Abstract
Background
The effect of daily prenatal and postnatal vitamin supplementation on concentrations of breast milk nutrients is not well characterized in HIV-infected women.
Objective
We examined the impact of vitamin supplementation during pregnancy and lactation on breast milk concentrations of retinol, carotenoids, and tocopherols during the first year post-partum among 626 HIV-infected Tanzanian women.
Design
We conducted a randomized, double-blind, placebo controlled trial. Women were assigned to one of four daily oral supplements: vitamin A + β-carotene (VA+BC); multivitamins (B, C, E (MV)); MV+VA+BC; or placebo. Concentrations of breast milk nutrients were determined by HPLC at birth and every 3 mo thereafter.
Results
Supplementation with VA+BC increased concentrations of retinol, β-carotene, and α-carotene at delivery by 4799, 1791, and 84 nmol/L, respectively, compared to no VA+BC (all p<0.0001). MV supplementation did not increase concentrations of α-tocopherol or δ-tocopherol at delivery but significantly decreased concentrations of breast milk γ-tocopherol and retinol. Although concentrations of all nutrients decreased significantly by 3 months postpartum, retinol, α-carotene, and β-carotene concentrations were significantly higher among those receiving VA+BC at 3, 6, and 12 mo compared to no VA+BC. Alpha tocopherol was significantly higher, while γ-tocopherol concentrations were significantly lower, among women receiving MV compared to no MV at 3, 6, and 12 mo post-partum.
Conclusions
Sustained supplementation of HIV-infected breastfeeding mothers with MV could be a safe and effective intervention to improve vitamin E concentrations in breast milk. VA+BC supplementation increases concentrations of breast milk retinol but it is not recommended in HIV-infected mothers due to the elevated risk of vertical transmission.
Keywords: breast milk, HIV, vitamin supplementation, retinol, carotenoids, tocopherol
INTRODUCTION
Approximately 90% of the nearly 530,000 new HIV infections annually in children under 15 y are attributed to mother to child transmission (UNAIDS, 2006). Five to twenty percent of these infections are transmitted during breastfeeding (Coutsoudis et al., 2004; Iliff et al., 2005). Although total avoidance of breast feeding eliminates the risk of transmission via breast milk, in many developing countries where HIV is a substantial public health burden this option often fails to meet the criteria of acceptability, feasibility, affordability, sustainability, and safety (AFASS). In such circumstances, the World Health Organization (WHO) recommends that HIV-infected mothers exclusively breastfeed their infants for up to 6 mo or until replacement feeding meets the criteria of AFASS (World Health Organization, 2006).
In many regions of Africa, HIV infection co-exists with malnutrition and the disease may further increase the nutrient requirements of mothers above those of normal pregnancy and lactation (Baum et al., 1997; Papathakis et al., 2007). If the nutrient requirements of an HIV-infected mother are not met, the redistribution of specific nutrients from body stores to breast milk may be impaired and could contribute to the development of nutrient deficiencies among exclusively breastfed infants. We have previously reported that the prevalence of vitamin A, vitamin E, and vitamin B12 deficiencies of breastfed infants born to unsupplemented HIV-infected women were high, between 12 and 29% (Baylin et al., 2005). These high prevalences suggest that breast milk concentrations of these nutrients were insufficient to meet the infants’ daily needs. Sub-optimal vitamin status among breastfed infants born to unsupplemented HIV-infected women could result in increased incidence of diarrhea (Fawzi et al., 2003) and impaired immunity, growth, and psychomotor development (Villamor et al., 2005; McGrath et al., 2006).
It is unclear whether improving the micronutrient status of HIV-infected mothers through micronutrient supplementation increases the concentrations of these nutrients in milk. Although one study in Malawi suggested that prenatal vitamin A supplementation increased breast milk in the early post-partum period (Semba et al., 2000), the effects of continued daily vitamin supplementation during pregnancy and throughout lactation on concentrations of vitamins in breast milk are not well known. We assessed the impact of daily vitamin supplementation during pregnancy and lactation on concentrations of retinol, β-carotene, α-carotene, α-tocopherol, γ-tocopherol, and δ-tocopherol in breast milk from delivery through the first year post-partum in a randomized clinical trial conducted among HIV-infected Tanzanian women.
