Abstract
A growing body of evidence supports the significant interplay between the immune system and glioma pathogenesis. Here we investigate whether the extent of local glioma-associated CD8+ T-cell infiltrate at initial presentation correlates with long-term survival in patients with glioblastoma multiforme (GBM). The study was conducted by the University of California San Francisco Brain Tumor Research Center as part of the San Francisco Bay Area Adult Glioma Study, which included over 519 patients with GBM. A central neuropathology review was performed and populations of infiltrating CD8+ T-cells were quantified histologically. Of 108 patients studied, 43 patients had poor survival (< 95 days) and 65 patients had extended long-term survival of > 403 days. Tumors from long-term survivors were more likely than short-term survivors to have intermediate or extensive T-cell infiltrates compared to focal or rare infiltrates, and this association appears to be most significant in Caucasian women (p < 0.006). Thus, CD8+ T-cell infiltrate is associated with prolonged survival. Our data provide the impetus for more sophisticated studies to further elucidate prospectively the specific T-cell subtypes associated with long-term survival.
Keywords: Clinical outcome, Glioma, Glioblastoma, Immune infiltrate, T-cell infiltrate
1 Introduction
A growing body of evidence supports the significant interplay between the immune system and glioma pathogenesis with clinical implications [1]. Long-term remission of malignant brain tumors secondary to post-operative infection has generated the hypothesis that a heightened immune status can confer some protection against intracranial tumors [2]. Several reports have also suggested that a significant allergy history lowers an individual's lifetime risk for developing an intracranial glioma [3, 4]. In contrast, an impaired immune system may generate a state in which intracranial tumors may more easily develop. This is suggested by the noted correlation between human immunodeficiency virus-mediated immunosuppression and intracranial glial tumors [5-7]. Furthermore, iatrogenic immunosuppression associated with transplant recipients have also been implicated in the development of intracranial glioma [8, 9].
Serologic analysis of antigens using recombinant cDNA expression cloning (SEREX) has identified several tumor-associated antigens that are able to generate a specific response in a variety of human cancers, including malignant glioma [10, 11]. These finding suggest that a T-cell dependant immune response may improve the outcome of glioma patients through an antigen-mediated immune response (Fig. 1).
Figure 1.
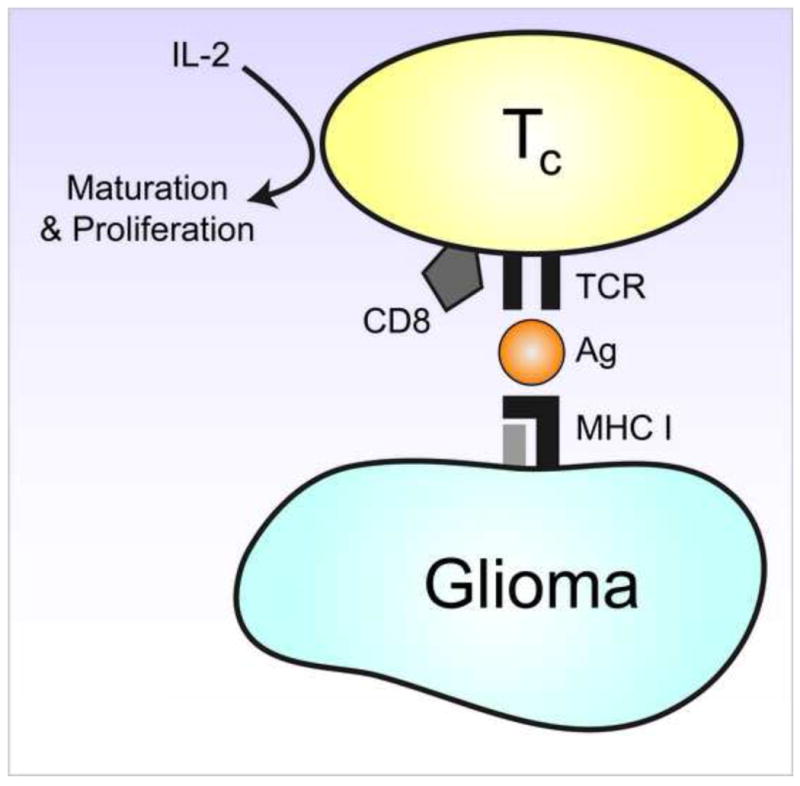
Diagram of cytotoxic CD8 T-cells showing the major histocompatibility complex (MHC) I=mediated antigen-dependant T-cell mechanism for cell killing and antigen recognition.
