Abstract
Idiopathic interstitial lung diseases (iILDs) are characterized by inflammation, hyperplasia of Type-II alveolar epithelial cells (AECs) and lung remodelling often with progressive fibrosis. It remains unclear which signals initiate iILD and/or maintain the disease processes. Using real-time RT-PCR and immunohistochemistry on archival biopsies of three patterns of iILD (usual interstitial pneumonitis/UIP, non-specific interstitial pneumonitis/NSIP and cryptogenic organizing pneumonia/COP) we investigated whether hedgehog signalling (previously associated with lung damage and repair) was functional and whether the damage associated extracellular matrix protein tenascin-C was present in activated Type-II AECs in all three iILDs. Using tissue culture, protein and mRNA detection we also determined how two signals (oxidative damage and TGF-β) associated with iILD pathogenesis affected Sonic hedgehog (SHH) and tenascin-C production by a Type-II AEC cell line. We report that SHH pathway and tenascin-C mRNA and proteins were found in UIP, NSIP and COP. SHH signalling was most active at sites of immature organizing fibrous tissue (fibroblastic foci) in UIP. In vitro Type-II AECs constitutively secrete SHH but not tenascin-C. Oxidative injury stimulated SHH release whereas TGF-β inhibited it. TGF-β and oxidative damage both upregulated tenascin-C mRNA but only TGF-β induced synthesis and release of a distinct protein isoform. SHH signalling is active in Type-II AECs from three types of ILD and all three express tenascin-C.
Keywords: fibrosis, interstitial lung disease, oxidative damage, remodelling, Sonic hedgehog signalling, tenascin-C, TGF-β
Idiopathic interstitial lung diseases (iILDs) are characterized by inflammation, remodelling and in many cases progressive fibrosis. Sub-classification within the iILDs is determined by what are frequently overlapping clinical observations and a very limited number of histological patterns which may relate to clinical progress (ATS/ERS guidelines 2000, 2002). The remodelling is thought to result from abnormal interactions between damaged alveolar epithelial cells (AECs) and fibroblasts which may be modulated by, and/or cause, associated inflammation (Wallace et al. 2007). The mechanisms involved in progressive fibrosis are not understood and it is unclear whether specific histological remodelling patterns reflect distinct molecular processes.
All iILDs have juxtaposed normal, remodelling and in most cases fibrotic lung areas. This implies a balance between damage and remodelling in these areas. Although initiating damage signals remain unknown these result in Type-I AEC injury and activate regenerative Type-II AECs. As patients only present with established disease it is unclear whether signals which initiate and those which maintain lung damage are the same. Oxidative stress is thought to be involved and patients have increased levels of exhaled H2O2 and ethane (Kanoh et al. 2005). The pro-fibrotic cytokine TGF-β can upregulate reactive oxygen species (Proell et al. 2007) and some iILD patients have been shown to have low levels of extracellular superoxide dismutase (Kinnula et al. 2006; Gao et al. 2008) suggesting greater susceptibility to oxidant damage.
In this study we hypothesized that a common remodelling signalling process might underlie three of the most common histological patterns of iILD, usual interstitial pneumonitis (UIP), non-specific interstitial pneumonitis (NSIP) and cryptogenic organizing pneumonia (COP) and therefore represent a common target for future therapeutic intervention.
UIP, the commonest iILD pattern, responds poorly to therapy with a median survival of 3–5 years. The histology is characterized by destructive fibrosis and immature ‘fibroblastic foci’. The fibroblastic foci are believed to represent active remodelling at sites of AEC injury (White et al. 2003; Selman & Pardo 2006). Hyperplastic Type-II AECs overlie fibroblastic foci and the alveolar surface in adjacent areas of mature collagenized fibrous tissue (Honda et al. 2002; Wallace et al. 2007). NSIP is an iILD with more even alveolar wall fibrosis and hyperplastic Type-II AECs but no fibroblastic foci (Katzenstein & Fiorelli 1994). NSIP has a variable outcome but prognosis is better than for UIP (Daniil et al. 1999). COP is an iILD characterized by hyperplastic Type-II AECs and nodular buds of organizing exudate in alveolar spaces. These buds, composed of myofibroblasts and immature, myxoid stromal elements, are morphologically similar to UIP fibroblastic foci. Patients with COP usually respond well to steroids and have a good prognosis.