METHODS
Study Design and Population
From 1995 to 1997, 1078 HIV-infected pregnant women were enrolled in a clinical trial of vitamin supplements in relation to vertical transmission and HIV disease progression in Dar es Salaam, Tanzania. Details of the trial design have been reported elsewhere (Fawzi et al., 2002). Briefly, eligible women between 12 and 27 weeks of gestation who consented to participate, were randomly assigned to receive a daily oral dose of one of four regimens: (1) vitamin A and β-carotene (VA+BC: 5000 IU (1500 μg retinol activity equivalents) of preformed vitamin A plus 30 mg of β-carotene), (2) multivitamins that did not include vitamin A and β-carotene (MV: 20 mg of thiamine, 20 mg of riboflavin, 25 mg of vitamin B6, 100 mg of niacin, 50 μg of vitamin B12, 500 mg of vitamin C (purified L-ascorbic acid), 30 mg of vitamin E (RRR-α-tocopherol acetate), and 0.8 mg of folic acid), (3) multivitamins that included vitamin A and β-carotene (same doses as above), or (4) placebo. The supplements were administered from enrollment throughout pregnancy and continued after delivery. All women, irrespective of the assigned experimental regimen, were given daily doses of iron and folate, and weekly doses of chloroquine as antimalarial prophylaxis during pregnancy. We assessed compliance with the study regimen at every monthly clinic visit by counting the number of pills absent from the bottles provided at the previous visit. We then estimated the proportion of tablets absent from the returned bottles over the total number of tablets the individual should have taken. Compliance was high, 90% during pregnancy and 83% by two years from randomization. It was similar in all treatment arms.
In accordance with the guidelines of the WHO at the time of the study (UNICEF/UNAIDS/WHO, 1998) and the Tanzanian Ministry of Health, HIV-infected women were provided with information about the benefits and risks associated with infant feeding options; however, the decision on whether to breast feed was ultimately made by the mother. Breast feeding was adopted by > 99% of participating women. At delivery and every 3 mo thereafter, breast milk samples were collected by manual expression from either breast and stored at −70° C.
Laboratory Methods
Prior to nutrient extraction, 250 μL of a breast milk internal standard consisting of 1 mg of trans-β-Apo-8′-Carotenal (Fluka) dissolved in 500 mL of EtOH was added to each 750 μL aliquot of breast milk sample. Samples were saponified twice in 750 μL EtOH, 500 μL 20% ascorbic acid and 2 mL of 60% KOH and incubated at 50º C for 5 minutes between each saponification. Samples were twice extracted with 2 mL of hexane and water-washed. The hexane layer was evaporated under nitrogen for 15 min at 30º C and reconstituted in 250 μL of a 3:1:1 mixture of acetonitrile:ethanol:dioxane.
The concentrations of retinol, total β-carotene, α-carotene, α-tocopherol, δ-tocopherol, and γ-tocopherol were quantitated by high performance liquid chromatography (HPLC) on a Restek Ultra C18 150 mm X 4.6 mm column, 3 μm particle size encased in a water-bath to prevent temperature fluctuations, and equipped with a trident guard cartridge system (Restek, Corp. Bellefonte, PA). The minimum detection limits in milk were 1.90, 1.65, and 3.12 nmol/L for α-carotene, β-carotene and retinol, respectively and 37.15, 40.71, and 67.78 nmol/L for γ-, δ-, and α-tocopherol, respectively.
Statistical analyses
We examined the impact of vitamin supplements during pregnancy and lactation on the concentrations of breast milk retinol, carotenoids, and tocopherols during the first year post-partum following the intent-to-treat principle. Curves of average nutrient concentrations over time were estimated using a mixed effects model (PROC MIXED; SAS Institute, Cary, NC, USA) with restricted cubic splines (Durrleman & Simon, 1989). In these models, each nutrient was the outcome while predictors included linear and spline terms for time post-partum (in mo), the treatment variable, and interaction terms between the treatment and time variables. These mixed models used random effects for the intercept and the linear term for time, which accounted for the within-person correlation of measurements in the estimation of the variance. These methods do not require the same number of observations on each subject or that the measurements be obtained at exactly the same time intervals between or within individuals; therefore all available breast milk measures on all women were used. We tested for interactions between the two treatment arms using the likelihood ratio test. Given that there were no significant interactions between the MV alone and VA+BC arms on any of the outcomes evaluated, we assessed the effect of VA+BC by comparing regimens that included VA+BC (groups (1) and (3)) against regimens that did not include VA+BC (groups (2) and (4)). Similarly, the effect of MV was assessed by comparing MV-containing groups ((2) and (3)) against non-MV-containing groups ((1) and (4)). Treatment effects were estimated as the difference in concentrations between supplemented and unsupplemented women at delivery, and 3, 6, and 12 mo post-partum. Confidence intervals (CIs) were constructed using robust estimates of the variance (White, 1980). The effects of supplementation did not differ after adjustment for total fat concentration of milk, thus results are presented unadjusted for fat content. Analyses were carried out with the Statistical Analyses System Software (SAS Institute).
Ethical clearance
The study protocol was approved by the Research and Publications Committee of Muhimbili University College of Health Sciences (Dar es Salaam, Tanzania), the Ethical Committee of the National AIDS Control Program of the Tanzanian Ministry of Health (Dar es Salaam, Tanzania), and the Institutional Review Board of the Harvard School of Public Health (Boston, MA, USA).
