Abstract
Several micro RNAs (miRNAs) have the ability to inhibit HIV replication in target cells. Thus, we investigated the impact of opioids (morphine and heroin), widely abused drugs among people infected with HIV, on the expression of cellular anti-HIV miRNAs in monocytes. We found that morphine-treated monocytes expressed lower levels of cellular anti-HIV miRNAs than untreated cells. In addition, morphine treatment of monocytes compromised type I interferon (IFN)–induced anti-HIV miRNA expression. These findings paralleled the observation that morphine treatment of monocytes enhanced HIV replication. These morphine-mediated actions on the anti-HIV miRNAs and HIV could be antagonized by the opioid receptor antagonists (naltrexone or Cys2, Tyr3, Arg5, Pen7-amide). Furthermore, the in vitro impact of morphine on miRNA expression was confirmed by the in vivo observation that heroin-dependent subjects had significantly lower levels of anti-HIV miRNAs (miRNA-28, 125b, 150, and 382) in peripheral blood mononuclear cells than the healthy subjects. These in vitro and in vivo findings indicate that opioid use impairs intracellular innate anti-HIV mechanism(s) in monocytes, contributing to cell susceptibility to HIV infection.
The HIV epidemic is being driven by drug abusers in the United States and other regions in the world. Opioids are widely abused drugs in the United States. Opioid absers have a higher incidence of infectious diseases, which may be directly related to impaired immune functions.1–6 Opioids, such as morphine, exert a profound influence on immunomodulatory activity. The administration of morphine to rodents suppresses a variety of immune responses that involve the major cell types of the immune system, including natural killer cells, T cells, B cells, monocytes, macrophages, and polymorphonuclear leukocytes.7 Morphine is known to inhibit specific immunocyte activities, such as monocyte respiratory burst,8 chemotaxis,9 and phagocytosis.10 Morphine induces apoptosis of macrophages and microglia.11,12 Morphine decreases the levels of IFN-γ and interleukin-2 in human T cells.13 Morphine, through the suppression of IFN-γ, compromised CD8+ T-cell–mediated noncytolytic anti-HIV activity in both acute and latently infected immune cells.14 Morphine induces the expression of HIV entry coreceptors (β-chemokine receptor 5 and α-chemokine receptor 4) in the immune cells and facilitates HIV replication in vitro.15–19 In addition, several in vivo studies suggested that opioid use may promote HIV disease progression.20–22 These in vitro and in vivo findings indicate that opioids can function as cofactors in the immunopathogenesis of HIV disease.
Although it is known that opioids, such as morphine, exert a profound influence on immunomodulating activity and enhance HIV infection and replication, the mechanism(s) of their actions remain to be determined. In particular, it is unknown whether opioid abuse has a negative impact on host cell intracellular innate immunity that plays a crucial role in the suppression of HIV replication in the target cells. A key facet of innate defense mechanisms against retroviruses, such as HIV, is the presence of intracellular viral restriction factors. The micro RNAs (miRNAs) belong to this group of restriction factors. Several cellular miRNAs (miRNA-28, miRNA-29a, miRNA-125b, miRNA-150, miRNA-198, miRNA-223, and miRNA-382) target a set of accessory genes of HIV.23–27 For example, these miRNAs can target the 3′-UTR of HIV transcripts,28 potentially rendering productive infection of HIV into latency in resting CD4+ T lymphocytes. Recently, it was reported that monocytes express significantly higher levels of anti-HIV miRNAs than donor-matched macrophages, thereby unraveling the refractory nature of monocytes to HIV infection.23 In addition to their direct effect on HIV replication, miRNAs also play crucial roles in regulating the host innate immune defense that relies on phagocytes, such as monocytes and macrophages.29 In this study, we investigated the in vitro effect of morphine on the expression of cellular anti-HIV miRNAs in monocytes. In parallel, we examined the in vitro effect of morphine on HIV infection of monocytes. Furthermore, we also determined the in vivo impact of heroin use on the expression of the anti-HIV miRNAs in peripheral blood mononuclear cells (PBMCs).
Materials and Methods
Study Subjects
The study subjects addicted to heroin were recruited by the Wuhan Center for Disease Prevention and Control in the People's Republic of China. Informed consent was obtained from the study subjects, and the Institutional Review Board of the Wuhan Center for Disease Prevention and Control approved this study. Peripheral blood mononuclear cells were obtained from seven heroin-dependent patients and eight healthy donors in the same age range (22 to 55 years). The heparinized blood samples from the subjects were identified as HIV antibody negative by anonymous testing on the basis of enzyme-linked immunosorbent assay (Beckman Coulter, Inc, Hialeah, FL). Subjects were excluded if they had a chronic systemic illness (cardiac, renal, pulmonary, hepatic, endocrine, metabolic, or autoimmune disorders) or a major psychiatric disorder or if they were abusing substances other than heroin (as determined by urine drug test result). For women, pregnancy was also a reason for exclusion. The control subjects were recruited using convenience sampling from the community in which the study site was located. Control subjects with no history of drug or alcohol abuse were also excluded if they had major medical or psychiatric disorders.
