Abstract
Human cytomegalovirus (CMV), one of the eight herpesviruses that commonly infect humans, is best known for its propensity to cause disease in immunocompromised patients, especially transplant recipients, patients with advanced AIDS, and congenitally infected newborns. Advances in molecular virology coupled with improvements in diagnostic methods and treatment options have vastly improved our understanding of and ability to manage CMV, but many uncertainties remain, including the mechanisms of persistence and pathogenesis and its hypothesized roles in a variety of human illnesses. Here we review recent advances that are reshaping our view and approach to this fascinating virus.
A century of progress
Human CMV infection is extremely common. A recent analysis in the United States revealed an overall 50% seroprevalence among adults (1), but rates in some populations are even higher. For example, approximately 90% of Mexican-Americans in the United States are seropositive by age 50, as are 88% of stem cell transplant patients in Italy and 96% of individuals in southern Brazil (1–4). Despite its high worldwide prevalence, CMV infections are generally inapparent, except in newborns and immunocompromised individuals, for whom they can cause life-threatening disease affecting many organ systems.
The virus was first detected in newborns during the early 20th century, when multiple reports described large cells in the urine of children with an often fatal systemic infection referred to as cytomegalic inclusion disease (5). The midcentury development of cell culture methods enabled propagation of CMV, but its detection in clinical specimens often required weeks of cultivation. Rapid diagnostic testing by centrifugation-enhanced inoculation combined with detection of CMV antigens in the 1980s was a transforming advance, enabling the diagnosis of CMV to be made in a clinically useful time frame (6). The emergence of effective antivirals in the 1990s was opportune, as CMV-associated disease was increasing in parallel with the AIDS epidemic and use of solid organ transplantation (SOT) and HSC transplantation (HCT). During the past two decades, further advances in diagnosis and treatment have greatly improved our ability to control CMV disease, but the virus still accounts for substantial morbidity, mortality, and cost.
Along with these clinical advances, remarkable progress has been made in understanding the molecular biology of CMV. Application of the nucleic acid and protein analytic methods led to an appreciation of the extraordinary complexity of CMV, a point solidified by the landmark report of the first complete sequence of a CMV strain in 1990 (7). Refinements to methods for studying CMV gene function have continued to reveal myriad mechanisms underlying CMV’s evolutionary success. However, understanding the pathogenesis of CMV diseases remains an enormous challenge, in large part because the virus only grows in human cells and it differs substantially from even its primate-infecting cousins (8–10).
Here we summarize the current understanding of CMV biology and disease. The topic is too broad to cover completely in this format, so the interested reader may wish to consult more comprehensive reviews (11, 12), as well as new reports that are certain to appear in the months and years ahead.
CMV replication: insights but limitations from the lab
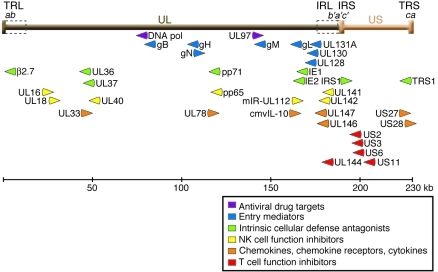
Human CMV is the prototypic member of the β herpesvirus subfamily, which also includes human herpes viruses 6 and 7 and many animal CMVs. Its DNA genome is approximately 230 kb in size, the largest among known human viruses, and consists of unique long (UL) and unique short (US) segments, each of which is flanked by inverted repeats (RL and RS; Figure 1). Most of the approximately 200 genes encode proteins, but some express only noncoding RNAs, including approximately 14 microRNAs (miRNAs; refs. 13–15). The central portion of the UL region contains clusters of core genes that have homologs in other herpesviruses, such as DNA polymerase, glycoprotein B (gB), and glycoprotein H (gH), whereas the remainder of the genome contain genes primarily found only in β herpesviruses or unique to human CMV (16, 17). In fact, considerable variation has been detected even among human CMV isolates (18). By convention, CMV genes are named by their position within the genome, although some also have additional descriptive names. For example, UL54 (the 54th gene in the UL region, according to the original report of the CMV, strain AD169, sequence; ref. 7) is the DNA polymerase gene.
Figure 1. CMV genome.
The genome of CMV clinical isolates, such as the Merlin strain depicted here (GenBank accession no. NC_006273; ref. 118), consists of long (brown) and short (orange) DNA segments, each of which has unique regions (UL and US) flanked by inverted repeats (TRL/IRL and IRS/TRS). These repeats contain segment-specific sequences (b, b′, c, and c′) as well as a variable number of shared a sequence repeats in direct orientation at the genomic ends and in an inverted orientation at the junction of the two segments. Laboratory-adapted strains often have deletions of multiple genes at the right end of the UL segment and their replacement with genes duplicated from the left end, resulting in longer TRL and IRL regions (dashed boxes) compared with clinical strains (21, 118). The gene names in this region are not always sequential because of historical precedence in nomenclature assignments and because of rearrangements among strains. The relative position and orientation of transcripts corresponding to several genes are shown, along with grouping by their putative functional classifications (24, 31, 43–45, 47). This diagram is a simplification, since some and possibly many of the genes shown here have more than one function and other genes that are not shown likely contribute to the indicated processes.
Propagation of CMV in cell culture has been an essential research tool but has definite limitations, in part because passage in the lab selects for mutants adapted for growth in this unnatural setting (19). As a result, commonly used lab strains have multiple mutations, deletions, and rearrangements (20). For example, the end of the UL region in isolates of the lab strains Towne and AD169 lacks approximately 13 kb of DNA, encoding 19 genes that are present in the Toledo strain (21, 22). Remarkably, inactivating mutations in the RL13 gene are detectable by sequence analyses of the viral genome immediately upon propagation of virus in cell culture (23), which suggests that this gene is necessary for success of the virus in humans but strongly inhibitory to replication in cell culture. The fact that most, if not all, prior laboratory-based studies have used only RL13 mutant viruses reinforces the importance of confirming results of laboratory studies with observations from the clinic. Advances in sequencing technology are sure to improve our understanding of the genetic variation of CMV without confounding artifacts that arise from propagation in the laboratory.