RESULTS
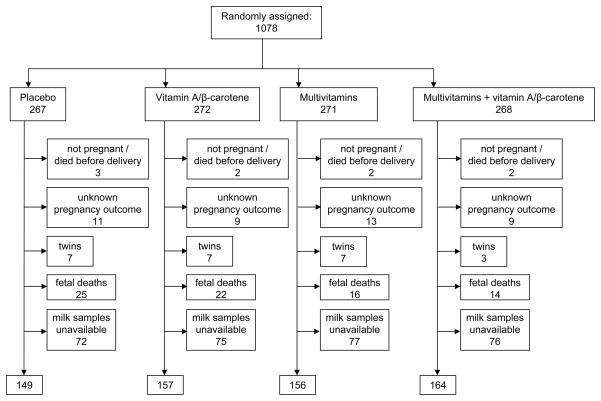
One thousand three hundred and seven breast milk samples were available from 626 women during the first year post-partum, for analysis of nutrients (Figure 1). The average (SD, interquartile range) number of samples per woman was 2.2 (1.2, 1–3) and did not differ by regimen assignment. The 626 women who had breast milk samples available did not differ from women without samples with respect to age, education level, HIV-disease stage, allocation to treatment regimen, or other baseline characteristics. Participants were 25 years of age, on average, and enrolled in the study at 20 weeks gestation. At baseline 6% of women had mid-upper arm circumference < 22 cm, 35% had serum retinol < 0.70 μmol/L, 28% had hemoglobin values below 85 g/L, 20% were beyond clinical stage 1 for HIV according to the WHO staging system (World Health Organization, 1993), and 12% had CD4 counts < 200/mm3. Women were similar with respect to baseline characteristics by treatment arm (Table 1).
Figure 1.
Trial profile.
Table 1.
Maternal characteristics at baseline according to treatment assignment
| Characteristic1 | Placebo (n=149) | Vitamin A + β carotene (n=157) | Multivitamins (n=156) | Multivitamins and Vitamin A+ β carotene (n=164) |
|---|---|---|---|---|
| Gestational age at entry, wk | 20.5 ± 3.7 | 20.1 ± 3.5 | 20.6 ± 3.2 | 20.1 ± 3.1 |
| Age, y | 24.9 ± 4.9 | 25.0 ± 5.3 | 24.9 ± 4.8 | 24.5 ± 4.3 |
| Complete primary education, % (n) | 85.9 (128) | 89.8 (141) | 85.9 (134) | 91.5 (150) |
| Primigravida, % (n) | 30.6 (45) | 33.6 (52) | 33.1 (51) | 31.3 (51) |
| Serum vitamin A, μmol/L | 0.87 ± 0.43 | 0.82 ± 0.34 | 0.85 ± 0.35 | 0.89 ± 0.30 |
| Serum vitamin E, μmol/L | 9.9 ± 2.9 | 9.9 ± 2.9 | 9.9 ± 3.1 | 10.1 ± 2.7 |
| Weight, kg | 56.9 ± 8.7 | 56.6 ± 10.2 | 58.2 ± 9.8 | 57.0 ± 7.8 |
| Height, cm | 156.2 ± 6.0 | 156.6 ± 6.0 | 156.9 ± 5.4 | 156.3 ± 5.6 |
| Mid upper arm circumference, cm | 25.5 ± 3.1 | 25.4 ± 3.0 | 25.8 ± 3.0 | 25.6 ± 2.7 |
| Hemoglobin, g/L | 95 ± 18 | 93 ± 17 | 95 ± 15 | 93 ± 15 |
| Baseline CD4 counts (cells/mm3) | 404 ± 176 | 397 ± 199 | 425 ± 197 | 412 ± 201 |
| HIV symptomatic2, % (n) | 19.5 (29) | 23.7 (37) | 19.4 (30) | 17.8 (29) |
| Infections, % (n) | ||||
| Malaria | 16.9 (25) | 19.9 (31) | 14.5 (22) | 21.0 (34) |
| Ascaris | 6.4 (8) | 3.7 (5) | 6.2 (8) | 8.4 (11) |
| Hookworm | 8.9 (12) | 9.3 (13) | 11.7 (16) | 11.3 (16) |
| Syphilis | 7.0 (9) | 3.8 (5) | 7.6 (10) | 3.6 (5) |
| Trichomonas Vaginalis | 26.2 (38) | 20.9 (32) | 24.2 (37) | 28.1 (45) |
Mean ± SD unless noted otherwise
Symptomatic women were in stages 2 or 3 at recruitment according to the WHO staging system of HIV disease (World Health Organization, 1993).
The concentrations of breast milk retinol, β-carotene and α-carotene decreased between 0 and 3 months postpartum irrespective of treatment arm. Despite these decreases, women who received VA+BC had significantly higher concentrations of breast milk retinol, β-carotene, and α-carotene at all time points during the first year post-partum compared to women who did not receive VA+BC (Table 2). Supplementation with VA+BC did not influence concentrations of α-, γ-, or δ-tocopherol from delivery to one year post-partum (data not shown).
