1. ABSTRACT
T cells tend to acquire a variety of cell surface molecules derived from antigen presenting cells (APCs). The molecule uptake occurs mainly during direct T/APC contact and is instigated by specific receptor/ligand interactions, such as T cell receptor (TCR) with a cognate peptide/MHC complex (pMHC) or CD28 with B7. The acquired molecules are targeted for internalization and degradation in the lysosome. Nevertheless, those molecules are expressed on the surface of T cells for a period of time. The presentation of APC-derived ligands by T cells exhibited a multitude of immunological effects via antigen-specific T/T interaction upon recognition of the absorbed antigens by contact with other T cells. Ligand uptake also occurs via absorption of membrane vesicles shed from APCs prior to contact (e.g., exosomes and plasma membrane-derived vesicles). As in ligand absorption via direct T/APC interaction, the absorption of pre-formed membrane vesicles is also dependent on specific receptor/ligand interactions. In this review, biological mechanisms underlying the ligand absorption process as well as the biological significance and application of the event will be discussed.
Keywords: T cell, ligand absorption, vesicle, T cell receptor, integrin
2. INTRODUCTION
Immune responses, both innate (1, 2) and adaptive (3, 4), generally begin as specific receptors on immune cells encounter cognate ligands either on the surface of cells or dissolved in a bodily fluid (i.e., blood and lymph). The receptor/ligand interactions trigger a myriad of intracellular signaling events leading to transcriptional activation of genes for their unique effector functions and/or cell cycle progression.
T cell activation followed by clonal expansion and acquisition of effector functions, requisite measures of productive T cell immunity, commences as a T cell receptor (TCR) encounters a cognate (processed) peptide in the context of a self major histocompatibility complex (MHC) presented by a specialized subset of immune cells called antigen presenting cells (APCs) (5). The interaction of TCR with peptide/MHC complex (pMHC) triggers key signaling events for T cell activation and induces a change in topology of cell membrane and rearrangement of cell surface molecules, assembling a unique molecular structure at the interface of T/APC contact termed immunological synapse (IS) (6). The formation of IS, which is highly dynamic in nature and features layers of distinct receptor/ligand pairs, not only promotes stability of T/APC contact but also allows more orchestrated T cell signaling for optimal T cell activation/proliferation (7).
The entire T cell activation/proliferation process is carefully regulated to avoid hypo- or hyper-immune responses, which may effect a fatal pathogenic infection as well as harmful immunopathologic diseases such as autoimmune and inflammatory diseases, respectively (8). Multiple regulatory mechanisms operate to achieve the goal. For example, T cells express a variety of cell surface molecules acting as either stimulatory or inhibitory receptors which are coordinately up- or down-regulated during immune responses. Similarly, several soluble factors (i.e., cytokines and chemokines) are produced by T cell themselves and neighboring cells (or tissues) to promote or suppress immune responses (9, 10). In addition, a subset of T cells (e.g., CD4+CD25+ regulatory T cells) devoted to control of immune responses are in place (11, 12).
An intriguing finding from studies for T cell activation is that T cells acquire cell surface molecules from APCs to present them on their own surface (13–15). While the molecule uptake occurs predominantly during physical T/APC contact, it may also occur, without the contact, by absorbing molecules already released from APCs. While the exact mechanism underlying the ligand uptake remains to be understood, it has become evident that T cell absorption of ligands of APC-origin occurs as T cells pick up membranous structures from APCs and specific receptor/ligand interactions, exemplified by TCR/pMHC interaction, plays a key role (16). The importance of the ligand absorption in regulating T cell immunity has been revealed in many recent studies. Application of the finding as experimental or clinical tools has been also explored. In this review, we will summarize the historical background as well as recent advances in the field and discuss potential applications of the findings.
3. HISTORICAL BACKGROUND
Transfer of cell surface molecules from APC to T cell was first suggested several decades ago as T cells activated in vivo were found to express non-T cell markers (i.e., IgM). Hudson et al. first reported the expression of IgM on the surface of activated donor T cells collected from thoracic duct lymph after injection of mouse splenocytes into irradiated allogeneic (MHC-mismatched) host to elicit graft versus host reaction. It was also found that IgM molecules on the surface of those T cells were specific toward host MHCs. It was speculated, based on those findings, that MHCs endogenously expressed in host cells were transferred to donor T cells during their activation and that the IgM molecules produced by co-injected B cells formed complexes with MHC molecules transferred onto the T cells (17–19).
Nagy and co-workers, later, made a similar observation in experiments for an in vitro mixed lymphocyte reaction (MLR). T cells cultured with irradiated allogeneic spleen cells turned positive for the expression of MHC molecules of stimulator cell origin. Specificity of the molecule transfer was, however, questioned due to the finding that not only class I but also class II molecules were transferred to T cells, even when MLR was directed selectively to class I MHC (20). The specificity issue became more perplexing as Lorber et al. reported T cell uptake of MHCs (i.e., class II MHCs) from syngeneic APCs (21, 22).
Sharrow et al. found that T cells in thymi of bone marrow chimeras, established by injecting bone marrow cells from a strain of mice to lethally irradiated MHC-mismatched host mice, expressed significant levels of both class I and II MHCs derived from host cells (23). In line, studies by Merkenschlager using fetal thymic organ culture showed that when thymocytes from a parental strain (H-2b or H-2k haplotype) were cultured with hybrid F1 stromal cells (H-2bxk), those T cells began to express both H-2b and H-2k MHC II determinants (24). When the same thymocytes were cultured with a mixed population of parental stromal cells, however, the T cells were found to express either a H-2b or H-2k MHC II determinant, implying that the molecule absorption occurred via direct contact of T cells with stromal cells.
Meanwhile, the molecule transfer mediated by membrane vesicles secreted by APCs was suggested. Studies by Mannie and his co-workers using a transformed rat T cell line recognizing a peptide derived from rat myelin basic proteins (MBPs) and electron microscopy indicated that T cells absorbed membrane vesicles shed from APCs during culture with APCs. Of note, the vesicle binding was found to occur only when the APCs were loaded with MBPs (25, 26).