Among the important recent advances has been the characterization of a previously unrecognized CMV entry pathway. Most laboratory research on CMV has used diploid human fibroblasts as a cellular host, which the virus enters by binding to and fusing with the plasma membrane in a process mediated by interactions of virion glycoproteins (gB, gH, and gL; Figure 1) with cell membrane receptors including integrins and possibly growth factor receptors (24, 25). However, in infected humans, CMV is commonly found in endothelial cells, epithelial cells, smooth muscle cells, and some HSCs in addition to fibroblasts (26). Entry into at least some of these other cells types follows an endocytic, pH-sensitive pathway and requires the UL128, UL130, and UL131A genes (Figure 1 and refs. 27–29). Many laboratory-adapted strains have mutations in one or more of these genes, resulting in their failure to replicate in endothelial and epithelial cells (27, 29).
Immune control
Following initial infection, a complex set of host responses conspires to limit CMV replication. Multiple defense systems sense the foreign nature of the virus very early after contact. The specific pathogen-associated molecular patterns likely include virion glycoproteins and the viral genome itself (24, 30). Among the earliest responses are elaboration of interferon and cytokines that help establish an antiviral state (31).
Complementing responses within the infected cell itself are defenses generated by cells of both the innate and the adaptive immune systems. Depletion of NK cells in mice results in higher titers of murine CMV in tissues and increased mortality (32). In humans, NK cell deficiency has been linked to severe CMV disease (33). Considerable evidence indicates that adaptive T cell responses are critical for keeping the virus inactive. For example, restoration of CD8+ and CD4+ T cells is a strong correlate of control of the virus after HSC transplant (HSCT; ref. 34). Moreover, adoptive transfer of CMV-specific T cells protects against clinical reactivation (35, 36).
In seropositive humans, a strikingly high fraction (10% or more) of circulating T lymphocytes target CMV (37). Use of tetramers has shown that pp65 is recognized by a high fraction T cells, but many other gene products are also recognized (37, 38). Moreover, the fraction of CMV-specific T cells tends to increase with age, which supports the hypothesis that CMV contributes to immune system exhaustion and dysfunction associated with aging (39).
Antibodies in CMV-infected individuals have been useful for establishing serostatus. Although it has long been thought that only antibodies to gB or gH neutralize the virus, the finding that other viral genes, including UL128, UL130, and UL131A, mediate entry into endothelial and epithelial cells raises new possibilities for therapeutic design. In fact, recent data indicate that neutralizing antibody titers in human sera may be two logs higher against these alternative entry pathway mediators compared with those targeting against gB and gH (40–42). Thus, future studies should revisit the possible role of antibody responses in controlling infections, and these genes will be important to consider in designing future vaccines.
Viral counterattack
Faced with such a breadth of host defense systems, the success of CMV in human infection has necessitated evolution of myriad viral evasion strategies. Proteins delivered with infecting virion (e.g., pp65 [UL83], pp71 [UL82], pTRS1, and pIRS1) and made very early after infection (UL36, UL37, IE1, and IE2) block intrinsic cellular defenses, including induction of apoptosis, production of interferon and interferon-stimulated genes, and shutoff of protein synthesis (31, 43). CMV also interferes with the cellular immune responses (44, 45). At least seven genes are able to modulate, and in many cases inhibit, NK cell function (Figure 1 and ref. 45). For example, the viral miRNA mIR-UL112 acts synergistically with a cellular miRNA to inhibit expression of the NK-activating ligand MICB (46). Several genes clustered in the US2-11 region prevent presentation of CMV peptides to T cells. US3 binds to and sequesters MHC class I in the ER, US6 inhibits loading of peptides onto the MHC complex, and US2 and US11 cause dislocation of class I molecules from the ER to the cytosol, where they are degraded.
CMV encodes chemokines, chemokine receptors, and cytokines that likely participate in immune evasion (Figure 1 and ref. 47). For example, cmvIL-10, which is only 27% identical to human IL-10 but binds to the same receptor and signals through a similar pathway, appears to be primarily immunosuppressive (48). It inhibits proliferation and cytokine production by peripheral blood mononuclear cells and inhibits maturation and stimulates apoptosis of dendritic cells. However, it also has potentially immunostimulatory effects, such as augmenting phagocytic activity of monocytes. Both cmvIL-10 and the CMV chemokine receptor US28 stimulate migration of some cell types, but inhibit migration of others. Although inhibition of cell migration might help the virus evade the host immune cells, enhanced cell migration might promote dissemination of virus to new sites, as has been shown for murine CMV (49, 50).
A major challenge for future research will be to dissect the bona fide functions of the immune modulators during infection in humans. Most of these genes are not essential for replication in cell culture (51, 52). Some general insights have and will continue to emerge from studies of animal CMVs, but immune evasion strategies differ in significant ways in different systems, even in human CMV’s well-studied close cousin, rhesus CMV (8–10). More sophisticated animal and cell culture models, along with deep sequencing technology applied to the analysis of gene expression and variation in both CMV strains and their hosts, should shed light on the key interactions of the host immune system and its viral targets.
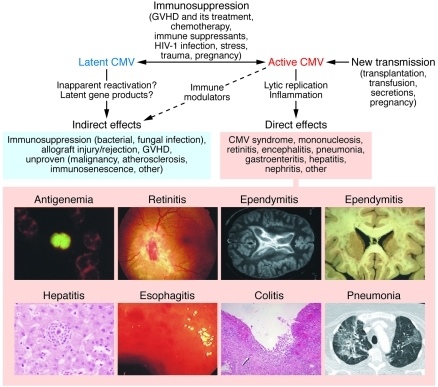
Millions of years of evolution have led CMV to establish an apparently benign relationship with its host, at least most of the time. However, CMV replication is poised in a delicate balance with host immune system controls, and even relatively minor perturbations, such as pregnancy or admission to an intensive care unit (ICU), allow CMV reactivation, often without overt CMV disease (Figure 2 and refs. 53, 54). In this sense, CMV seems to be a sentinel chicken of immune system dysfunction. Moreover, subtle effects of subclinical reactivation or quiescent CMV infection might contribute to chronic immune system dysfunction and predispose to other illnesses, including infections by other microbes (55, 56). On the other hand, interactions among infectious agents are exceedingly complex (57); in fact, murine CMV infection has been reported to protect mice against subsequent pathogenic bacterial infection (58). Thus, our understanding of the full spectrum of risks and benefits of CMV infection and its interactions with the host immune system is far from complete.