Type-II AECs can influence remodelling and fibrosis via direct contacts with fibroblasts, cytokine and growth factor secretion, and extracellular matrix (ECM) protein production. Tenascin-C is an ECM protein associated with epithelial damage, inflammation and wound healing (Chiquet-Ehrismann & Chiquet 2003). Tenascin-C protein was found in UIP (Wallace et al. 1995) and COP (Kuhn & Mason 1995) and the gene was upregulated in UIP and NSIP (Yang et al. 2007). The tenascin-C promoter contains a SMAD recognition site (Jinnin et al. 2004) and its expression is upregulated by TGF-β (Hau et al. 2006; Liu et al. 2006). We previously showed that cross linking of a UIP-associated autoantigen on Type-II AECs upregulated tenascin-C and TGF-β secretion in vitro (Wallace & Howie 2001). Although TGF-β induces experimental lung fibrosis (Chua et al. 2005; Ask et al. 2006; Decologne et al. 2007) and is present in iILD lungs (Khalil et al. 2001; Stewart et al. 2003) there is relatively little direct information on its contribution to iILD. Fibroblasts from UIP patients are reported to be hyper-responsive to TGF-β (Murray et al. 2008) and patients with NSIP and UIP have enhanced levels of serum thrombospondin which is known to activate TGF-β (Ide et al. 2008).
Like both TGF-β (Chen et al. 2005; Warburton et al. 2005) and tenascin-C (Roth-Kleiner et al. 2004; Cardoso & Lu 2006), Sonic hedgehog (SHH) signalling is essential for lung morphogenesis (Pepicelli et al. 1998; Warburton et al. 2005; White et al. 2006) and is upregulated by AECs after acute injury (Watkins et al. 2003). We previously demonstrated that SHH and its receptor Patched1 (PTC1) are expressed focally by Type-II AECs and inflammatory cells in UIP (Stewart et al. 2003) and others have confirmed SHH mRNA expression in UIP and NSIP (Coon et al. 2006). When SHH binds to PTC1 the GLI zinc-finger family of transcription factors is activated (Riobo & Manning 2007), GLI1 expression is upregulated (Ahn & Joyner 2005; Lamm et al. 2002) and nuclear GLI1 staining is reported to correlate with active SHH signalling in human biopsies (Mori et al. 2006).
In this study we wished to determine whether functional SHH signalling in Type-II AECs was a common feature of different histological patterns of iILDs and whether or not oxidative damage or exposure to TGF-β might affect SHH and/or tenascin-C release by activated Type-II AECs.
Materials and methods
Ethical permission
The study had ethical and managerial approval from NHS Lothian for the use of anonymized tissue blocks from the pathology department archive at the Royal Infirmary of Edinburgh.
Study cases
Formalin-fixed, paraffin-embedded thoracoscopic lung biopsies from 15 archival, iILD cases were selected for disease-specific histology where diagnosis matched clinical and radiological features (UIP 3M, 3F, ages 61–73; COP 3M, 3F, ages 35–68; NSIP 1M, 2F, ages 37–57). All biopsies were taken prior to any treatment. Control lung tissue was obtained from macroscopically and microscopically normal lung blocks from cancer resections taken as far away as possible from the tumour (6F, age range 61–72).
Reagents
Unless otherwise stated, reagents came from Sigma Aldrich, Poole, UK.
Immunohistochemistry
Sections (3 μm) were dewaxed, rehydrated and microwaved in citric-acid antigen unmasking solution (Vector Laboratories, Peterborough, UK). Non-specific binding was blocked with 3% H2O2 followed by avidin–biotin blocking (Vector Laboratories). Sections were stained with anti-SHH, cat No. sc-1194, anti-PTC1, cat No. sc-6147, (both affinity purified goat polyclonal IgG, Santa Cruz Biotechnology Inc., CA, USA), anti-tenascin-C (cat No. NCL-TENAS-C mouse monoclonal, Leica Microsystems, Milton Keynes, UK) or anti-GLI1 (cat No. ab49314, affinity purified rabbit polyclonal IgG, Abcam, Cambridge, UK) antibodies overnight at 4 C and detected with affinity purified, biotinylated secondary antibodies (all from Dako Cytomation, Ely, UK). Staining was visualized with ABC-peroxidase (Vector) followed by diaminobenzidene (Dako). Stained sections were examined by a specialist lung pathologist (WAHW). % GLI1 nuclear positivity in Type-II AECs was estimated by counting at least 200 cells in at least 10 different fields at 400× magnification. Antibody specificity was confirmed by using relevant peptides and by Western blotting (not shown). Control (no primary antibody) sections were included in every run and were always negative (not shown).