Despite the recurring findings, the ambiguity in underlying mechanisms and scarcity in plausible experimental evidence for immunological effects of the molecule transfer had undermined the enthusiasm for the event among immunologists. Recent studies by Sprent and his colleagues attracted a renewed attention to the subject and lead to an expansion in the number of publications related to the topic, advancing our knowledge in the mechanism as well as the biological significance of the molecule transfer. We discuss those studies next.
4. RENEWED INTEREST IN T CELL ABSORPTION OF APC-DERIVED MOLECULES
In studies using artificial APCs constructed by expressing defined mouse immuno-molecules of interest in insect (Drosophila S2) cells, Hwang et al. made a surprising observation. Culture of freshly purified resting naive TCR transgenic (Tg) CD8+ T cells expressing 2C αβ TCR with Drosophila (Dros) APCs (27), expressing Ld (a mouse class I MHC) and B7-1 (LdB7-1 Dros APCs) or Ld and B7-1 plus ICAM-1 (LdB7-1ICAM-1 Dros APCs), led to prompt expression of B7-1 as well as Ld on the T cells (28). (Note: For 2C TCR Tg mice carry H-2b haplotype and the level of intrinsic B7-1 expression in resting T cells is undetectably low, flow cytometric analysis of T cells for expression of Ld and B7-1 after culture with APCs provides a highly sensitive and quantitative assay system for the molecule transfer.)
Even if a marginal level of B7-1 (and Ld) uptake was detected as 2C T cells were cultured with the APCs loaded with a non-cognate peptide (P1A), the level of ligand uptake increased to a great extent when they were cultured with the APCs loaded with a cognate peptide (p2Ca or QL9). This validated a critical role of TCR/pMHC interaction in the process. The predominant role of the TCR/pMHC interaction was further supported by experiments using a mAb blocking interaction of 2C TCR with cognate pMHCs on Dros APCs (28).
The molecule transfer was facilitated when T cells were activated. The level of the B7-1 (and Ld) transfer increased to a considerable extent when 2C T cells were activated by PMA (phorbol ester) + Ionomycin (Ca2+ ionophore) before culture with QL9-loaded LdB7-1ICAM-1 (or LdB7-1) Dros APCs. In parallel, the role of CD28/B7-1 interaction (29) also became more prominent when T cells were activated. Thus, PMA + Ionomycin-treated normal (non-Tg) C57BL/6 (B6) T cells (both CD4+ and CD8+) acquired a significant level of B7-1 (and Ld) during culture with LdB7-1 or LdB7-1ICAM-1 Dros APCs. Regardless of the activation state of the T cells, the LFA-1/ICAM-1 interaction seemed to play a minimal role for culture of either activated or resting normal T cells deficient in CD28 expression (CD28−/−) with LdB7-1ICAM-1 Dros APCs resulted in little molecule transfer. The results indicated that selected receptor/ligand interactions (e.g., CD28/B7-1) other than TCR/pMHC interaction instigated T cells to absorb cell-surface molecules from APCs. These observations provide a mechanistic insight for earlier observations by others; for example, the finding by Lorber et al. that molecule absorption could occur when T cells were cultured with syngeneic APCs (20) and an observation by Lipsky’s group that activated T cells absorbed a variety of molecules during migration on human umbilical vein endothelial cells (30).
The finding that activated normal B6 T cells absorbed not only B7-1 but also Ld from LdB7-1 Dros APCs was intriguing. Similarly, resting 2C T cells deficient in CD28 expression (2C.CD28−/−) absorbed B7-1 as well as Ld from QL9 (or p2Ca)-loaded LdB7-1 Dros APCs. As CD28/B7-1 interaction was the sole receptor/ligand pair present during culture of the B6 T cells with the Dros APCs, Ld molecules were thought to be transferred to T cells as bystanders without pairing with a tangible receptor; on the other hand, B7-1 molecules must have been transferred as bystanders during culture of 2C.CD28−/− T cells with QL9 (or p2Ca)-loaded LdB7-1 Dros APCs. Taken together, it implied that ligand uptake proceeded via absorption of membranous objects containing bystander molecules as well as ligands physically interacting with receptor(s) directing the process.
Here, a question was whether the molecule transfer occurred during direct T/APC contact or via uptake of membrane vesicles shed from APCs prior to the contact (e.g., exosomes (31)). That issue was first tackled using Transwell™ that keeps T cells and APCs separate during the culture but permits free traffic between the two chambers of soluble materials or particles smaller than the pore size of its membrane (0.4 μm in diameter) (32). Results from the Transwell experiments concluded that the T cell uptake of APC-derived ligands occurred mainly during direct T/APC contact. That is, 2C T cells cultured with QL9-loaded LdB7-1 Dros APCs in Transwell showed expression of neither B7-1 nor Ld while 2C T cells in direct contact with the same APCs promptly expressed high levels of both ligands. The conclusion drawn from experiments using Transwell was confirmed later in experiments using exosomes prepared from culture supernatant of LdB7-1Dros APC; culture of 2C T cells with QL9 (or p2Ca)-loaded LdB7-1 Dros exosomes resulted in a lack of B7-1 (or Ld) expression on the T cells. In addition, culture of activated normal B6 T cells with the same exosomes resulted in no B7-1 expression in T cells (33).
Most studies described above were performed using flow cytometry to detect APC-derived molecules on T cells, giving rise to the criticism that the molecule transfer might occur artifactually during flow cytometry. Namely, the T/APC conjugate might be torn apart by mechanical force applied during the analysis, resulting in a rip in the plasma membrane and the molecule transfer without an underlying biological basis. In order to address that criticism, Huang et al. used Dros APCs expressing Ld-green fluorescence protein (GFP) fusion protein to monitor by confocal microscopy live 2C T cells in contact with the peptide-loaded Dros APCs. Uisng that technology, they documented transfer of the GFP-fusion proteins to 2C T cells in situ (34). As in flow cytometric analysis, the molecule transfer was dependent on TCR/pMHC interaction so that it occurred only when the APCs were loaded with a cognate peptide (QL9 or p2Ca). Another relevant finding from studies by Huang et al. was that the transferred GFP-fusion proteins were internalized by 2C T cells and merged with lysosomes.