Cell Culture
Purified monocytes were purchased from the Center for AIDS Research, University of Pennsylvania, Philadelphia, PA. The center has Institutional Review Board approval for blood collection from healthy donors. Monocytes were isolated by elutriation, and the purity of isolated monocytes was higher than 95%. Specifically, the purified monocyte population (CD14+) contained a low level of other cell types: CD19+ B cells, 0.1%; CD3+ T cells, 0.4%; and CD56+ natural killer cells, 2%. Blood samples were screened for the common blood-borne pathogens and certified to be pathogen free. Freshly isolated monocytes were cultured in 96-well culture plates at a density of 105 cells per well or in 48-well culture plates at a density of 2.5 × 105 cells per well in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. For in vivo study, PBMCs were isolated from the study participants (the heroin-dependant and control subjects) by standard Ficoll-Paque density gradient centrifugation, according to a previously described technique.14 Total cellular RNA extracted from PBMCs (2 × 106 cells) with a reagent (Tri-reagent; Molecular Research Center, Cincinnati, OH) was subjected to miRNA analysis by real-time RT-PCR.23
Reagents
Morphine sulfate was obtained from Elkins-Sinn, Inc, Cherry Hill, NJ; and naltrexone was obtained from Sigma, St. Louis, MO. The CTAP (Cys2, Tyr3, Arg5, Pen7-amide), a specific μ-opioid receptor antagonist, was obtained from Phoenix Pharmaceutical, Inc, Mountain View, CA; and recombinant human IFN-α and IFN-β were obtained from PBL Biomedical Laboratories, Piscataway, NJ.
Pseudotyped HIV Infection Assay
The HIV virions pseudotyped with the envelope from amphotropic murine leukemia virus (MLV) (HIV entry receptor independent) were used to study the impact of morphine on viral replication. The plasmid encoding the MLV envelop (Env) gene was provided by John Moore (Aaron Diamond AIDS Research Center, New York, NY). The Env-deleted luciferase reporter gene containing plasmid (PNL-Luc-E-R+) was cotransfected into 293 T cells along with the plasmids encoding the MLV Env genes, as previously described.30 Pseudotyped HIV was prepared as previously described.31 Fresh monocytes plated in 48-well plates (5 × 105 cells per well) were pretreated with morphine (10−12 to 10−8 mol/L) for 3 hours. The cells were then infected with the pseudotyped HIV (p24, 20 ng/ml) for 8 hours in the presence of polybrene (4 μg/ml). After washing with plain Dulbecco's modified Eagle's medium, morphine was added in the culture. The cells were lysed in 100 μL of ×1 reporter lysis buffer (Promega Corp, Madison, WI) 72 hours after infection. Lysate (50 μL) was mixed with 100 μL of luciferase substrate (Promega Corp), and luciferase activity was then determined (TD-20/20 Luminometer; Turner Designs, Sunnyvale, CA).
In Vitro HIV Infection of Monocytes
For in vitro HIV infection experiments, freshly isolated monocytes were treated with or without morphine at different concentrations for 3 hours before HIV (Bal strain) infection (p24, 60 ng/106 cells) for 16 hours. In the experiments designed to determine whether naltrexone or CTAP antagonized the morphine action, naltrexone (10−8 mol/L) or CTAP (10−8 mol/L) was added to monocyte cultures 1 hour before morphine treatment. Culture supernatants were collected for HIV RT activity31,32 and RNA extraction for real-time RT-PCR of HIV GAG and LTR at 72 hours after infection.
