1038/sj.emboj.7601344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gari K, Decaillet C, Delannoy M, Wu L, Constantinou A. Remodeling of DNA replication structures by the branch point translocase FANCM. Proc Natl Acad Sci USA. 2008;105:16107–16112. doi: 10.1073/pnas.0804777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang M, Kim JM, Shiotani B, Yang K, Zou L, D'Andrea AD. The FANCM/FAAP24 complex is required for the DNA interstrand crosslink-induced checkpoint response. Mol Cell. 2010;39:259–268. doi: 10.1016/j.molcel.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreassen PR, D'Andrea AD, Taniguchi T. ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev. 2004;18:1958–1963. doi: 10.1101/gad.1196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 60.Kim JM, Kee Y, Gurtan A, D'Andrea AD. Cell cycle-dependent chromatin loading of the Fanconi anemia core complex by FANCM/FAAP24. Blood. 2008;111:5215–5222. doi: 10.1182/blood-2007-09-113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mosedale G, Niedzwiedz W, Alpi A, Perrina F, Pereira-Leal JB, Johnson M, et al. The vertebrate Hef ortholog is a component of the Fanconi anemia tumor-suppressor pathway. Nat Struct Mol Biol. 2005;12:763–771. doi: 10.1038/nsmb981. [DOI] [PubMed] [Google Scholar]
- 62.Bakker ST, van de Vrugt HJ, Rooimans MA, Oostra AB, Steltenpool J, Delzenne-Goette E, et al. Fancm-deficient mice reveal unique features of Fanconi anemia complementation group M. Hum Mol Genet. 2009;18:3484–3495. doi: 10.1093/hmg/ddp297. [DOI] [PubMed] [Google Scholar]
- 63.Wood RD. Mammalian nucleotide excision repair proteins and interstrand crosslink repair. Environ Mol Mutagen. 2010;51:520–526. doi: 10.1002/em.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Silva IU, McHugh PJ, Clingen PH, Hartley JA. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Mol Cell Biol. 2000;20:7980–7990. doi: 10.1128/mcb.20.21.7980-7990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suhasini AN, Rawtani NA, Wu Y, Sommers JA, Sharma S, Mosedale G, et al. Interaction between the helicases genetically linked to Fanconi anemia group J and Bloom's syndrome. EMBO J. 2011;30:692–705. doi: 10.1038/emboj.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergstralh DT, Sekelsky J. Interstrand crosslink repair: can XPF-ERCC1 be let off the hook? Trends Genet. 2008;24:70–76. doi: 10.1016/j.tig.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Kratz K, Schopf B, Kaden S, Sendoel A, Eberhard R, Lademann C, et al. Deficiency of FANCD2-associated nuclease KIAA1018/FAN1 sensitizes cells to interstrand crosslinking agents. Cell. 2010;142:77–88. doi: 10.1016/j.cell.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 68.MacKay C, Declais AC, Lundin C, Agostinho A, Deans AJ, MacArtney TJ, et al. Identification of KIAA1018/FAN1, a DNA repair nuclease recruited to DNA damage by monoubiquitinated FANCD2. Cell. 2010;142:65–76. doi: 10.1016/j.cell.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smogorzewska A, Desetty R, Saito TT, Schlabach M, Lach FP, Sowa ME, et al. A genetic screen identifies FAN1, a Fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair. Mol Cell. 2010;39:36–47. doi: 10.1016/j.molcel.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cannavo E, Gerrits B, Marra G, Schlapbach R, Jiricny J. Characterization of the interactome of the human MutL homologues MLH1, PMS1 and PMS2. J Biol Chem. 2007;282:2976–2986. doi: 10.1074/jbc.M609989200. [DOI] [PubMed] [Google Scholar]
- 72.Peng M, Litman R, Xie J, Sharma S, Brosh RM, Jr, Cantor SB. The FANCJ/MutLalpha interaction is required for correction of the cross-link response in FA-J cells. EMBO J. 2007;26:3238–3249. doi: 10.1038/sj.emboj.7601754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 74.Wu Q, Christensen LA, Legerski RJ, Vasquez KM. Mismatch repair participates in error-free processing of DNA interstrand crosslinks in human cells. EMBO Rep. 2005;6:551–557. doi: 10.1038/sj.embor.7400418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, et al. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- 76.Knipscheer P, Raschle M, Smogorzewska A, Enoiu M, Ho TV, Scharer OD, et al. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;326:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.French CA, Masson JY, Griffin CS, O'Regan P, West SC, Thacker J. Role of mammalian RAD51L2 (RAD51C) in recombination and genetic stability. J Biol Chem. 2002;277:19322–19330. doi: 10.1074/jbc.M201402200. [DOI] [PubMed] [Google Scholar]
- 78.Godthelp BC, Wiegant WW, van Duijn-Goedhart A, Scharer OD, van Buul PP, Kanaar R, et al. Mammalian Rad51C contributes to DNA cross-link resistance, sister chromatid cohesion and genomic stability. Nucleic Acids Res. 2002;30:2172–2182. doi: 10.1093/nar/30.10.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]