Table 2.
Effect of supplementation with vitamin A and β-carotene (VA+BC) during pregnancy and lactation on concentrations of breast milk retinol and carotenoids from birth to 12 mo post-partum.1
| Nutrient (nmol/L) | 0 mo | 3 mo | 6 mo | 12 mo |
|---|---|---|---|---|
| Retinol | ||||
| No VA+BC | 4317 ± 301 | 2246 ± 118 | 2244 ± 124 | 2210 ± 118 |
| VA+BC | 9116 ± 456 | 4903 ± 194 | 4737 ± 166 | 4549 ± 214 |
| Between-group difference | 4799 (3727, 5871) | 2656 (2210, 3102) | 2493 (2086, 2899) | 2339 (1859, 2818) |
| β-Carotene | ||||
| No VA+BC | 223 ± 33 | 49 ± 9 | 46 ± 5 | 50 ± 6 |
| VA+BC | 2013 ± 180 | 495 ± 48 | 430 ± 41 | 465 ± 45 |
| Between-group difference | 1791 (1433, 2149) | 446 (351, 541) | 384 (304, 465) | 414 (326, 503) |
| α-Carotene | ||||
| No VA+BC | 46 ± 5 | 10 ± 2 | 10 ± 2 | 10 ± 1 |
| VA+BC | 130 ± 10 | 31 ± 3 | 28 ± 2 | 27 ± 3 |
| Between-group difference | 84 (62, 105) | 21 (15, 28) | 18 (12, 24) | 17 (12, 23) |
Mean ± SE for nutrient concentrations and the mean (95% confidence interval) for the effect of treatment were estimated from cubic spline models. The effect of VA+BC was estimated by comparing mothers who received VA+BC (assigned to VA+BC+MV or VA+BC without MV) vs. mothers who did not receive VA+BC (assigned to MV alone or placebo).
The concentrations of breast milk tocopherols also decreased significantly between 0 and 3 months postpartum. However, supplementation with MV during pregnancy and lactation resulted in significantly increased concentrations of α-tocopherol at 3, 6, and 12 mo post-partum (Table 3). MV supplementation significantly decreased concentrations of breast milk γ-tocopherol at all time points and reduced retinol concentrations at delivery. MV supplementation was not associated with changes in concentrations of β-carotene, or α-carotene (data not shown). There were no significant interactions between the VA+BC and the MV treatment arms on the concentrations of nutrients in breast milk (all p ≥ 0.10).
Table 3.
Effect of supplementation with vitamins B, C and E (MV) during pregnancy and lactation on concentrations of breast milk tocopherols and retinol from birth to 12 mo post-partum.1
| Nutrient (nmol/L) | 0 mo | 3 mo | 6 mo | 12 mo |
|---|---|---|---|---|
| α-Tocopherol | ||||
| no MV | 28467 ± 1639 | 10281 ± 517 | 8826 ± 375 | 8378 ± 466 |
| MV | 30707 ± 2200 | 12481 ± 673 | 11480 ± 537 | 10261 ± 739 |
| Between-group difference | 2240 (−3138, 7617) | 2199 (535, 3863) | 2654 (1370, 3938) | 1882 (170, 3594) |
| δ-Tocopherol | ||||
| no MV | 1243 ± 69 | 529 ± 30 | 461 ± 25 | 481 ± 31 |
| MV | 1080 ± 75 | 458 ± 29 | 431 ± 24 | 435 ± 34 |
| Between-group difference | −163 (−362, 36) | −70 (−154, 13) | −30 (−98, 39) | −46 (−136, 44) |
| γ-Tocopherol | ||||
| no MV | 1852 ± 128 | 949 ± 47 | 822 ± 36 | 690 ± 52 |
| MV | 1329 ± 116 | 653 ± 38 | 625 ± 30 | 547 ± 34 |
| Between-group difference | −522 (−861, −184) | −296 (−414, −178) | −197 (−289, −104) | −144 (−265, −22) |
| Retinol | ||||
| no MV | 7522 ± 456 | 3686 ± 191 | 3522 ± 164 | 3485 ± 192 |
| MV | 5990 ± 390 | 3646 ± 198 | 3616 ± 182 | 3487 ± 215 |
| Between-group difference | −1533 (−2709, −356) | −43 (−582, 497) | 93 (−387, 574) | 2 (−563, 566) |
Mean ± SE for nutrient concentrations and the mean (95% confidence interval) for the effect of treatment were estimated from cubic spline models. The effect of MV was estimated by comparing mothers who received MV (assigned to VA+BC+MV or MV without VA+BC) vs. mothers who did not receive MV (assigned to VA+BC alone or placebo).