Figure 2. CMV disease mechanisms.
Many humans harbor clinically quiescent or latent CMV. Even when asymptomatic, the virus may cause indirect effects, possibly by altering immune system function after subclinical reactivation episodes or by expression of viral genes (e.g., LAcmvIl-10) during latency. Immune system dysfunction resulting from a variety of iatrogenic or natural causes or from a new transmission event can lead to active CMV replication. Depending on the clinical setting, active replication may contribute to indirect effects, but also leads to direct tissue damage, resulting in an inflammatory response and dysfunction of various organ systems. In addition to CMV antigenemia, a common indicator of active infection, examples of end CMV organ disease commonly occurring in AIDS patients and in transplant recipients are shown. Image credits: antigenemia, pp65+ cell in a leukocyte cytospin preparation (M. Boeckh); retinitis, ophthalmoscopic view of retinal hemorrhage and inflammation (E. Chuang); ependymitis, periventricular inflammation detected by MRI (left; reproduced from ref. 119 with permission from McGraw Hill) and postmortem brain specimen (right; C. Marra); hepatitis, microabscesses associated with CMV hepatitis (A. Limaye); esophagitis, endoscopic view of shallow esophageal ulcers (G. McDonald); colitis, deep ulcer in a colonic biopsy (G. McDonald); pneumonia, chest CT scan of CMV pneumonia (M. Boeckh).
The latency puzzle
Like other herpesviruses, CMV becomes clinically quiescent after the primary infection is brought under control by the host immune response. The virus is generally believed to persist in a state of cellular latency, in which infected cells are not producing any infectious virus, but retain the complete genome and have the potential to start producing virus at a later time (59). However, it remains possible that clinical quiescence is often a state in which CMV is replicating at a very low level, below the threshold of current detection methods. True cellular latency has been quite convincingly established in the case of murine CMV (60) and likely occurs with human CMV; however, as illustrated by the extraordinarily high frequency of shedding of viral DNA by asymptomatic herpes simplex virus–2–infected individuals (61), extrapolating from animal models to the human setting requires caution.
The site — or, more likely, sites — of CMV latency or persistence in the human are not yet clear. The risk of acquiring CMV by transfusion from asymptomatic donors implicates blood as one site. Indeed, many studies of CMV latency have focused on circulating leukocytes, in large part because of their being relatively accessible for research, and these studies have revealed latent infection in immature cells of the myeloid lineage (59). However, these results do not rule out other sites of latency. SOT from seropositive to seronegative donors can transmit CMV (62). Although these organs are undoubtedly contaminated with leukocytes, the fact that the virus has a propensity to reactivate within the transplanted organ suggests that parenchymal cells may harbor latent virus.
At least in cell culture models of latency, CMV expresses only a small number of genes (59), as is true of latency in other herpesvirus systems. A particularly intriguing one of these is an alternatively spliced, latency-associated form of cmvIL-10 (LAcmvIL-10), which retains some but not all of the activities of cmvIL-10. Interestingly, LAcmvIL-10 inhibits MHC class II recognition of infected cells and might thus assist them in avoiding elimination by the immune system (48). Moreover, since LAcmvIL-10 has immunomodulatory properties and might be secreted from latently infected cells for decades, it has the potential to cause chronic immune system dysfunction in otherwise healthy individuals. Clarification of the genes expressed during bona fide latency and elucidating their effects on the host will require much additional research.
CMV infection and disease
The common manifestations of CMV infection depend to a large extent on the particular clinical setting. Overt disease is limited primarily to patients with significant immune system dysfunction that can result from other illnesses or iatrogenic causes (Figure 2).
Congenital and neonatal infection.
CMV is the most frequent congenital viral infection, occurring in as many as 40,000 cases in the United States each year. A recent review of multiple studies found that of infected infants, 13% had symptoms at birth, and in 0.5%, the infection proved fatal (63). Approximately 20% of the total — primarily, but not exclusively, those symptomatic at birth — suffer from permanent sequelae, commonly sensorineural hearing loss (64). Seronegative mothers who become infected during pregnancy have a much higher risk of transmitting the virus and of bearing an affected infant compared with women who are seropositive at conception, but infants of the latter group can also suffer sequelae (65). It now seems likely that at least some cases result from infection of the pregnant woman with a new virus, rather than from reactivation of a latent virus the women had acquired earlier (66). Antiviral treatment of congenital CMV disease with ganciclovir or valganciclovir has been shown to be beneficial (67). The development of preventative strategies before pregnancy (i.e., immunization, ref. 68), during pregnancy (i.e., CMV-specific immunoglobulin, ref. 69, and valacyclovir), and after birth (i.e., newborn CMV screening, screening for late-onset hearing loss, ref. 70, and preemptive treatment) are high-priority research areas.
CMV can also be acquired in the neonatal period via breast milk (71). Reported transmission rates from mothers to preterm infants vary widely (6%–60%) as do disease rates (0%–35%), so the burden and determinants of breast milk transmission are not yet clear (71).
Immunocompetent hosts.
Judging from data showing increasing seroprevalence with age, acquisition of CMV may occur at any time during life (1). In early childhood, CMV can be acquired via saliva in family or day care settings. During adulthood, CMV is transmitted sexually and via saliva (e.g., from children), and occasionally via blood transfusions or transplanted organs (11, 72). Primary infection is typically asymptomatic, but may present clinically as a mononucleosis-like illness. Very occasionally, CMV seems to cause pneumonia or gastrointestinal disease in immunocompetent hosts (73).
Hematologic malignancies and HCT.