Real-Time RT-PCR
Total cellular RNA, including miRNA, was extracted from cells using a kit (miRNeasy Mini Kit; QIAGEN, Valencia, CA). Total RNA (1 μg) was reverse transcribed with another kit (miScript Reverse Transcription Kit; QIAGEN). The real-time RT-PCR for the quantification of a subset of miRNAs (miRNA-28, miRNA-125b, miRNA-150, miRNA-382, miRNA-223, miRNA-122, and miRNA-296) was performed with a kit (miScript Primer Assays and miScript SYBR Green PCR Kit; QIAGEN), as previously described.23 Real-time RT-PCR for the quantification of glyceraldehyde-3-phosphate dehydrogenase mRNA was performed with a mix (iQ SYBR Green Supermix; Bio-Rad Laboratories, Hercules, CA), as previously described.33,34 The levels of glyceraldehyde-3-phosphate dehydrogenase mRNA were used as an endogenous reference to normalize the quantities of target mRNA. Extracellular HIV GAG and LTR expression levels were also detected by real-time RT-PCR.28,35 The specific oligonucleotide primers used were as follows: GAG, 5′-ATAATCCACCTATCCCAGTAGGAGAAA-3′ (sense) and 5′-TTTGGTCCTTGTCTTATGTCCAGAATGC-3′ (antisense); and LTR, 5′-TCTCTCTGGTTAGACCAGATCTG-3′ (sense) and 5′-ACTGCTAGAGATTTTCCACACTG-3′ (antisense).
Statistical Analysis
Where appropriate, data were expressed as mean ± SD of triplicate cultures. For comparison of the mean of two groups (treated versus untreated), statistical significance was assessed by Student's t test. If there were more than two groups, one-way repeated measures of analysis of variance were used. Statistical analyses were performed with computer software (Graphpad Instat Statistical Software). Statistical significance was defined as P < 0.05.
Results
Morphine Enhances HIV Replication in Monocytes
We first determined the effect of morphine on HIV infection of monocytes. To minimize the involvement of monocyte-derived macrophages in the morphine action, the experiments with HIV infection were completed at 72 hours after infection. Morphine treatment enhanced the susceptibility of monocytes to infection with HIV Bal strain, as evidenced by increased RT activity at 72 hours after infection (Figure 1A). We also examined HIV GAG and LTR expression in monocytes treated with or without morphine. As demonstrated in Figure 1, B and C, morphine treatment increased the expression of these viral elements. The pretreatment of monocytes with the pan-opioid receptor antagonist (naltrexone) or the specific μ-opioid receptor antagonist (CTAP) completely abrogated the enhancing effect of morphine on HIV RT activity and the expression of GAG and LTR (Figure 1, A–C). To rule out the impact of morphine on HIV entry, we used an MLV (HIV receptor–independent) Env-pseudotyped HIV encoding a luciferase gene to infect monocytes. When infected with an MLV Env-pseudotyped virus, a significant increase in luciferase activity was observed in morphine-treated monocytes compared with untreated monocytes (Figure 1D).
Figure 1.
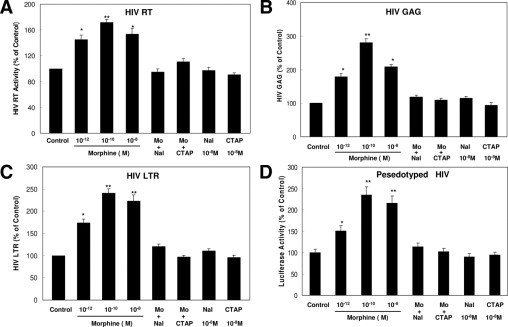
Effect of morphine (Mo) on HIV infection of monocytes. A to C: Morphine enhances HIV (Bal) infection of monocytes. Freshly isolated monocytes from healthy donors were incubated with or without Mo at indicated concentrations for 3 hours before HIV infection (p24, 60 ng/106 cells). An opioid receptor antagonist, naltrexone (Nal) (10−8 mol/L) or CTAP (10−8 mol/L), was added to monocyte cultures 1 hour before morphine (10−10 mol/L) treatment. The HIV RT activity (A), GAG (B), and LTR (C) expression levels in culture supernatant were determined at 72 hours after infection. D: Morphine enhances single-round HIV (MLV Env-pseudotyoped HIV) infection of monocytes. Freshly isolated monocytes were cultured in the presence or absence of morphine at indicated concentrations for 3 hours and then challenged with recombinant luciferase-encoding HIV pseudotype with MLV Env. An opioid receptor antagonist, Nal (10−8 mol/L) or CTAP (10−8 mol/L), was added to monocyte cultures 1 hour before Mo (10−10 mol/L) treatment. Luciferase activity was quantitated in the cell lysates 72 hours after infection. Data are given as mean ± SD of triplicate cultures representative of three experiments using cells from three different donors (*P < 0.05 and **P < 0.01 for Mo versus control, Mo, plus Nal or CTAP versus Mo only).