Cytotoxic T-cell (CD8+ T-cell) infiltrates found in glioma tissue may represent a local antigen-dependant immune activation against the glioma. Our hypothesis in this investigation was that the extent of CD8+ T-cell infiltrate at initial presentation and diagnosis correlates with long-term survival in patients with GBM. CD8+ T-cell infiltration in newly diagnosed glioma patients have not been well characterized in relation to long-term survival; here we report our specific analysis of 108 patients evaluated for CD8+ T-cell infiltrate and long-term survival.
2 Materials and Methods
2.1. Patient population
The study was conducted by the University of California San Francisco (UCSF) Brain Tumor Research Center as part of a population-based study, the San Francisco Bay Area Adult Glioma Study from 1991 to 1994 and 1997 to 1999, which included over 519 patients with GBM [4, 12, 13]. During these time frames, this study attempted to enroll every adult patient with glioma in the San Francisco Bay Area (as classified by the International Classification of Disease for Oncology [4, 12, 13], morphology codes 9380-9481). Utilizing the Northern California Cancer Center's rapid case ascertainment system, adult patients with glioma in the Bay Area were ascertained within 2 to 8 weeks after diagnosis. The Northern Californa Cancer Center is part of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program. Treatments, vital status, and other factors were determined utilizing registry, medical records, interviews, and active follow-up data [13]. These protocols have been previously described [4, 13, 14]. From these survival data, those patients with a survival < 95 days (n = 43) and those > 403 days (n = 65) were all further evaluated for prolific CD8+ T-cell infiltration. All study protocols were reviewed by the Committee on Human Research at UCSF.
2.2. Immunohistochemistry and Histological Analysis
All pathology records and specimens were obtained and reviewed by a central neuropathology review [13]. Central neuropathology review was performed based on the World Health Organization (WHO) II Classification [13, 15]. Quantification of proliferating CD8+ T-cell infiltration was performed histologically by systematically screening the entire tumor area from at least 3 sections obtained from different portions of the glioma. Ten microscopic fields were then chosen and examined with histological fluoroscopy. The average numbers of > 50 CD8+ T-cell infiltrates were noted as infiltration and these groups were classified into minimal, intermediate and extensive infiltration according to this distinction. CD8+ T-cell infiltrates were identified through immunohistochemistry using acetone-fixed cryostat sections stained with hematoxylin and eoin (H&E) followed by monoclonal antibodies against human CD8+ (AbCam; Cambridge, MA, USA). Cutoffs for survival quartiles included 95 days, 214 days, and 403 days. Mitigating factors known to affect survival were controlled for, including age, extent of resection, and adjuvant therapies. The neuropathology reviews of the tissue samples were performed in a blinded fashion in regards to long-term survival. Because the specific hypothesis of this study was to compare long-term survivors against short-term survivors, we specifically analyzed the CD8+ T-cell infiltrate in a bimodal manner by evaluating the short-term survivors and the long-term survivors based on our previously published quartile survival data.
2.3. Statistical analysis
The raw data were tabulated using Microsoft Excel (Microsoft; Seattle, WA, USA). All results were analyzed using the chi-squared (χ2) test, Fisher's exact probability test or a t-test when appropriate for statistical evaluation of the data. A multivariate analysis of CD8+ T-cell infiltration adjusting for known prognostic and treatment factors was performed using the logistic regression model. For all statistical investigations, tests for significance were 2-sided, with a (2-tailed) p -value threshold of 0.05 considered statistically significant. Unless otherwise stated, all continuous values were presented as mean ± standard deviation (SD).