DISCUSSION
In this study, daily prenatal and postnatal supplementation with VA+BC to HIV-infected women doubled the concentrations of retinol in breast milk and increased the concentrations of β-carotene and α-carotene by approximately nine-fold and three-fold, respectively, during the first year post-partum. Daily supplementation with MV (vitamins B, C, and E) increased α-tocopherol concentrations in breast milk by approximately 20% while MV supplementation was associated with a ~25% reduction in breast milk concentrations of γ-tocopherol. In the placebo group, the concentrations of retinol, β-carotene, α-carotene, and the tocopherols were comparable to those reported from studies in nonsupplemented, presumably HIV-uninfected women at similar times post-partum (Canfield et al., 2003; Gossage et al., 2002; Sakurai et al., 2005; Schweigert et al., 2004).
The magnitude of the effect of supplementation with either retinol or β-carotene on breast milk retinol concentrations has been highly variable as reported by 9 randomized controlled trials in presumably HIV-uninfected women (Bahl et al., 2002; Basu et al., 2003; Canfield et al., 2001; Dijkhuizen et al., 2004; Muslimatun et al., 2001; Rice et al., 1999; Roy et al., 1997; Stoltzfus et al., 1993; Vinutha et al., 2000) and one randomized controlled trial in HIV infected Malawian women (Semba et al., 2000). This variation likely depends on differences in the dose and timing of supplementation. In our study, daily prenatal and postnatal supplementation with VA+BC approximately doubled breast milk concentrations of retinol at all time points. The average magnitude of effect reported by other studies, in which the majority of vitamin A supplementation regimens were single, large doses provided in the early post-partum period, has been reported to be approximately 30% (minimum = −37%, maximum=337%). In the majority of studies that used large doses administered once in the early postpartum period, increases in retinol concentrations were not sustained beyond 6 months (Bahl et al., 2002; Basu et al., 2003; Rice et al., 1999). In our study, however, daily prenatal and postnatal supplementation with VA+BC resulted in sustained increases in concentrations of breast milk retinol for up to one year postpartum.
We found that VA+BC supplementation increased concentrations of β-carotene in breast milk from delivery to 1 year post-partum. This finding is consistent with randomized controlled studies of single dose (Canfield et al., 1997) and daily β-carotene supplementation (Canfield et al., 1998; Canfield et al., 2001; Canfield et al., 1999; Dijkhuizen et al., 2004; Rice et al., 1999) as well as supplementation with β-carotene-rich red palm oil (Lietz et al., 2001; Lietz et al., 2006). VA+BC supplementation was also associated with sustained increases in breast milk α-carotene concentrations. These findings are comparable to those observed in supplementation studies in cancer patients in which daily β-carotene supplementation increased α-carotene concentrations in serum (Micozzi et al., 1992; Pappalardo et al., 1997; Wahlqvist et al., 1994; Willett et al., 1983). It has been hypothesized that in populations with depressed antioxidant capacity, such as cancer and HIV patients, β-carotene supplementation preserves α-carotene concentrations by reducing its use in antioxidant activities (Pappalardo et al., 1997).
Enhancing the concentrations of retinol in breast milk could improve the health outcomes of breastfeeding infants. It has been estimated that approximately 20% of supplemental vitamin A is transferred from the mother to the infant via breastfeeding (Ross & Harvey, 2003). Two studies reported that single dose maternal vitamin A supplementation (200,000 IU of preformed retinol) during the early post-partum period reduced the incidence and duration of febrile illnesses, diarrheal diseases, and respiratory illnesses (Basu et al., 2003; Roy et al., 1997), and the incidence of measles (Basu et al., 2003) of breastfeeding infants up to 6 mo post-partum. We had previously reported that VA+BC supplementation of mothers during pregnancy and lactation increased serum retinol of breastfeeding infants at 6 wk and 6 mo of age (Baylin et al., 2005). The observed increases in serum retinol could be the result of the effects of supplementation on retinol concentrations in breast milk as we currently report. Despite the ability of maternal VA+BC supplementation to improve health outcomes in breastfeeding infants, supplementation with VA+BC to HIV-infected women during pregnancy and lactation is not recommended due to the risk of increased mother to child transmission through breast feeding (Fawzi et al., 2002). Since the majority of women in African countries do not know their HIV status, the potential risks of unknowingly supplementing HIV-infected women with vitamin A and β-carotene need to be considered.