Morphine Inhibits Anti-HIV miRNA Expression in Monocytes
A recent study23 demonstrated that freshly isolated monocytes from human blood expressed significantly higher levels of cellular anti-HIV miRNAs (miRNA-28, miRNA-150, miRNA-223, and miRNA-382) than donor-matched macrophages. These miRNAs play a key role in suppressing HIV replication in monocytes and macrophages.23 Thus, we examined whether morphine had the ability to suppress these anti-HIV miRNAs in monocytes. Among the five known anti-HIV miRNAs, expression of four (miRNA-28, miRNA-125b, miRNA-150, and miRNA-382) was lower in monocytes treated with morphine compared with untreated cells (Figure 2, A and B). The highest inhibition by morphine was observed at a concentration of 10−10 mol/L (Figure 2B). In contrast, morphine treatment of monocytes had little effect on the expression of miRNA-223 (anti-HIV miRNA), miRNA-296 (anti–hepatitis C virus miRNA), and miRNA-122 (hepatitis C virus replication–required miRNA) (Figure 2A). Because morphine-mediated enhancement of HIV replication could be blocked by the opioid receptor antagonists (Figure 1), we hypothesized that the negative effect of morphine on anti-HIV miRNA expression in monocytes was also mediated through the opioid receptor. We showed that both naltrexone and CTAP completely abrogated the suppressing effect of morphine on anti-HIV miRNA expression (Figure 2B), whereas naltrexone or CTAP alone had little effect on anti-HIV miRNA expression (Figure 2B).
Figure 2.
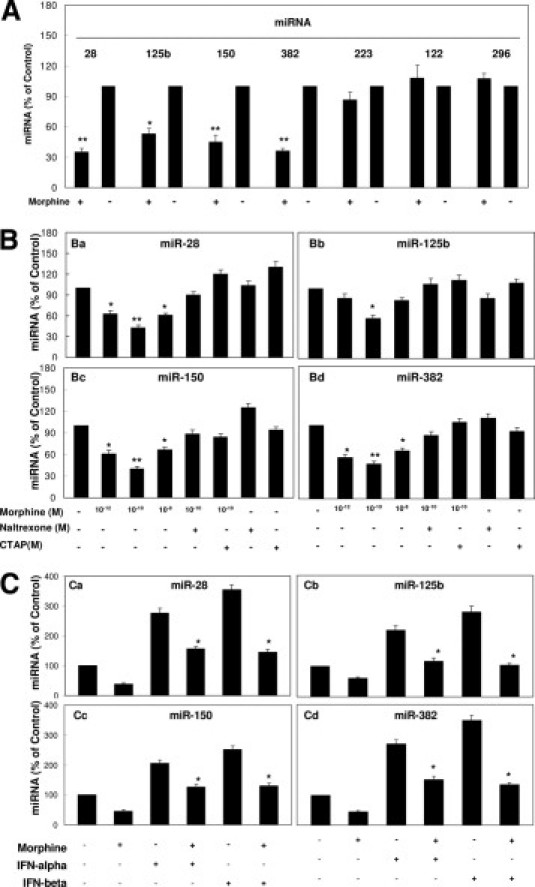
Effect of morphine on anti-HIV miRNA expression. A: Effect of morphine on miRNA expression of monocytes. Freshly isolated monocytes were cultured in the presence or absence of morphine at indicated concentrations for 3 hours. Total cellular RNA was then extracted from cell cultures and subjected to real-time RT-PCR for miRNA-28, miRNA-125b, miRNA-150, miRNA-382, miRNA-223, miRNA-122, and miRNA-296 expression. Data are given as mean ± SD of triplicate cultures representative of three experiments using cells from three different donors (*P < 0.05 and **P < 0.01 for morphine versus control). B: Morphine suppresses anti-HIV miRNA expression. Freshly isolated monocytes were cultured in the presence or absence of morphine at indicated concentrations for 3 hours. Naltrexone (10−8 mol/L) or CTAP (10−8 mol/L) was added to monocyte cultures for 1 hour before morphine (10−10 mol/L) treatment. Total cellular RNA was then extracted from cell cultures 3 hours after treatment and subjected to real-time RT-PCR for miRNA expression: miR-28 (a), miR-125b (b), miR-150 (c), and miR-382 (d). Data are given as mean ± SD of triplicate cultures representative of three experiments using cells from three different donors (*P < 0.05 and **P < 0.01 for morphine versus control, morphine plus naltrexone versus morphine only, or morphine plus CTAP versus morphine only). C: Morphine suppresses IFN-α/β–induced anti-HIV miRNA expression. Freshly isolated peripheral blood monocytes were cultured in the presence or absence of morphine (10−10 mol/L) for 24 hours before IFN-α/β (100 U/ml) treatment for 6 hours. RNA extracted from cells was subjected to real-time RT-PCR for miRNA expression, as indicated. Data are given as the mean ± SD of triplicate cultures representative of three experiments using cells from three different donors (*P < 0.05 and **P < 0.01 for morphine versus control or morphine plus IFN-α/β versus IFN-α/β only).
