Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease. The gradual, irreversible loss of dopamine neurons in the substantia nigra isthe signature lesion of PD. Clinical symptoms of PD become apparent when 50–60% of nigral dopamine neurons are lost. PD progresses insidiously for 5–7 years (preclinical period) and then continues to worsen even under the symptomatic treatment. To determine what triggers the disease onset and what drives the chronic, self-propelling neurodegenerative process becomes critical and urgent, since lack of such knowledge impedes the discovery of effective treatments to retard PD progression. At present, available therapeutics only temporarily relieve PD symptoms. While the identification of causative gene defects in familial PD uncovers important genetic influences in this disease, the majority of PD cases are sporadic and idiopathic. The current consensus suggests that PD develops from multiple risk factors including aging, genetic predisposition, and environmental exposure. Here, we briefly review research on the genetic and environmental causes of PD. We also summarize very recent genome-wide association studies on risk gene polymorphisms in the emergence of PD. We highlight the new converging evidence on gene-environment interplay in the development of PD with an emphasis on newly developed multiple-hit PD models involving both genetic lesions and environmental triggers.
1. Introduction
Parkinson’s disease (PD), an age-related neurodegenerative movement disorder, affects more than 1 million Americans and 4 million people worldwide at any one time. While 5–10% of cases are considered of early-onset (PD occurring before 50 years of age), the average age of onset of PD is around 60 years. PD patients suffer from resting tremor, bradykinesia (slowed movements), muscle rigidity, and postural instability. These symptoms result from the degeneration of dopamine-containing neurons in the substantia nigra (SN) and the consequent loss of dopamine, a neurotransmitter involved in the fine modulation of motor function. Dopamine replacement therapy (the main treatment for PD) using the metabolic precursor of dopamine, L-DOPA, or dopamine receptor agonists, can temporarily alleviate motor symptoms rather than retard PD progression. This treatment cannot relieve concomitant nonmotor symptoms (e.g. autonomic dysfunction, depression, anxiety and dementia) that also have a high impact on PD patients. Unsuccessful translation of encouraging preclinical research to clinical therapeutics pinpoints the urgent need for better approaches and better animal models to unravel mechanisms of PD neurodegeneration and to discover disease-modifying therapies for PD.
Both genetic defects and exposure to environmental risk factors are linked to PD. The occurrence of PD-causative genetic defects in apparently sporadic cases of PD and even in healthy carriers as well as the highly variable onset ages and considerable phenotypic variations in inherited PD underscore a crucial role of gene-environment interactions in the development of PD. The lack of overt loss of nigral dopamine neurons—the signature lesion of PD—in most gene-based PD animal models and the paucity of α-synuclein-containing Lewy body inclusions—the pathological hallmark of PD—in most toxin-based PD models further support the essential role of gene-environment interplay in PD pathogenesis. Newly developed two-hit PD models, created with combinations of genetic lesions and environmental triggers, are invaluable for better defining precise disease mechanisms and screening putative disease-modifying therapeutics. Epidemiological studies aimed at identifying gene-environment interactions in PD development may also prove to be fruitful.
2. The genetic causes of PD
2.1 Familial aggregation and twin studies of PD
Approximately 10–30% of patients with PD report a first-degree relative with parkinsonism (Farrer, 2006; Rocca et al., 2004; Sveinbjornsdottir et al., 2000). Such familial aggregation of PD used to be considered as a consequence of shared exposure to a common environment. PD was traditionally considered a non–genetic disorder of ‘sporadic’ origin, since heritability estimates in twin studies revealed conflicting results that were heavily weighted against a genetic basis for this disease. For instance, multiple studies show low concordance in both monozygotic and dizygotic twins (Duvoisin et al., 1981; Marttila et al., 1988; Tanner, 2003; Ward et al., 1983; Wirdefeldt et al., 2004). However, a higher risk ratio for concordance in monozygotic versus dizygotic twins was reported in early-onset PD patients (Tanner et al., 1999). Striatal 18F-dopa positron emission tomography (PET) scan, a presymptomatic detection marker of PD, show three times higher concordance rate for PD in monozygotic twins (55%) than in dizygotic twins (18%), implying a significant genetic contribution (Piccini et al., 1999). By minimizing methodological problems in ascertainment and recall bias, twin studies can achieve more consistent results and advance our understanding of genetic influence on PD.
2.2 Monogenically inherited PD
Recent advances in molecular genetics have unveiled that 5–20% of PD patients from different geographical areas have monogenic forms of PD. Numerous genetic defects (~82% simple mutations and ~18% copy number variations) in more than 13 loci and 9 genes have been identified in inherited PD patients and families (Dawson et al., 2010; Lesage and Brice, 2009; Nuytemans et al., 2010). To date, a causal role for PD has been confirmed with five genes: SNCA (PARK1) encoding α-synuclein, Parkin (PARK2) encoding Parkin, PINK1 (PARK6) encoding PTEN-induced putative kinase 1 (PINK1), DJ-1 (PARK7) encoding DJ-1, and LRRK2 (PARK8) encoding Leucine-rich repeat kinase 2 (LRRK2). Extensive mutation screening of these five causative genes has discovered approximately 330 confirmed or possible pathogenic mutations in over 1900 families (Nuytemans et al., 2010).
2.3 Dominantly inherited mutations
2.3.1 SNCA (PARK1) and α-synuclein
SNCA encodes α-synuclein that consists of 140 amino acids and is a natively unfolded, soluble protein in the cytoplasm or associated with lipid membranes (Davidson et al., 1998). Although physiological functions of α-synuclein is still not fully understood, experimental evidence implicates α-synuclein in neurotransmitter release, vesicle turnover, synaptic plasticity, and intracellular trafficking within the endoplasmic reticulum/Golgi network (Abeliovich et al., 2000; Chandra et al., 2004; Cooper et al., 2006; Gitler et al., 2008; Liu et al., 2004). A recent elegant genetic study illustrates that α-synuclein directly binds to the soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE)-protein synaptobrevin-2/vesicle-associated membrane protein 2 and promotes SNARE-complex assembly in vivo and in vitro. Thus, α-synuclein may function to maintain normal SNARE-complex assembly and participates in repeated neurotransmitter release in presynaptic terminals (Burre et al., 2010).
Three missense mutations in α-synuclein, Ala53Thr, Ala30Pro and Glu46Lys, cause a autosomal dominant PD in rare kindreds (Kruger et al., 1998; Polymeropoulos et al., 1997; Zarranz et al., 2004). de novo genomic multiplications of SNCA are linked to familial PD; age of onset as well as duration and severity of disease are gene copy number-dependent (Chartier-Harlin et al., 2004; Farrer et al., 2004; Ibanez et al., 2004; Singleton et al., 2003). Interestingly, SNCA duplications were also reported in four apparently sporadic PD patients (Ahn et al., 2008; Nishioka et al., 2009). Common variability within the promoter or 3′-UTR of SNCA is associated with increased α-synuclein expression and a greater risk of sporadic PD (Mueller et al., 2005; Pals et al., 2004; Winkler et al., 2007).
Under pathological condition, α-synuclein undergoes conformational changes including oligomerization and fibrillogenesis; unstructured monomeric α-synuclein self-assembles into protofibrils (heterogeneous and metastable assembly intermediates) and mature fibrils (Conway et al., 2000; Conway et al., 2001). While both α-synuclein protofibrils and fibrils are cytotoxic (Lee and Trojanowski, 2006; Volles et al., 2001), some studies suggest that sequestration of protofibrillar intermediates, more toxic form of α-synuclein, into mature fibrils may be protective (Forman et al., 2005; Volles et al., 2001). Filamentous aggregates of misfolded α-synuclein protein are the major components of Lewy bodies in both familial and sporadic PD (Spillantini et al., 1997). α-synuclein carrying missense substitutions often presents reduced affinity for lipids and adopts a propensity for misfolding and accelerated fibril formation (Conway et al., 2000) (Figure 1). The identity of the most toxic α-synuclein species and the better understanding of the structural basis of α-synuclein aggregation and toxicity are crucial for the development of better diagnosis and prognosis tools as well as effective therapeutics for PD. Age-associated increases of α-synuclein in monkeys and humans are related to nigrostriatal dopamine depletion (Chu and Kordower, 2007). The formation of α-synuclein-containing inclusions in grafted fetal mesencephalic neurons in PD patients strongly indicates a prominent role of α-synuclein in PD progression and even host-to-graft propagation (Li et al., 2008; Maries et al., 2003; Mendez et al., 2008).
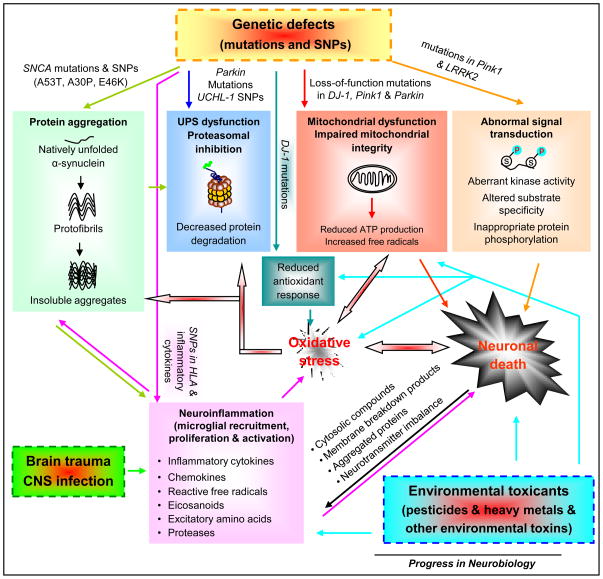
Figure 1. PD develops from complex gene-environment interactions and involves multiple molecular pathways that crosstalk and converge leading to PD neurodegeneration.
The identification of causative gene defects in PD has defined four major pathways leading to neuronal demise: protein aggregation, ubiquitin-proteasome system (UPS) dysfunction and impaired protein degradation, mitochondrial dysfunction and integrity impairment, and aberrant signal transduction. SNCA mutations and single-nucleotide polymorphisms (SNPs) make α-synuclein adopt a propensity for misfolding and accelerated aggregate formation. Excessive α-synuclein aggregates may overwhelm UPS protein degeneration. Accumulated α-synuclein can translocate to the mitochondria and impair mitochondrial activity. Parkin mutations and UCHL-1 SNPs prevent the proteolytic degradation of excessive toxic proteins (e.g. misfolded α-synuclein) in proteasomal machinery. PINK1, Parkin, and DJ-1 functionally interact to maintain mitochondrial integrity and functionality and to protect cells against adverse effects of multiple stressors. Mutations in these genes cause mitochondrial dysfunction and subsequent decline in ATP production and increase in free radical generation, which results in oxidative stress and energy deficiency. Impaired mitochondria can release cytochrome c and other ‘pro-apoptotic factors’ triggering apoptotic cascades and cell death. Mitochondria in at least some forms of PD reveal abnormal morphology, impaired fission-fusion balance, and metabolic malfunction. DJ-1 mutations reduce antioxidant response of cells, aggravating oxidative stress. Oxidative stress engages in diverse cellular processes and plays a prominent role in the induction of neuronal death. For instance, excessive production of free radicals can damage proteins (e.g. abnormal modification of α-synuclein and inactivation of Parkin), lipids, DNA, or RNA, leading to cell dysfunction (e.g. UPS and mitochondrial impairment) and eventual death. Mutations in Pink1 and LRRK2 induced aberrant kinase activity, altered substrate specificity, leading to inappropriate protein phosphorylation (e.g. increased α-synuclein phosphorylation at serine 129 by LRRK2 in vitro) and thereby affecting cell survival. Environmental toxins and brain trauma can trigger neuronal lesions by damaging mitochondria, causing oxidative stress, inducing inflammation in the central nervous system (CNS), and compromising defence mechanisms of cells. Some environmental risk factors can directly activate microglia (the resident immune cells in the CNS) or cause systemic inflammation, which in turn affects CNS inflammation. Genetic variation and polymorphisms in the HLA region and several inflammatory cytokines may become risk factors for PD. Activated microglia produce and secrete a spectrum of inflammatory and cytotoxic molecules, such as cytokines, chemokines, reactive free radicals, eicosanoids, and proteases. In addition to modulating microglial activity, these molecules influence the fate of surrounding neurons. Excessive inflammatory reaction usually becomes exaggerated and destructive, and turns into chronic inflammation that drives progressive neurodegenerative process. Injured neurons activate the surrounding microglia through the release or leakage of noxious self-compounds into the extracellular milieu, such as membrane breakdown products, abnormally processed or aggregated proteins (e.g. α-synuclein and β-amyloid), imbalanced neurotransmitters (e.g. elevated glutamate) and cytosolic compounds (e.g. α-synuclein, ATP, HMGB1 and neuromelanin). Thus, gene-environment interplay induces complex crosstalk among multiple signal cascades, forming a network and culminating in neuronal death and PD development.
2.3.2 LRRK2 (PARK8) and LRRK2 (dardarin)
The LRRK2 transcript contains 51 exons encoding for a 2527 amino acid protein, LRRK2 (also known as Dardarin). Several predicted functional domains (e.g. Roc GTPase, Roc-COR tandem domain and protein kinase domains) in LRRK2 suggest this protein have multiple functions, including protein–protein interactions (scaffold protein function), substrate binding, and protein phosphorylation (Deng et al., 2008b; Liu et al.,; Mata et al., 2006; Xiong et al., 2010). Under physiological conditions, LRRK2 is found to form dimers (Greggio et al., 2008) and may play a role in neuronal outgrowth and guidance (MacLeod et al., 2006; Sakaguchi-Nakashima et al., 2007). Wildtype LRRK2, but not its mutants, attenuates oxidative stress-induced cell death, indicating a protective role for LRRK2 (Liou et al., 2008). A recent study using LRRK2 knockout mice hints at an essential role of LRRK2 during aging in the regulation of protein homeostasis and, specifically, α-synuclein through the regulation of protein degradation pathways. Although the dopaminergic system of LRRK2 null mice appears normal, the loss of LRRK2 in kidneys of aged mice leads to impairment of autophagy-lysosomal pathways, age-dependent accumulation and aggregation of α-synuclein (60-fold) and ubiquitinated proteins, inflammatory responses, oxidative damage, and apoptotic cell death (Tong et al., 2010). Moreover, LRRK2 has been reported to interact with the microRNA pathway to regulate protein synthesis in Drosophila, and pathogenic LRRK2 carrying I2020T or G2019S mutations suppresses microRNA-mediated translational repression of E2F1/DP, transcription factors implicated in cell cycle and survival control (Gehrke et al., 2010). However, biological functions, physiological substrates, binding partners, and regulators of kinase activity of LRRK2 merit further exploration.