5. T CELL ABSORPTION OF APC-DERIVED LIGANDS DURING DIRECT T/APC CONTACT
5.1. Current views on mechanisms (Figure 1)
Figure 1.
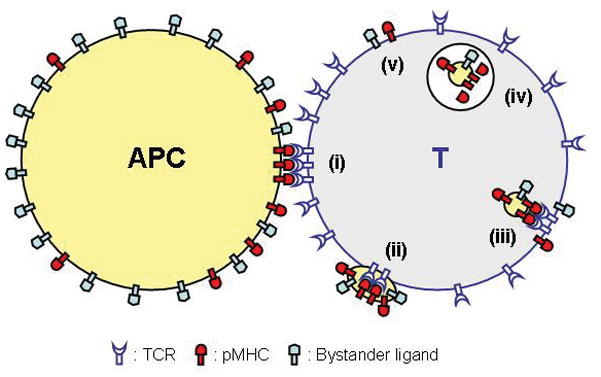
Schematic representation of the process for T cell absorption of APC-derived molecules via direct T/APC contact. Encounter of a specific receptor in T cell (e.g., TCR) with its cognate ligands in APC (e.g., pMHCs) leads to recruitment of those receptors and ligands to the contact site, resulting in formation of the immunological synapse at the junction (i). The T cell then absorbs, exploiting a mechanism still to be understood, microscopic pieces of plasma membrane from the APC (ii). The absorbed vesicles express not only ligands directly engaged in the receptor/ligand interaction but also adjacent bystander molecules. The absorbed ligands are internalized by the T cell (iii) and merge with lysosomes (iv). It is also speculated that some of the internalized molecules are recycled to the cell surface (v).
Studies by Hudrisier and her co-workers, using CD8+ TCR Tg T cells specific for a LCMV virus-derived peptide (gp33) and mouse APCs labeled with a lipophilic fluorescence dye incorporated into plasma membrane, substantiated the notion that TCR-mediated T cell uptake of ligands proceeded via absorption of microscopic pieces of plasma membrane from APCs (35). A study using small molecule inhibitors for tyrosine kinases, PI3-kinase, Ca2+ signaling and actin metabolism, respectively, also indicated that membrane-proximal T cell signaling played a critical role in T cell uptake of ligands from APCs (36). Supporting this idea, it was also found that membrane-proximal signaling molecules (i.e., p56lck, phosphotyrosines) and F-actin were heavily recruited to the area where APC-derived molecules were found (37).
Studies by Sabzevari’s group detailed CD28-mediated ligand absorption. They showed, in a study using cycloheximide (a small molecule inhibitor for protein synthesis) and T cells genetically deficient in CD80 (B7-1) expression, that both mouse and human T cells stimulated by anti-CD3 mAb actively absorbed CD80 (B7-1) (38). The level of CD80 absorption had a strong correlation with the extent of T cell activation and the level of CD80 expression in APCs (39). The finding that CD28 could invoke T cell absorption of APC-derived molecules led to an effort to identify other receptor-ligand pairs capable of initiating the event and the importance of CD2, CD4, CD8, CD9, and CD27 in the process has been documented (40).
An intriguing issue regarding T cell uptake of APC-derived ligands is the directionality of the molecule transfer; namely, whether the molecule transfer proceeds unidirectionally only from APCs to T cells or if T cell molecules are also transferred to APCs as T cells acquire ligands from APCs. In this regard, several studies are notable. He et al. showed, in a study using the DC2.4 DC cell line pulsed with ovalbumin (OVA) and OT II CD4+ TCR Tg T cells, that antigen-specific T/APC contact resulted in not only transfer of APC-derived molecules (i.e., IAb, CD11c, CD40, CD80) to T cells but also T cell-derived molecules (CD4, CD25, CD69, TCR) to APCs (41). Busch et al. also made a similar observation in a study using several TCR Tg T cells plus purified mouse splenic DCs and human peripheral blood mononuclear cells (PBMCs) plus human monocyte-derived DCs. Further, they noticed that molecule transfer from T cell to APC took place in two separate phases. The first phase occurred rapidly following T/APC contact and the transferred T cell molecules bound firmly to DCs (acid-wash resistant) while the second phase occurred slowly and the transferred molecules bound loosely (acid-wash sensitive) (42). While the results from studies by He et al. and Busch et al. indicated that the molecule transfer was a bidirectional process, it is yet to be clarified whether the molecule transfer from T cell to APC is directed by receptors expressed on T cells (e.g., TCR, CD28) or separate receptors expressed on APCs during their interaction with ligands on T cells (43).
Another topic being debated is the actual biological mechanism for T cells to extract microscopic membrane fragments from APCs. Several potential mechanisms have been implicated. Stinchcomb et al. made a surprising observation in an experiment using electron microscopy that when a cytotoxic CD8+ T cell came in contact with its target cell to form a conjugate, IS-like structure was promptly established at the junction and the apposed plasma membranes fused at a certain site to make a bridge connecting the two cells (44). That observation led to the idea that T cell absorption of APC-derived ligands might occur as the membrane bridge dissolved in the midst of a dynamic membrane rearrangement (13, 15).
It is known that in certain circumstances T cells project a thin and long membrane structure out of plasma membrane called nanotubes, which are generally several hundred nm in diameter and reach 10–30 μm in length. Their structural integrity is maintained by actin cytoskeleton lining the inner wall. It was shown in studies using fluorescence confocal microscopy that a T cell made long-range contact with another T cell through a nanotube. Membrane nanotubes are highly dynamic and elastic in nature so it was postulated that T cell absorption of APC-derived ligands occurred via nanotubes connecting the two cells (13, 45, 46).