CMV pneumonia remains one of the most feared infectious complications following HCT. Even with treatment, mortality remains high (74). CMV gastrointestinal disease, which can affect the upper and lower tracts, is presently the most prevalent manifestation of CMV disease in HCT recipients (74). Retinitis, hepatitis, and encephalitis occur infrequently.
The most important pretransplant risk factor for CMV disease is the serological status of the donor and recipient, with seropositive recipients being at highest risk, followed by CMV-seronegative patients receiving stem cells from a CMV-seropositive donor (referred to as D+/R– patients); seronegative recipients of stem cells from seronegative donors have a very low risk of primary infection if CMV-safe blood products are used (75). Other risk factors for CMV infection include the use of high-dose corticosteroids, acute and chronic graft-versus-host disease (GVHD), and use of mismatched or unrelated donors (56). Stem cell source and conditioning regimen only minimally affect the risk of CMV infection and disease, with the exception of umbilical cord blood transplantation, which is associated with reactivation and high disease rates in the absence of antiviral prophylaxis (76). Alemtuzumab, an anti-CD52 monoclonal antibody that results in prolonged CD4+ and CD8+ lymphopenia, may also lead to high reactivation rates in both transplant and nontransplant patients (77). CMV disease is very rare after autologous transplantation, unless CD34+ selected stem cells are used.
Today, the use of preemptive antiviral therapy or prophylaxis is the standard of care in HCT recipients (78). These strategies have reduced the incidence of CMV disease during the first 3 months from approximately 25%–30% to 5% in seropositive recipients; however, late CMV disease may occur and requires continued surveillance in high-risk patients (79).
SOT.
CMV can cause a febrile syndrome with leukopenia and/or transaminitis (CMV syndrome) as well as other end-organ disease. There seems to be a predilection of clinical disease manifestations for the transplanted organ, possibly caused by minor HLA mismatches promoting local CMV reactivation and replication (62). Therefore, the end-organ disease manifestations differ according to the type of organ transplantation.
The highest risk for CMV disease occurs in CMV D+/R– patients. In contrast to HCT recipients, reactivation disease (in the seropositive recipients) is less common in SOT recipients. Antiviral prophylaxis and preemptive therapy strategies are widely used in SOT recipients and have led to a reduction of CMV disease during the time they are applied (typically for at least 3 months; refs. 80–82). Despite these advances, late CMV disease continues to be a clinical problem in D+/R– patients receiving antiviral prophylaxis (83). In addition to CMV syndrome and end-organ disease, also called direct effects, CMV causes indirect effects including allograft rejection, decreased graft and patient survival, and predisposition to opportunistic infections and perhaps malignancies (Figure 2 and ref. 55).
HIV.
CMV disease occurs in HIV-1–infected persons with advanced immunosuppression (CD4+ counts <50 cells/mm3, HIV load >100,000 copies/ml, and/or prior opportunistic infections). Retinitis is the most common clinical manifestation, followed by gastrointestinal disease and encephalitis. Since the introduction of combination antiretroviral therapy (ART), the incidence of new cases of CMV end-organ disease has declined dramatically, now occurring most commonly in persons who are not receiving ART or who have failed to respond (84).
Other disease associations.
In recent years, the ease and sensitivity of diagnostic capabilities have revealed the presence of CMV (or its immunologic correlates) in settings not traditionally known to manifest CMV. Numerous studies have shown that CMV reactivates in immunocompetent patients admitted to ICUs, and there appears to be an association with prolonged hospital and ICU stay (53, 85). Moreover, associations of CMV with inflammatory bowel disease, new-onset diabetes, and tumors such as glioblastoma multiforme have been suggested (86–88). Perhaps most intriguing are reports that CMV may play a role in immunosenescence (89) and in the pathogenesis of atherosclerosis (90), possibly through actions of its many immunomodulatory genes (Figure 1). Whether CMV is causative in these diseases and conditions or a bystander is presently the subject of intense research.
Treatments
Current therapies.
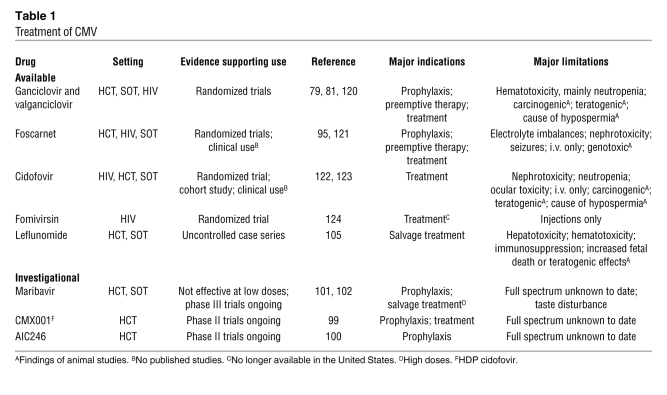
Ganciclovir, foscarnet, and cidofovir are currently available drugs for CMV treatment and prevention (Table 1). Ganciclovir (and its orally available formulation, valganciclovir) is a guanosine analog that, after phosphorylation by the CMV UL97 kinase, acts as a chain terminator during viral DNA replication. The nucleoside monophosphate analog cidofovir and the pyrophosphate analog foscarnet also inhibit viral DNA polymerase activity, but neither requires prior activation by any other viral protein (91). Ganciclovir products have been tested most widely in randomized controlled trials in both transplant and HIV-infected subjects (92, 93). In addition to i.v. and oral formulations, ganciclovir can be given locally to the eye in patients with sight-threatening retinitis (94). Systemic ganciclovir’s principal toxicity is neutropenia. Although foscarnet is as effective as ganciclovir, its main side effects are renal toxicity and electrolyte imbalances (95). Although cidofovir has been shown to be effective in the treatment of CMV retinitis (94), no randomized trial has been performed in transplant recipients. High-dose acyclovir or valacyclovir have been shown to reduce indirect effects of CMV in D+/R– renal transplant recipients (96). Fomivirsen, an injectable antisense antiviral for CMV retinitis, is no longer marketed, because advances in antiretroviral therapy have reduced the incidence of CMV retinitis in patients with HIV-1 (97).
Table 1 .