3. Results
3.1. Patient Demographic Results and Censoring
A total of 519 patients were enrolled in the San Francisco Bay Area Glioma SEER study with central neuropathology review. The median age at diagnosis in this group was 63 years of age. The median length of survival was 7.2 months with a 95% confidence limit of 6.6 to 7.9 months (Table 1). Not all ptient slides could be stained and evaluated, and were censored from analysis. Using the determined cutoffs of poor long-term survival at < 95 days (n = 43) and the extended long-term survival of > 403 days (n = 65), 108 samples were analyzed. Of the patients whose samples were analysed, the mean age was 65.2 years in the short-term survivor group and 54.3 years in the long-term survivor group (Table 1).
Table 1. Comparison of patients with glioblastoma with CD8+ T-cell infiltrate results compared to those on whom staining could not be performed.
| Poor long-term survival group | Extended long-term survival group | |||
|---|---|---|---|---|
| With results | Without staining | With results | Without staining | |
| n = 43 | n = 30 | n = 65 | n = 21 | |
| Mean age (yrs) | 65.2 | 71.6 | 54.3 | 54 |
| Median age (yrs) | 67 | 74.5 | 54 | 53 |
| Mean survival (days) | 49.2 | 43.1 | 799.2 | 604 |
| % Male | 48.8 | 63.3 | 63.1 | 76.2 |
| % White | 93 | 80 | 83.1 | 81 |
The characteristics of our patient population in this study reflected the regional demographics of the study population in the San Francisco Bay Area. Of the study samples, 44% were female, and 83% of the patients enrolled in this study were White or Caucasian in ethnicity. This demographic pattern appropriately reflects the adult population of the San Francisco Bay Area.
3.2. CD8+ T-cell analysis results
Tissue sections were subsequently evaluated for the degree of CD8+ T-cell infiltrate and analyzed for correlation between survival and CD8+ T-cell infiltration (Fig. 2). Tumors from long-term survivors were more likely than those from short-term survivors to have intermediate or extensive T-cell infiltrates compared to focal or rare infiltrates (38% for long-term; 20% for short-term survivors, p = 0.06). Our analysis did not note any significant differences between the invasive margin of the tumor and more central areas. Tumor heterogeneity was not suggestive or correlated with an improved outcome.
Figure 2.
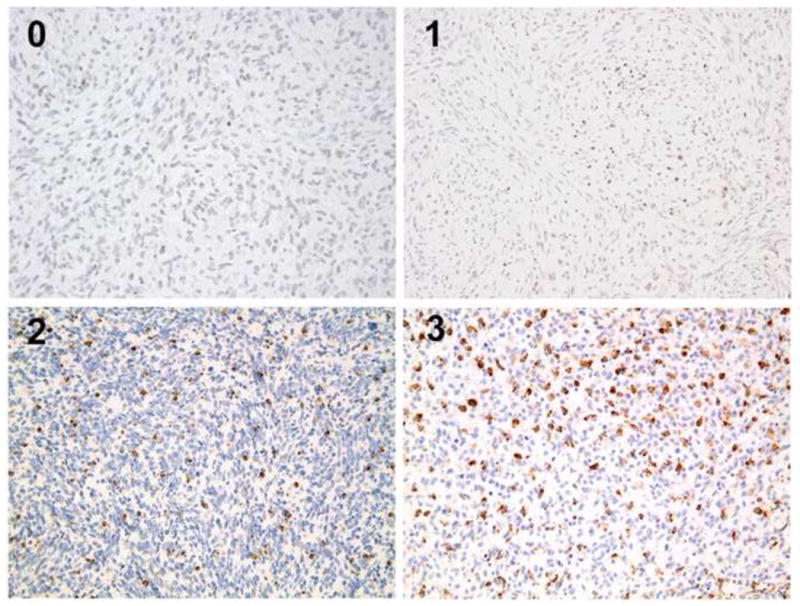
Glioma tumor cells under light microscopy with varying levels of cytotoxic T-cell (CD8+ T-cell) infiltrate with human CD8 antibody and eosin counterstain (magnification ×20).