Antibody purification
Mouse-IgG1-monoclonal-anti-SHH (5E1; Developmental Studies Hybridoma Cell Bank, Iowa City, IA, USA) was purified from culture supernatant using protein-G columns (Amersham Pharmacia Biotech, Bucks, UK), aliquoted and stored at −20 °C (Lowrey et al. 2002; Stewart et al. 2002).
Cell culture
All cultures were kept at 37 °C in a humidified, 5% CO2 incubator. The well-characterized human lung-carci-noma-derived Type-II AEC line A549 (Health Protection Agency Culture Collections, Salisbury, UK) was passaged in RPMI1640, supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 5% FCS. For experiments cells were plated at 1.3 × 105/ml in serum-containing medium for 24 h; for oxidative injury cells were washed with fresh serum-free medium, incubated for 90 min in serum-free medium alone or containing various concentrations of H2O2; washed and fresh medium added. For tenascin-C Western blots, supernatants were concentrated to approx 50 μl using 100 kDa cut-off Centricon filters (Millipore Ltd, Watford, UK). All experiments were repeated three to six times with two to four replicates/group/experiment.
MTT assay
Fifty-μl 5 mg/ml MTT in PBS was added to 450 μl serum-free cultures of A549 cells in 24-well plates and incubated at 37 °C for 45 min. Medium was removed and incorporated MTT solubilized by addition of 350 μl DMSO. 100 μl from each well was transferred to a 96-well plate and absorbance read at 490 nm with a 630 nm reference filter.
RNA analysis
All primers and probes used are detailed in Table 1.
Table 1.
Primers and probes for RT-PCR and real-time RT-PCR
| Gene (method) | Forward primer | Reverse primer | Probe | Amplicon size (bp) |
|---|---|---|---|---|
| Tenascin C (real-time PCR) | CTGCTCCCAAGCAATGCTGAA | CTTCCAGCGCCTGAGCCTTATCACCAT | AGACACGACCTCTGGCCTCTACACCATTTATC | 89 |
| GLI1 (real-time PCR) | GGGCACCATCCATTTCTACAGT | TCAGTCTGCTTTCCTCCCTGAT | AGCCCAAGAGGGAGCGGGAAGG | 77 |
| GAPDH (real-time PCR) | AAGGACTCATGACCACAGTCCAT | CCATCACGCCACAGTTTCC | CCATCACTGCCACCCAGAAGACTGTG | 84 |
| SHH (conventional PCR) | ACTGGGTGTACTACGAGTCCAAGG | AAAGTGAGGAAGTCGCTGTAGAGC | n/a | 211 |
| PTC1 (conventional PCR) | TCCTCGTGTGCGCTGTCTTCCTTC | CGTCAGAAAGGCCAAAGCAACGTGA | n/a | 202 |
| SMO (conventional PCR) | CTGGTACGAGGACGTGGAGG | AGGGTGAAGAGCGTGCAGAG | n/a | 140 |
| GLI1 (conventional PCR) | ACTGAAGACCTCTCCAGC | GCTGACAGTATAGGCAGA | n/a | 244 |
| GLI2 (conventional PCR) | TGGCCGCTTCAGATGACAGATGTTG | CGTTAGCCGAATGTCAGCCGTGAAG | n/a | 200 |
Nucleotide sequences of the primers and probes used and the expected sizes of the amplicons.
(a) Extraction
Formalin fixed material: Paraffin wax embedded sections (10 μm) were stored in 1.5 ml Eppendorf tubes at room temperature in the dark. Preliminary experiments established that storage of pre-cut material for up to 6 weeks had no effect on RNA quality. Material was microfuged (2 min 13,000 g), 1 ml xylene added for 10 min and tubes spun (1 min, 13,000 g). Supernatant was removed and this process repeated with 100%, 70% and 50% ethanol followed twice by 1 ml RNase free water. 150 μl of RLT containing 2-mercapto-ethanol, 290 μl DEPC water and 10 μl proteinase-K (proteinase-K digestion buffer) was then added to each pellet. Tubes were incubated overnight in a shaking incubator at 55 °C. The sample was then either frozen at −70 °C or applied directly to a Qiagen (Crawley, UK) RNeasy Microkit as per manufacturer’s instructions.
(b) Cells
A549 Cells were lysed with RLT. Samples were diluted and incubated overnight with proteinase-K digestion buffer. RNA was extracted with Qiagen mini columns following manufacturer’s instructions.