Morphine Inhibits IFN-α/β–Induced Anti-HIV miRNAs
Type I IFNs (IFN-α and IFN-β) play a crucial role in the control of viral replication because they have the ability to induce several antiviral cellular factors, including miRNAs.36 We next examined the impact of morphine on the expression of IFN-α/β–induced miRNAs in monocytes. When treated with IFN-α or IFN-β, monocytes expressed higher levels of anti-HIV miRNAs (miRNA-28, miRNA-125b, miRNA-150, and miRNA-382) compared with untreated cells (Figure 2C). However, IFN-mediated induction of the miRNAs (miRNA-28, miRNA-125b, miRNA-150, and miRNA-382) in monocytes was inhibited by morphine treatment (Figure 2C). The IFN-α/β had little effect on anti-HIV miRNA-223 expression in monocytes (data not shown).
In Vivo Impact of Heroin Use on Anti-HIV miRNA Expression in PBMCs
To validate the in vitro observation that morphine suppressed expression of anti-HIV miRNA, we examined the anti-HIV miRNA expression in PBMCs isolated from heroin-dependant subjects and healthy control donors. We observed that PBMCs isolated from heroin-dependant subjects (n = 8) had significantly lower levels of anti-HIV miRNAs (miRNA-28, miRNA-125b, miRNA-150, and miRNA-382) compared with the control subjects (n = 7), and the anti-HIV miRNA-223 expression level was insignificant between heroin-dependant subjects and healthy control donors (Figure 3).
Figure 3.
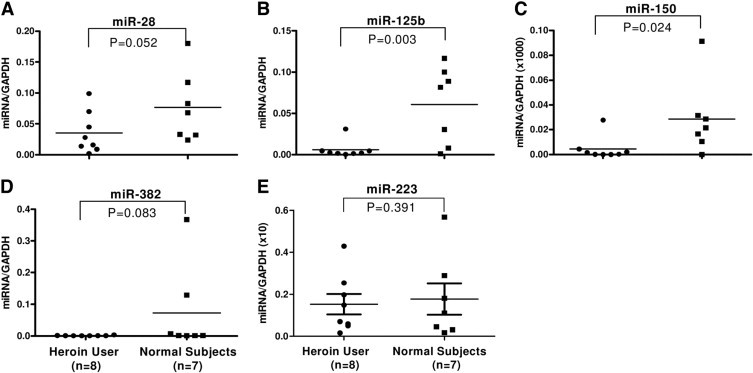
Effect of heroin use on anti-HIV miRNA expression in PBMCs. Total RNA was extracted from PBMCs isolated from the heroin-dependant subjects (n = 8) or healthy control subjects (n = 7). The levels of five anti-HIV miRNAs (A, miRNA-28; B, miRNA-125b; C, miRNA-150; D, miRNA-382 and E, miRNA-223) as indicated were measured by real-time PCR with the specific primers. The results are expressed as relative transcript abundance of miRNA/glyceraldehyde-3-phosphate dehydrogenase. The levels of miRNA expression in PBMCs between two groups were compared using the Student's t test (Graphpad Instat Statistical Software).