MV supplementation during pregnancy and lactation increased breast milk α-tocopherol concentrations from 3 to 12 mo post-partum and decreased concentrations of γ-tocopherol at all time points and of retinol at delivery. Although additional studies of the effects of vitamin E supplementation on tocopherol concentrations in breast milk were not available for comparison, the findings from our study are consistent with those from Baylin and colleagues who reported that MV supplementation was associated with increased serum vitamin E in breastfed infants at 6 wk and 6 mo of age and reduced serum retinol in infants at 6 wk of age (Baylin et al., 2005). Changes in nutrient concentrations with MV supplementation, including a decrease in retinol, might be attributed to the α-tocopherol content of the supplement. In well-nourished men, serum retinol concentrations were significantly decreased after 16 weeks of daily high-dose α-tocopherol supplementation (800 IU) (Willett et al., 1983), consistent with our findings in breastmilk. Other studies have also reported a negative impact of α-tocopherol supplementation on serum and adipose tissue concentrations of γ-tocopherol (Handelman et al., 1994; Handelman et al., 1985; Huang & Appel, 2003; Traber & Kayden, 1989), likely via competition during absorption and the promotion of metabolic degradation of γ-tocopherol by high concentrations of α-tocopherol (Wolf, 2006). Because γ-tocopherol possess approximately one third the antioxidant capacity of α–tocopherol (Bieri et al., 1976), reductions in γ-tocopherol that are accompanied by equivalent increases in α-tocopherol concentrations are unlikely to impair the total antioxidant capacity.
Additional studies in this population of HIV-infected mothers and their infants showed that daily prenatal and postnatal supplementation with vitamins B, C, and E was associated with child health outcomes such as improved growth (Villamor et al., 2005), increased CD4 cell counts, reduced risk of diarrhea (Fawzi et al., 2003), and improved psychosocial and motor development (McGrath et al., 2006). Our present findings lend mechanistic support to these previous studies, in that the observed benefits on the infants’ nutritional and health status could be mediated by increases in the concentration of nutrients in breast milk. Specifically, increased concentrations of vitamins in breast milk including α-tocopherol, B vitamins, and vitamin C may have mediated improvements in child health outcomes.
Current infant guidelines recommend that in situations where replacement feeding options do not meet AFASS criteria, the infants of HIV-infected mothers should be exclusively breastfed for the first 6 months of life or until these criteria are met. Because supplementation with VA+BC is associated with increased mother to child transmission, interventions that promote enhanced intakes of vitamin A to recommended levels by increasing overall dietary intakes and diversity rather than VA+BC supplementation may be indicated in pregnant and lactating HIV-infected women. Daily prenatal and postnatal supplementation with vitamins E, C, and B could be an effective and low cost intervention to increase the concentrations of other micronutrients available to the infants through breastfeeding, and improve the health of breastfeeding infants born to HIV-infected mothers.
Acknowledgments
The authors thank the mothers and children, field teams including nurses, midwives, supervisors, laboratory staff, and the administrative staff who made the study possible. The authors also thank Muhimbili Medical Centre, Muhimbili University College of Health Sciences, and the National AIDS Control Program in Dar es Salaam for their institutional support. This work was supported by grants R01HD045134 and T32DK07703 from the National Institutes of Health. Clinical Trials Identifier: NCT00197756
Footnotes
Contribution of Authors: AW carried out the data analyses and wrote the initial draft of the manuscript. SA, WWF (Principal Investigator of the “Tanzania Trial of Vitamins” study), and EV (Principal Investigator of the “Vitamins, Breast milk HIV shedding, and Child Health” study) participated in the study design and implementation in the field. JF, CM, and HC carried out the analyses of breast milk samples and contributed to the study implementation. All authors participated in the interpretation of the data and in writing the final draft of the manuscript.
References
- Bahl R, Bhandari N, Wahed MA, Kumar GT, Bhan MK. Vitamin A supplementation of women postpartum and of their infants at immunization alters breast milk retinol and infant vitamin A status. J Nutr. 2002;132(11):3243–8. doi: 10.1093/jn/132.11.3243. [DOI] [PubMed] [Google Scholar]