Treatment of CMV
Drug resistance can develop with all available drugs (91). Mutations affecting the viral UL97 kinase or, less often, the viral DNA polymerase can cause ganciclovir resistance. Since foscarnet and cidofovir do not require phosphorylation by UL97, resistance arises only by mutations of the DNA polymerase gene (91). Some DNA polymerase mutations cause resistance to more than one of these agents. Resistance is most frequently seen in D+/R– SOT recipients (probably as a result of prolonged drug exposure and incomplete suppression of CMV); however, it may also occur in other clinical situations when antiviral drug is given for a prolonged period of time at levels that incompletely suppress CMV infection (98). An increase in viral load should trigger molecular testing for mutations that are associated with resistance and empiric switching to another drug.
Experimental therapies.
Several new anti-CMV compounds are presently in phase II clinical development (Table 1). These include CMX001, a lipid derivative of cidofovir (99), and AIC246, which blocks a late step (possibly CMV terminase activity) in CMV replication (100). The UL97 kinase inhibitor maribavir has little serious toxicity and showed some efficacy in one controlled trial (101, 102), but appeared to be ineffective in an as-yet-unpublished phase III trial, and plans for its further development are currently unclear. In addition, there are licensed drugs that have anti-CMV activity in vitro, including leflunomide (FDA approved for arthritis treatment; ref. 103), which inhibits a late step in virion assembly (104), and imatinib, a tyrosine kinase inhibitor used to treat chronic myelogenous leukemia that blocks CMV entry into cells (25). Leflunomide has been used in salvage situations for CMV disease (105); however, no randomized trials have been conducted to evaluate its efficacy and toxicity as either monotherapy or combination therapy. Imatinib does not appear to be active in vivo (106). Donor-derived CMV-specific T cell therapy has been used in selected patients in salvage situations and is a field of active research (107).
CMV-specific and pooled immunoglobulin prophylaxis have had little success in transplant recipients (108, 109), although a meta-analysis of studies performed in the 1990s in SOT recipients suggested a beneficial effect (110). One recent uncontrolled trial suggested it might be useful as a prenatal therapy to prevent infection and disease in infants whose mothers acquired CMV during pregnancy (69). Randomized trials are ongoing to test this potential application more rigorously.
Prevention
The transmission patterns of CMV suggest several ways to prevent primary CMV acquisition. Transmission by sexual secretions can be prevented by use of condoms (111). The risk of transmission via saliva (e.g., child-to-mother transmission) can be reduced by handwashing and gloves (112). Transmission via blood transfusion or organ transplantation is almost completely preventable by leukocyte reduction techniques applied to the blood products or by donor selection, respectively (75). The strength of the evidence that these measures are effective and feasible on a population base varies, with the strongest evidence existing for reducing CMV transmission via the blood supply.
The development of vaccines has been a primary goal for controlling CMV. Indeed, an Institute of Medicine report declared that development of a CMV vaccine should be a top priority (113). After decades of development and incremental advances (114), the recent report that a subunit vaccine, consisting of CMV gB with MF59 adjuvant, reduced acquisition of CMV in seronegative mothers who had recently given birth (68) was a major advance. However, this vaccine reduced CMV acquisition by only 50%, possibly because the vaccine could not induce antibodies that prevent entry into endothelial and epithelial cells (40–42). Other approaches to vaccine development are being studied, including chimeric live-attenuated vaccines, DNA vaccines, and alphavirus replicons encoding CMV proteins (115). The recent finding that rhesus macaques mount immune responses to antigens delivered by repeated sequential inoculation of rhesus CMV recombinants highlights a potential role for live CMV vaccine vectors, but also illustrates the challenges in developing a vaccine that can block CMV infection itself (116).
Future directions
Decades of CMV research have resulted in remarkable advances in our understanding of basic CMV biology and mechanisms of immunologic control. However, many questions remain about the functions of numerous CMV genes, the mechanism of latency, and the pathogenetic processes that account for differing disease manifestations in various clinical settings. Nonetheless, enormous progress has been made in diagnostics, drug therapy, immunotherapy, and vaccine development. At the same time, it has become apparent that the spectrum of CMV morbidity may be much larger than originally appreciated. One task for the future will be to assess conclusively whether CMV is a pathogen or bystander in critically ill pediatric and adult patients as well as in other diseases such as atherosclerosis, inflammatory bowel disease, and tumors, including glioblastoma multiforme. The possible role of CMV in immunosenescence is particularly interesting and will be a field of active research for years to come. New therapeutics with improved toxicity profiles are urgently needed, not only for transplant recipients, but also for congenital disease and possibly for future new indications in immunocompetent persons. Meanwhile, management strategies with currently available drugs should be optimized. The field of adoptive T cell immunotherapy is also well on its way to overcoming obstacles that have prevented widespread application, including the time needed to generate sufficient numbers of T cells and failure to restore persisting T cell immunity in the presence of high-dose steroids (117). Finally, exciting developments toward a CMV vaccine, arguably the holy grail of prevention, are underway (68). Thus, myriad challenges remain, but judging from recent progress, we suspect that major advances are on the horizon.
Acknowledgments
The authors thank Elaine Chuang, Christina Marra, Ajit Limaye, and George McDonald for providing images and David Myerson for helpful discussions. We also acknowledge the NIH for grant support (HL093294, HL102547, and CA18029 to M. Boeckh; AI26672 to A.P. Geballe).
Footnotes
Conflict of interest: M. Boeckh received research funding from Roche Laboratories, Vical Inc., Viropharma Inc., and Chimerix Inc.
Citation for this article: J Clin Invest. 2011;121(5):1673–1680. doi:10.1172/JCI45449.