Discussion
In this study, we examined the impact of opioids (morphine and heroin) on the expression of anti-HIV cellular miRNAs and HIV infection of monocytes. We demonstrated that when exposed to morphine, monocytes became more susceptible to HIV infection than unexposed cells, which was evidenced by increased HIV RT activity and HIV GAG and LTR expression levels (Figure 1). The impact of morphine on HIV replication was also confirmed in the experiments in which MLV Env-pseudotyped HIV was used (Figure 1D). Because MLV Env-pseudotyped HIV has the ability to infect primary monocytes without using CD4 and β-chemokine receptor 5, the key entry receptor for HIV infection of monocytes, we can rule out the entry impact of morphine on HIV. The morphine action on HIV was mediated through an opioid receptor because the opioid receptor antagonist blocked the morphine effect (Figure 1). It is well-known that monocytes are refractory to HIV infection in vitro and in vivo.37–41 This refractory property to HIV infection could be because monocytes express high levels of intracellular innate anti-HIV factors, including miRNAs.23 We were particularly interested in miRNA-28, miRNA-125b, miRNA-150, miRNA-223, and miR-382 because these miRNAs can target a highly conserved region of HIV, present in all HIV clades.25 These miRNAs are highly expressed in resting CD4+ T cells28 and monocytes.23 The levels of these miRNAs correlate with the susceptibility of monocytes/macrophages to HIV infection.23 Thus, the demonstration of inhibition of these miRNAs by morphine provides a plausible mechanism for morphine-mediated enhancement of HIV infection of monocytes. This opioid-mediated inhibition of anti-HIV miRNAs was demonstrated in both in vitro experiments using monocytes and in vivo investigations using PBMCs from the heroin-dependent and control subjects. The morphine effect on miRNAs is specific because miR-223 (anti-HIV miRNA), miRNA-296 (anti–hepatitis C virus miRNA), and miRNA-122 (hepatitis C virus replication–required miRNA) were not affected by morphine (Figure 2A). This in vitro observation is supported by the in vivo finding that there was no significant difference in anti-HIV miRNA-223 expression in PBMCs between the heroin-dependant subjects and control individuals. It is not entirely clear why anti-HIV miRNA-223 was not affected by opioid use. However, we demonstrated that, although IFN-α/β could induce the expression of anti-HIV RNAs (miRNA-28, miRNA-125b, miRNA-150, and miRNA-382) (Figure 2C), they failed to induce anti-HIV miR-223 expression in monocytes (data not shown). This finding is in agreement with a previous publication42 showing that IFN-α/β had little effect on miRNA-223 expression in macrophages. Thus, it is likely that the adverse effect of morphine on anti-HIV miRNAs may be through its negative impact on endogenous IFN-α/β expression in monocytes.
Type I IFNs have a key role in the host innate defense mechanism against viral infections, including HIV. A recent study36 has shown that type I IFNs modulate miRNA expression as an antiviral mechanism. We demonstrated that IFN-α/β treatment of monocytes induced the expression of anti-HIV miRNAs (Figure 2C). This finding supports the studies by others36,43,44 showing that IFN-α/β is the potent inducer of miRNAs in several cell systems. However, this IFN-mediated anti-HIV innate immunity in monocytes was dampened by morphine treatment (Figure 2C). Although the mechanism(s) for morphine action remain to be determined, it is likely that morphine suppresses IFN signaling pathways, resulting in the down-regulation of IFN-stimulating genes. Morphine suppressed IFN-α expression in PBMCs and lymphocytes.2,45 Morphine suppresses Sendai virus-induced IFN-α production by PBMCs.2 Morphine also inhibits the release of IFN-α by Sindbis virus-infected monocytes.46 In support of these findings, we demonstrated that morphine treatment inhibited the expression of both IFN-α and IFN-β in monocytes (data not shown). Because type I IFNs have the ability to induce the expression of antiviral miRNAs,36 it is likely that the down-regulation of anti-HIV miRNAs by morphine is because of its negative impact on endogenous type I IFNs in monocytes.
In summary, given the essential functions of the miRNAs in regulating host innate immunity against viral infections, including HIV, down-regulation of anti-HIV miRNA expression in monocytes constitutes a critical mechanism for opioid-mediated enhancement of HIV infection. Thus, to identify factors that have the ability to block the negative action of opioids on the intracellular innate HIV restriction factors in monocytes could aid in the development of new therapeutic strategies for treating opioid-mediated suppression of host cell innate immunity. Nevertheless, future studies with a large sample size are necessary to determine the impact of opioid use on miRNA expression in the in vivo microenvironment where multiple cell types and cellular factors interact.
Footnotes
Supported by the National Institutes of Health grants DA12815, DA22177, and DA27550 (W.-Z.H.).