Mutations in the LRRK2 gene, the most common known cause of PD, are associated with autosomal dominant and sporadic forms of PD (Correia Guedes et al., 2010; Di Fonzo et al., 2005; Farrer, 2006; Nichols et al., 2005; Paisan-Ruiz et al., 2004). More than 80 missense mutations identified so far spread across the entire LRRK2 protein and affect all predicted functional domains. The clinical manifestations of homozygous carriers seem not different from those of heterozygous carriers (Lesage et al., 2005). Unlike other PD-causative genes, mutations in LRRK2 have been detected in up to 2% sporadic PD patients (Correia Guedes et al., 2010; Gilks et al., 2005; Tomiyama et al., 2008). Some of the missense mutations are also found in healthy control individuals (Carmine Belin et al., 2006; Kay et al., 2006; Lesage et al., 2008a). Gly2019Ser, the most frequent mutation in LRRK2, was reported worldwide with an average frequency of 1% in patients with sporadic PD and 4% in patients with hereditary PD (Chu and Kordower, 2007; Healy et al., 2008; Paisan-Ruiz et al., 2009). A heterogeneous distribution of this mutation in different ethnic groups was observed. The frequencies of this mutation is very low in Asian (<0.5%), higher in Caucasians (0.5–2.0% of sporadic PD and 5% of familial PD), and the highest (18–30%) in North African Arab and Ashkenazi Jewish populations (Benamer and de Silva, 2010; Chu and Kordower, 2007; Healy et al., 2008; Hulihan et al., 2008; Lesage et al., 2006; Ozelius et al., 2006; Zhang et al., 2009). The disease penetrance in carriers of LRRK2 Gly2019Ser mutation is age-dependent. An international case-control consortium study conducted in 1045 people with LRRK2 mutations from 133 families has reported the cumulative risk for carriers of Gly2019Ser mutation rises from 28% at age 59 years to 74% at age 79 years (Healy et al., 2008). Interestingly, the penetrance of Gly2019Ser in North African population is low and does not exceed 14% (Troiano et al., 2010). The worldwide and possible population-specific genetic penetrance of LRRK2 mutations merit further exploration.
Multiple LRRK2 mutations (e.g. Arg1441Cys, Arg1441Gly, Tyr1699Cys, Gly2019Ser and Ile2020Thr) have been confirmed to be pathogenic. The substitutions of Gly2019Ser and Ile2020Thr increase the catalytic activity of the MAPKKK domain (Gloeckner et al., 2006; West et al., 2005) (Figure 1). In cellular models, overexpression of disease-causing LRRK2 mutants is toxic; the toxicity is associated with kinase activity, GTP-binding, and mitochondria-dependent apoptosis (Greggio et al., 2006; Iaccarino et al., 2007; Smith et al., 2006; West et al., 2005; West et al., 2007). The presence of mutations in healthy carriers and the high frequency in sporadic PD patients, together with the highly variable onset ages and disease phenotypes, render the determination of pathogenicity of the identified LRRK2 mutations extremely challenging. post mortem studies of PD patients with LRRK2 mutations show pleomorphic pathology with typical Lewy bodies in most cases (Gilks et al., 2005; Ross et al., 2006; Zimprich et al., 2004).
2.4 Recessively inherited mutations
Loss-of-function mutations in genes encoding Parkin, PINK1, and DJ-1 cause early-onset autosomal recessive PD. All these forms of PD are relatively rare and are L-DOPA responsive. Simple mutations (e.g. missense, nonsense and splice site mutations), exonic rearrangements, small insertions and deletions, as well as copy number variations of the promoter region and exon(s) have been identified in these recessive genes. While homozygous or compound heterozygous mutations in these recessive genes have a complete penetrance, the pathogenic effect of a single heterozygous gene defect in these genes is far less clear. The higher prevalence of the heterozygous variants in PD patients than in control individuals (Lesage et al., 2008b; Marongiu et al., 2008) implies that a heterozygous recessive mutation may predispose the carriers to PD. To date, no neuropathological studies have been reported for patients with PINK1 and DJ-1 mutations.
2.4.1 Parkin (PARK2) and Parkin
The transcript of Parkin spans approximately 1.38Mb of genomic DNA and comprises 12 exons. The 465 amino acid protein, Parkin, harbors an N-terminal ubiquitin-like domain, an IBR (in-between-ring) domain, and two RING-finger domains. Parkin functions as an E3-ligase, conjugating ubiquitin to excessive or dysfunctional proteins to target them for degradation by the proteasome (Chung et al., 2004; Imai et al., 2000; Shimura et al., 2000; Zhang et al., 2000) (Figure 1). Parkin modifies proteins through monoubiquitination and polyubiqutination using both lysine-48 and lysine-63 linkages (Dawson and Dawson, 2010). Under physiological conditions, Parkin also participates in mitochondrial maintenance (Berger et al., 2009; Exner et al., 2007; Park et al., 2006; Poole et al., 2008) and clearance of dysfunctional mitochondria by autophagy (Narendra et al., 2008) (Figure 1).
Loss-of function mutations in Parkin (>100 different mutations identified) are a major cause of early-onset autosomal recessive familial PD in different ethnic groups (Dawson and Dawson, 2010; Kitada et al., 1998). These mutations account for ~50% of early-onset familial PD cases and at least 20% of early-onset sporadic PD (Lucking et al., 2000). A complete penetrance appears in individuals who have two disease-causing mutations (homozygous or compound heterozygous) in Parkin. Individuals carrying a single heterozygous Parkin mutation present with minor Parkinsonian symptoms (Khan et al., 2005; Lesage et al., 2008b; Pramstaller et al., 2002), susceptibility to late-onset PD, as implied by the reduced uptake of 18F-labelled l-DOPA detected by PET scans (Klein et al., 2007; Pavese et al., 2009), or no neurological impairment in healthy control individuals (Bruggemann et al., 2009; Kay et al., 2007). Heterozygous promoter and coding polymorphisms in Parkin gene are associated with increased susceptibility to late-onset PD (Hu et al., 2000; Lucking et al., 2003).
Most PD-causing mutations in Parkin abolish its E3 ubiquitin ligase activity, while some missense mutations reduce this activity and affect substrate specificity (Dawson and Dawson, 2010; Sriram et al., 2005). Although numerous potential substrates for Parkin have been reported, it remains unclear which of these substrates mediate the pathogenicity of Parkin deficiency. Dopaminergic, oxidative, and nitrosative stress, key players in PD, can inactivate Parkin (Chung et al., 2004; Dawson and Dawson, 2010; LaVoie et al., 2005; Yao et al., 2004). About half of examined PD patients carrying causative Parkin mutations have Lewy bodies, while the other half do not (Dawson et al., 2010).
2.4.2 PINK1 (PARK6) and PINK1
PINK1 comprises 8 exons that code for 581 amino acids. PINK1 contains an N-terminal mitochondrial targeting motif and a highly conserved serine/threonine kinase domain (Valente et al., 2004). PINK1 is mainly localized to the mitochondria and its kinase domain appears to face the cytosol, which suggests that physiologic substrates of PINK1 may reside in the cytosol (Silvestri et al., 2005; Zhou et al., 2008). However, the submitochondrial localization, the action site, and the authentic physiologic/pathophysiologic substrates of PINK1 require further investigations. PINK1 has been reported to participate in multiple mitochondrial functions, such as calcium dynamics, trafficking, respiration efficacy, free radical formation, and opening of the mitochondrial permeability transition pore (Gandhi et al., 2009; Liu et al., 2009; Plun-Favreau and Hardy, 2008; Weihofen et al., 2009; Yang et al., 2008). PINK1 may regulate mitochondrial response to cellular and oxidative stress, protecting cells from mitochondrial dysfunction (Pridgeon et al., 2007; Valente et al., 2004) (Figure 1).
Approximately 6.5% of known mutation carriers carry a homozygous recessive or compound heterozygous mutation in PINK1 (Nuytemans et al., 2010). The PINK1 mutation spectrum locates across the entire gene. Penetrance appears to be complete in individuals carrying two disease-causing mutations in PINK1, whereas the role of heterozygous mutations in PINK1 is unsettled. Many putative pathogenic mutations were also observed in heterozygous state in familial and sporadic patients as well as in healthy control individuals (Bonifati, 2007; Djarmati et al., 2006; Healy et al., 2004; Klein et al., 2007). Patients with such mutations reveal a broad phenotypic spectrum ranging from early-onset parkinsonism with atypical features to clinical manifestations indistinguishable from sporadic late-onset PD (Farrer, 2006; Gelmetti et al., 2008). PET studies using 18F-l-DOPA have demonstrated nigrostriatal abnormalities in heterozygous carriers and possibly early-onset disease (Khan et al., 2002; Valente et al., 2004). It is believed that loss-of-function mutations in PINK1 influences its stability, localization, and kinase activity (Beilina et al., 2005; Petit et al., 2005; Valente et al., 2004).
2.4.3 DJ-1 (PARK7) and DJ-1
DJ-1 contains 8 exons that span 24 kb. DJ-1, a member of the ThiJ/PfpI family of molecular chaperones, is expressed widely throughout the body (Cookson, 2010; Farrer, 2006; Moore et al., 2006). It primarily exists as a dimer and is subcellularly localized to the cytosol, mitochondrial matrix, and mitochondrial intermembrane space (Cookson, 2010; Tao and Tong, 2003; Zhang et al., 2005). DJ-1 is a redox-sensitive molecular chaperone with diverse functions (Kahle et al., 2009; Moore et al., 2006). It regulates redox-dependent kinase signaling pathways and antioxidant gene expression (Kahle et al., 2009) (Figure 1). Acting as an atypical peroxiredoxin-like peroxidase, DJ-1 protects mitochondria against oxidative stress (Andres-Mateos et al., 2007).
Recessively inherited DJ-1 mutations are rare overall, causing less than 1% of early-onset parkinsonism (Clark et al., 2004; Lockhart et al., 2004). Both missense mutations and copy number variants of DJ-1 have been identified in PD patients (Bonifati et al., 2003; Hague et al., 2003). Individuals carrying homozygous or compound heterozygous mutations in DJ-1 present with reduced dimmer formation or lack of expression of DJ-1 (Macedo et al., 2003; Moore et al., 2003). Clinically unaffected DJ-1 mutation carriers who carry heterozygous variants seem normal in brain-imaging studies, indicating a requirement of a complete loss of DJ-1 function for PD (Dekker et al., 2004).
2.5 Crosstalk among PD-associated genetic pathways
Striking similarities in the phenotypes among familial PD patients carrying various PD-related gene mutants and sporadic PD patients strongly suggest shared molecular pathways and genetic interactions in the pathogenesis of both familial and sporadic PD (Figure 1). In support of this notion, PINK1, Parkin and DJ-1 have been reported to functionally interact to maintain mitochondrial integrity and functionality and to protect against adverse effects of multiple stressors (Deng et al., 2008a; Exner et al., 2007; Narendra et al., 2010; Park et al., 2006; Poole et al., 2008; Vives-Bauza et al., 2010b) (Figure 1). Transgenic expression of Parkin attenuates PINK1 loss-of-function phenotypes in flies, whereas transgenic expression of PINK1 had no effect on Parkin loss-of-function phenotypes (Clark et al., 2006; Park et al., 2006). PINK1 mediates the translocation of Parkin to mitochondria, which directs mitochondria to autophagy (Vives-Bauza et al., 2010a). Furthermore, PINK1 also interacts with mitochondrial serine protease HtrA2 (PARK13); PINK1-dependent phosphorylation of HtrA2 might modulate the proteolytic activity of HtrA2, thereby contributing to an increased resistance of cells to mitochondrial stress (Plun-Favreau et al., 2007). Coexpression of PINK1, Parkin or DJ-1, but not PD-associated mutants of these genes, rescues cells from mitochondrial fusion elicited by α-synuclein overexpression (Kamp et al., 2010). It has been proposed that the functional interaction among different PD-associated genes does not necessarily result from a direct physical interaction among corresponding proteins; alternatively, the convergence at the level of mitochondrial integrity of different pathways appears, at least partially, responsible for the overall outcome of such interactions. Moreover, DJ-1, Parkin, and PINK1 have been reported to form a ubiquitin E3 ligase complex promoting unfolded protein degradation (Xiong et al., 2009). In double transgenic Drosophila, the coexpression of PINK1, DJ-1 or Parkin alters various phenotypes of the eye and dopaminergic system elicited by LRRK2 expression in a complex fashion (Venderova et al., 2009). In α-synuclein/LRRK2 double mutant mice, overexpression of LRRK2 promoted and knockout of LRRK2 suppressed the abnormal aggregation and somatic accumulation of α-synuclein as well as the progressive loss of cortical and striatal neurons of the forebrain induced by overexpression of Ala53Thr α-synuclein (Lin et al., 2009). These findings reveal an important functional interplay between LRRK2 and α-synuclein and suggest these two dominant PD gene products may share common pathogenic mechanisms. Certainly, further investigation of detailed molecular cascades of genetic interactions will glean many fresh insights into PD pathogenesis (Figure 1).