An important topic directly related to the biological significance of molecule transfer is the duration of absorbed molecules on the surface of T cells. Studies by Huang et al. (34) and Hwang et al. (28, 32) demonstrated that APC molecules taken up by T cells were internalized and merged with the lysosome, an organelle where impaired biomolecules are destined to be degraded by specific hydrolyzing enzymes. Nevertheless, the absorbed molecules are detected by flow cytometry for a period of time and also recognized by other T cells to mediate an antigen-specific T/T interaction, implying that molecule internalization progresses gradually. It was reported that expression of CD8 on T cells had an effect on the rate of the molecule internalization. Studies by Zhang et al. showed that 2C T cells deficient in CD8 expression (2C.CD8−/−) expressed Ld MHC I molecules transferred from allogeneic APCs for an extended period of time compared to normal 2C (2C.CD8+) T cells (47). Intact actin regulation was found critical for internalization of APC-derived molecules. Thus, while activated (PMA + Ionomycin treated) 2C T cells treated with an inhibitory actin drug (i.e., Latruculin B or Cytochalsin D) still exerted ligand absorption from QL9-loaded Ld-expressing Dros APCs to a reduced but significant level, internalization of those molecules was completely abrogated by the same treatment (32).
The fate of the internalized molecules is also an ongoing topics of investigation. Studies by Huang et al. clearly showed migration of the internalized molecules to lysosome, indicating degradation of the internalized molecules. Nevertheless, the possibility remains that a fraction of the internalized molecules are recycled to the cell surface and expressed along with endogenously expressed molecules.
5.2. In vivo T cell absorption of APC-derived molecules
As noted above, T cell absorption of cell-surface ligands from APCs was first hinted by Hudson et al. in experiments for adoptive transfer of T cells into irradiated allogeneic hosts (17, 18). The ligand absorption by T cells in vivo was also reported in later studies using bone marrow chimeras (23). Those studies provided evidence for the ligand transfer so that donor T cells expressed host-specific MHC molecules.
Hwang et al. also examined in vivo T cell absorption of APC-derived ligands by analyzing rat T cells residing in SCID mice. Both activated and resting rat T cells, obtained after adoptive transfer of purified mature resting rat T cells into SCID mice and after injection of rat embryonic hematopoietic stem cells into neonatal SCID mice, respectively, were found to express a variety of mouse molecules including mB7-1, mB7-2 and mICAM-1 as well as mMHCs (28). Similarly, adoptive transfer of purified mouse T cells deficient in expression of specific cell-surface molecule (i.e., B7-1, B7-2 or ICAM-1) into wild-type syngeneic host mice led to the expression of the missing molecules on the surface of donor T cells (author’s unpublished data).
In addition, Kedl et al. found that APCs carrying specific pMHCs tended to lose those complexes after contact with T cells expressing cognate TCRs (48). While the mechanism underlying the depletion of antigens was not sought, it was implied that the antigen depletion resulted from T cell uptake of pMHCs.
5.3. Biological significance of the ligand-absorption event
One of the most intensely debated subjects concerning T cell absorption of APC-derived ligands is physiological significance of the event. Many groups have independently investigated biological (immunological) significance of the event in their own experimental systems and introduced plausible results, which can be generally grouped into two categories.
A group of reports regarding the biological significance of the event are based on the fact that APC-derived ligands picked up by T cells remained structurally and functionally intact. Thus, class I and II pMHCs transferred from APCs to T cells are recognized by TCRs expressed on other T cells, mediating antigen-specific T cell/T cell (T/T) interaction. Many studies have uncovered immuno-regulatory effects of the T/T interaction, which appeared quite versatile and depended on the nature of absorbed ligands as well as effector functions of the engaged T cells.
Studies by Huang et al. showed that Ld/QL9 complexes picked up by 2C CTLs from Dros APCs were re-presented to other 2C CTLs, leading to homotypic T/T interaction and inducing cross-killing (fratricidal killing) to limit the size of clonal expansion (34). Similarly, many studies illustrated suppression of specific T cell (both CD4+ and CD8+) immune responses by the T/T interaction mediated by the absorbed pMHCs (37, 49–55). Actual mechanisms implied for the immuno-suppression, however, varied from cytotoxic killing of encountered cells and directional delivery of immunosuppressive cytokines to presentation of immuno-suppressive ligands (e.g., HLA-G) (56).
Here, studies by Zhang’s group are of special note (47, 51). Microchimerism is an immunological phenomenon where injection of donor spleen cells into MHC-mismatched recipient mice prior to tissue graft results in prevention of the graft rejection by the recipient host. While the effectiveness of microchimerism is well known, the underlying mechanism had not been clearly understood. In the study using class I MHC mismatched host and recipient mice, they pinpointed an importance of CD4−CD8− double negative (DN) αβ TCR+ T cells in the process. The study showed that while both CD8+ and DN T cells absorbed allo MHCs from the donor spleen cells, the absorbed MHCs stayed for an extended period of time on DN T cells. As a result, the DN T cells continued to make contact with existing or newly developed T cells that recognize the allo-antigens to suppress their immune responses against the allo-antigens.
Other studies illustrated promotion of T cell immune responses via the T/T interaction. That is, APC-derived class I pMHCs, taken up by activated CD4+ T cells from APCs, were re-presented to CD8+ T cells expressing cognate TCRs, mediating antigen-specific CD4+/CD8+ T/T interaction and facilitating directional CD4+ T cell help for CD8+ T cell activation (57–61).
An additional immunological significance of T cell absorption of APC-derived ligands is based on the observation that interaction of T cells with APCs carrying cognate pMHCs resulted in selective depletion of those pMHCs from the APCs. Kedl et al. found that Tg T cells expressing a high affinity TCR for a set of pMHCs made preferential contact with the APCs, depleting those pMHCs from APCs (48). They also provided evidence that as a result of antigen-depletion the high-affinity T cells could separate from the APCs, allowing other Tg T cells, expressing a low affinity TCR for another set of pMHCs, to come into contact with the APCs. Based on those observations, it was suggested that the antigen-depletion mechanism limit the size of clonal expansion of those T cells exhibiting high affinity contact with APCs and allow activation/proliferation of those T cells exhibiting low affinity contact, helping to maintain TCR diversity in a pool of T cells.
