Abstract
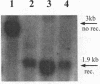
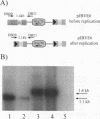
Vectors for gene transfer and gene therapy were developed which combine the advantages of the integrase and recombinase systems. This was achieved by inserting two loxP sites for specific DNA excision into an MESV based retroviral vector. We show that this 'retroviral lox system' allows the infection of cells and the expression of transferred genes. In addition, we constructed an efficient retrovirus-based expression system for a modified Cre recombinase. Functional tests for DNA excision from integrated retroviral lox vectors were performed by the use of a negative selectable marker gene (thymidine kinase). Cre expression in cells infected with retroviral lox vectors and subsequent BrdU selection for cells in which site-specific recombination has occurred results in large numbers of independent cell clones. These results were confirmed by detailed molecular analysis. In addition we developed retroviral suicide vectors in which the enhancer/promoter elements of both LTRs were replaced by lox sequences. We show that lox-sequences located in the LTRs of retroviral vectors are stable during retroviral replication. Potential applications of this system would be the establishment of revertants of retrovirus-infected cells by controlled excision of nearly the complete proviral DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abremski K., Hoess R. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein. J Biol Chem. 1984 Feb 10;259(3):1509–1514. [PubMed] [Google Scholar]
- Abremski K., Hoess R., Sternberg N. Studies on the properties of P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell. 1983 Apr;32(4):1301–1311. doi: 10.1016/0092-8674(83)90311-2. [DOI] [PubMed] [Google Scholar]
- Albert H., Dale E. C., Lee E., Ow D. W. Site-specific integration of DNA into wild-type and mutant lox sites placed in the plant genome. Plant J. 1995 Apr;7(4):649–659. doi: 10.1046/j.1365-313x.1995.7040649.x. [DOI] [PubMed] [Google Scholar]
- Baubonis W., Sauer B. Genomic targeting with purified Cre recombinase. Nucleic Acids Res. 1993 May 11;21(9):2025–2029. doi: 10.1093/nar/21.9.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C., Forster P., Hegewisch-Becker S., Harbers K. An optimized electroporation protocol applicable to a wide range of cell lines. Biotechniques. 1994 Dec;17(6):1058–1062. [PubMed] [Google Scholar]
- Beck-Engeser G., Stocking C., Just U., Albritton L., Dexter M., Spooncer E., Ostertag W. Retroviral vectors related to the myeloproliferative sarcoma virus allow efficient expression in hematopoietic stem and precursor cell lines, but retroviral infection is reduced in more primitive cells. Hum Gene Ther. 1991 Spring;2(1):61–70. doi: 10.1089/hum.1991.2.1-61. [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Madden K. R. Use of site-specific recombination to regenerate selectable markers. Mol Gen Genet. 1989 Oct;219(1-2):320–323. doi: 10.1007/BF00261194. [DOI] [PubMed] [Google Scholar]
- Dale E. C., Ow D. W. Intra- and intermolecular site-specific recombination in plant cells mediated by bacteriophage P1 recombinase. Gene. 1990 Jul 2;91(1):79–85. doi: 10.1016/0378-1119(90)90165-n. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Müller W., Guy-Grand D., Fischer A., Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic K. G., Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989 Nov 3;59(3):499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Grez M., Zörnig M., Nowock J., Ziegler M. A single point mutation activates the Moloney murine leukemia virus long terminal repeat in embryonal stem cells. J Virol. 1991 Sep;65(9):4691–4698. doi: 10.1128/jvi.65.9.4691-4698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Marth J. D., Orban P. C., Mossmann H., Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994 Jul 1;265(5168):103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Hwang L. H., Gilboa E. Expression of genes introduced into cells by retroviral infection is more efficient than that of genes introduced into cells by DNA transfection. J Virol. 