References
- 1.McCarthy L., Wetzel M., Sliker J.K., Eisenstein T.K., Rogers T.J. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 2.Nair M.P., Schwartz S.A., Polasani R., Hou J., Sweet A., Chadha K.C. Immunoregulatory effects of morphine on human lymphocytes. Clin Diagn Lab Immunol. 1997;4:127–132. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novick D.M., Ochshorn M., Ghali V., Croxson T.S., Mercer W.D., Chiorazzi N., Kreek M.J. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J Pharmacol Exp Ther. 1989;250:606–610. [PubMed] [Google Scholar]
- 4.Ochshorn M., Novick D.M., Kreek M.J. In vitro studies of the effect of methadone on natural killer cell activity. Isr J Med Sci. 1990;26:421–425. [PubMed] [Google Scholar]
- 5.Peterson P.K., Molitor T.W., Chao C.C. Mechanisms of morphine-induced immunomodulation. Biochem Pharmacol. 1993;46:343–348. doi: 10.1016/0006-2952(93)90508-t. [DOI] [PubMed] [Google Scholar]
- 6.Risdahl J.M., Khanna K.V., Peterson P.K., Molitor T.W. Opiates and infection. J Neuroimmunol. 1998;83:4–18. doi: 10.1016/s0165-5728(97)00216-6. [DOI] [PubMed] [Google Scholar]
- 7.Bayer B.M., Daussin S., Hernandez M., Irvin L. Morphine inhibition of lymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology. 1990;29:369–374. doi: 10.1016/0028-3908(90)90096-a. [DOI] [PubMed] [Google Scholar]
- 8.Peterson P.K., Sharp B., Gekker G., Brummitt C., Keane W.F. Opioid-mediated suppression of cultured peripheral blood mononuclear cell respiratory burst activity. J Immunol. 1987;138:3907–3912. [PubMed] [Google Scholar]
- 9.Stefano G.B., Scharrer B., Smith E.M., Hughes T.K., Magazine H.I., Bilfinger T.V., Hartman A.R., Fricchione G.L., Liu Y., Makman M.H. Opioid and opiate immunoregulatory processes. Crit Rev Immunol. 1996;16:109–144. doi: 10.1615/critrevimmunol.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- 10.Rojavin M., Szabo I., Bussiere J.L., Rogers T.J., Adler M.W., Eisenstein T.K. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53:997–1006. doi: 10.1016/0024-3205(93)90122-j. [DOI] [PubMed] [Google Scholar]
- 11.Hu S., Sheng W.S., Lokensgard J.R., Peterson P.K. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 12.Singhal P.C., Sharma P., Kapasi A.A., Reddy K., Franki N., Gibbons N. Morphine enhances macrophage apoptosis. J Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- 13.Nyland S.B., Cao C., Bai Y., Loughran T.P., Ugen K.E. Modulation of infection and type 1 cytokine expression parameters by morphine during in vitro coinfection with human T-cell leukemia virus type I and HIV-1. J Acquir Immune Defic Syndr. 2003;32:406–416. doi: 10.1097/00126334-200304010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Tan N., Douglas S.D., Zhang T., Wang Y.J., Ho W.Z. Morphine inhibits CD8+ T cell-mediated, noncytolytic, anti-HIV activity in latently infected immune cells. J Leukoc Biol. 2005;78:772–776. doi: 10.1189/jlb.0305167. [DOI] [PubMed] [Google Scholar]
- 15.Guo C.J., Li Y., Tian S., Wang X., Douglas S.D., Ho W.Z. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta chemokines and CCR5 receptor. J Investig Med. 2002;50:435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Merrill J.D., Mooney K., Song L., Wang X., Guo C.J., Savani R.C., Metzger D.S., Douglas S.D., Ho W.Z. Morphine enhances HIV infection of neonatal macrophages. Pediatr Res. 2003;54:282–288. doi: 10.1203/01.PDR.0000074973.83826.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Wang X., Tian S., Guo C.J., Douglas S.D., Ho W.Z. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185:118–122. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson P.K., Sharp B.M., Gekker G., Portoghese P.S., Sannerud K., Balfour H.H., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Steele A.D., Henderson E.E., Rogers T.J. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 20.Alcabes P., Friedland G. Injection drug use and human immunodeficiency virus infection. Clin Infect Dis. 1995;20:1467–1479. doi: 10.1093/clinids/20.6.1467. [DOI] [PubMed] [Google Scholar]
- 21.Ronald P.J., Robertson J.R., Elton R.A. Continued drug use and other cofactors for progression to AIDS among injecting drug users. AIDS. 1994;8:339–343. doi: 10.1097/00002030-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Specter S. Drugs of abuse and infectious diseases. J Fla Med Assoc. 1994;81:485–487. [PubMed] [Google Scholar]
- 23.Wang X., Ye L., Hou W., Zhou Y., Wang Y.J., Metzger D.S., Ho W.Z. Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood. 2009;113:671–674. doi: 10.1182/blood-2008-09-175000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung T.L., Rice A.P. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 2009;5:e1000263. doi: 10.1371/journal.ppat.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariharan M., Scaria V., Pillai B., Brahmachari S.K. Targets for human encoded microRNAs in HIV genes. Biochem Biophys Res Commun. 2005;337:1214–1218. doi: 10.1016/j.bbrc.2005.09.183. [DOI] [PubMed] [Google Scholar]