References
- 1.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: The national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: Implications for congenital CMV. Rev Med Virol. 2010;20(5):311–326. doi: 10.1002/rmv.659. [DOI] [PubMed] [Google Scholar]
- 3.Ljungman P, Brandan R. Factors influencing cytomegalovirus seropositivity in stem cell transplant patients and donors. Haematologica. 2007;92(8):1139–1142. doi: 10.3324/haematol.11061. [DOI] [PubMed] [Google Scholar]
- 4.Souza MA, Passos AM, Treitinger A, Spada C. Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil. Rev Soc Bras Med Trop. 2010;43(4):359–361. doi: 10.1590/S0037-86822010000400004. [DOI] [PubMed] [Google Scholar]
- 5.Rippert H. Uber protozoenartige Zellen in der Niere eines syphilitischen Neugoborenen und in der Parotis von Kindern. Zentralbl Allg Pathol. 1904;15:945–948. [Google Scholar]
- 6.Gleaves CA, Smith TF, Shuster EA, Pearson GR. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chee MS, et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. In: McDougall, JK ed.Cytomegaloviruses . New York, New York, USA: Springer-Verlag; 1990:125–169. [DOI] [PubMed] [Google Scholar]
- 8.DeFilippis V, Fruh K. Rhesus cytomegalovirus particles prevent activation of interferon regulatory factor 3. J Virol. 2005;79(10):6419–6431. doi: 10.1128/JVI.79.10.6419-6431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. Complete sequence and genomic analysis of rhesus cytomegalovirus. J Virol. 2003;77(12):6620–6636. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue Y, Barry PA. Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus. Adv Virus Res. 2008;72:207–226. doi: 10.1016/S0065-3527(08)00405-3. [DOI] [PubMed] [Google Scholar]
- 11. Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, eds.Field’s Virology . 5th ed. Philadelphia, Pennsylvania, USA: Lippincott/Williams and Wilkins Co.; 2006:2701–2772. [Google Scholar]
- 12. Shenk TE, Stinski MF, eds.Human Cytomegalovirus (Current Topics in Microbiology and Immunology) . Heidelberg, Germany: Springer; 2008. [PubMed] [Google Scholar]
- 13.Fannin Rider PJ, Dunn W, Yang E, Liu F. Human cytomegalovirus microRNAs. Curr Top Microbiol Immunol. 2008;325:21–39. doi: 10.1007/978-3-540-77349-8_2. [DOI] [PubMed] [Google Scholar]
- 14.Kulesza CA, Shenk T. Human cytomegalovirus 5-kilobase immediate-early RNA is a stable intron. J Virol. 2004;78(23):13182–13189. doi: 10.1128/JVI.78.23.13182-13189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McSharry BP, Tomasec P, Neale ML, Wilkinson GW. The most abundantly transcribed human cytomegalovirus gene (beta 2.7) is non-essential for growth in vitro. J Gen Virol. 2003;84(pt 9):2511–2516. doi: 10.1099/vir.0.19298-0. [DOI] [PubMed] [Google Scholar]
- 16.McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006;117(1):90–104. doi: 10.1016/j.virusres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Rawlinson WD, Farrell HE, Barrell BG. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70(12):8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lurain NS, et al. Analysis of the human cytomegalovirus genomic region from UL146 through UL147A reveals sequence hypervariability, genotypic stability, and overlapping transcripts. Virol J. 2006;3:4. doi: 10.1186/1743-422X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dargan DJ, et al. Sequential mutations associated with adaptation of human cytomegalovirus to growth in cell culture. J Gen Virol. 2010;91(pt 6):1535–1546. doi: 10.1099/vir.0.018994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley AJ, et al. High-throughput sequence analysis of variants of human cytomegalovirus strains Towne and AD169. J Gen Virol. 2009;90(pt 10):2375–2380. doi: 10.1099/vir.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70(1):78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prichard MN, Penfold ME, Duke GM, Spaete RR, Kemble GW. A review of genetic differences between limited and extensively passaged human cytomegalovirus strains. Rev Med Virol. 2001;11(3):191–200. doi: 10.1002/rmv.315. [DOI] [PubMed] [Google Scholar]
- 23.Stanton RJ, et al. Reconstruction of the complete human cytomegalovirus genome in a bac reveals RL13 to be a potent inhibitor of replication. J Clin Invest. 2010;120(9):3191–3208. doi: 10.1172/JCI42955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaacson MK, Juckem LK, Compton T. Virus entry and innate immune activation. Curr Top Microbiol Immunol. 2008;325:85–100. doi: 10.1007/978-3-540-77349-8_5. [DOI] [PubMed] [Google Scholar]
- 25.Soroceanu L, Akhavan A, Cobbs CS. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature. 2008;455(7211):391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 26.Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 27.Hahn G, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78(18):10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80(2):710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol. 2005;79(16):10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol. 2010;84(1):585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall EE, Geballe AP. Multifaceted evasion of the interferon response by cytomegalovirus. J Interferon Cytokine Res. 2009;29(9):609–619. doi: 10.1089/jir.2009.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scalzo AA, Yokoyama WM. CMV1 and natural killer cell responses to murine cytomegalovirus infection. Curr Top Microbiol Immunol. 2008;321:101–122. doi: 10.1007/978-3-540-75203-5_5. [DOI] [PubMed] [Google Scholar]
- 33.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 34.Lilleri D, Fornara C, Chiesa A, Caldera D, Alessandrino EP, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in adult allogeneic hematopoietic stem cell transplant recipients and immune control of viral infection. Haematologica. 2008;93(2):248–256. doi: 10.3324/haematol.11912. [DOI] [PubMed] [Google Scholar]
- 35.Emery VC, Einsele H, Atabani S, Haque T. Immunotherapy and vaccination after transplant: The present, the future. Infect Dis Clin North Am. 2010;24(2):515–529. doi: 10.1016/j.idc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Walter EA, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 37.Sylwester AW, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manley TJ, Luy L, Jones T, Boeckh M, Mutimer H, Riddell SR. Immune evasion proteins of human cytomegalovirus do not prevent a diverse CD8+ cytotoxic T-cell response in natural infection. Blood. 2004;104(4):1075–1082. doi: 10.1182/blood-2003-06-1937. [DOI] [PubMed] [Google Scholar]
- 39.Moss P. The emerging role of cytomegalovirus in driving immune senescence: A novel therapeutic opportunity for improving health in the elderly. Curr Opin Immunol. 2010;22(4):529–534. doi: 10.1016/j.coi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine. 2008;26(45):5760–5766. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerna G, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89(pt 4):853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 42.Macagno A, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol. 2010;84(2):1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormick AL. Control of apoptosis by human cytomegalovirus. Curr Top Microbiol Immunol. 2008;325:281–295. doi: 10.1007/978-3-540-77349-8_16. [DOI] [PubMed] [Google Scholar]