2.6 Genome-wide association study (GWAS) of PD
Multiple GWASs conducted in PD patients and controls have independently identified polymorphisms in the SNCA (4q22), MAPT (17q21.1) and LRRK2 (12q12) regions as risk factors for PD, confirming the known associations of these genes with PD (Edwards et al., 2010; Maraganore et al., 2005; Pankratz et al., 2009; Satake et al., 2009; Simon-Sanchez et al., 2009; Tan et al., 2010). A GWAS and two replication studies conducted in a total of 2,011 PD patients and 18,381 controls from Japan have detected two new risk loci: 1q32 (PARK16) and 4p15 (BST1) (Satake et al., 2009). While SNCA polymorphisms as a major risk locus across populations have been confirmed by several GWASs, BST1 and MAPT as risk loci for PD show population differences (Satake et al., 2009; Simon-Sanchez et al., 2009). Thus, an unequivocal role for common genetic variants in the etiology of typical PD implies population-specific genetic heterogeneity in PD. Additionally, a recent GWAS of 2,000 PD patients and 1,986 controls from the NeuroGenetics Research Consortium Common has identified an association of genetic variation in the HLA region with late-onset sporadic PD (Hamza et al., 2010), which will be discussed in detail later (see Section 4.3). Certainly, GWASs in a population sample may prove to be productive in identifying association of genetic variability with disease risk. However, association does not imply causation and can not be used to predict disease risk of an individual for a certain disease. The pathogenic effects of specific mutations and polymorphisms must be investigated.
3. Genetically engineered PD animal models
Transgenic overexpression of mutant genes for autosomal-dominant genes (SNCA and LRRK2) and knockout/knockdown of autosomal-recessive genes (Parkin, DJ-1, and PINK1) in different organisms, such as mice, Drosophila and C. elegans have been used to model PD. These models not only yield fruitful insights into pathogenic mechanisms of causative gene mutations in neuronal dysfunction in familial PD, but also provide fresh insights into sporadic PD. Several excellent reviews have introduced in detail these animal models and discussed controversies around these models (Chesselet, 2008; Dawson et al., 2010; Lim and Ng, 2009; Lu and Vogel, 2009; Pienaar et al.). Here, we briefly summarize the genetically engineered animal models of PD, with focus on future work on refinement and optimization of these models.
3.1 Overexpression of α-synuclein
A variety of transgenic animals overexpressing either wildtype or mutant human α-synuclein variably recapitulate neurodegeneration and α-synuclein pathology (e.g. aggregation, truncation, phosphorylation and ubiquitination) in different brain regions (Farrer, 2006). For example, α-synuclein transgenic Drosophila (Feany and Bender, 2000) and virus-infected mice, rats, and primates (Kirik et al., 2002; Klein et al., 2002; Lo Bianco et al., 2002; Yamada et al., 2004) revealed loss of dopamine neurons and formation of Lewy body-like inclusions. However, of the many α-synuclein transgenic mouse models, a few showed alterations in the nigrostriatal dopamine system (Masliah et al., 2000; Richfield et al., 2002; Rockenstein et al., 2002; Tofaris et al., 2006), and only very few of them developed overt loss of dopamine neurons in the SN. For instance, when human wildtype or doubly mutated α-synuclein (A53T and A30P) was expressed in mice by using rat tyrosine hydroxylase promoter, the number of nigral dopamine neurons declines with age (Thiruchelvam et al., 2004). Although the reason for the lack of overt loss of nigral dopamine neurons in most human α-synuclein transgenic mice is unclear, we speculate the following reasons may be partially responsible for such a phenomenon. Firstly, while A53T mutant α-synuclein is pathogenic in humans, the threonine at position 53 is the wildtype amino acid of rodent α-synuclein; this difference and additional five amino acid differences between mouse and human α-synuclein may affect the oligomerization and fibrillogenesis as well as consequent pathogenicity of α-synuclein. Secondly, the coexistence of human α-synuclein and mouse α-synuclein is postulated to influence the phenotypes of human α-synuclein transgenic mice (Cabin et al., 2005; Rochet et al., 2000). Thirdly, abnormalities in genetically engineered mice carrying PD-related genetic defects (e.g. mutant SNCA and LRRK2), probably at the very best only mimic the earliest dysfunction of the nigrostriatal dopaminergic system and the initial preclinical stage of PD. Fourthly, the short lifespan of mice (< 3 years) makes it strictly difficult, if not impossible, to replicate the decades-long neurodegenerative process of PD patients in these animals. Fifthly, the presence of neuromelanin in dopamine neurons in humans and non-human primates, but not in rodents, may render primates more vulnerable than rodents to diverse PD-associated insults (e.g. genetic mutations and environmental toxins). Sixthly, one single genetic defect is not sufficient to produce overt loss of nigral dopamine neurons, and an environmental trigger may be required for PD neurodegeneration. These potential reasons except the first two may be also partially responsible for the paucity of the full-spectrum of PD in other genetic mouse models.
3.2 Overexpression of LRRK2
Drosophila and C. elegans overexpressing mutant LRRK2 display death of dopamine neurons and increased sensitivity to rotenone and paraquat (Liu et al., 2008; Ng et al., 2009; Saha et al., 2009; Venderova et al., 2009). However, the short lifespan and the lack of endogenous α-synuclein expression render it potentially problematic to study mechanisms of LRRK2-mediated neurodegeneration in Drosophila and C. elegans, since a prominent feature of PD caused by LRRK2 mutations is α-synuclein pathology. Bacterial artificial chromosome (BAC) transgenic mice overexpressing Arg1441Gly mutant LRRK2 show diminished striatal dopamine, axonal pathology of nigrostriatal dopamine pathway, and age-dependent, levodopa-responsive motor impairment (Li et al., 2009). BAC transgenic mice overexpressing Gly2019Ser mutant LRRK2 exhibited an age-dependent striatal dopamine deficiency but not degeneration of nigrostriatal dopamine neurons or terminals at 12 months of age (Li et al., 2010). Conditional expression of wildtype and Gly2019Ser mutant LRRK2 in dopamine neurons or knockin of the Arg1441Cys mutant in mice failed to produce dopaminergic neurodegeneration (Lin et al., 2009; Tong et al., 2009). So far, the most consistent phenotypes in various LRRK2 transgenic mice are abnormalities in the nigrostriatal dopamine neurotransmission. These abnormalities probably indicate early dysfunction of the nigrostriatal dopamine system. However, the lack of substantial neurodegeneration in LRRK2 transgenic mice limits their utility in testing neuroprotective agents. Interestingly, co-overexpression of either wildtype or Gly2019Ser LRRK2 with A53T α-synuclein promoted abnormal somatic accumulation and aggregation formation of α-synuclein and dramatically accelerated neurodegeneration in cortical and striatal neurons of the forebrain in the double-transgenic mice (Lin et al., 2009). However, the expression of the transgene in less than 5 percent of midbrain dopaminergic neurons and the occurrence of forebrain neurodegeneration in these double-transgenic mice limit their relevance to PD pathogenesis. Crossbreeding of inducible dopamine neuron-specific α-synuclein transgenic mice with LRRK2 mutant mice may provide an innovative and valuable mouse model to investigate how LRRK2 interacts with α-synuclein in the modulation of dopaminergic function and degeneration (Tong and Shen, 2009).
3.3 Knockout of α-synuclein or LRRK2
α-synuclein knockout mice exhibit a subtle electrophysiological phenotype but no neurodegenerative pathology (Perez and Hastings, 2004). Knockout of LRRK2 in mice is not neurotoxic per se and does not exacerbate MPTP-elicited dopaminergic neurodegeneration (Andres-Mateos et al., 2009). Deletion of LRRK homolog in Drosophila causes dopaminergic impairment and locomotive deficit (Lee et al., 2007). Knockdown of LRRK homolog in C. elegans does not affect on baseline viability but enhances their vulnerability to rotenone toxicity (Saha et al., 2009).
3.4 Knockout of DJ-1
Drosophila possesses two orthologs of DJ-1: DJ-1a and DJ-1b. Knockout of both DJ-1 homologs in Drosophila does not alter fertility, lifespan, or dopamine neurons but sensitizes Drosophila to PD-associated environmental toxins, paraquat and rotenone (Meulener et al., 2005). Selective knockdown of DJ-1 by RNAi in dopamine neurons leads to age-dependent loss of dopamine neurons in the dorsomedial cluster in Drosophila (Yang et al., 2005). DJ-1 knockout mice exhibit abnormal dopamine neurotransmission in the nigrostriatal pathway and motor deficits but no observable neurodegeneration (Andres-Mateos et al., 2007; Chen et al., 2005b; Goldberg et al., 2005; Kim et al., 2005).
3.5 Knockout of Parkin or overexpression of mutant Parkin
Parkin-knockout Drosophila reveals male sterility, reduced lifespan, and severe defects in flight and climbing abilities. These phenotypes may be associated with mitochondrial defects and consequent apoptotic cell death in muscle and sperm. Aged Parkin-null flies show dopamine-responsive locomotion deficits and degeneration of a subset of dopamine neurons (Greene et al., 2003; Whitworth et al., 2005). Parkin-null mice display either subtle alterations in the nigrostriatal dopamine circuit or no overt dopaminergic or behavioral abnormalities (Goldberg et al., 2003; Itier et al., 2003; Perez and Palmiter, 2005). Interestingly, overexpression of mutant human Parkin in Drosophila and mice causes progressive degeneration of dopamine neurons (Lu et al., 2009; Sang et al., 2007; Wang et al., 2007).
3.6 Knockout of PINK1
PINK1-null Drosophila and Parkin-null Drosophila share marked phenotypic similarities including male sterility, behavioral deficits, fewer dopamine neurons, mitochondrial defects, and apoptosis in muscle (Clark et al., 2006; Gautier et al., 2008; Park et al., 2006). In the Drosophila loss-of-function models of PINK1 and Parkin, the dopaminergic system is mildly affected, while the most dramatic phenotypes occur outside the nervous system. PINK1 knockout mice show subtle deficits in nigrostriatal dopamine neurotransmission (Gautier et al., 2008; Gispert et al., 2009; Kitada et al., 2007). Because deficiency in Parkin, PINK1, or DJ-1 in mice does not cause substantial nigrostriatal pathology, these mice at the very best only mimic the earliest dysfunction of the nigrostriatal dopaminergic system. Thus, usage of current autosomal-recessive animal models to study mechanisms of dopamine neurodegeneration and to screen neuroprotective agents is potentially problematic or impossible. Overall, gene-based Drosophila and C. elegans models offer powerful tools to rapidly screen genetic and pharmacologic modifiers of known PD-associated genes, but the challenge is to verify the evolutionary conservation of these potential modifiers in human PD.
4. The environmental causes of PD
4.1 Pesticides and other environmental toxins
Although modern genetics uncovers important genetics basis in familial PD, it is worth emphasizing that only about 10%–20% of PD cases result from genetic causes. The majority of PD cases are sporadic and idiopathic. The accidental use of 1-methyl-4-phenyl-1,2,3-tetrahydropyridine (MPTP), a by-product of illicit heroin synthesis, leads to a Parkinson syndrome that is clinically indistinguishable from PD (Langston et al., 1983). This milestone finding has stimulated the search for environmental factors as potential causes of PD. The structural similarity between paraquat (1,1′-dimethyl-4,4′-bypyridinium), a common herbicide, and 1-methyl-4-phenylpyridinium ion (MPP+), the active metabolite of MPTP, prompted speculation that paraquat might be a dopaminergic neurotoxicant and exposure to paraquat may be related to the development of PD. Epidemiological studies have reported that exposure to pesticides including paraquat correlates with increased incidence of PD (Ascherio et al., 2006; Liou et al., 1997; Rajput and Uitti, 1987). Case reports have also associated development of Parkinsonism with exposure to maneb (a widely used fungicide) and organophosphorus insecticides (Bhatt et al., 1999; Liu et al., 2003; Meco et al., 1994). Elevated levels of residual dieldrin (an organochlorine pesticide) and polychlorinated biphenyls (widely used industrial chemicals) have been detected in the brains of PD patients (Corrigan et al., 1998; Corrigan et al., 2000; Fleming et al., 1994). Chronic occupational exposure to high levels of manganese in manganese miners causes accumulation of this metal in the basal ganglia, resulting in tremors, rigidity and psychosis that resemble PD (Mergler and Baldwin, 1997). However, elevated levels of certain pesticides or chemicals in the brains of PD patients do not indicate causal relationship between toxin exposure and PD development. Experimental exposure of animal models to several classes of pesticides, such as paraquat, dithiocarbamate-based fungicides (e.g. maneb), insecticide rotenone, and organochlorine pesticides (e.g. dieldrin) leads to dopaminergic neurotoxicity. These pesticides have hence been proposed as potential PD risk factors in humans (Liu et al., 2003). Furthermore, epidemiological and case-control studies have suggested rural residence, well-water consumption, pesticide use, and certain occupations (e.g. farming, mining and welding) are associated with an increased risk of PD (Dhillon et al., 2008; Elbaz et al., 2009; Engel et al., 2001; Gorell et al., 1998; Semchuk et al., 1992). Several specific pesticides including dieldrin, maneb, paraquat, and rotenone have recently been implied to increase PD risk by recent epidemiological studies (Brown et al., 2006; Dhillon et al., 2008; Kamel et al., 2007; Ritz et al., 2009). However, overall epidemiological evidence is fragmentary and insufficient to establish a specific association between exposure to specific pesticides/toxicants and increased risk for PD.
4.2 Brain trauma
Numerous case reports have associated episodes of traumatic brain injuries with the occurrence of an acute-phase Parkinsonism or later-life development of PD (Doder et al., 1999; Nayernouri, 1985). Several epidemiological studies including an analysis of the medical histories of a large group of World War II veterans have identified antecedent head injuries as a major risk factor for the development of PD in later life (Goldman et al., 2006; Stern, 1991; Taylor et al., 1999). However, contradiction epidemiological results suggest no such a correlation (Goetz and Pappert, 1992; Spangenberg et al., 2009; Williams et al., 1991). Boxers who have professionally related higher chance of suffering head injuries appear to have a higher incidence of Parkinson-like movement disorders (Friedman, 1989; Guterman and Smith, 1987).