- 26.Ahluwalia J.K., Khan S.Z., Soni K., Rawat P., Gupta A., Hariharan M., Scaria V., Lalwani M., Pillai B., Mitra D., Brahmachari S.K. Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology. 2008;5:117. doi: 10.1186/1742-4690-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathans R., Chu C.Y., Serquina A.K., Lu C.C., Cao H., Rana T.M. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell. 2009;34:696–709. doi: 10.1016/j.molcel.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J., Wang F., Argyris E., Chen K., Liang Z., Tian H., Huang W., Squires K., Verlinghieri G., Zhang H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13:1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 29.Lodish H.F., Zhou B., Liu G., Chen–CZ Micromanagement of the immune system by microRNAs. Nat Rev Immunol. 2008;8:120–130. doi: 10.1038/nri2252. [DOI] [PubMed] [Google Scholar]
- 30.Connor R.I., Chen B.K., Choe S., Landau N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., Douglas S.D., Peng J.S., Metzger D.S., O'Brien C.P., Zhang T., Ho W.Z. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. J Leukoc Biol. 2006;79:1166–1172. doi: 10.1189/jlb.1105642. [DOI] [PubMed] [Google Scholar]
- 32.Wang X., Douglas S.D., Lai J.P., Tuluc F., Tebas P., Ho W.Z. Neurokinin-1 receptor antagonist (aprepitant) inhibits drug-resistant HIV-1 infection of macrophages in vitro. J Neuroimmune Pharmacol. 2007;2:42–48. doi: 10.1007/s11481-006-9059-6. [DOI] [PubMed] [Google Scholar]
- 33.Hou W., Wang X., Ye L., Zhou L., Yang Z.Q., Riedel E., Ho W.Z. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol. 2009;83:3834–3842. doi: 10.1128/JVI.01773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y., Ye L., Wan Q., Zhou L., Wang X., Li J., Hu S., Zhou D., Ho W. Activation of Toll-like receptors inhibits herpes simplex virus-1 infection of human neuronal cells. J Neurosci Res. 2009;87:2916–2925. doi: 10.1002/jnr.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds J.L., Mahajan S.D., Sykes D.E., Schwartz S.A., Nair M.P. Proteomic analyses of methamphetamine (METH)-induced differential protein expression by immature dendritic cells (IDC) Biochim Biophys Acta. 2007;1774:433–442. doi: 10.1016/j.bbapap.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen I.M., Cheng G., Wieland S., Volinia S., Croce C.M., Chisari F.V., David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewin S.R., Kirihara J., Sonza S., Irving L., Mills J., Crowe S.M. HIV-1 DNA and mRNA concentrations are similar in peripheral blood monocytes and alveolar macrophages in HIV-1-infected individuals. AIDS. 1998;12:719–727. doi: 10.1097/00002030-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Orenstein J.M., Fox C., Wahl S.M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 39.Rich E.A., Chen I.S., Zack J.A., Leonard M.L., O'Brien W.A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu T. HIV-1 in peripheral blood monocytes: an underrated viral source. J Antimicrobial Chemother. 2002;50:309–311. doi: 10.1093/jac/dkf143. [DOI] [PubMed] [Google Scholar]
- 41.Zhu T., Mo H., Wang N., Nam D.S., Cao Y., Koup R.A., Ho D.D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Wang X., Liu M., Hu Q., Song L., Ye L., Zhou D., Ho W. A critical function of toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology. 2010;131:40–49. doi: 10.1111/j.1365-2567.2010.03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connell R.M., Taganov K.D., Boldin M.P., Cheng G., Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohno M., Natsume A., Kondo Y., Iwamizu H., Motomura K., Toda H., Ito M., Kato T., Wakabayashi T. The modulation of microRNAs by type I IFN through the activation of signal transducers and activators of transcription 3 in human glioma. Mol Cancer Res. 2009;7:2022–2030. doi: 10.1158/1541-7786.MCR-09-0319. [DOI] [PubMed] [Google Scholar]
- 45.Homan J.W., Steele A.D., Martinand-Mari C., Rogers T.J., Henderson E.E., Charubala R., Pfleiderer W., Reichenbach N.L., Suhadolnik R.J. Inhibition of morphine-potentiated HIV-1 replication in peripheral blood mononuclear cells with the nuclease-resistant 2-5A agonist analog, 2-5A(N6B) J Acquir Immune Defic Syndr. 2002;30:9–20. doi: 10.1097/00042560-200205010-00002. [DOI] [PubMed] [Google Scholar]
- 46.Stoll-Keller F., Schmitt C., Thumann C., Schmitt M.P., Caussin C., Kirn A. Effects of morphine on purified human blood monocytes: modifications of properties involved in antiviral defences. Int J Immunopharmacol. 1997;19:95–100. doi: 10.1016/s0192-0561(97)00017-9. [DOI] [PubMed] [Google Scholar]