- 44.Powers C, DeFilippis V, Malouli D, Fruh K. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol. 2008;325:333–359. doi: 10.1007/978-3-540-77349-8_19. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson GW, et al. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol. 2008;41(3):206–212. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nachmani D, Lankry D, Wolf DG, Mandelboim O. The human cytomegalovirus microrna mir-UL112 acts synergistically with a cellular microRNA to escape immune elimination. Nat Immunol. 2010;11(9):806–813. doi: 10.1038/ni.1916. [DOI] [PubMed] [Google Scholar]
- 47.Beisser PS, Lavreysen H, Bruggeman CA, Vink C. Chemokines and chemokine receptors encoded by cytomegaloviruses. Curr Top Microbiol Immunol. 2008;325:221–242. doi: 10.1007/978-3-540-77349-8_13. [DOI] [PubMed] [Google Scholar]
- 48.Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J Virol. 2009;83(19):9618–9629. doi: 10.1128/JVI.01098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noda S, et al. Cytomegalovirus MCK-2 controls mobilization and recruitment of myeloid progenitor cells to facilitate dissemination. Blood. 2006;107(1):30–38. doi: 10.1182/blood-2005-05-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vomaske J, et al. Differential ligand binding to a human cytomegalovirus chemokine receptor determines cell type-specific motility. PLoS Pathog. 2009;5(2):e1000304. doi: 10.1371/journal.ppat.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunn W, et al. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A. 2003;100(24):14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu D, Silva MC, Shenk T. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc Natl Acad Sci U S A. 2003;100(21):12396–12401. doi: 10.1073/pnas.1635160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Limaye AP, Boeckh M. Cmv in critically ill patients: Pathogen or bystander? Rev Med Virol. 2010;20(6):372–379. doi: 10.1002/rmv.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier J, Lienicke U, Tschirch E, Kruger DH, Wauer RR, Prosch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005;43(3):1318–1324. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fishman JA, et al. Cytomegalovirus in transplantation - challenging the status quo. Clin Transplant. 2007;21(2):149–158. doi: 10.1111/j.1399-0012.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 56.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543–558. doi: 10.1016/S1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 57.Telfer S, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330(6001):243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barton ES, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447(7142):326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 59.Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:297–313. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- 60.Kurz S, Steffens HP, Mayer A, Harris JR, Reddehase MJ. Latency versus persistence or intermittent recurrences: Evidence for a latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71(4):2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wald A, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342(12):844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 62.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338(24):1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 63.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 64.Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: A quantitative assessment. J Clin Virol. 2008;41(2):57–62. doi: 10.1016/j.jcv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 66.Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis. 2010;201(3):386–389. doi: 10.1086/649903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliver SE, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol. 2009;46 suppl 4:S22–S26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pass RF, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353(13):1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 70.Boppana SB, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurath S, Halwachs-Baumann G, Muller W, Resch B. Transmission of cytomegalovirus via breast milk to the prematurely born infant: A systematic review. Clin Microbiol Infect. 2010;16(8):1172–1178. doi: 10.1111/j.1469-0691.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 72. Hakki M, Geballe AP. Cytomegalovirus. In: Holmes, KK, et al., eds.Sexually Transmitted Diseases . 4th ed. New York, New York, USA: McGraw-Hill; 2008:439–451. [Google Scholar]
- 73.Cunha BA. Cytomegalovirus pneumonia: Community-acquired pneumonia in immunocompetent hosts. Infect Dis Clin North Am. 2010;24(1):147–158. doi: 10.1016/j.idc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24(2):319–337. doi: 10.1016/j.idc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Nichols WG, Price TH, Gooley T, Corey L, Boeckh M. Transfusion-transmitted cytomegalovirus infection after receipt of leukoreduced blood products. Blood. 2003;101(10):4195–4200. doi: 10.1182/blood-2002-10-3143. [DOI] [PubMed] [Google Scholar]
- 76.Matsumura T, et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biol Blood Marrow Transplant. 2007;13(5):577–583. doi: 10.1016/j.bbmt.2006.12.454. [DOI] [PubMed] [Google Scholar]
- 77.O’Brien S, et al. Valganciclovir prevents cytomegalovirus reactivation in patients receiving alemtuzumab-based therapy. Blood. 2008;111(4):1816–1819. doi: 10.1182/blood-2007-03-080010. [DOI] [PubMed] [Google Scholar]
- 78.Pollack M, et al. An international comparison of current strategies to prevent herpesvirus and fungal infections in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2010.07.026. [published online ahead of print August 7, 2010]. doi:10.1016/j.bbmt.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: A randomized double-blind study. Blood. 1996;88(10):4063–4071. [PubMed] [Google Scholar]
- 80.Palmer SM, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: A randomized, controlled trial. Ann Intern Med. 2010;152(12):761–769. doi: 10.7326/0003-4819-152-12-201006150-00003. [DOI] [PubMed] [Google Scholar]
- 81.Paya C, et al. Efficacy and safety of valganciclovir vs. Oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4(4):611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 82.Sun HY, Cacciarelli TV, Wagener MM, Singh N. Preemptive therapy for cytomegalovirus based on real-time measurement of viral load in liver transplant recipients. Transpl Immunol. 2010;23(4):166–169. doi: 10.1016/j.trim.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Limaye AP, et al. Late-onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation. 2004;78(9):1390–1396. doi: 10.1097/01.TP.0000145989.22373.03. [DOI] [PubMed] [Google Scholar]
- 84.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS:1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. 2009;37(8):2350–2358. doi: 10.1097/CCM.0b013e3181a3aa43. [DOI] [PubMed] [Google Scholar]