5.4. Application of the ligand-absorption event
The finding that a specific TCR/pMHC interaction evokes the absorption of various ligands from APCs provides a way to identify T cells expressing TCRs recognizing specific pMHCs presented by APCs. Indeed, Tomaru et al. attempted to use an immortalized B cell line transfected with a full length green fluorescence protein (GFP)-HLA-A*210 (a human class I MHC) fusion protein to detect a population of CD8+ T cells, in bulk PBMCs, recognizing a peptide derived from a human T-cell lymphotropic virus type 1 (HTLV-1) - or cytomegalo virus (CMV) (62). Flow cytometric analysis of the expression of GFP on T cells cultured with the peptide-loaded APCs in conjunction with MHC-tetramer staining (63) revealed the effectiveness of the method not only for indentifying antigen-specific T cells but also for assessing the functional competency of the antigen-specific T cells in patients infected with the virus; the latter differentiates the GFP absorption assay from the MHC-tetramer binding assay.
6. T CELL ABSORPTION OF PRE-FORMED MEMBRANE VESICLES SHED FROM APCS
6.1. Overview
Cells tend to release membrane vesicles to the extracellular matrix. It is known that a wide variety of cells form an organelle-like structure called the multivesiclular body (MVB) which is filled with a number of small membrane vesicles. These cells secrete those vesicles through MVB fusion to plasma membrane (31). In addition, cells undergoing apoptotic cell death form plasma membrane blebs which eventually shed from the cells (64). It is generally thought that vesicle release is a means of long-range intercellular communication.
The roles of exosomes in T cell immunity have been revealed (31). Studies by Zitvogel’s group showed that injection of exosomes prepared from culture supernatants of tumor cells or dendritic cells (DCs) loaded with tumor antigens mounted a strong tumor-specific CD8+ T cell immune response to eradicate tumor cells in mice (65). Despite the prominent effect of exosomes, mechanisms underlying the action of exosomes are largely unknown, while cross-priming by DCs of tumor-specific antigens along with an adjuvant effect of heat shock proteins expressed in exosomes have been implicated (66, 67).
Studies by Hwang et al. involving Transwell (32) and exosomes (33) isolated from culture supernatants of Dros APCs documented that T cells also absorbed soluble pre-formed membrane vesicles (e.g., exosomes) to express APC-derived ligands. Thus, culture of resting 2C T cells with intact LdB7-1ICAM-1 Dros APCs in Transwell or exosomes released from those cells resulted in expression of both Ld and B7-1. As in the absorption of APC-derived ligands through direct T/APC contact, absorption of LdB7-1ICAM-1 exosomes by resting 2C T cells occurred only when the exosomes were loaded with a cognate peptide (QL9 or p2Ca), demonstrating that TCR/pMHC interaction was requisite for vesicle absorption. Different from absorption through direct T/APC contact, however, TCR/pMHC interaction alone was not sufficient for the absorption of Dros exosomes by resting 2C T cells. Instead, the LFA-1/ICAM-1 interaction was also critically required. Thus, wild type 2C T cells pre-treated with anti-LFA-1 mAb or 2C T cells deficient in LFA-1 expression failed to absorb LdB7-1ICAM-1 Dros exosomes loaded with QL9 (or p2Ca) peptide.
Resting 2C T cells picked up nanometer-scale membrane vesicles prepared by homogenizing plasma membrane of LdB7-1ICAM-1 Dros APCs as well (68). In accord with the results obtained with Dros exosomes, dual receptor/ligand interactions of TCR/pMHC plus LFA-1/ICAM-1 were mandatory for 2C T cell absorption of plasma membrane-derived membrane vesicles (pMVs).
According to the study by Hwang et al., CD28 played a minimal role in resting 2C T cell absorption of Dros exosomes. Its role, however, became apparent as T cells were activated. Thus, PMA + Ionomycin-treated normal B6 T cells, but not the same T cells deficient in CD28 expression (CD28−/−), absorbed LdB7-1 ICAM-1 Dros exosomes to a significant level without peptide loading, which was abrogated by anti-CD28 mAb treatment (32, 33). LFA-1/ICAM-1 interaction also played a pivotal role in vesicle absorption by activated normal T cells, denoting that dual receptor/ligand interactions of CD28/B7-1 plus LFA-1/ICAM-1 were requisite for exosome absorption.
Other studies have also reported T cell uptake of pre-formed membrane vesicles. A study by Prakken et al. using artificial membrane vesicles (liposomes) engineered to express high concentration of class II MHCs (i.e., IAd) showed that liposomes loaded with a cognate peptide (i.e., OVA323–339) bound to Tg T cells expressing a cognate TCRs (DO11.10) (69). In contrast to the results from study by Hwang et al., uptake of the liposomes by DO11.10 Tg T cells took place without the LFA-1/ICAM-1 interaction. While it is yet to be proven, the inconsistency might be a result of the difference in levels of pMHC expression in those vesicles; the level (density) of pMHC expression in liposomes was roughly 100 fold higher than that in LdB7-1ICAM-1 Dros pMVs.
T cell absorption of exosomes derived from physiological APCs (DCs) was shown in a study by Nolte-‘t Hoen et al. as well (70). According to their study, exosome binding occurred only when T cell were activated via treatment with anti-CD3 plus anti-CD28 mAbs and was dependent on LFA-1/ICAM-1 interaction.
6.2. Underlying mechanism(s) for pre-formed vesicle absorption (Figure 2)
Figure 2.