Reverse transcription
Absence of DNA contamination was always confirmed by β-Actin PCR as described (Lowrey et al. 2002; Stewart et al. 2002). RNA was reverse transcribed using Applied Biosystems (Warrington, UK) reagents in a reaction mix of Taq Buffer 2 μl, MgCl2 4.4 μl, dNTPs 4 μl, random hexamers 1 μl, RNase Inhibitors 0.4 μl, Multiscribe 0.5 μl, and RNA. For formalin fixed material, 7.7 μl total RNA solution was added. For cell line derived material, 400 ng RNA in 7.7 μl was added. Samples were run at 25 °C: 10 min; 48 °C: 40 min; 95 °C: 5 min, 4 °C hold step in a Px2 thermal cycler (Thermo Electron Corporation, Basingstoke, UK).
Conventional PCR
cDNA was placed in 20 μl PCR reactions with primers for either SHH (63 °C, 2 nM dNTP, 125 nM Mg), SMO (62 °C, 2 nM dNTP, 62.5 nM Mg), PTC1, GLI1 or GLI2 (62 °C, 4 nM dNTP, 62.5 nM Mg) for 40 cycles with gold taq polymerase (BioGene, Kimbolton, UK) as reported previously (Stewart et al. 2003). Products were visualized after 2% agarose gel electrophoresis using a Versadoc 4000 imaging system (Bio-Rad Laboratories, Hemel Hempstead, UK). No sample negative controls were always run.
Real-time PCR
Probes, primers and reagents came from Applied Biosystems. Reactions were multiplexed with an internal control (18S or GAPDH). 25 μl reaction mixes contained 4.25 μl cDNA; gene of interest primers (300 nM each) and FAM labelled probe (200 nM); and, GAPDH primers (300 nM each) and VIC labelled probe (200 nM), or 18S endogenous control mix. Reactions were run on an ABI Prism Detector (Applied Biosystems) at 50 °C 2 min, 95 °C 10 min followed by 95 °C 15 s and 60 °C 1 min for 40 cycles. All samples tested for each set of primers and probes and no sample negative controls were always run in triplicate on the same plates.
SHH ELISA
Culture supernatants were coupled to EIA plates (Corning-Costar, Fisher Scientific, Loughborough, UK) 1:1 vol:vol in 0.05 M carbonate–bicarbonate buffer, pH 9.6. Each plate had an 8-point standard curve of recombinant SHH (R&D Systems) diluted 1:1 in culture medium carbonate–bicarbonate buffer starting at 200 ng/ml. All standards and samples were plated at 50 μl per well, plates were incubated overnight at 4 °C, washed, blocked with 1% BSA for 2 h, washed and 30 ng/well 5E1 antibody in 0.1% BSA/PBS-T added for 2 h at RT. Plates were washed and 12.5 ng/well biotinylated-goat-anti-mouse-IgG antibody (R&D Systems) added for 2 h. Antibody binding was visualized with peroxidase-streptavidin/biotin and substrate A&B (R&D Systems). The colorimetric reaction was stopped with 2 N H2SO4. Specificity was shown by blocking the detection antibody with varying concentrations of rSHH (not shown). From multiple repeats (>50) the lower limit of detection was 6 ng/ml (not shown).
Western blotting
Samples were electrophoresed using NuPage™ precast gels (Invitrogen, Paisley, UK), transferred to Hybond PDVF membranes (Amersham Pharmacia Biotech), blocked with 5% milk powder and detected using goat anti-Shh (Santa-Cruz), rat-monoclonal anti-tenascin-C (R&D Systems) and rabbit polyclonal anti-β-actin (Abcam). Secondary antibodies were horseradish-peroxidase-conjugated-goat-anti-rat-IgG (Santa Cruz), or rabbit-anti-goat-IgG or goat-anti-rabbit-IgG (Dako Cytomation). Bands were visualized by chemiluminescence using ‘Supersignal’ (Thermo Scientific, Cramlington, UK) for SHH and tenascin-C, and ECL-plus (GE-Healthcare, Little Chalfont, UK) for β-actin, on the Versadoc 4000 imaging system.
Statistical analysis
Mann–Whitney U-test was used to compare biopsies and Student’s t-test [unpaired with Welch’s correction] was used to compare in vitro treatments with GraphPad Prism™ software. P values < 0.05 were considered significant.