3.3. Multivariate Regression Analysis
In the multivariate regression analysis, utilizing the logistic regression model, the association between long-term survival and increased CD8+ T-cell infiltrate was the most significant beneficial risk factor for Caucasian women (p < 0.006). Thus, prolific CD8+ T-cell infiltrate appears to correlate with partitioned long-term survival in newly diagnosed GBM patients.
4 Discussion
Tumor-related antigens can be recognized by cytotoxic CD8+ T-cells in the context of major histocompatibility complex (MHC) class I expressing tumors [16-18]. In the present study, we analyzed prolific CD8+ T-cell infiltration within newly diagnosed glioma neoplasms to demonstrate that increased CD8+ T-cell infiltration is an associated prognostic factor for improved long-term survival. Our findings are one of the largest analysis reported to date that characterize the results specific for CD8+ T-cells in the setting of newly diagnosed glioblastoma.
Additional cancer studies into the presence of infiltrating T-cells have been investigated in several neoplasms such as melanoma [19-21], ovarian cancer [22-24], and colorectal cancer [25-28], but unlike in these lesions, the clinical outcome of glioma patients with CD8+ T-cell infiltrate has remained uncertain [1]. Early studies into T-cell infiltrates into glioma indicated that there was a subset of gliomas with T-cell infiltrate, but no clinical correlation was observed in these studies [29, 30]. These early investigations did establish, however, that 28% to 60% of gliomas demonstrated tumor infiltration with lymphocytes. Several studies since then have confirmed the varying degrees of lymphocytic infiltraion in human gliomas with differing infiltration rates [31-34]. Other studies of glioma lymphocyte infiltration have characterized the mix of lymphocyte-infiltrating gliomas as a mixture of different lymphocytes including CD4+ and CD8+ cells [35, 36]. Although these papers report varying presence and degree of lymphocytic infiltration, they confirm the presence of lymphocytic infiltration rather than any correlation with long-term clinical outcome.
Several studies have evaluated the relationship between infiltrating lymphocytes and clinical outcome. Brooks et al. report that 45% of gliomas expressed lymphocyte infiltration and that these findings correlated with survival for the patients [37]. Several other studies have suggested a positive correlation between lymphocyte infiltration and survival, but these studies did not evaluate the T-cell subset in these infiltrating lymphocytes [38, 39]. Conversely Schiffer et al. in a study of 324 patients found no correlation with infiltrating lymphocytes and clinical outcomes [40]. In a study investigating the lymphocyte subsets, Rossi et al. found no correlation between clinical survival and CD8+ T-cell infiltration [41]. Furthermore, Safdari et al. in their study of 342 glioma patients report a negative correlation between infiltrating lymphocytes and clinical survival, as those patients with infiltrating lymphocytes had shorter survival times [42]. These studies represent disconcordant results and do not support a clear relationship between lymphocytes and clinical survival.
This poorly characterized association between lymphocytes and clinical outcome may be due to the lack of lymphocyte subset classification such as CD4, CD8 and regulatory T-cells performed in these reports. Recent studies have demonstrated the presence of a T-cell subset, the regulatory T-cells, which can be found in gliomas exerting an overall immunosuppressive and anergic response [43-47]. Furthermore, due to tumor heterogeneity, differing methodologies, short length of follow-up, and inconsistent sampling, these reports investigating lymphocyte infiltration may contain ambiguity in their clinical correlation.