- Basu S, Sengupta B, Paladhi PK. Single megadose vitamin A supplementation of Indian mothers and morbidity in breastfed young infants. Postgrad Med J. 2003;79 (933):397–402. doi: 10.1136/pmj.79.933.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Shor-Posner G, Zhang G, Lai H, Quesada JA, Campa A, Jose-Burbano M, Fletcher MA, Sauberlich H, Page JB. HIV-1 infection in women is associated with severe nutritional deficiencies. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(4):272–8. doi: 10.1097/00042560-199712010-00008. [DOI] [PubMed] [Google Scholar]
- Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi W. Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. Eur J Clin Nutr. 2005;59:960–68. doi: 10.1038/sj.ejcn.1602201. [DOI] [PubMed] [Google Scholar]
- Bieri JG, Evarts RP, Gart JJ. Relative activity of alpha-tocopherol and gamma-tocopherol in preventing oxidative red cell hemolysis. J Nutr. 1976;106(1):124–7. doi: 10.1093/jn/106.1.124. [DOI] [PubMed] [Google Scholar]
- Canfield LM, Giuliano AR, Neilson EM, Yap HH, Graver EJ, Cui HA, Blashill BM. Beta-Carotene in breast milk and serum is increased after a single beta-carotene dose. Am J Clin Nutr. 1997;66(1):52–61. doi: 10.1093/ajcn/66.1.52. [DOI] [PubMed] [Google Scholar]
- Canfield LM, Giuliano AR, Neilson EM, Blashil BM, Graver EJ, Yap HH. Kinetics of the response of milk and serum beta-carotene to daily beta-carotene supplementation in healthy, lactating women. Am J Clin Nutr. 1998;67(2):276–83. doi: 10.1093/ajcn/67.2.276. [DOI] [PubMed] [Google Scholar]
- Canfield LM, Taren DL, Kaminsky RG, Mahal Z. Short-term beta-carotene supplementation of lactating mothers consuming diets low in vitamin A. J Nutr Biochem. 1999;10(9):532–8. doi: 10.1016/s0955-2863(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Canfield LM, Kaminsky RG, Taren DL, Shaw E, Sander JK. Red palm oil in the maternal diet increases provitamin A carotenoids in breastmilk and serum of the mother-infant dyad. Eur J Nutr. 2001;40(1):30–8. doi: 10.1007/pl00007383. [DOI] [PubMed] [Google Scholar]
- Canfield LM, Clandinin MT, Davies DP, Fernandez MC, Jackson J, Hawkes J, Goldman WJ, Pramuk K, Reyes H, Sablan B, Sonobe T, Bo X. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr. 2003;42(3):133–41. doi: 10.1007/s00394-003-0403-9. [DOI] [PubMed] [Google Scholar]
- Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Msellati P, Newell ML, Nsuati R, Read JS, Wiktor S. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189(12):2154–66. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen MA, Wieringa FT, West CE, Muhilal Zinc plus beta-carotene supplementation of pregnant women is superior to beta-carotene supplementation alone in improving vitamin A status in both mothers and infants. Am J Clin Nutr. 2004;80 (5):1299–307. doi: 10.1093/ajcn/80.5.1299. [DOI] [PubMed] [Google Scholar]
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, Manji K, Kapiga S, Mwakagile D, Essex M, Spiegelman D. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. 2002;16(14):1935–44. doi: 10.1097/00002030-200209270-00011. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Wei R, Spiegelman D, Antelman G, Villamor E, Manji K, Hunter D. Effect of providing vitamin supplements to human immunodeficiency virus-infected, lactating mothers on the child’s morbidity and CD4+ cell counts. Clin Infect Dis. 2003;36(8):1053–62. doi: 10.1086/374223. [DOI] [PubMed] [Google Scholar]
- Gossage CP, Deyhim M, Yamini S, Douglass LW, Moser-Veillon PB. Carotenoid composition of human milk during the first month postpartum and the response to beta-carotene supplementation. Am J Clin Nutr. 2002;76(1):193–7. doi: 10.1093/ajcn/76.1.193. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J Nutr. 1985;115(6):807–13. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Epstein WL, Peerson J, Spiegelman D, Machlin LJ, Dratz EA. Human adipose alpha-tocopherol and gamma-tocopherol kinetics during and after 1 y of alpha-tocopherol supplementation. Am J Clin Nutr. 1994;59(5):1025–32. doi: 10.1093/ajcn/59.5.1025. [DOI] [PubMed] [Google Scholar]
- Huang HY, Appel LJ. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J Nutr. 2003;133(10):3137–40. doi: 10.1093/jn/133.10.3137. [DOI] [PubMed] [Google Scholar]
- Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, Moulton LH, Ward BJ, Humphrey JH. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. Aids. 2005;19(7):699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- Lietz G, Henry CJ, Mulokozi G, Mugyabuso JK, Ballart A, Ndossi GD, Lorri W, Tomkins A. Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. Am J Clin Nutr. 2001;74(4):501–9. doi: 10.1093/ajcn/74.4.501. [DOI] [PubMed] [Google Scholar]
- Lietz G, Mulokozi G, Henry JC, Tomkins AM. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J Nutr. 2006;136(7):1821–7. doi: 10.1093/jn/136.7.1821. [DOI] [PubMed] [Google Scholar]
- McGrath N, Bellinger D, Robins J, Msamanga GI, Tronick E, Fawzi WW. Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics. 2006;117(2):e216–25. doi: 10.1542/peds.2004-1668. [DOI] [PubMed] [Google Scholar]
- Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR, Smith JC., Jr Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr. 1992;55(6):1120–5. doi: 10.1093/ajcn/55.6.1120. [DOI] [PubMed] [Google Scholar]