Results
GLI1 and tenascin mRNA is expressed in UIP and NSIP
To confirm previous reports that GLI1 and tenascin message could be detected in lung tissue we extracted RNA from whole formalin fixed paraffin embedded sections of each type of iILD and from non-ILD lung tissue obtained from cancer resection cases which was microscopically normal. To avoid inconsistencies encountered using amplification of isolated mRNA, we used non-amplified samples for analysis. Three cases of each iILD and three non-ILD cases were compared by real-time RT-PCR. To take into account differences in the amounts of tissue present and RNA extracted, real-time reactions were multiplexed to include the gene of interest detected and a housekeeping gene, GAPDH, selected on the basis of proven efficiency using formalin cross-linked material (data not shown). In light of publications (Chambers 2002; Kriegova et al. 2008) regards variability with GAPDH we use Figure 1 as evidence of expression of transcript rather than comparison of expression levels. Figure 1b illustrates that COP sections had significantly less disease involved lung tissue than those from UIP or NSIP which both had very similar levels and it had to be borne in mind that this might skew the COP data. Laser microdissection of diseased areas was tried but without amplification of mRNA insufficient material for analysis was obtained.
Figure 1.

GLI1 and tenascin-C in mRNA extracted from paraffin sections. (a) Real-time PCR as evidence of expression of genes in COP, UIP, NSIP and microscopically normal lung sections (three cases of each condition were analysed by determining the mean value per case from three separate 10 μm sections). Data are expressed as means ± SEM relative to GAPDH levels multiplexed in the same samples. (b) Percentage of disease involved tissue on biopsy sections. COP biopsies (n = 7 cases) had significantly less involved tissue per section than UIP (n = 7 cases) or NSIP (n = 3 cases) sections.
Tenascin-C is expressed in UIP, COP and NSIP
To confirm our findings of mRNA expression, immunohistochemical analysis was performed. Tenascin-C was expressed in biopsies from patients with all three patterns of iILD. The pattern of expression was very consistent between the cases of the different types of iILD. In UIP expression was in all the fibroblastic foci (Figure 2a); in COP there was focal expression in the buds of organizing exudate that characterize the condition (Figure 2b) whilst in NSIP there was very focal expression within the thickened fibrotic alveolar walls (Figure 2c).
Figure 2.
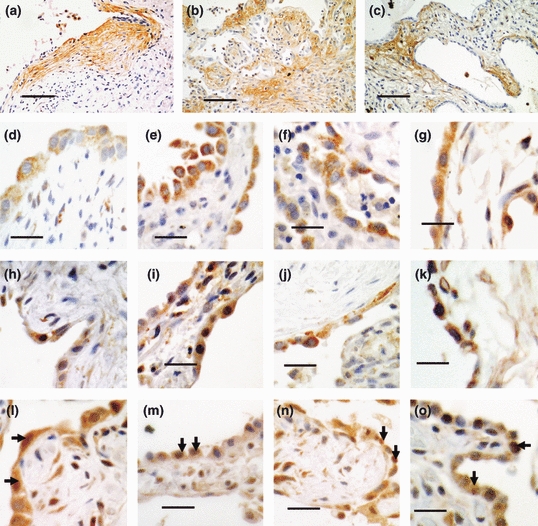
Immunohistochemistry of iILDs. Pictures representative of 6 UIP, 6 COP and 3 NSIP cases are shown. (a–c) Tenascin-C staining in fibroblastic foci of UIP (a); in buds of organizing exudate in COP (b); and, focal expression within thickened, fibrotic alveolar walls in NSIP (c). Black bars = 200 μm. (d–o) SHH signalling components in Type-II AECs in iILD. SHH (d–g), PTC1 (h–k) and GLI1 (l–o) staining in fibroblastic foci of UIP (d, h, l), non-fibroblastic foci of UIP (e, i, m), COP (f, j, n) and NSIP (g, k, o). Note the GLI1 positive nuclei in i–o. Black bars = 50 μm.
SHH signalling pathway proteins in remodelling Type-II alveolar epithelial cells in different types of human ILD
We previously reported that SHH and its PTC1 receptor are expressed in Type-II AECs in UIP (Stewart et al. 2003). To determine whether this was common to other iILDs and whether active signalling was present, we stained sections for SHH, PTC1 and the downstream transcription factor GLI1. All the proteins were present in Type-II AECs in all the biopsies (Figure 2d–o). Nuclear GLI1 staining (examples arrowed) indicated active SHH signalling in Type-II AECs in UIP, NSIP and COP.