- 86.Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: Pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16(9):1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 87.Mitchell DA, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10(1):10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116(1):79–86. doi: 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pawelec G, et al. Immunosenescence and cytomegalovirus: Where do we stand after a decade? Immun Ageing. 2010;7:13. doi: 10.1186/1742-4933-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caposio P, Orloff SL, Streblow DN. The role of cytomegalovirus in angiogenesis. Virus Res. doi: 10.1016/j.virusres.2010.09.011. [published online ahead of print October 1, 2010]. doi:10.1016/j.virusres.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23(4):689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hodson EM, Craig JC, Strippoli GF, Webster AC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008;(2):CD003774. doi: 10.1002/14651858.CD003774.pub3. [DOI] [PubMed] [Google Scholar]
- 93.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: The efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143(12):870–880. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 94.Jabs DA. The ganciclovir implant plus oral ganciclovir versus parenteral cidofovir for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: The ganciclovir cidofovir cytomegalovirus retinitis trial. Am J Ophthalmol. 2001;131(4):457–467. doi: 10.1016/S0002-9394(01)00840-6. [DOI] [PubMed] [Google Scholar]
- 95.Reusser P, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002;99(4):1159–1164. doi: 10.1182/blood.V99.4.1159. [DOI] [PubMed] [Google Scholar]
- 96.Lowance D, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International valacyclovir cytomegalovirus prophylaxis transplantation study group. N Engl J Med. 1999;340(19):1462–1470. doi: 10.1056/NEJM199905133401903. [DOI] [PubMed] [Google Scholar]
- 97. Isis Pharmaceuticals. Inflammation and Other Diseases Website. http://www.isispharm.com/Pipeline/Therapeutic-Areas/Inflammatory-Disease.htm#Vitravene . Accessed February 17, 2011. [Google Scholar]
- 98.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bidanset DJ, Beadle JR, Wan WB, Hostetler KY, Kern ER. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J Infect Dis. 2004;190(3):499–503. doi: 10.1086/421912. [DOI] [PubMed] [Google Scholar]
- 100.Lischka P, et al. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother. 2010;54(3):1290–1297. doi: 10.1128/AAC.01596-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winston DJ, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111(11):5403–5410. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. ViroPharma, Inc. Viropharma Reports Results of Phase 3 Clinical Trial for Maribavir in Bone Marrow Transplant Patients Web Site. http://phx.corporate-ir.net/phoenix.zhtml?c=92320&P=irol-newsArticle&ID=1254507&highlight . Accessed February 17, 2011. [Google Scholar]
- 103.Eid AJ, Razonable RR. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010;70(8):965–981. doi: 10.2165/10898540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 104.Waldman WJ, et al. Novel mechanism of inhibition of cytomegalovirus by the experimental immunosuppressive agent leflunomide. Transplantation. 1999;68(6):814–825. doi: 10.1097/00007890-199909270-00014. [DOI] [PubMed] [Google Scholar]
- 105.Avery RK, et al. Utility of leflunomide in the treatment of complex cytomegalovirus syndromes. Transplantation. 2010;90(4):419–426. doi: 10.1097/TP.0b013e3181e94106. [DOI] [PubMed] [Google Scholar]
- 106.Travi G, et al. The effect of imatinib on cytomegalovirus reactivation in hematopoietic cell transplantation. Clin Infect Dis. 2009;49(11):e120–e123. doi: 10.1086/648120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feuchtinger T, et al. Adoptive transfer of pp–65 specific T–cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 108.Hodson EM, Jones CA, Strippoli GF, Webster AC, Craig JC. Immunoglobulins, vaccines or interferon for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2007;(2):CD005129. doi: 10.1002/14651858.CD005129.pub2. [DOI] [PubMed] [Google Scholar]
- 109.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: Systematic review and meta-analysis. J Clin Oncol. 2009;27(5):770–781. doi: 10.1200/JCO.2008.16.8450. [DOI] [PubMed] [Google Scholar]
- 110.Bonaros N, Mayer B, Schachner T, Laufer G, Kocher A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: A meta-analysis. Clin Transplant. 2008;22(1):89–97. doi: 10.1111/j.1399-0012.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 111.Robain M, Carre N, Dussaix E, Salmon-Ceron D, Meyer L. Incidence and sexual risk factors of cytomegalovirus seroconversion in HIV-infected subjects. The SEROCO study group. Sex Transm Dis. 1998;25(9):476–480. doi: 10.1097/00007435-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 112.Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J Pediatr. 2004;145(4):485–491. doi: 10.1016/j.jpeds.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 113. Stratton K, Durch JS, Lawrence RS, eds.Vaccines for the 21st Century: A Tool for Decision Making . Washington, DC, USA: National Academy Press; 2000. [PubMed] [Google Scholar]
- 114.Schleiss MR. Cytomegalovirus vaccine development. Curr Top Microbiol Immunol. 2008;325:361–382. doi: 10.1007/978-3-540-77349-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schleiss MR. Cytomegalovirus vaccines: At last, a major step forward. Herpes. 2009;15(3):44–45. [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen SG, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328(5974):102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr Opin Immunol. 2009;21(2):224–232. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dolan A, et al. Genetic content of wild-type human cytomegalovirus. J Gen Virol. 2004;85(pt 5):1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 119. Holmes K, et al., eds.Sexually Transmitted Diseases. 3rd ed. New York, New York, USA: McGraw Hill; 1999. [Google Scholar]
- 120.Martin DF, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346(15):1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 121.Palestine AG, et al. A randomized, controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Ann Intern Med. 1991;115(9):665–673. doi: 10.7326/0003-4819-115-9-665. [DOI] [PubMed] [Google Scholar]
- 122.Lalezari JP, et al. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. A randomized, controlled trial. Ann Intern Med. 1997;126(4):257–263. doi: 10.7326/0003-4819-126-4-199702150-00001. [DOI] [PubMed] [Google Scholar]
- 123.Ljungman P, et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The infectious diseases working party of the European group for blood and marrow transplantation. Blood. 2001;97(2):388–392. doi: 10.1182/blood.V97.2.388. [DOI] [PubMed] [Google Scholar]
- 124.The Vitravene Study Group. A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol. 2002;133(4):467–474. doi: 10.1016/S0002-9394(02)01327-2. [DOI] [PubMed] [Google Scholar]