- Muslimatun S, Schmidt MK, West CE, Schultink W, Hautvast JG, Karyadi D. Weekly vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. J Nutr. 2001;131(10):2664–9. doi: 10.1093/jn/131.10.2664. [DOI] [PubMed] [Google Scholar]
- Papathakis PC, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am J Clin Nutr. 2007;85(1):182–92. doi: 10.1093/ajcn/85.1.182. [DOI] [PubMed] [Google Scholar]
- Pappalardo G, Maiani G, Mobarhan S, Guadalaxara A, Azzini E, Raguzzini A, Salucci M, Serafini M, Trifero M, Illomei G, Ferro-Luzzi A. Plasma (carotenoids, retinol, alpha-tocopherol) and tissue (carotenoids) levels after supplementation with beta-carotene in subjects with precancerous and cancerous lesions of sigmoid colon. Eur J Clin Nutr. 1997;51(10):661–6. doi: 10.1038/sj.ejcn.1600457. [DOI] [PubMed] [Google Scholar]
- Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed MA. Maternal vitamin A or beta-carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr. 1999;129(2):356–65. doi: 10.1093/jn/129.2.356. [DOI] [PubMed] [Google Scholar]
- Ross JS, Harvey PWJ. Contribution of breastfeeding to vitamin A nutrition of infants: a simulation model. Bull World Health Organ. 2003;81:80–86. [PMC free article] [PubMed] [Google Scholar]
- Roy SK, Islam A, Molla A, Akramuzzaman SM, Jahan F, Fuchs G. Impact of a single megadose of vitamin A at delivery on breastmilk of mothers and morbidity of their infants. Eur J Clin Nutr. 1997;51(5):302–7. doi: 10.1038/sj.ejcn.1600398. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Furukawa M, Asoh M, Kanno T, Kojima T, Yonekubo A. Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. J Nutr Sci Vitaminol (Tokyo) 2005;51(4):239–47. doi: 10.3177/jnsv.51.239. [DOI] [PubMed] [Google Scholar]
- Schweigert FJ, Bathe K, Chen F, Buscher U, Dudenhausen JW. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur J Nutr. 2004;43(1):39–44. doi: 10.1007/s00394-004-0439-5. [DOI] [PubMed] [Google Scholar]
- Semba RD, Kumwenda N, Taha TE, Mtimavalye L, Broadhead R, Miotti PG, Eisinger W, Hoover D, Chiphangwi JD. Plasma and breast milk vitamin A as indicators of vitamin A status in pregnant women. Int J Vitam Nutr Res. 2000;70(6):271–7. doi: 10.1024/0300-9831.70.6.271. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Hakimi M, Miller KW, Rasmussen KM, Dawiesah S, Habicht JP, Dibley MJ. High dose vitamin A supplementation of breast-feeding Indonesian mothers: effects on the vitamin A status of mother and infant. J Nutr. 1993;123(4):666–75. doi: 10.1093/jn/123.4.666. [DOI] [PubMed] [Google Scholar]
- Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr. 1989;49(3):517–26. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Report on the global AIDS epidemic 2006. Geneva: UNAIDS; 2006. Feb 20, http://www.unaids.org/en/Publications/default.asp. 2007. [Google Scholar]
- UNICEF/UNAIDS/WHO. A review of HIV transmission through breastfeeding. Geneva: WHO, UNAIDS, and UNICEF; 1998. HIV and infant feeding: Guidelines for decision-makers. A guide for health care managers and supervisors. [Google Scholar]
- Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, Msamanga G, Hunter DJ, Fawzi WW. Vitamin supplementation of HIV-infected women improves postnatal child growth. Am J Clin Nutr. 2005;81(4):880–8. doi: 10.1093/ajcn/81.4.880. [DOI] [PubMed] [Google Scholar]
- Vinutha B, Mehta MN, Shanbag P. Vitamin A status of pregnant women and effect of post partum vitamin a supplementation. Indian Pediatr. 2000;37(11):1188–93. [PubMed] [Google Scholar]
- Wahlqvist ML, Wattanapenpaiboon N, Macrae FA, Lambert JR, MacLennan R, Hsu-Hage BH. Changes in serum carotenoids in subjects with colorectal adenomas after 24 mo of beta-carotene supplementation. Australian Polyp Prevention Project Investigators. Am J Clin Nutr. 1994;60(6):936–43. doi: 10.1093/ajcn/60.6.936. [DOI] [PubMed] [Google Scholar]
- White H. A heteroskedasticity-consistent covariance matric estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–30. [Google Scholar]
- World Health Organization. Proposed World Health Organization staging system for HIV infection and disease: preliminary testing by an international collaborative cross-sectional study. AIDS. 1993;7:711–8. [PubMed] [Google Scholar]
- World Health Organization. WHO HIV and infant feeding technical consultation consensus statement. Geneva, Switzerland: WHO; 2006. Feb 20, http://www.who.int/child-adolescent-health/publications/NUTRITION/consensus_statement.htm. 2007. [Google Scholar]
- Willett WC, Stampfer MJ, Underwood BA, Taylor JO, Hennekens CH. Vitamins A, E, and carotene: effects of supplementation on their plasma levels. Am J Clin Nutr. 1983;38(4):559–66. doi: 10.1093/ajcn/38.4.559. [DOI] [PubMed] [Google Scholar]
- Wolf G. How an increased intake of alpha tocopherol can suppress the bioavailability of gamma tocopherol. Nutr Rev. 2006;64(6):295–99. doi: 10.1111/j.1753-4887.2006.tb00213.x. [DOI] [PubMed] [Google Scholar]