4.3 Inflammation
Early-life occurrence of brain inflammation resulting from either brain injury or exposure to infectious agents may modulate PD risk (Figure 1). Epidemiologic studies pointed to an association between early-life viral infections and postencephalitic PD, while case reports tend to relate viral infections to the development of acute Parkinsonism (Duvoisin et al., 1972; Elizan and Casals, 1991; Ghaemi et al., 2000; Henry et al., 2010; Liu et al., 2003). Experimental exposure of neonatal Fisher 344 rats to Japanese encephalitis virus can induce degeneration of nigral dopamine neurons, gliosis in the SN, and movement disorders resembling human PD (Ogata et al., 1997). Moreover, prospective studies suggest that higher plasma concentration of proinflammatory cytokine interleukin-6 correlates with an increased risk for PD (Chen et al., 2008). Epidemiological and case–control studies on associations between chronic use of nonsteroidal anti-inflammatory drugs and PD risk generate mixed results, with some studies suggesting a reduced incidence and others finding no association (Chen et al., 2005a; Chen et al., 2003; Powers et al., 2008; Ton et al., 2006). DNA polymorphisms of several inflammatory cytokines might become risk factors for PD (Wahner et al., 2007). A recent GWAS of 2,000 PD patients and 1,986 controls from the NeuroGenetics Research Consortium Common identifies an association of genetic variation in the HLA region with late-onset sporadic PD (Hamza et al., 2010). This hypothesis-free GWAS lends strong and independent support to the participation of inflammation in PD pathogenesis (Figure 1). HLA (human leukocyte antigen) region contains a large number of genes related to immune function in humans. HLA class II histocompatibility antigen plays a central role in inflammation by presenting peptide antigens to the immune system. While additional studies are needed to establish a causal role for inflammation in PD pathogenesis, the increased awareness of the involvement of inflammation in PD will stimulate research toward new therapeutic targets. Collectively, environmental risk factors, including toxins, early-life infections in the brain, and traumatic brain injuries may contribute to the cause of PD (Figure 1). However, specific causative environmental toxicants or infectious agents (DNA or antigens) for PD have not yet been identified.
4.4 Protective environmental factors
Inverse associations of PD with cigarette smoking, caffeine intake, and high plasma urate concentrations have been noted (Checkoway et al., 2002; Chen et al., 2010; Hernan et al., 2002; Powers et al., 2008; Weisskopf et al., 2007). However, whether such associations reflect true biologic protection needs further investigation. Interestingly, a few randomized controlled clinical trials suggest beneficial effects of exercise (e.g. improvements in postural stability and balance task performance) in PD patients (Dibble et al., 2009; Gobbi et al., 2009; Morris et al., 2009). Larger number of studies, more outcomes used, and longer term follow-up are needed to confirm that excise-related improvements in PD motor dysfunction are long-lasting. A few prospective cohort studies suggest that moderate to vigorous exercise may protect against PD (Xu et al., 2010). In rodents, exercise reduces the behavioral impairments and the loss of dopamine neurons elicited by the dopaminergic neurotoxins MPTP or 6-hydroxydopamine (6-OHDA) (Gerecke et al., 2010; Zigmond et al., 2009). The trigger of endogenous neuroprotective mechanisms (e.g. increased expression of neurotrophic factors) and the preconditioning (mild stress before more intensive stress) have been proposed to be responsible for beneficial effects of exercise (Zigmond et al., 2009).
5. Toxin-based models of PD
5.1 MPTP model
Several toxin-based models of PD have provided the framework for symptomatic improvements in medical and surgical therapies for PD. MPTP model is the most widely used PD model for the study of molecular cascades of death of dopamine neurons and for the screening of neuroprotective strategies. MPTP can cross the blood-brain barrier after subcutaneous or intraperitoneal injection. Once inside the brain, MPTP is converted into MPP+ by the enzyme monoamine oxidase B in astroglia. Released MPP+ is then taken up by the dopamine transporter and concentrated in dopamine neurons where it inhibits mitochondrial complex I, resulting in ATP depletion, increased free-radical generation, and death of dopamine neurons. MPTP is the only known dopaminergic neurotoxin inducing Parkinsonism in both humans and monkeys (Jackson-Lewis and Przedborski, 2007). Mice and monkeys treated with MPTP develop reliable and reproducible nigrostriatal lesion and gliosis (Jackson-Lewis and Przedborski, 2007). MPTP-injected monkeys revealed increased brain levels of α-synuclein, PD-like modifications of α-synuclein (e.g. aggregation, nitration and phosphorylation), or intraneuronal inclusions (Forno et al., 1986; McCormack et al., 2008; Purisai et al., 2005). However, it seems that unless MPTP is chronically infused by osmotic minipump or is administered with probenecid, a uricosuric agent that is used to block the rapid clearance of MPTP, proteinaceous inclusions will not be produced in dopamine neurons in mice (Fornai et al., 2005; Meredith et al., 2002; Shimoji et al., 2005). A few drawbacks, such as strain-specificity of animals, lack of classical and convincing α-synuclein pathology, relatively rapid kinetics of neurodegeneration and partial lesion recovery, make MPTP mouse models less ideal for studying PD progression and for screening drugs to slow down the disease progression.
5.2 6-OHDA model
6-OHDA is the first catecholaminergic neurotoxin that was used to generate animal models of PD. Because of its preferential uptake by dopamine and noradrenergic transporters, 6-OHDA induced relatively selective neurotoxicity to monoaminergic neurons (Ungerstedt, 1968). Inside these neurons, 6-OHDA produces reactive oxygen species and quinones that inactivate biological macromolecules. Being unable to cross the blood-brain barrier, 6-OHDA must be administered by local stereotaxic injection to target the nigrostriatal dopaminergic pathway (Javoy et al., 1976). When injected into the SN or median forebrain bundle that carries ascending dopaminergic and serotonergic projections to the forebrain, 6-OHDA causes acute loss of dopamine neurons within 24 hr (Jeon et al., 1995); when injected into the striatum, it induces retrograde degeneration of nigral dopamine neurons, which lasts for 1–3 weeks (Przedborski et al., 1995; Sauer and Oertel, 1994). To date, no Lewy body-like inclusion has been described in 6-OHDA model. The paucity of classical PD pathology, the relatively acute neurotoxicity, and the requirement of stereotaxic injection make 6-OHDA model less popular in recent years.
5.3 Rotenone model
Rotenone, a mitochondrial complex I inhibitor (Earley and Ragan, 1984), is commonly used as a pesticide and fish poison. Although it remains to be determined whether typical human exposure to rotenone alone is likely to increase risk for PD, rotenone has been employed to model PD. Intracerebral application of rotenone led to damage to the nigrostriatal dopaminergic pathway in rats (Heikkila et al., 1985). Initial attempts to systemically administer rotenone, acutely or sub-chronically, to rodents produced only minimal damage to the nigrostriatal dopaminergic system (Ferrante et al., 1997; Thiffault et al., 2000). Later, continuous systemic administration of rotenone to rats and mice reproduces cardinal features of PD, including the degeneration of the nigrostriatal dopaminergic pathway, the formation of Lewy body-like inclusions containing ubiquitin and filamentous α-synuclein in remaining nigral neurons, and movement impairments (Alam and Schmidt, 2002; Betarbet et al., 2000; Hoglinger et al., 2003; Sherer et al., 2003). Mechanistically, inhibition of mitochondrial complex I activity with consequent ATP depletion and oxidative damage, facilitation of α-synuclein aggregation and inflammation-induced injury may be responsible for the dopaminergic neurotoxicity of rotenone (Betarbet et al., 2000; Gao et al., 2002a; Sherer et al., 2002; Sherer et al., 2003). A high mortality has limited an extensive utility of rotenone model.
5.4 Paraquat model
Intracerebral injection of paraquat resulted in loss of nigral dopamine neurons, depletion of dopamine in the striatum, and an elevated response to apomorphine-induced rotational behavior (Liou et al., 1996). Effects of systemic administration of paraquat on dopamine neurons have been less consistent. Paraquat does not easily penetrate the blood brain barrier (Shimizu et al., 2001), and its distribution in the CNS is not region-specific or cell-type-specific. Mice that underwent a single or repeated systemic administration of paraquat exhibited a selective loss of nigral dopamine neurons with or without degeneration of striatal dopamine fibers or a reduction in striatal dopamine content (Brooks et al., 1999; Manning-Bog et al., 2002). Free radical generation and redox cycling (Bonneh-Barkay et al., 2005; Fukushima et al., 1995; Wu et al., 2005; Yumino et al., 2002), α-synuclein upregulation and fibrillation (Manning-Bog et al., 2002; Uversky et al., 2001), as well as apoptotic cell death (Chun et al., 2001) have been associated with paraquat-induced dopaminergic neurotoxicity. Furthermore, repeated systemic administration of maneb and paraquat to mice induced synergistic nigrostriatal dopaminergic neurodegeneration and motor behavioral abnormalities (Thiruchelvam et al., 2000). Neonatal exposure to both maneb and paraquat sensitized the nigrostriatal dopaminergic system to rechallenge with the same agents during adulthood (Thiruchelvam et al., 2002).
5.5 Inflammation-associated model
Whether bacterial or viral infection can act as an initiating factor in human PD is unclear, but intracranial or systemic administration of bacterial endotoxin or virus into rodents has been used to produce inflammation-associated PD model. Direct nigral injection of lipopolysaccharide (LPS), the active immunostimulant in the cell wall of Gram-negative bacteria, causes robust microglial activation and acute loss of nigral dopamine neurons in rodents (Castano et al., 1998; Kim et al., 2000; Liu et al., 2000; Tomas-Camardiel et al., 2004). Chronic infusion of LPS for 2 weeks into the rat SN triggered a rapid activation of microglia, followed by a selective and gradual loss of nigral dopamine neurons that began at between 4 and 6 weeks and reached 70% by 10 weeks (Gao et al., 2002b). This is the first report that microglial activation induced by chronic exposure to inflammogen was capable of producing a delayed, progressive and selective degeneration of nigral dopamine neurons. Microglia-originated free radicals play a critical role in dopaminergic neurotoxicity in this inflammation-mediated model of PD (Gao et al., 2002b). Additionally, in utero exposure of developing rat fetuses to LPS results in lesions of nigrostriatal dopaminergic system in neonates (Ling et al., 2002). Adult rats with prenatal exposure to LPS developed progressive dopaminergic neurodegeneration following chronic nigral LPS infusion (Ling et al., 2006). Moreover, an intraperitoneal injection of LPS (5 mg/kg) led to significant loss of nigral dopamine neurons in C57BL/6 mice 7 months after the injection; such neurodegeneration progressed further overtime (Qin et al., 2007). These findings together indicate that once reaching certain threshold, the inflammatory process is capable of initiating neurodegeneration. The higher density of microglia in the SN than other brain regions (Kim et al., 2000; Lawson et al., 1990) may be partially responsible for the foremost loss of dopamine neurons in the inflammation-induced neurodegenerative process.
Although humans infected by highly pathogenic H5N1 influenza virus show acute neurological impairments ranging from mild encephalitis to motor disturbances to coma (de Jong et al., 2005; Gambotto et al., 2008), the long-term neurological manifestations of H5N1 infection among surviving hosts remain unknown. A recent study has reported that after intranasal inoculation in C57BL/6 mice, H5N1 virus travelled from the peripheral nervous system into the CNS. Microglial activation and α-synuclein phosphorylation and aggregation persists long after resolution of the infection in infected brain regions. While H5N1 virus was no longer detectable in the CNS 21 days post-inoculation, a significant loss of nigral dopamine neurons was observed 60 days after the virus infection (Jang et al., 2009). This study provides important information on the role of virus-associated inflammation in potential long-term impairment to the CNS. However, the requirement of a biosafety level 3+ laboratory will limit the usage of this virus-based PD model.
6. Two-hit PD models created by genetic lesions and environmental insults
As described above, animal models of PD created by using individual neurotoxins or genetic manipulation have been extremely valuable for studying the pathogenesis of PD. However, most of the current genetic PD models failed to reproduce overt nigrostriatal dopaminergic loss, suggesting that a single genetic risk factor is not sufficient and an environmental trigger may be required for producing nigral dopamine neuronal loss. Thus, two-hit animal models are generated to test whether genetic deficiency increases vulnerability of dopamine neurons to potential environmental risk factors (Horowitz and Greenamyre, 2010). The concept of “model fusion” in the creation of a new generation of animal models to study gene-environment interactions in PD has been proposed by Drs. Manning-Bog and Langston (Manning-Bog and Langston, 2007). These new models would be of tremendous importance to a more in-depth understanding of the pathogenesis of PD and to the discovery of therapeutics that can retard PD progression.