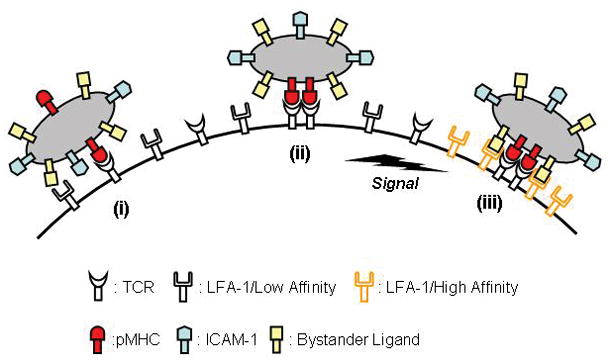
Schematic representation of the process for T cell absorption of pre-formed membrane vesicles shed from APCs. Encounter of a specific receptor in T cell (e.g., TCR) and its cognate ligands in APC-derived vesicles triggers intracellular signaling cascades resulting in activation of LFA-1 function. Then, both affinity and avidity of LFA-1 for ICAM-1 increase via structural change and relocation of the molecules, respectively. As a result, LFA-1s can exhibit firm interaction with ICAM-1s in the vesicles, promoting stability of T/vesicle contact.
The functionality of LFA-1, a T cell integrin, is carefully regulated. LFA-1 exists in low affinity/avidity state in resting T cells. When neighboring signaling receptors, represented by TCR, are triggered to activate intracellular signaling events, LFA-1 is transformed into high affinity/avidity state. But, the transition from low affinity/avidity to high affinity/avidity state is temporary and LFA-1 returns to low affinity/avidity state as the cell signal responsible for LFA-1 activation, called “inside-out” signal, decays (71).
Based on the well-known properties of LFA-1, Kim et. al postulated that T cell absorption of APC-derived pre-formed membrane vesicles was facilitated by “inside-out” signaling. That is, it was proposed that interaction of TCR with cognate pMHCs in those vesicles, or interaction of CD28 in activated T cells with B7-1, induces “inside-out” signaling to promote LFA-1/ICAM-1 interaction and thereby enhance overall avidity and stability of T/vesicle contact. Supporting the idea, the vesicle absorption only occurred when T cells were cultured with Dros pMVs at physiological temperature (37oC) and treatments of T cells with various signaling inhibitors abrogated vesicle absorption. Further, induction of multiple membrane-proximal signaling events was observed promptly following culture of 2C T cells with Dros vesicles expressing cognate pMHCs, including polymerization/reorganization of actin cytoskeleton, activation of the PI3K/Akt signaling axis and activation of the Ca2+ signaling cascade. Entry of extracellular Ca2+ and dynamic redistribution of TCRs following contact of T cells with liposomes expressing cognate pMHCs were also observed in studies by Prakken et al. (69).
Several studies also reported activation/proliferation of resting naive Tg T cells (i.e., 2C CD8+ T cells, Marylin CD4+ T cells) after culture with APC-derived vesicles expressing cognate pMHCs (i.e., exosomes and pMVs from Dros APCs or exosomes from a activated DC cell line) and highlighted the importance of LFA-1/ICAM-1 interaction in T cell activation/proliferation by APC-derived vesicles (33, 72).
6.3. Application of pre-formed vesicle absorption
The finding that nanometric membrane vesicles derived from APCs (exosomes and pMVs) bind to T cells in an antigen-specific manner to induce intracellular signaling cascades for T cell activation/proliferation reveals potential applications of those vesicles.
MAbs specific to signaling receptors (e.g., TCR, CD28, LFA-1) in conjunction with secondary Abs cross-linking the mAb/receptor complexes have been widely used for investigating T cell signaling (73, 74). Investigation of T cell signaling events using mAbs, instead of cells (i.e., physiological and artificial APCs) expressing natural ligands, have several advantages. Firstly, as each mAb targets a specific receptor, it is easy to probe the function(s) of individual receptor in T cell signaling. Secondly, biochemical changes induced upon mAb treatment can be interrogated immediately; when APCs are used for T cell activation, however, T cells must be separated from APCs before biochemical analysis, which is often time-consuming and requires a complex methodology. Thirdly, real-time analysis of physiological change in a population of T cells is feasible using flow cytometry. Despite those advantages, the physiological relevance of results from experiments using mAbs are often questioned as binding affinities of mAbs to their corresponding receptors are exceptionally high and cross-linking of mAb/receptor complexes with secondary Abs tends to lead to massive capping of receptors.
Micro-vesicles prepared from plasma membrane of Dros APCs (pMVs) have the same advantages as mAbs. Namely, (i) Dros pMVs expressing a set of defined ligand(s) interact with only selected receptors, (ii) T cells can be analyzed immediately after culture with pMVs without a purification process, (iii) real-time analysis using flow cytometry of a population of T cells for a specific signaling process is feasible during culture with pMVs. Further, as physiological ligands are used for triggering receptors of interest, data obtained with Dros pMVs may have superior physiological relevance. In an attempt to use Dros pMVs to investigate Ca2+ signaling induced upon TCR triggering, Kim et al. found a critical role of LFA-1 in the Ca2+ signaling and proposed a novel mechanism for entry of extracellular Ca2+ (75).
Efforts to use exosomes derived from tumor cells or DCs loaded with tumor antigens as a cell-free vaccine have been made extensively by Zitvogel’s group, proving their clinical efficacy in mounting tumor-specific T cell immunity. Yet, the difficulty in obtaining a large quantity of exosomes hampers their clinical use (65). As an alternative approach, Kovar et al. prepared nanometer-scale membrane vesicles from plasma membrane of activated DCs (DC2.4 cell line) and used them as a cell-free tumor vaccine. Prakken et al. also attempted to use liposomes expressing cognate pMHCs as a cell-free vaccine for promoting antigen-specific T cell immunity and showed their in vivo efficacy (76).
Acknowledgments
We thank Cookie Santamaria for her administrative assistance. This work was supported by grants from National Institute of Heath to I.H. (AI066146, MH085707-01, MH084690-3R74G).