Our study of newly diagnosed gliomas represents one of the largest studies reported to date that specifically evaluates the subset of CD8+ T-cell infiltration in newly diagnosed human glioblastomas, utilizing a systematically consistent analysis of newly diagnosed glioblastomas with consistent pathologic review and sampling. Our results here show that for patients who have intermediate or extensive CD8+ T-cell infiltrate at the time of new diagnosis with glioma were more likely to have long-term survival than patients with rare or focal CD8+ T-cell infiltrates (p = 0.06). Our data analysis is further bolstered by our specific comparison of patients with CD8+ infiltrates against patients who did not have CD8+ infiltrate. Furthermore, our analysis comprehensively evaluated long-term survival of patients, and suggests that patients surviving for longer than 1 year had an increased statistical association with infiltrating CD8+ T-cells. Our analysis also did not reveal significant differences inT-cell densities in infiltrative glioma tissues nor any change in CD8+ T-cell densities in the adjacent neuronal tissues. This suggests that perhaps the infiltrative nature of these CD8+ T-cells may have a critical role in elucidating the mechanism for this positive correlation. Future studies evaluating the immune cell density in adjacent normal brain tissue may reveal important characteristics of the immune environment of adjacent normal brain tissue surrounding glioblastomas. Our evaluation of newly diagnosed glioblastoma in this adult population suggests a significant prognostic factor for newly diagnosed glioblastoma prior to any treatment effect, and demonstrates a clear immunohistochemically identified difference in the long-term survivors compared to the short-term survivors regarding CD8+ T-cell infiltrate.
A limitation of these results is that these data represent a retrospective neuropathologic review establishing the correlation specifically between CD8+ T-cell infiltrates and long-term survival, but this neither demonstrates causation nor provides a clear mechanism [18]. Hence, these data cannot provide an actuarial or time-dependant analysis of T-cell infiltrate if it varied at different time points in the disease. It was our aim that the large nature of this analysis, utilizing a specific subset characterization of the lymphocyte infiltration, and the rigorous standardized adherence to WHO histopathologic diagnosis with central neuropathologic review would help mitigate these retrospective limitations. Future histopathologic and prospective studies may include monitoring the activation of T-cell infiltrates using specific markers, and the molecular profiling of known immunosuppressive pathways of glioma tissue in individual patients [48]. This final limitation suggests that prospective analysis of T-cell infiltrates may be informative with further CD4+ T-cell or regulatory T-cell characterization and other immune cell infiltrates that could not be performed in a retrospective analysis.
The demonstrated relationship between the degree of CD8+ T-cell infiltrates and clinical outcomes continues to provide support and promise for T-cell based immunotherapies that are currently in clinical investigation [16, 49-51]. In the setting of ongoing clinical immunotherapuetics, modifications of the infiltrating T-cell populations are observed alongside successful responses [52, 53]. Thus, continuing to elucidate the intricacies of the intimate interaction between glioma and infiltrating T-cells will be necessary for optimizing immunotherapeutic treatment modalities.
Conclusion
Here we report one of the largest series to date specifically evaluating CD8+ T-cell infiltration with long-term clinical survival in newly diagnosed human glioblastoma that systematically analyzes CD8+ infiltrate in newly diagnosed glioma with central neuropathologic evaluation. Our data here indicate that CD8+ T-cell infiltrate in patients with newly diagnosed glioblastoma is significantly associated with prolonged clinical outcome. This association appears to be most significant in women of Caucasian ethnicity. Prolific CD8+ T-cell infiltration may prove to be an important clinical prognostic factor in the treatment of newly diagnosed human glioblastomas.