6.1 LPS and α-synuclein
The fact that only very few of many different α-synuclein transgenic mouse models developed loss of dopamine neurons in the SN reveals the limitations of rodent models in the replication of all aspects of age-related neurodegeneration. Alternatively, such a fact might also suggest that other factors participate in the development of PD. Stereotaxic injection of LPS into the SN of wildtype mice and transgenic mice overexpressing either wildtype or A53T mutant human α-synuclein induced similar nigral inflammatory reactions. Transgenic expression of human α-synuclein renders dopamine neurons more vulnerable to LPS-induced inflammation, which in turn leads to accumulation of insoluble α-synuclein aggregates in nigral neurons and death of nigral dopamine neurons. Nitrated/oxidized α-synuclein was detected in Lewy body-like cytoplasmic inclusions in LPS-injected transgenic mice (Gao et al., 2008). This two-hit (neuroinflammation and overexpression of wildtype or mutant human α-synuclein) animal model not only provides better tool to advance our understanding of the role of neuroinflammation and α-synuclein dysfunction in the pathogenesis of PD, but also underscores synergistic effects of genetic predisposition and environmental exposures in the development of PD. Furthermore, a two-hit, chronic, progressive model has been created by an intraperitoneal injection of a relatively low dose of LPS (3 × 106 EU/kg) into 7-month-old transgenic mice overexpressing A53T mutant human α-synuclein (Gao et al., 2011). Such a LPS injection induced similar systemic inflammation and a comparatively mild neuroinflammatory response in both wildtype and α-synuclein transgenic mice. However, only in LPS-injected transgenic mice, the low-grade neuroinflammation sustains; α-synuclein accumulates and forms aggregates; Lewy body-like neuronal inclusions containing aggregated α-synuclein occur; and dopaminergic neurodegeneration in the nigrostriatal pathway progresses over time. Thus, synergistic effects of low-grade neuroinflammation and α-synuclein dysfunction drive chronic PD neurodegeneration, while neither factor alone is sufficient to cause neuronal death (Gao et al., 2011). This new model mimics PD multifactorial etiology and recapitulates the key features of PD. More importantly, the chronic progressive nature of dopaminergic neurodegeneration makes this model invaluable for the study of mechanisms of PD progression and for the screening of disease-modifying therapeutics.
6.2 LPS and Parkin
Although loss-of-function mutations in the parkin gene cause early-onset familial PD, Parkin-deficient mice do not display nigrostriatal degeneration. The intraperitoneal administration of low-dose of LPS (7.5 × 105 EU/kg) twice a week into Parkin-null or wildtype mice for 3 to 6 months indicated that genetic deficiency in Parkin sensitizes nigral dopamine neurons to inflammation-mediated neurotoxicity. Specifically, although persistent neuroinflammation and oxidative stress responses triggered by LPS are not different between two genotypes, only Parkin-null mice displayed selective loss of nigral dopamine neurons and subtle fine-motor deficits (Frank-Cannon et al., 2008). This model may enable identification of early biomarkers of neurodegeneration and preclinical screening of potential compounds that can delay the progressive degeneration of the nigrostriatal pathway.
6.3 Neurotoxins and α-synuclein
The possible interaction between genetic factors and neurotoxins has been investigated by examining the sensitivity of α-synuclein transgenic mice to secondary neurotoxic insults, such as paraquat, maneb, MPTP, or rotenone. In general, either dopaminergic neurodegeneration or α-synuclein pathology is missing or has not been determined in those neurotoxin-exposed α-synuclein transgenic mice. For instance, intraperitoneal injection twice a week for 3 weeks into transgenic mice overexpressing A53T mutant human α-synuclein with maneb and paraquat, but not with either pesticide alone, drastically exacerbates neuronal α-synuclein pathology (e.g. accumulation of aggregated α-synuclein and formation of α-synuclein-containing inclusions) throughout the CNS. However, no noticeable neuron loss in the SN or other brain regions was observed in transgenic mice after treatment with maneb and/or paraquat (Norris et al., 2007). Whether the exacerbated α-synuclein pathology in pesticide-exposed transgenic mice represents early disease stage that precedes neuronal degeneration remains to be determined. In addition, weekly intraperitoneal injections of paraquat (10 mg/kg, 3 times) into wildtype and transgenic mice expressing human wildtype or A53T mutant human α-synuclein driven by the tyrosine hydroxylase promoter resulted in an upregulation of α-synuclein and the formation of α-synuclein-positive inclusions. However, only wildtype mice, but not transgenic mice, developed nigral degeneration after paraquat injection (Manning-Bog et al., 2003). The mechanism underlying the protective effect of transgenic overexpression of human α-synuclein against pesticide-induced neurodegeneration in transgenic mice warrants further investigation. Interestingly, recent evidence also indicates that transgenic expression of α-synuclein abolishes the lethality and neurodegeneration induced by ablation of cysteine-string protein-α (CSPα, an abundant synaptic vesicle protein with a cochaperone function), whereas deletion of endogenous synucleins exacerbates these phenotypes. Here, transgenic α-synuclein attenuates CSPα-deficiency-elicited inhibition of SNARE-complex assembly, in which binding of α-synuclein to synaptic vesicle phospholipids is requires (Chandra et al., 2005).
Chronic treatment with MPTP (daily intraperitoneal injection of 16 or 30 mg/kg MPTP for 5 consecutive days), but not with rotenone (0.125 mg/kg twice a week for 3 consecutive weeks), led to greater deterioration of the nigrostriatal dopamine system in transgenic mice expressing human A30P mutant α-synuclein than in wildtype littermates (Nieto et al., 2006). Transgenic mice expressing wildtype or a doubly mutated (A53T and A30P) α-synuclein show increased density of the dopamine transporter and elevated vulnerability to MPTP neurotoxicity. The doubly mutated mice exhibit reduced locomotor responses to repeated doses of amphetamine (Richfield et al., 2002). Collectively, although neurotoxin-exposed α-synuclein transgenic mice reveal either exacerbated α-synuclein pathology or greater deterioration of nigrostriatal dopamine pathway, it seems there is dissociation between toxicant-induced α-synuclein pathology and neurodegeneration
6.4 Neurotoxins and LRRK2
The ubiquitous expression of LRRK2 increases lifespan and fertility of the Drosophila but enhances the sensitivity of these flies to rotenone (Venderova et al., 2009). Similar to α-synuclein knockout mice, LRRK2 knockout mice that lack the kinase domain of LRRK2 have no observable neurodegenerative pathology. Different from α-synuclein knockout mice that are more resistant to neurotoxicity induced by LPS or MPTP (Dauer et al., 2002; Gao et al., 2008), LRRK2 knockout mice display the same susceptibility to MPTP as wildtype mice (Andres-Mateos et al., 2009).
6.5 Glial cell line-derived neurotrophic factor (GDNF) and methamphetamine
The importance of GDNF for maintenance of dopamine neurons has been well established. Enhanced dopaminergic cell survival and fiber outgrowth as well as improved behavioral recovery in rodent and monkey models of PD have encouraged the use of GDNF in clinical trials. Safety concerns for CNS delivery of GDNF and mixed results from limited human studies make it impossible to draw a firm conclusion about the efficacy and potential usage of GDNF in PD treatment. The genetic reduction of Gdnf and the reduced availability of GDNF protein in young Gdnf+/− mice increase the vulnerability of dopamine systems to methamphetamine exposure (Boger et al., 2010). This dual-hit model will extend current knowledge of the impact of GDNF on progressive dopaminergic degeneration and motor dysfunction in PD. Experimental exposure of these Gdnf+/− mice to more relevant environmental toxins (e.g. rotenone or paraquat) may provide fruitful information on PD etiology and pathogenesis.
7. Ideal models of PD
To date, none of existing animal models of PD mirrors the full-spectrum of the disease, even though they reproduce many of the features of PD (Chesselet et al., 2008). What are ideal animal models of PD? Despite the paucity of Lewy bodies in some PD patients carrying mutant LRRK2 or Parkin, all PD patients ultimately develop gradual loss of dopamine neurons in the nigrostriatal pathway. Therefore, any “reasonable” model of PD should replicate the loss of nigral dopamine neurons. Ideal PD models should be age-dependent and progressive. They should reproduce the gradual loss of dopamine neurons and the dopamine-responsive motor impairment (e.g. slowness of movement, rigidity, rest tremor and postural instability). These models should also exhibit Lewy bodies and Lewy neurites that contain aggregated α-synuclein and ubiquitin-proteasomal proteins. Chronic neuroinflammation, a common feature shared by neurodegenerative diseases, should be another key feature of ideal PD models. More importantly, ideal models should mimic the multifactorial etiology of PD, in which sub-toxic concentrations of relevant environmental toxins are administrated into genetically engineered animals with PD-associated genetic defects. In addition, the pathological alterations in the autonomic and/or enteric nervous systems in PD patients are also desirable characteristics of ideal models. Collectively, ideal animal models of PD should recapitulate the dopamine-responsive motor impairment, the autonomic dysfunction, and the full pathological spectrum seen in PD patients.
8. Gene-environment interactions influence PD susceptibility
The concept that gene-environment interactions affect PD susceptibility has been proposed for more than a decade (Ross and Smith, 2007; Vance et al., 2010). Only a few human association studies have examined gene-environment interactions, although many studies have described positive associations between genetic polymorphisms and increased risk for PD. To date, dozens of single-nucleotide polymorphisms (SNPs) in several genes have shown gene–environment interactions in relation to PD (Table 1). Cumulative and interactive effects of cigarette smoking and gene polymorphisms in NOS1 (neuronal nitric oxide synthase), NOS2A (inducible nitric oxide synthase) or SNCA may modulate risk for PD (Hancock et al., 2006; Hancock et al., 2008; Levecque et al., 2003; McCulloch et al., 2008). Occupational pesticide exposure as well as high exposure to paraquat and maneb in carriers of DAT (dopamine transporter) genetic variants increased PD risk (Kelada et al., 2006; Ritz et al., 2009). SNPs in NOS1 and GSTP1 (glutathione S-transferase pi 1) have been linked to an increased risk for PD in pesticide-exposed individuals (Hancock et al., 2008; Menegon et al., 1998). However, the association between GSTP1 and pesticide exposure has not been supported by a large cohort study conducted later (Dick et al., 2007). Additional interactions between GSTM1 (glutathione S-transferase Mu 1) null genotype and heavy solvent exposure (Dick et al., 2007) and between APOE (apolipoprotein E) SNPs and coffee consumption (McCulloch et al., 2008) have been found to affect PD susceptibility. However, the majority of gene-environment analyses in a case-control study of 767 PD patients and 1989 controls across five European centers did not show significant interactions between polymorphisms in 15 genes that influence metabolism of foreign chemicals or dopamine and exposure to solvents, pesticides and metals (Dick et al., 2007). Overall, results from human association studies should be interpreted with caution as most of these studies are based on a relatively small number of exposed subjects. Certainly, more large-scale human association studies aimed at identifying gene-environment interactions in the development PD may prove to be fruitful.
Table 1.
Human association studies identifying influence of gene-environment interactions on PD susceptibility
| SNP | protein product of gene | environmental factors | references |
|---|---|---|---|
| DAT | dopamine transporter | pesticides | Ritz et al, 2009; Kelada et al, 2006 |
| NOS1 (nNOS) | neuronal nitric oxide synthase | pesticides | Hancock et al, 2008 |
| NOS1 (nNOS) | neuronal nitric oxide synthase | cigarette smoking | Levecque et al, 2003; Hancock et al, 2008 |
| NOS2A (iNOS) | inducible nitric oxide synthase | cigarette smoking | Hancock et al, 2008 |
| APOE | apolipoprotein E | caffeinated coffee | McCulloch et al, 2008 |
| SNCA | α-synuclein | cigarette smoking | McCulloch et al, 2008 |
| GSTP1 | glutathione S-transferase pi 1 | pesticide | Menegon et al, 1998 |
| CYP2D6 | cytochrome P-450 2D6 | solvent exposure | De Palma et al, 1998 |
| GSTM1 null | glutathione S-transferase Mu 1 | heavy solvent exposure | Dick et al, 2007 |
9. Oxidative stress serves as a central mechanism of chronic PD progression
Aging represents an unequivocal risk factor for PD, and oxidative stress is believed to be a major component of age-related disorders such as cancer, strokes, osteoarthritis, cardiovascular and neurodegenerative diseases. Oxidative stress engages in diverse cellular processes and plays a prominent role in the induction of neuronal death. For instance, excessive free radicals can damage proteins (e.g. abnormal modification of α-synuclein and inactivation of Parkin), lipids, DNA, or RNA, leading to cell dysfunction (e.g. ubiquitin-proteasome system [UPS] and mitochondrial impairment) and eventual cell death. While overwhelming evidence underlines the importance of oxidative stress in dopaminergic neurodegeneration elicited by inflammation or various environmental toxins (e.g. MPTP, rotenone, paraquat and 6-OHDA), growing experimental findings indicate that all known PD-associated genetic defects (i.e., mutations in SNCA, Parkin, PINK1, DJ-1, and LRRK2) are related to oxidative stress directly or indirectly, which is discussed in each corresponding section above.
As illustrated in Figure 1, oxidative stress interacts with every aspect of major PD pathological events including protein misfolding and aggregation, mitochondrial dysfunction and impairment, UPS perturbation, and aberrant signal transduction. Given their intimate relationship, oxidative stress and mitochondrial impairment/dysfunction become integral and inseparable elements of PD pathogenesis. Emerging evidence has also demonstrated a detrimental role of oxidative stress in the disruption of intracellular protein homeostasis. For instance, free radicals can either cause oxidation of α-synuclein and parkin, thereby exacerbating their misfolding and accumulation, or attack protein surveillance machineries by oxidizing their subunits or key enzymes (e.g. ubiquitin-activating [E1], -conjugating [E2], and -ligating [E3] enzymes, three enzymes responsible for protein ubiquitination). When the accumulation of misfolded proteins exceeds the capacity for the chaperone, ubiquitin–proteasome, and autophagy systems to detect, to repair, and eventually to destroy those faulty proteins (Box 1), especially when these protein surveillance machineries are malfunction under oxidative condition, the build-up of toxic misfolded proteins in the cell will precipitate cellular demise. Although the precise mechanism of UPS dysfunction in PD merits further investigation, this ATP-driven degradation machinery is sensitive to age-related energy depletion and oxidative stress (Tan et al., 2009) as well as environmental toxins, particularly those that inhibit mitochondrial function or generate reactive free radicals (Betarbet et al., 2006; Wang et al., 2006). Oxidative stress can also affect the function of other key PD-linked gene products (e.g. DJ-1, PINK1 and LRRK2) and other signaling molecules, leading to abnormal signal transduction. Thus, oxidative stress appears as a converging point of multiple pathways underlying the PD pathogenesis and plays a central role in the chronic progression of PD.
Text box 1. protein surveillance machineries.