1984 May;50(2):417–424. doi: 10.1128/jvi.50.2.417-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilby N. J., Snaith M. R., Murray J. A. Site-specific recombinases: tools for genome engineering. Trends Genet. 1993 Dec;9(12):413–421. doi: 10.1016/0168-9525(93)90104-p. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M., Sauer B., Mosinger B., Jr, Lee E. J., Manning R. W., Yu S. H., Mulder K. L., Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. M., Davis R. W. Functional expression of the yeast FLP/FRT site-specific recombination system in Nicotiana tabacum. Mol Gen Genet. 1994 Mar;242(6):653–657. doi: 10.1007/BF00283419. [DOI] [PubMed] [Google Scholar]
- Lyznik L. A., Mitchell J. C., Hirayama L., Hodges T. K. Activity of yeast FLP recombinase in maize and rice protoplasts. Nucleic Acids Res. 1993 Feb 25;21(4):969–975. doi: 10.1093/nar/21.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeser S., Kahmann R. The Gin recombinase of phage Mu can catalyse site-specific recombination in plant protoplasts. Mol Gen Genet. 1991 Nov;230(1-2):170–176. doi: 10.1007/BF00290665. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McLachlin J. R., Cornetta K., Eglitis M. A., Anderson W. F. Retroviral-mediated gene transfer. Prog Nucleic Acid Res Mol Biol. 1990;38:91–135. doi: 10.1016/s0079-6603(08)60709-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolten F. L., Cupples L. A. A model for predicting the risk of cancer consequent to retroviral gene therapy. Hum Gene Ther. 1992 Oct;3(5):479–486. doi: 10.1089/hum.1992.3.5-479. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell. 1992 Jun 12;69(6):939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- Mooslehner K., Karls U., Harbers K. Retroviral integration sites in transgenic Mov mice frequently map in the vicinity of transcribed DNA regions. J Virol. 1990 Jun;64(6):3056–3058. doi: 10.1128/jvi.64.6.3056-3058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. C., Schaub T. L., James A. A. FLP-mediated recombination in the vector mosquito, Aedes aegypti. Nucleic Acids Res. 1991 Nov 11;19(21):5895–5900. doi: 10.1093/nar/19.21.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- O'Gorman S., Fox D. T., Wahl G. M. Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science. 1991 Mar 15;251(4999):1351–1355. doi: 10.1126/science.1900642. [DOI] [PubMed] [Google Scholar]
- Odell J., Caimi P., Sauer B., Russell S. Site-directed recombination in the genome of transgenic tobacco. Mol Gen Genet. 1990 Sep;223(3):369–378. doi: 10.1007/BF00264442. [DOI] [PubMed] [Google Scholar]
- Onouchi H., Yokoi K., Machida C., Matsuzaki H., Oshima Y., Matsuoka K., Nakamura K., Machida Y. Operation of an efficient site-specific recombination system of Zygosaccharomyces rouxii in tobacco cells. Nucleic Acids Res. 1991 Dec 11;19(23):6373–6378. doi: 10.1093/nar/19.23.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. The retrovirus pol gene encodes a product required for DNA integration: identification of a retrovirus int locus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7885–7889. doi: 10.1073/pnas.81.24.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud G., Roux J., Pictet R., Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991 Nov 29;67(5):977–986. doi: 10.1016/0092-8674(91)90370-e. [DOI] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jun;7(6):2087–2096. doi: 10.1128/mcb.7.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Cre-stimulated recombination at loxP-containing DNA sequences placed into the mammalian genome. Nucleic Acids Res. 1989 Jan 11;17(1):147–161. doi: 10.1093/nar/17.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Vile R., Russell S. J. Gene transfer technologies for the gene therapy of cancer. Gene Ther. 1994 Mar;1(2):88–98. [PubMed] [Google Scholar]
- Whitcomb J. M., Hughes S. H. Retroviral reverse transcription and integration: progress and problems. Annu Rev Cell Biol. 1992;8:275–306. doi: 10.1146/annurev.cb.08.110192.001423. [DOI] [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]