Abbreviations
- APC
antigen presenting cell
- B6
C57BL/6
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- Dros
Drosophila
- GFP
green fluorescence protein
- ICAM-1
intercellular adhesion molecule-1
- IS
immunological synapse
- LFA-1
lymphocyte function-associated antigen-1
- MBP
myelin basic protein
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- MVB
multivesicular body
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cell
- pMHC
peptide/MHC complex
- PMA
phorbol 12-myristate 13-acetate
- pMV
plasma membrane-derived vesicle
- TCR
T cell receptor
- Tg
transgenic
References
- 1.Beutler B. Innate immune responses to microbial poisons: discovery and function of the Toll-like receptors. Annu Rev Pharmacol Toxicol. 2003;43:609–28. doi: 10.1146/annurev.pharmtox.43.100901.135729. [DOI] [PubMed] [Google Scholar]
- 2.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 3.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–86. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 4.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–74. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 5.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 6.Yuseff MI, Lankar D, Lennon-Dumenil AM. Dynamics of membrane trafficking downstream of B and T cell receptor engagement: impact on immune synapses. Traffic. 2009;10:629–36. doi: 10.1111/j.1600-0854.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dustin ML. Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu Rev Cell Dev Biol. 2008;24:577–96. doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 9.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 10.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 12.Randolph DA, Fathman CG. CD4+CD25+ regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 13.Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Lett. 2009;583:1792–9. doi: 10.1016/j.febslet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Smyth LA, Afzali B, Tsang J, Lombardi G, Lechler RI. Intercellular transfer of MHC and immunological molecules: molecular mechanisms and biological significance. Am J Transplant. 2007;7:1442–9. doi: 10.1111/j.1600-6143.2007.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wetzel SA, Parker DC. MHC transfer from APC to T cells following antigen recognition. Crit Rev Immunol. 2006;26:1–21. doi: 10.1615/critrevimmunol.v26.i1.10. [DOI] [PubMed] [Google Scholar]
- 16.Sprent J. Swapping molecules during cell-cell interactions. Sci STKE. 2005;2005:pe8. doi: 10.1126/stke.2732005pe8. [DOI] [PubMed] [Google Scholar]
- 17.Hudson L, Sprent J. Specific adsorption of IgM antibody onto H-2-activated mouse T lymphocytes. J Exp Med. 1976;143:444–9. doi: 10.1084/jem.143.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson L, Sprent J, Miller JF, Playfair JH. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature. 1974;251:60–2. doi: 10.1038/251060a0. [DOI] [PubMed] [Google Scholar]
- 19.Krammer PH, Hudson L, Sprent J. Fc-receptors, Ia-antigens, and immunoglobulin on normal and activated mouse T lymphocytes. J Exp Med. 1975;142:1403–15. doi: 10.1084/jem.142.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorber MI, Loken MR, Stall AM, Fitch FW. I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J Immunol. 1982;128:2798–803. [PubMed] [Google Scholar]
- 21.Nagy Z, Elliott BE, Nabholz M. Specific binding of K- and I-region products of the H-2 complex to activated thymus-derived (T) cells belonging to different Ly subclasses. J Exp Med. 1976;144:1545–53. doi: 10.1084/jem.144.6.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy Z, Elliott BE, Nabholz M, Krammer PH, Pernis B. Specific binding of alloantigens to T cells activated in the mixed lymphocyte reaction. J Exp Med. 1976;143:648–59. doi: 10.1084/jem.143.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharrow SO, Mathieson BJ, Singer A. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J Immunol. 1981;126:1327–35. [PubMed] [Google Scholar]
- 24.Merkenschlager M. Tracing interactions of thymocytes with individual stromal cell partners. Eur J Immunol. 1996;26:892–6. doi: 10.1002/eji.1830260426. [DOI] [PubMed] [Google Scholar]
- 25.Arnold PY, Mannie MD. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur J Immunol. 1999;29:1363–73. doi: 10.1002/(SICI)1521-4141(199904)29:04<1363::AID-IMMU1363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol. 1999;163:5201–10. [PubMed] [Google Scholar]
- 27.Cai Z, Brunmark A, Jackson MR, Loh D, Peterson PA, Sprent J. Transfected Drosophila cells as a probe for defining the minimal requirements for stimulating unprimed CD8+ T cells. Proc Natl Acad Sci U S A. 1996;93:14736–41. doi: 10.1073/pnas.93.25.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–48. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 30.Brezinschek RI, Oppenheimer-Marks N, Lipsky PE. Activated T cells acquire endothelial cell surface determinants during transendothelial migration. J Immunol. 1999;162:1677–84. [PubMed] [Google Scholar]
- 31.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 32.Hwang I, Sprent J. Role of the actin cytoskeleton in T cell absorption and internalization of ligands from APC. J Immunol. 2001;166:5099–107. doi: 10.4049/jimmunol.166.8.5099. [DOI] [PubMed] [Google Scholar]
- 33.Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci U S A. 2003;100:6670–5. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 35.Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166:3645–9. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- 36.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111:5621–8. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetzel SA, McKeithan TW, Parker DC. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174:80–9. doi: 10.4049/jimmunol.174.1.80. [DOI] [PubMed] [Google Scholar]
- 38.Sabzevari H, Kantor J, Jaigirdar A, Tagaya Y, Naramura M, Hodge J, Bernon J, Schlom J. Acquisition of CD80 (B7-1) by T cells. J Immunol. 2001;166:2505–13. doi: 10.4049/jimmunol.166.4.2505. [DOI] [PubMed] [Google Scholar]
- 39.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169:6162–9. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 40.Hudrisier D, Aucher A, Puaux AL, Bordier C, Joly E. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J Immunol. 2007;178:3637–47. doi: 10.4049/jimmunol.178.6.3637. [DOI] [PubMed] [Google Scholar]
- 41.He T, Tang C, Liu Y, Ye Z, Wu X, Wei Y, Moyana T, Xiang J. Bidirectional membrane molecule transfer between dendritic and T cells. Biochem Biophys Res Commun. 2007;359:202–8. doi: 10.1016/j.bbrc.2007.05.099. [DOI] [PubMed] [Google Scholar]