Under physiological condition, the chaperone, ubiquitin–proteasome, and autophagy systems function in concert to effectively counterbalance the threat of protein misfolding and aggregation and to maintain protein homeostasis. Among these three major protein surveillance machineries, the chaperone system (i.e., molecular chaperones that include members of the heat-shock protein family) plays a major role in correctly folding and refolding proteins (Libereket al., 2008), sensing misfolded proteins, and directing misfolded proteins for proteolysis when a native folding state can not be achieved. In this case, misfolded proteins are usually tagged with ubiquitin(s) and degraded by the proteasome, the major intracellular protein degradation machinery that destroys unneeded or damaged proteins by proteolysis. A polyubiquitin chain on a substrate typically acts as a signal for degradation by the proteasome. Polyubiquitin modification of proteins regulates many essential cellular processes including protein degradation, membrane trafficking, cell cycle, gene transcription, DNA repair, and responses to oxidative stress. Impaired USP and disrupted ubiquitin signaling may cause broad consequences for neuronal function and survival. Under conditions of cellular stress, especially when the UPS is impaired, autophagy serves as a compensatory degradation system; polyubiquitinated proteins can also be removed via the autophagy–lysosomal pathway (Pandeyet al., 2007). This autophagy–lysosomal pathway appears as a final line of defense against the accumulation of toxic misfolded proteins in the cell, and it participates in the maintenance of a balance between the synthesis, degradation, and subsequent recycling of cellular products. This protein degradation process involves the sequestration of damaged organelles and unwanted protein substrates into a double-membrane vesicle called autophagosome. The autophagosome then fuses with a lysosome to form autolysosomes, where their contents are degraded by acidic lysosomal hydrolases. This form of autophagy is called macroautophagy. Another form of autophagy is chaperone-mediated autophagy, which involves the direct translocation of substrate/chaperone complex—composed of unfolded proteins and a cytosolic chaperone hsc70—across the lysosomal membrane (Martinez-Vicenteet al., 2008; Mizushimaet al., 2008).
10. Conclusion
Emerging evidence has demonstrated an intimate connection between genetic and environmental causes of PD neurodegeneration. Environmental risk factors and PD-associated gene mutations act in series and/or in parallel pathways, likely sharing some common molecular mechanisms. Animal models of PD created by using individual neurotoxins or genetic manipulation have been extremely valuable for further studying the pathogenesis of PD. However, although available PD animal models reproduce many features of this disease, none of them replicates the full-spectrum of the disease. On the one hand, most of genetically engineered animals carrying PD-associated genetic defects failed to mimic the overt nigrostriatal dopaminergic neurodegeneration, suggesting that a single genetic risk factor is not sufficient to produce PD neurodegeneration. On the other hand, most of toxin-based PD models are generated by acute or subacute experimental exposure of young animals to relatively high doses of environmental toxins, and these models are often lack of classical α-synuclein pathology (e.g. abnormal α-synuclein aggregation and inclusion formation), a pathological hallmark of PD. There is tremendous hope that chronic progressive two-hit animal models involving both genetic mutations and environmental exposure will provide invaluable tool to advance our understanding of PD pathogenesis and to ultimately develop therapeutics that enable to retard PD progression. Clearly, additional animal studies as well as GWAS and epidemiologic studies aimed at identifying gene-environment interactions will glean many fresh insights into PD pathogenesis.
Acknowledgments
The authors thank Dr. Honglei Chen for the helpful comments on the manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences.
Abbreviations
- PD
Parkinson’s disease
- SN
substantia nigra
- LPS
lipopolysaccharide
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NOS
nitric oxide synthase
- LRRK2
Leucine-rich repeat kinase 2
- PINK1
PTEN-induced putative kinase 1
- GWAS
genome-wide association study
- PET
positron emission tomography
- GDNF
glial cell line-derived neurotrophic factor
- SNPs
single-nucleotide polymorphisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, Kim YK, Kim SE, Kim JM, Kim HJ, Cho J, Jeon BS. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002;136:317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Mejias R, Sasaki M, Li X, Lin BM, Biskup S, Zhang L, Banerjee R, Thomas B, Yang L, Liu G, Beal MF, Huso DL, Dawson TM, Dawson VL. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J Neurosci. 2009;29:15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, Schwarzschild MA, Thun MJ. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamer HT, de Silva R. LRRK2 G2019S in the North African Population: A Review. Eur Neurol. 2010;63:321–325. doi: 10.1159/000279653. [DOI] [PubMed] [Google Scholar]
- Berger AK, Cortese GP, Amodeo KD, Weihofen A, Letai A, LaVoie MJ. Parkin selectively alters the intrinsic threshold for mitochondrial cytochrome c release. Hum Mol Genet. 2009;18:4317–4328. doi: 10.1093/hmg/ddp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bhatt MH, Elias MA, Mankodi AK. Acute and reversible parkinsonism due to organophosphate pesticide intoxication: five cases. Neurology. 1999;52:1467–1471. doi: 10.1212/wnl.52.7.1467. [DOI] [PubMed] [Google Scholar]
- Boger HA, Granholm AC, McGinty JF, Middaugh LD. A dual-hit animal model for age-related parkinsonism. Prog Neurobiol. 2010;90:217–229. doi: 10.1016/j.pneurobio.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V. Genetics of parkinsonism. Parkinsonism Relat Disord. 2007;13(Suppl 3):S233–241. doi: 10.1016/S1353-8020(08)70008-7. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52–56. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease--is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann N, Mitterer M, Lanthaler AJ, Djarmati A, Hagenah J, Wiegers K, Winkler S, Pawlack H, Lohnau T, Pramstaller PP, Klein C, Lohmann K. Frequency of heterozygous Parkin mutations in healthy subjects: need for careful prospective follow-up examination of mutation carriers. Parkinsonism Relat Disord. 2009;15:425–429. doi: 10.1016/j.parkreldis.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, Gispert-Sanchez S, Murphy D, Auburger G, Myers RR, Nussbaum RL. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol Aging. 2005;26:25–35. doi: 10.1016/j.neurobiolaging.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Carmine Belin A, Westerlund M, Sydow O, Lundstromer K, Hakansson A, Nissbrandt H, Olson L, Galter D. Leucine-rich repeat kinase 2 (LRRK2) mutations in a Swedish Parkinson cohort and a healthy nonagenarian. Mov Disord. 2006;21:1731–1734. doi: 10.1002/mds.21016. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol. 2002;155:732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A, Blair A. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74:878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol. 2005a;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J Biol Chem. 2005b;280:21418–21426. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Fleming S, Mortazavi F, Meurers B. Strengths and limitations of genetic mouse models of Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(Suppl 2):S84–87. doi: 10.1016/j.parkreldis.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. Dopaminergic cell death induced by MPP(+), oxidant and specific neurotoxicants shares the common molecular mechanism. J Neurochem. 2001;76:1010–1021. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Clark LN, Afridi S, Mejia-Santana H, Harris J, Louis ED, Cote LJ, Andrews H, Singleton A, Wavrant De-Vrieze F, Hardy J, Mayeux R, Fahn S, Waters C, Ford B, Frucht S, Ottman R, Marder K. Analysis of an early-onset Parkinson’s disease cohort for DJ-1 mutations. Mov Disord. 2004;19:796–800. doi: 10.1002/mds.20131. [DOI] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Cookson MR. DJ-1, PINK1, and their effects on mitochondrial pathways. Mov Disord. 2010;25(Suppl 1):S44–48. doi: 10.1002/mds.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia Guedes L, Ferreira JJ, Rosa MM, Coelho M, Bonifati V, Sampaio C. Worldwide frequency of G2019S LRRK2 mutation in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2010;16:237–242. doi: 10.1016/j.parkreldis.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S32–39. doi: 10.1002/mds.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, Beld M, Le TP, Truong HK, Nguyen VV, Tran TH, Do QH, Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- Dekker MC, Eshuis SA, Maguire RP, Veenma-van der Duijn L, Pruim J, Snijders PJ, Oostra BA, van Duijn CM, Leenders KL. PET neuroimaging and mutations in the DJ-1 gene. J Neural Transm. 2004;111:1575–1581. doi: 10.1007/s00702-004-0165-4. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008a;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR. Structure of the ROC domain from the Parkinson’s disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci U S A. 2008b;105:1499–1504. doi: 10.1073/pnas.0709098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Tarbutton GL, Levin JL, Plotkin GM, Lowry LK, Nalbone JT, Shepherd S. Pesticide/environmental exposures and Parkinson’s disease in East Texas. J Agromedicine. 2008;13:37–48. doi: 10.1080/10599240801986215. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Rohe CF, Ferreira J, Chien HF, Vacca L, Stocchi F, Guedes L, Fabrizio E, Manfredi M, Vanacore N, Goldwurm S, Breedveld G, Sampaio C, Meco G, Barbosa E, Oostra BA, Bonifati V. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson’s disease. Lancet. 2005;365:412–415. doi: 10.1016/S0140-6736(05)17829-5. [DOI] [PubMed] [Google Scholar]
- Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson’s disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33:14–26. doi: 10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- Dick FD, De Palma G, Ahmadi A, Osborne A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A. Gene-environment interactions in parkinsonism and Parkinson’s disease: the Geoparkinson study. Occup Environ Med. 2007;64:673–680. doi: 10.1136/oem.2006.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djarmati A, Hedrich K, Svetel M, Lohnau T, Schwinger E, Romac S, Pramstaller PP, Kostic V, Klein C. Heterozygous PINK1 mutations: a susceptibility factor for Parkinson disease? Mov Disord. 2006;21:1526–1530. doi: 10.1002/mds.20977. [DOI] [PubMed] [Google Scholar]
- Doder M, Jahanshahi M, Turjanski N, Moseley IF, Lees AJ. Parkinson’s syndrome after closed head injury: a single case report. J Neurol Neurosurg Psychiatry. 1999;66:380–385. doi: 10.1136/jnnp.66.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RC, Eldridge R, Williams A, Nutt J, Calne D. Twin study of Parkinson disease. Neurology. 1981;31:77–80. doi: 10.1212/wnl.31.1.77. [DOI] [PubMed] [Google Scholar]
- Duvoisin RC, Lobo-Antunes J, Yahr MD. Response of patients with postencephalitic Parkinsonism to levodopa. J Neurol Neurosurg Psychiatry. 1972;35:487–495. doi: 10.1136/jnnp.35.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley FG, Ragan CI. Photoaffinity labelling of mitochondrial NADH dehydrogenase with arylazidoamorphigenin, an analogue of rotenone. Biochem J. 1984;224:525–534. doi: 10.1042/bj2240525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, Alperovitch A, Tzourio C. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- Elizan TS, Casals J. Astrogliosis in von Economo’s and postencephalitic Parkinson’s diseases supports probable viral etiology. J Neurol Sci. 1991;105:131–134. doi: 10.1016/0022-510x(91)90135-t. [DOI] [PubMed] [Google Scholar]
- Engel LS, Checkoway H, Keifer MC, Seixas NS, Longstreth WT, Jr, Scott KC, Hudnell K, Anger WK, Camicioli R. Parkinsonism and occupational exposure to pesticides. Occup Environ Med. 2001;58:582–589. doi: 10.1136/oem.58.9.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Schulz JB, Kowall NW, Beal MF. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997;753:157–162. doi: 10.1016/s0006-8993(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson’s disease: looking for the way out of a quagmire. Neuron. 2005;47:479–482. doi: 10.1016/j.neuron.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol. 1986;20:449–455. doi: 10.1002/ana.410200403. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JH. Progressive parkinsonism in boxers. South Med J. 1989;82:543–546. doi: 10.1097/00007611-198905000-00002. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Tawara T, Isobe A, Hojo N, Shiwaku K, Yamane Y. Radical formation site of cerebral complex I and Parkinson’s disease. J Neurosci Res. 1995;42:385–390. doi: 10.1002/jnr.490420313. [DOI] [PubMed] [Google Scholar]
- Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002a;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002b;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and alpha-Synuclein Dysfunction Potentiate Each Other Driving Chronic Progression of Neurodegeneration in a Mouse Model of Parkinson’s Disease. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke S, Imai Y, Sokol N, Lu B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 2010;466:637–641. doi: 10.1038/nature09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmetti V, Ferraris A, Brusa L, Romano F, Lombardi F, Barzaghi C, Stanzione P, Garavaglia B, Dallapiccola B, Valente EM. Late onset sporadic Parkinson’s disease caused by PINK1 mutations: clinical and functional study. Mov Disord. 2008;23:881–885. doi: 10.1002/mds.21960. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemi M, Rudolf J, Schmulling S, Bamborschke S, Heiss WD. FDG- and Dopa-PET in postencephalitic parkinsonism. J Neural Transm. 2000;107:1289–1295. doi: 10.1007/s007020070018. [DOI] [PubMed] [Google Scholar]
- Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, Lynch J, Healy DG, Holton JL, Revesz T, Wood NW. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Muller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, Garcia-Arencibia M, Fernandez-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert AS, Rub U, Chen A, Nussbaum RL, Auburger G. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O’Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- Gobbi LT, Oliveira-Ferreira MD, Caetano MJ, Lirani-Silva E, Barbieri FA, Stella F, Gobbi S. Exercise programs improve mobility and balance in people with Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S49–52. doi: 10.1016/S1353-8020(09)70780-1. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Pappert EJ. Trauma and movement disorders. Neurol Clin. 1992;10:907–919. [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, Shen J. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson’s disease risk in twins. Ann Neurol. 2006;60:65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniels V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem. 2008;283:16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman A, Smith RW. Neurological sequelae of boxing. Sports Med. 1987;4:194–210. doi: 10.2165/00007256-198704030-00004. [DOI] [PubMed] [Google Scholar]
- Hague S, Rogaeva E, Hernandez D, Gulick C, Singleton A, Hanson M, Johnson J, Weiser R, Gallardo M, Ravina B, Gwinn-Hardy K, Crawley A, St George-Hyslop PH, Lang AE, Heutink P, Bonifati V, Hardy J, Singleton A. Early-onset Parkinson’s disease caused by a compound heterozygous DJ-1 mutation. Ann Neurol. 2003;54:271–274. doi: 10.1002/ana.10663. [DOI] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Fujiwara K, Stacy MA, Scott BL, Stajich JM, Jewett R, Li YJ, Hauser MA, Vance JM, Scott WK. NOS2A and the modulating effect of cigarette smoking in Parkinson’s disease. Ann Neurol. 2006;60:366–373. doi: 10.1002/ana.20915. [DOI] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Vance JM, Scott WK. Nitric oxide synthase genes and their interactions with environmental factors in Parkinson’s disease. Neurogenetics. 2008;9:249–262. doi: 10.1007/s10048-008-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DG, Abou-Sleiman PM, Ahmadi KR, Muqit MM, Bhatia KP, Quinn NP, Lees AJ, Latchmann DS, Goldstein DB, Wood NW. The gene responsible for PARK6 Parkinson’s disease, PINK1, does not influence common forms of parkinsonism. Ann Neurol. 2004;56:329–335. doi: 10.1002/ana.20206. [DOI] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Nicklas WJ, Vyas I, Duvoisin RC. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci Lett. 1985;62:389–394. doi: 10.1016/0304-3940(85)90580-4. [DOI] [PubMed] [Google Scholar]
- Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord. 2010;16:566–571. doi: 10.1016/j.parkreldis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52:276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem. 2003;84:491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- Horowitz MP, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: the importance of animal modeling. Clin Pharmacol Ther. 2010;88:467–474. doi: 10.1038/clpt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Sung SM, Liu HC, Lee CC, Tsai CH, Chang JG. Polymorphisms of the parkin gene in sporadic Parkinson’s disease among Chinese in Taiwan. Eur Neurol. 2000;44:90–93. doi: 10.1159/000008203. [DOI] [PubMed] [Google Scholar]
- Hulihan MM, Ishihara-Paul L, Kachergus J, Warren L, Amouri R, Elango R, Prinjha RK, Upmanyu R, Kefi M, Zouari M, Sassi SB, Yahmed SB, El Euch-Fayeche G, Matthews PM, Middleton LT, Gibson RA, Hentati F, Farrer MJ. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a case-control genetic study. Lancet Neurol. 2008;7:591–594. doi: 10.1016/S1474-4422(08)70116-9. [DOI] [PubMed] [Google Scholar]
- Iaccarino C, Crosio C, Vitale C, Sanna G, Carri MT, Barone P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum Mol Genet. 2007;16:1319–1326. doi: 10.1093/hmg/ddm080. [DOI] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- Itier JM, Ibanez P, Mena MA, Abbas N, Cohen-Salmon C, Bohme GA, Laville M, Pratt J, Corti O, Pradier L, Ret G, Joubert C, Periquet M, Araujo F, Negroni J, Casarejos MJ, Canals S, Solano R, Serrano A, Gallego E, Sanchez M, Denefle P, Benavides J, Tremp G, Rooney TA, Brice A, Garcia de Yebenes J. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–2291. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, Smeyne RJ. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009;106:14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javoy F, Sotelo C, Herbet A, Agid Y. Specificity of dopaminergic neuronal degeneration induced by intracerebral injection of 6-hydroxydopamine in the nigrostriatal dopamine system. Brain Res. 1976;102:201–215. doi: 10.1016/0006-8993(76)90877-5. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, Comyns K, Goldman S, Korell M, Langston J, Ross G, Sandler D. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. Embo J. 2010 doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DM, Moran D, Moses L, Poorkaj P, Zabetian CP, Nutt J, Factor SA, Yu CE, Montimurro JS, Keefe RG, Schellenberg GD, Payami H. Heterozygous parkin point mutations are as common in control subjects as in Parkinson’s patients. Ann Neurol. 2007;61:47–54. doi: 10.1002/ana.21039. [DOI] [PubMed] [Google Scholar]
- Kay DM, Zabetian CP, Factor SA, Nutt JG, Samii A, Griffith A, Bird TD, Kramer P, Higgins DS, Payami H. Parkinson’s disease and LRRK2: frequency of a common mutation in U.S. movement disorder clinics. Mov Disord. 2006;21:519–523. doi: 10.1002/mds.20751. [DOI] [PubMed] [Google Scholar]
- Kelada SN, Checkoway H, Kardia SL, Carlson CS, Costa-Mallen P, Eaton DL, Firestone J, Powers KM, Swanson PD, Franklin GM, Longstreth WT, Jr, Weller TS, Afsharinejad Z, Costa LG. 5′ and 3′ region variability in the dopamine transporter gene (SLC6A3), pesticide exposure and Parkinson’s disease risk: a hypothesis-generating study. Hum Mol Genet. 2006;15:3055–3062. doi: 10.1093/hmg/ddl247. [DOI] [PubMed] [Google Scholar]
- Khan NL, Horta W, Eunson L, Graham E, Johnson JO, Chang S, Davis M, Singleton A, Wood NW, Lees AJ. Parkin disease in a Brazilian kindred: Manifesting heterozygotes and clinical follow-up over 10 years. Mov Disord. 2005;20:479–484. doi: 10.1002/mds.20335. [DOI] [PubMed] [Google Scholar]
- Khan NL, Valente EM, Bentivoglio AR, Wood NW, Albanese A, Brooks DJ, Piccini P. Clinical and subclinical dopaminergic dysfunction in PARK6-linked parkinsonism: an 18F-dopa PET study. Ann Neurol. 2002;52:849–853. doi: 10.1002/ana.10417. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Lohmann-Hedrich K, Rogaeva E, Schlossmacher MG, Lang AE. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol. 2007;6:652–662. doi: 10.1016/S1474-4422(07)70174-6. [DOI] [PubMed] [Google Scholar]
- Klein RL, King MA, Hamby ME, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum Gene Ther. 2002;13:605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee SB, Kim W, Lee S, Chung J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem Biophys Res Commun. 2007;358:534–539. doi: 10.1016/j.bbrc.2007.04.156. [DOI] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Lesage S, Belarbi S, Troiano A, Condroyer C, Hecham N, Pollak P, Lohman E, Benhassine T, Ysmail-Dahlouk F, Durr A, Tazir M, Brice A. Is the common LRRK2 G2019S mutation related to dyskinesias in North African Parkinson disease? Neurology. 2008a;71:1550–1552. doi: 10.1212/01.wnl.0000338460.89796.06. [DOI] [PubMed] [Google Scholar]
- Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Lesage S, Durr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, Pollak P, Brice A. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N Engl J Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- Lesage S, Ibanez P, Lohmann E, Pollak P, Tison F, Tazir M, Leutenegger AL, Guimaraes J, Bonnet AM, Agid Y, Durr A, Brice A. G2019S LRRK2 mutation in French and North African families with Parkinson’s disease. Ann Neurol. 2005;58:784–787. doi: 10.1002/ana.20636. [DOI] [PubMed] [Google Scholar]
- Lesage S, Lohmann E, Tison F, Durif F, Durr A, Brice A. Rare heterozygous parkin variants in French early-onset Parkinson disease patients and controls. J Med Genet. 2008b;45:43–46. doi: 10.1136/jmg.2007.051854. [DOI] [PubMed] [Google Scholar]
- Levecque C, Elbaz A, Clavel J, Richard F, Vidal JS, Amouyel P, Tzourio C, Alperovitch A, Chartier-Harlin MC. Association between Parkinson’s disease and polymorphisms in the nNOS and iNOS genes in a community-based case-control study. Hum Mol Genet. 2003;12:79–86. doi: 10.1093/hmg/ddg009. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li X, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD, Elder GA, Rice ME, Yue Z. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J Neurosci. 2010;30:1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K, Lewandowska A, Zietkiewicz S. Chaperones in control of protein disaggregation. Embo J. 2008;27:328–335. doi: 10.1038/sj.emboj.7601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KL, Ng CH. Genetic models of Parkinson disease. Biochim Biophys Acta. 2009;1792:604–615. doi: 10.1016/j.bbadis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, Carvey PM. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Liou AK, Leak RK, Li L, Zigmond MJ. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis. 2008;32:116–124. doi: 10.1016/j.nbd.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou HH, Chen RC, Tsai YF, Chen WP, Chang YC, Tsai MC. Effects of paraquat on the substantia nigra of the wistar rats: neurochemical, histological, and behavioral studies. Toxicol Appl Pharmacol. 1996;137:34–41. doi: 10.1006/taap.1996.0054. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293:607–617. [PubMed] [Google Scholar]
- Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Dobson B, Glicksman MA, Yue Z, Stein RL. Kinetic mechanistic studies of wild-type leucine-rich repeat kinase 2: characterization of the kinase and GTPase activities. Biochemistry. 49:2008–2017. doi: 10.1021/bi901851y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. Embo J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Vives-Bauza C, Acin-Perez R, Yamamoto A, Tan Y, Li Y, Magrane J, Stavarache MA, Shaffer S, Chang S, Kaplitt MG, Huang XY, Beal MF, Manfredi G, Li C. PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and alpha-synuclein aggregation in cell culture models of Parkinson’s disease. PLoS One. 2009;4:e4597. doi: 10.1371/journal.pone.0004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang X, Yu Y, Li X, Wang T, Jiang H, Ren Q, Jiao Y, Sawa A, Moran T, Ross CA, Montell C, Smith WW. A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart PJ, Lincoln S, Hulihan M, Kachergus J, Wilkes K, Bisceglio G, Mash DC, Farrer MJ. DJ-1 mutations are a rare cause of recessively inherited early onset parkinsonism mediated by loss of protein function. J Med Genet. 2004;41:e22. doi: 10.1136/jmg.2003.011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XH, Fleming SM, Meurers B, Ackerson LC, Mortazavi F, Lo V, Hernandez D, Sulzer D, Jackson GR, Maidment NT, Chesselet MF, Yang XW. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J Neurosci. 2009;29:1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking CB, Chesneau V, Lohmann E, Verpillat P, Dulac C, Bonnet AM, Gasparini F, Agid Y, Durr A, Brice A. Coding polymorphisms in the parkin gene and susceptibility to Parkinson disease. Arch Neurol. 2003;60:1253–1256. doi: 10.1001/archneur.60.9.1253. [DOI] [PubMed] [Google Scholar]
- Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denefle P, Wood NW, Agid Y, Brice A. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- Macedo MG, Anar B, Bronner IF, Cannella M, Squitieri F, Bonifati V, Hoogeveen A, Heutink P, Rizzu P. The DJ-1L166P mutant protein associated with early onset Parkinson’s disease is unstable and forms higher-order protein complexes. Hum Mol Genet. 2003;12:2807–2816. doi: 10.1093/hmg/ddg304. [DOI] [PubMed] [Google Scholar]
- MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, Langston JW. Model fusion, the next phase in developing animal models for Parkinson’s disease. Neurotox Res. 2007;11:219–240. doi: 10.1007/BF03033569. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, Pant PV, Frazer KA, Cox DR, Ballinger DG. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005;77:685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson’s disease: insights from animal models. Nat Rev Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- Marongiu R, Ferraris A, Ialongo T, Michiorri S, Soleti F, Ferrari F, Elia AE, Ghezzi D, Albanese A, Altavista MC, Antonini A, Barone P, Brusa L, Cortelli P, Martinelli P, Pellecchia MT, Pezzoli G, Scaglione C, Stanzione P, Tinazzi M, Zecchinelli A, Zeviani M, Cassetta E, Garavaglia B, Dallapiccola B, Bentivoglio AR, Valente EM. PINK1 heterozygous rare variants: prevalence, significance and phenotypic spectrum. Hum Mutat. 2008;29:565. doi: 10.1002/humu.20719. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marttila RJ, Kaprio J, Koskenvuo M, Rinne UK. Parkinson’s disease in a nationwide twin cohort. Neurology. 1988;38:1217–1219. doi: 10.1212/wnl.38.8.1217. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Mak SK, Shenasa M, Langston WJ, Forno LS, Di Monte DA. Pathologic modifications of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J Neuropathol Exp Neurol. 2008;67:793–802. doi: 10.1097/NEN.0b013e318180f0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch CC, Kay DM, Factor SA, Samii A, Nutt JG, Higgins DS, Griffith A, Roberts JW, Leis BC, Montimurro JS, Zabetian CP, Payami H. Exploring gene-environment interactions in Parkinson’s disease. Hum Genet. 2008;123:257–265. doi: 10.1007/s00439-008-0466-z. [DOI] [PubMed] [Google Scholar]
- Meco G, Bonifati V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate) Scand J Work Environ Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG. Parkinson’s disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352:1344–1346. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res. 2002;956:156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Dawson VL, Dawson TM. Lessons from Drosophila models of DJ-1 deficiency. Sci Aging Knowledge Environ. 2006;2006:pe2. doi: 10.1126/sageke.2006.2.pe2. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Dawson TM, Dawson VL. A missense mutation (L166P) in DJ-1, linked to familial Parkinson’s disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem. 2003;87:1558–1567. doi: 10.1111/j.1471-4159.2003.02265.x. [DOI] [PubMed] [Google Scholar]