Acknowledgments
IY, the first author, was partially supported by an NIH training grant, F32 CA132489-01A1, a Congress of Neurological Surgeons Dandy Clinical Research Fellowship and a UCSF Clinical and Translational Scientist Training Research Award in performing this investigation. ATP, senior author, is partially funded by the Reza and Georgianna Khatib Endowed Chair in Skull Base Tumor Surgery. The authors have no conflict of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunn GP, Dunn IF, Curry WT. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- 2.Bowles AP, Jr, Perkins E. Long-term remission of malignant brain tumors after intracranial infection: a report of four cases. Neurosurgery. 1999;44:636–642. doi: 10.1097/00006123-199903000-00110. discussion 642-633. [DOI] [PubMed] [Google Scholar]
- 3.Brenner AV, Linet MS, Fine HA, Shapiro WR, Selker RG, Black PM, Inskip PD. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99:252–259. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 4.Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609–615. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 5.Gervasoni C, Ridolfo AL, Rocca A, Vago L, d'Arminio Monforte A. Cerebral astrocytoma in HIV-infected patients. AIDS. 1995;9:403–404. [PubMed] [Google Scholar]
- 6.Moulignier A, Mikol J, Pialoux G, Eliaszewicz M, Thurel C, Thiebaut JB. Cerebral glial tumors and human immunodeficiency virus-1 infection. More than a coincidental association Cancer. 1994;74:686–692. doi: 10.1002/1097-0142(19940715)74:2<686::aid-cncr2820740222>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Neal JW, Llewelyn MB, Morrison HL, Jasani B, Borysiewicz LK. A malignant astrocytoma in a patient with AIDS: a possible association between astrocytomas and HIV infection. J Infect. 1996;33:159–162. doi: 10.1016/s0163-4453(96)92105-2. [DOI] [PubMed] [Google Scholar]
- 8.Schiff D, O'Neill B, Wijdicks E, Antin JH, Wen PY. Gliomas arising in organ transplant recipients: an unrecognized complication of transplantation? Neurology. 2001;57:1486–1488. doi: 10.1212/wnl.57.8.1486. [DOI] [PubMed] [Google Scholar]
- 9.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 10.Struss AK, Romeike BF, Munnia A, Nastainczyk W, Steudel WI, Konig J, Ohgaki H, Feiden W, Fischer U, Meese E. PHF3-specific antibody responses in over 60% of patients with glioblastoma multiforme. Oncogene. 2001;20:4107–4114. doi: 10.1038/sj.onc.1204552. [DOI] [PubMed] [Google Scholar]
- 11.Prins RM, Odesa SK, Liau LM. Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res. 2003;63:8487–8491. [PubMed] [Google Scholar]
- 12.International classification of disease for oncology. 2nd. Geneva: World Health Organization; 1990. [Google Scholar]
- 13.Wrensch M, Rice T, Miike R, McMillan A, Lamborn KR, Aldape K, Prados MD. Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro Oncol. 2006;8:12–26. doi: 10.1215/S1522851705000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrensch M, Lee M, Miike R, Newman B, Barger G, Davis R, Wiencke J, Neuhaus J. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997;145:581–593. doi: 10.1093/oxfordjournals.aje.a009154. [DOI] [PubMed] [Google Scholar]
- 15.Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3:255–268. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 16.Liau LM, Black KL, Martin NA, Sykes SN, Bronstein JM, Jouben-Steele L, Mischel PS, Belldegrun A, Cloughesy TF. Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case Report Neurosurg Focus. 2000;9:e8. doi: 10.3171/foc.2000.9.6.9. [DOI] [PubMed] [Google Scholar]
- 17.Prins RM, Liau LM. Immunology and immunotherapy in neurosurgical disease. Neurosurgery. 2003;53:144–152. doi: 10.1227/01.neu.0000068865.34216.3a. discussion 152-143. [DOI] [PubMed] [Google Scholar]
- 18.Yang I, Kremen TJ, Giovannone AJ, Paik E, Odesa SK, Prins RM, Liau LM. Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg. 2004;100:310–319. doi: 10.3171/jns.2004.100.2.0310. [DOI] [PubMed] [Google Scholar]
- 19.Clark WH, Jr, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 20.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 22.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 23.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 24.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 26.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 27.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 28.Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4. [PMC free article] [PubMed] [Google Scholar]