- 42.Busch T, Sirbu H, Zenker D, Dalichau H. Vascular complications related to intraaortic balloon counterpulsation: an analysis of ten years experience. Thorac Cardiovasc Surg. 1997;45:55–9. doi: 10.1055/s-2007-1013687. [DOI] [PubMed] [Google Scholar]
- 43.Daubeuf S, Lindorfer MA, Taylor RP, Joly E, Hudrisier D. The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. J Immunol. 184:1897–908. doi: 10.4049/jimmunol.0901570. [DOI] [PubMed] [Google Scholar]
- 44.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5:261–9. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–6. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6:782–9. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 48.Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 49.Cox JH, McMichael AJ, Screaton GR, Xu XN. CTLs target Th cells that acquire bystander MHC class I-peptide complex from APCs. J Immunol. 2007;179:830–6. doi: 10.4049/jimmunol.179.2.830. [DOI] [PubMed] [Google Scholar]
- 50.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A. Isolation and characterization of human antigen-specific TCR αβ+ CD4−CD8− double-negative regulatory T cells. Blood. 2005;105:2828–35. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- 51.Ford McIntyre MS, Young KJ, Gao J, Joe B, Zhang L. Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J Immunol. 2008;181:2271–5. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- 52.Hao S, Yuan J, Xu S, Munegowda MA, Deng Y, Gordon J, Xing Z, Xiang J. Antigen specificity acquisition of adoptive CD4+ regulatory T cells via acquired peptide-MHC class I complexes. J Immunol. 2008;181:2428–37. doi: 10.4049/jimmunol.181.4.2428. [DOI] [PubMed] [Google Scholar]
- 53.Helft J, Jacquet A, Joncker NT, Grandjean I, Dorothee G, Kissenpfennig A, Malissen B, Matzinger P, Lantz O. Antigen-specific T-T interactions regulate CD4 T-cell expansion. Blood. 2008;112:1249–58. doi: 10.1182/blood-2007-09-114389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, Carosella ED. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–8. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 55.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101:2704–10. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 56.Wischhusen J, Waschbisch A, Wiendl H. Immune-refractory cancers and their little helpers--an extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin Cancer Biol. 2007;17:459–68. doi: 10.1016/j.semcancer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Adamopoulou E, Diekmann J, Tolosa E, Kuntz G, Einsele H, Rammensee HG, Topp MS. Human CD4+ T cells displaying viral epitopes elicit a functional virus-specific memory CD8+ T cell response. J Immunol. 2007;178:5465–72. doi: 10.4049/jimmunol.178.9.5465. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy R, Undale AH, Kieper WC, Block MS, Pease LR, Celis E. Direct cross-priming by th lymphocytes generates memory cytotoxic T cell responses. J Immunol. 2005;174:3967–77. doi: 10.4049/jimmunol.174.7.3967. [DOI] [PubMed] [Google Scholar]
- 59.Shi M, Hao S, Chan T, Xiang J. CD4 (+) T cells stimulate memory CD8 (+) T cell expansion via acquired pMHC I complexes and costimulatory molecules, and IL-2 secretion. J Leukoc Biol. 2006;80:1354–63. doi: 10.1189/jlb.0506321. [DOI] [PubMed] [Google Scholar]
- 60.Umeshappa CS, Huang H, Xie Y, Wei Y, Mulligan SJ, Deng Y, Xiang J. CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses. J Immunol. 2009;182:193–206. doi: 10.4049/jimmunol.182.1.193. [DOI] [PubMed] [Google Scholar]
- 61.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- 62.Tomaru U, Yamano Y, Nagai M, Maric D, Kaumaya PT, Biddison W, Jacobson S. Detection of virus-specific T cells and CD8+ T-cell epitopes by acquisition of peptide-HLA-GFP complexes: analysis of T-cell phenotype and function in chronic viral infections. Nat Med. 2003;9:469–76. doi: 10.1038/nm845. [DOI] [PubMed] [Google Scholar]
- 63.McHeyzer-Williams MG, Altman JD, Davis MM. Enumeration and characterization of memory cells in the TH compartment. Immunol Rev. 1996;150:5–21. doi: 10.1111/j.1600-065x.1996.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 64.Nusbaum P, Laine C, Seveau S, Lesavre P, Halbwachs-Mecarelli L. Early membrane events in polymorphonuclear cell (PMN) apoptosis: membrane blebbing and vesicle release, CD43 and CD16 down-regulation and phosphatidylserine externalization. Biochem Soc Trans. 2004;32:477–9. doi: 10.1042/BST0320477. [DOI] [PubMed] [Google Scholar]
- 65.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 66.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 68.Kim K, Wang L, Hwang I. A novel flow cytometric high throughput assay for a systematic study on molecular mechanisms underlying T cell receptor-mediated integrin activation. PLoS One. 2009;4:e6044. doi: 10.1371/journal.pone.0006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prakken B, Wauben M, Genini D, Samodal R, Barnett J, Mendivil A, Leoni L, Albani S. Artificial antigen-presenting cells as a tool to exploit the immune ‘synapse’. Nat Med. 2000;6:1406–10. doi: 10.1038/82231. [DOI] [PubMed] [Google Scholar]
- 70.Nolte-‘t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–81. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 71.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–62. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–23. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 73.June C, Moore J. Measurement of Intracellular Ions by Flow Cytometry. Current protocol in cytometry. 2004 doi: 10.1002/0471142735.im0505s64. [DOI] [PubMed] [Google Scholar]
- 74.Moretta A, Ciccone E, Pantaleo G, Tambussi G, Bottino C, Melioli G, Mingari MC, Moretta L. Surface molecules involved in the activation and regulation of T or natural killer lymphocytes in humans. Immunol Rev. 1989;111:145–75. doi: 10.1111/j.1600-065x.1989.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 75.Kim K, Wang L, Hwang I. LFA-1-dependent Ca2+ entry following suboptimal T cell receptor triggering proceeds without mobilization of intracellular Ca2+ J Biol Chem. 2009;284:22149–54. doi: 10.1074/jbc.M109.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kovar M, Boyman O, Shen X, Hwang I, Kohler R, Sprent J. Direct stimulation of T cells by membrane vesicles from antigen-presenting cells. Proc Natl Acad Sci U S A. 2006;103:11671–6. doi: 10.1073/pnas.0603466103. [DOI] [PMC free article] [PubMed] [Google Scholar]