- Morris ME, Iansek R, Kirkwood B. A randomized controlled trial of movement strategies compared with exercise for people with Parkinson’s disease. Mov Disord. 2009;24:64–71. doi: 10.1002/mds.22295. [DOI] [PubMed] [Google Scholar]
- Mueller JC, Fuchs J, Hofer A, Zimprich A, Lichtner P, Illig T, Berg D, Wullner U, Meitinger T, Gasser T. Multiple regions of alpha-synuclein are associated with Parkinson’s disease. Ann Neurol. 2005;57:535–541. doi: 10.1002/ana.20438. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayernouri T. Posttraumatic parkinsonism. Surg Neurol. 1985;24:263–264. doi: 10.1016/0090-3019(85)90035-7. [DOI] [PubMed] [Google Scholar]
- Ng CH, Mok SZ, Koh C, Ouyang X, Fivaz ML, Tan EK, Dawson VL, Dawson TM, Yu F, Lim KL. Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci. 2009;29:11257–11262. doi: 10.1523/JNEUROSCI.2375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols WC, Pankratz N, Hernandez D, Paisan-Ruiz C, Jain S, Halter CA, Michaels VE, Reed T, Rudolph A, Shults CW, Singleton A, Foroud T. Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet. 2005;365:410–412. doi: 10.1016/S0140-6736(05)17828-3. [DOI] [PubMed] [Google Scholar]
- Nieto M, Gil-Bea FJ, Dalfo E, Cuadrado M, Cabodevilla F, Sanchez B, Catena S, Sesma T, Ribe E, Ferrer I, Ramirez MJ, Gomez-Isla T. Increased sensitivity to MPTP in human alpha-synuclein A30P transgenic mice. Neurobiol Aging. 2006;27:848–856. doi: 10.1016/j.neurobiolaging.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Ross OA, Ishii K, Kachergus JM, Ishiwata K, Kitagawa M, Kono S, Obi T, Mizoguchi K, Inoue Y, Imai H, Takanashi M, Mizuno Y, Farrer MJ, Hattori N. Expanding the clinical phenotype of SNCA duplication carriers. Mov Disord. 2009;24:1811–1819. doi: 10.1002/mds.22682. [DOI] [PubMed] [Google Scholar]
- Norris EH, Uryu K, Leight S, Giasson BI, Trojanowski JQ, Lee VM. Pesticide exposure exacerbates alpha-synucleinopathy in an A53T transgenic mouse model. Am J Pathol. 2007;170:658–666. doi: 10.2353/ajpath.2007.060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat. 2010;31:763–780. doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata A, Tashiro K, Nukuzuma S, Nagashima K, Hall WW. A rat model of Parkinson’s disease induced by Japanese encephalitis virus. J Neurovirol. 1997;3:141–147. doi: 10.3109/13550289709015803. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, Deligtisch A, Tagliati M, Hunt AL, Klein C, Henick B, Hailpern SM, Lipton RB, Soto-Valencia J, Risch N, Bressman SB. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Washecka N, Nath P, Singleton AB, Corder EH. Parkinson’s disease and low frequency alleles found together throughout LRRK2. Ann Hum Genet. 2009;73:391–403. doi: 10.1111/j.1469-1809.2009.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pals P, Lincoln S, Manning J, Heckman M, Skipper L, Hulihan M, Van den Broeck M, De Pooter T, Cras P, Crook J, Van Broeckhoven C, Farrer MJ. alpha-Synuclein promoter confers susceptibility to Parkinson’s disease. Ann Neurol. 2004;56:591–595. doi: 10.1002/ana.20268. [DOI] [PubMed] [Google Scholar]
- Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Pavese N, Khan NL, Scherfler C, Cohen L, Brooks DJ, Wood NW, Bhatia KP, Quinn NP, Lees AJ, Piccini P. Nigrostriatal dysfunction in homozygous and heterozygous parkin gene carriers: an 18F-dopa PET progression study. Mov Disord. 2009;24:2260–2266. doi: 10.1002/mds.22817. [DOI] [PubMed] [Google Scholar]
- Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RG, Hastings TG. Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J Neurochem. 2004;89:1318–1324. doi: 10.1111/j.1471-4159.2004.02423.x. [DOI] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ. The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol. 1999;45:577–582. doi: 10.1002/1531-8249(199905)45:5<577::aid-ana5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Pienaar IS, Gotz J, Feany MB. Parkinson’s Disease: Insights from Non-Traditional Model Organisms. Prog Neurobiol. doi: 10.1016/j.pneurobio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H, Hardy J. PINK1 in mitochondrial function. Proc Natl Acad Sci U S A. 2008;105:11041–11042. doi: 10.1073/pnas.0805908105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KM, Kay DM, Factor SA, Zabetian CP, Higgins DS, Samii A, Nutt JG, Griffith A, Leis B, Roberts JW, Martinez ED, Montimurro JS, Checkoway H, Payami H. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Mov Disord. 2008;23:88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- Pramstaller PP, Kis B, Eskelson C, Hedrich K, Scherer M, Schwinger E, Breakefield XO, Kramer PL, Ozelius LJ, Klein C. Phenotypic variability in a large kindred (Family LA) with deletions in the parkin gene. Mov Disord. 2002;17:424–426. doi: 10.1002/mds.10071. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–647. doi: 10.1016/0306-4522(95)00066-r. [DOI] [PubMed] [Google Scholar]
- Purisai MG, McCormack AL, Langston WJ, Johnston LC, Di Monte DA. Alpha-synuclein expression in the substantia nigra of MPTP-lesioned non-human primates. Neurobiol Dis. 2005;20:898–906. doi: 10.1016/j.nbd.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput AH, Uitti RJ. Paraquat and Parkinson’s disease. Neurology. 1987;37:1820–1821. doi: 10.1212/wnl.37.11.1820. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca WA, McDonnell SK, Strain KJ, Bower JH, Ahlskog JE, Elbaz A, Schaid DJ, Maraganore DM. Familial aggregation of Parkinson’s disease: The Mayo Clinic family study. Ann Neurol. 2004;56:495–502. doi: 10.1002/ana.20228. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Conway KA, Lansbury PT., Jr Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry. 2000;39:10619–10626. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Ross CA, Smith WW. Gene-environment interactions in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S309–315. doi: 10.1016/S1353-8020(08)70022-1. [DOI] [PubMed] [Google Scholar]
- Ross OA, Toft M, Whittle AJ, Johnson JL, Papapetropoulos S, Mash DC, Litvan I, Gordon MF, Wszolek ZK, Farrer MJ, Dickson DW. Lrrk2 and Lewy body disease. Ann Neurol. 2006;59:388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH, Segal L, Raghavan K, Matsumoto K, Hisamoto N, Kuwahara T, Iwatsubo T, Moore L, Goldstein L, Cookson M, Wolozin B. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci. 2009;29:9210–9218. doi: 10.1523/JNEUROSCI.2281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi-Nakashima A, Meir JY, Jin Y, Matsumoto K, Hisamoto N. LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr Biol. 2007;17:592–598. doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- Sang TK, Chang HY, Lawless GM, Ratnaparkhi A, Mee L, Ackerson LC, Maidment NT, Krantz DE, Jackson GR. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 2007;27:981–992. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, Suno M, Ogawa K, Hayase N, Kimura K, Shiono H. Carrier-mediated processes in blood--brain barrier penetration and neural uptake of paraquat. Brain Res. 2001;906:135–142. doi: 10.1016/s0006-8993(01)02577-x. [DOI] [PubMed] [Google Scholar]
- Shimoji M, Zhang L, Mandir AS, Dawson VL, Dawson TM. Absence of inclusion body formation in the MPTP mouse model of Parkinson’s disease. Brain Res Mol Brain Res. 2005;134:103–108. doi: 10.1016/j.molbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- Spangenberg S, Hannerz H, Tuchsen F, Mikkelsen KL. A nationwide population study of severe head injury and Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:12–14. doi: 10.1016/j.parkreldis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sriram SR, Li X, Ko HS, Chung KK, Wong E, Lim KL, Dawson VL, Dawson TM. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- Stern MB. Head trauma as a risk factor for Parkinson’s disease. Mov Disord. 1991;6:95–97. doi: 10.1002/mds.870060202. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Hicks AA, Jonsson T, Petursson H, Gugmundsson G, Frigge ML, Kong A, Gulcher JR, Stefansson K. Familial aggregation of Parkinson’s disease in Iceland. N Engl J Med. 2000;343:1765–1770. doi: 10.1056/NEJM200012143432404. [DOI] [PubMed] [Google Scholar]
- Tan EK, Kwok HK, Tan LC, Zhao WT, Prakash KM, Au WL, Pavanni R, Ng YY, Satake W, Zhao Y, Toda T, Liu JJ. Analysis of GWAS-linked loci in Parkinson disease reaffirms PARK16 as a susceptibility locus. Neurology. 2010;75:508–512. doi: 10.1212/WNL.0b013e3181eccfcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JM, Wong ES, Lim KL. Protein misfolding and aggregation in Parkinson’s disease. Antioxid Redox Signal. 2009;11:2119–2134. doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- Tanner CM. Is the cause of Parkinson’s disease environmental or hereditary? Evidence from twin studies. Adv Neurol. 2003;91:133–142. [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins: an etiologic study. Jama. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Tao X, Tong L. Crystal structure of human DJ-1, a protein associated with early onset Parkinson’s disease. J Biol Chem. 2003;278:31372–31379. doi: 10.1074/jbc.M304221200. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Saint-Hilaire MH, Cupples LA, Thomas CA, Burchard AE, Feldman RG, Myers RH. Environmental, medical, and family history risk factors for Parkinson’s disease: a New England-based case control study. Am J Med Genet. 1999;88:742–749. [PubMed] [Google Scholar]
- Thiffault C, Langston JW, Di Monte DA. Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res. 2000;885:283–288. doi: 10.1016/s0006-8993(00)02960-7. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson’s disease phenotype. Neurotoxicology. 2002;23:621–633. doi: 10.1016/s0161-813x(02)00092-x. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL. Minocycline reduces the lipopolysaccharide-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis. 2004;16:190–201. doi: 10.1016/j.nbd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, Mizuta I, Li Y, Funayama M, Yoshino H, Li L, Murata M, Yamamoto M, Kubo S, Mizuno Y, Toda T, Hattori N. LRRK2 P755L variant in sporadic Parkinson’s disease. J Hum Genet. 2008;53:1012–1015. doi: 10.1007/s10038-008-0336-5. [DOI] [PubMed] [Google Scholar]
- Ton TG, Heckbert SR, Longstreth WT, Jr, Rossing MA, Kukull WA, Franklin GM, Swanson PD, Smith-Weller T, Checkoway H. Nonsteroidal anti-inflammatory drugs and risk of Parkinson’s disease. Mov Disord. 2006;21:964–969. doi: 10.1002/mds.20856. [DOI] [PubMed] [Google Scholar]
- Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Shen J. alpha-synuclein and LRRK2: partners in crime. Neuron. 2009;64:771–773. doi: 10.1016/j.neuron.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, 3rd, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of {alpha}-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano AR, Elbaz A, Lohmann E, Belarbi S, Vidailhet M, Bonnet AM, Lesage S, Pollak P, Cazeneuve C, Borg M, Feingold J, Durr A, Tazir M, Brice A. Low disease risk in relatives of north african lrrk2 Parkinson disease patients. Neurology. 2010;75:1118–1119. doi: 10.1212/WNL.0b013e3181f39a2e. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Vance JM, Ali S, Bradley WG, Singer C, Di Monte DA. Gene-environment interactions in Parkinson’s disease and other forms of parkinsonism. Neurotoxicology. 2010 doi: 10.1016/j.neuro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Venderova K, Kabbach G, Abdel-Messih E, Zhang Y, Parks RJ, Imai Y, Gehrke S, Ngsee J, Lavoie MJ, Slack RS, Rao Y, Zhang Z, Lu B, Haque ME, Park DS. Leucine-Rich Repeat Kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease. Hum Mol Genet. 2009;18:4390–4404. doi: 10.1093/hmg/ddp394. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, de Vries RL, Tocilescu M, Przedborski S. PINK1/Parkin direct mitochondria to autophagy. Autophagy. 2010a;6:315–316. doi: 10.4161/auto.6.2.11199. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010b;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Wang C, Lu R, Ouyang X, Ho MW, Chia W, Yu F, Lim KL. Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J Neurosci. 2007;27:8563–8570. doi: 10.1523/JNEUROSCI.0218-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Li S, Chou AP, Bronstein JM. Inhibitory effects of pesticides on proteasome activity: implication in Parkinson’s disease. Neurobiol Dis. 2006;23:198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Ward CD, Duvoisin RC, Ince SE, Nutt JD, Eldridge R, Calne DB. Parkinson’s disease in 65 pairs of twins and in a set of quadruplets. Neurology. 1983;33:815–824. doi: 10.1212/wnl.33.7.815. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DB, Annegers JF, Kokmen E, O’Brien PC, Kurland LT. Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology. 1991;41:1554–1557. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- Winkler S, Hagenah J, Lincoln S, Heckman M, Haugarvoll K, Lohmann-Hedrich K, Kostic V, Farrer M, Klein C. alpha-Synuclein and Parkinson disease susceptibility. Neurology. 2007;69:1745–1750. doi: 10.1212/01.wnl.0000275524.15125.f4. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63:305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, Xia K, Jiang W, Ronai Z, Zhuang X, Zhang Z. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009;119:650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Coombes CE, Kilaru A, Li X, Gitler AD, Bowers WJ, Dawson VL, Dawson TM, Moore DJ. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010;6:e1000902. doi: 10.1371/journal.pgen.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson’s disease. J Neurochem. 2004;91:451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumino K, Kawakami I, Tamura M, Hayashi T, Nakamura M. Paraquat- and diquat-induced oxygen radical generation and lipid peroxidation in rat brain microsomes. J Biochem. 2002;131:565–570. doi: 10.1093/oxfordjournals.jbchem.a003135. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Burgunder JM, An X, Wu Y, Chen W, Zhang J, Wang Y, Xu Y, Gou Y, Yuan G, Mao X, Peng R. LRRK2 R1628P variant is a risk factor of Parkinson’s disease among Han-Chinese from mainland China. Mov Disord. 2009;24:1902–1905. doi: 10.1002/mds.22371. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Shao Y, May J, Prou D, Perier C, Dauer W, Schon EA, Przedborski S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proc Natl Acad Sci U S A. 2008;105:12022–12027. doi: 10.1073/pnas.0802814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, Smith AD. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord. 2009;15(Suppl 3):S42–45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]