- 29.Ridley A, Cavanagh JB. Lymphocytic infiltration in gliomas: evidence of possible host resistance. Brain. 1971;94:117–124. doi: 10.1093/brain/94.1.117. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi J, Barnard RO. Perivascular lymphocytic cuffing in astrocytomas. Acta Neuropathol. 1976;35:265–271. [PubMed] [Google Scholar]
- 31.von Hanwehr RI, Hofman FM, Taylor CR, Apuzzo ML. Mononuclear lymphoid populations infiltrating the microenvironment of primary CNS tumors. Characterization of cell subsets with monoclonal antibodies. J Neurosurg. 1984;60:1138–1147. doi: 10.3171/jns.1984.60.6.1138. [DOI] [PubMed] [Google Scholar]
- 32.Hitchcock ER, Morris CS. Mononuclear cell infiltration in central portions of human astrocytomas. J Neurosurg. 1988;68:432–437. doi: 10.3171/jns.1988.68.3.0432. [DOI] [PubMed] [Google Scholar]
- 33.Farmer JP, Antel JP, Freedman M, Cashman NR, Rode H, Villemure JG. Characterization of lymphoid cells isolated from human gliomas. J Neurosurg. 1989;71:528–533. doi: 10.3171/jns.1989.71.4.0528. [DOI] [PubMed] [Google Scholar]
- 34.Stevens A, Kloter I, Roggendorf W. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer. 1988;61:738–743. doi: 10.1002/1097-0142(19880215)61:4<738::aid-cncr2820610417>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 35.Kuppner MC, Hamou MF, de Tribolet N. Immunohistological and functional analyses of lymphoid infiltrates in human glioblastomas. Cancer Res. 1988;48:6926–6932. [PubMed] [Google Scholar]
- 36.Sawamura Y, Hosokawa M, Kuppner MC, Kobayashi H, Aida T, Abe H, de Tribolet N. Antitumor activity and surface phenotypes of human glioma-infiltrating lymphocytes after in vitro expansion in the presence of interleukin 2. Cancer Res. 1989;49:1843–1849. [PubMed] [Google Scholar]
- 37.Brooks WH, Markesbery WR, Gupta GD, Roszman TL. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol. 1978;4:219–224. doi: 10.1002/ana.410040305. [DOI] [PubMed] [Google Scholar]
- 38.Palma L, Di Lorenzo N, Guidetti B. Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas. Incidence, fate, and relevance to prognosis in 228 operated cases. J Neurosurg. 1978;49:854–861. doi: 10.3171/jns.1978.49.6.0854. [DOI] [PubMed] [Google Scholar]
- 39.Boker DK, Kalff R, Gullotta F, Weekes-Seifert S, Mohrer U. Mononuclear infiltrates in human intracranial tumors as a prognostic factor. Influence of preoperative steroid treatment. I. Glioblastoma. Clin Neuropathol. 1984;3:143–147. [PubMed] [Google Scholar]
- 40.Schiffer D, Cavicchioli D, Giordana MT, Palmucci L, Piazza A. Analysis of some factors effecting survival in malignant gliomas. Tumori. 1979;65:119–125. doi: 10.1177/030089167906500114. [DOI] [PubMed] [Google Scholar]
- 41.Rossi ML, Jones NR, Candy E, Nicoll JA, Compton JS, Hughes JT, Esiri MM, Moss TH, Cruz-Sanchez FF, Coakham HB. The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. Acta Neuropathol. 1989;78:189–193. doi: 10.1007/BF00688208. [DOI] [PubMed] [Google Scholar]
- 42.Safdari H, Hochberg FH, Richardson EP., Jr Prognostic value of round cell (lymphocyte) infiltration in malignant gliomas. Surg Neurol. 1985;23:221–226. doi: 10.1016/0090-3019(85)90086-2. [DOI] [PubMed] [Google Scholar]
- 43.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, Cummings T, Allison JP, Bigner DD, Sampson JH. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13:2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 47.Learn CA, Fecci PE, Schmittling RJ, Xie W, Karikari I, Mitchell DA, Archer GE, Wei Z, Dressman H, Sampson JH. Profiling of CD4+, CD8+, and CD4+CD25+CD45RO+FoxP3+ T cells in patients with malignant glioma reveals differential expression of the immunologic transcriptome compared with T cells from healthy volunteers. Clin Cancer Res. 2006;12:7306–7315. doi: 10.1158/1078-0432.CCR-06-1727. [DOI] [PubMed] [Google Scholar]
- 48.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 49.Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, Sampson JH. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19:713–723. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, 2nd, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8:2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9:1087–1098. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 53.Akasaki Y, Black KL, Yu JS. T cell immunity in patients with malignant glioma: recent progress in dendritic cell-based immunotherapeutic approaches. Front Biosci. 2005;10:2908–2921. doi: 10.2741/1747. [DOI] [PubMed] [Google Scholar]