In UIP SHH signalling pathway is differentially active in Type-II alveolar epithelial cells
To determine any differences in SHH signalling activity, the % of nuclear GLI1 + ve Type-II AECs in diseased lung was counted for each case. There was no overall significant difference between COP, NSIP and UIP (Figure 3a). However, there was a significantly greater % of nuclear GLI1 + ve Type-II AECs overlying the fibroblastic foci in UIP cases compared to those overlying mature collagenized fibrous tissue in the same sections (Figure 3b).
Figure 3.

Nuclear GLI1 is increased in active fibrosis. (a) No statistically significant difference in the percentage of type-II AECs showing nuclear staining for GLI1 was detected between the UIP, COP and NSIP cases studied. (b) There was however a significant difference between the % of type-II AECs expressing nuclear GLI1 overlying fibroblastic foci (FF) compared with those overlying mature fibrous tissue (non-FF) in UIP biopsies (P = 0.0152, Mann–Whitney U-test, n = 6 cases of UIP).
SHH, but not Tenascin-C, protein is released by oxidative damage to human Type-II AECs
We used the well characterized human Type-II AEC cell line, A549 to model the response of activated Type-II AECs. A549 cells expressed SHH signalling pathway genes (Figure 4a). Exposure to H2O2 for 90 min at varying concentrations resulted in damage measured by decreased viability (Figure 4b). A549 cells constitutively secreted SHH (Figure 4c) which was increased by oxidative damage (Figure 4d) peaking after 6 h. The rapidity of this in absence of mRNA upregulation (not shown) suggests that release is from intracellular stores.
Figure 4.
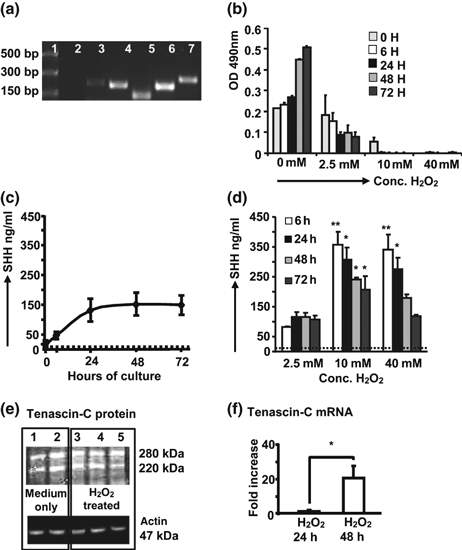
Effects of oxidative damage on A549 cells. (a) RT-PCR representative of seven separate experiments for SHH (211 bp, lane 3), PTC1 (206 bp, lane 4), SMO (140 bp, lane 5), GLI2 (200 bp, lane 6) and GLI1 (244 bp, lane 7). Lane 1= DNA markers, lane 2 = mastermix only. See Table 1 for details of primers. (b–d) Data pooled from three separate experiments. (b) MTT assay showing that H2O2 induces damage to A549 cells. (c, d) ELISA estimation of SHH in A549 cell supernatants, dotted line = limit of detection, *P < 0.05, **P > 0.005, compared to values for cells cultured in medium alone, (Student’s t-test); (c) Constitutive secretion of SHH by A549 cells. (d) SHH release induced by H2O2. Monolayers of A549 cells in 24-well plates were washed and exposed to different concentrations of H2O2 for 90 min, washed and fresh medium added. Supernatants collected after 6, 24, 48 and 72 h. (e) Tenascin-C protein isoforms in cell lysates after 72 h culture in medium only (lanes 1 and 2) or following exposure to 2.5 mM H2O2 (lanes 3–5, three separate experiments); equal protein loading was confirmed by probing with anti-actin antibody. Gel representative of five separate experiments. (f) Real-time PCR for tenascin-C mRNA after exposure to 2.5 mM H2O2. Data pooled from four separate experiments [normalized to medium only control = 1 at each time point], *P = 0.0407, unpaired t-test with Welch’s correction.
Tenascin-C was not secreted at any timepoint after exposure to various H2O2 concentrations (not shown). To investigate tenascin-C further it was decided to look at regulation after exposure to 2.5 mM H2O2, a concentration which induced cell damage but where viable cells remained. Figure 4e shows A549 cells constitutively contain two isoforms of approximately 250 and 220 kDa (lanes 1 and 2). The tenascin-C level did not change after 24 h (not shown) or 48 h exposure (Figure 4e, lanes 3–5). In contrast, tenascin-C mRNA was significantly upregulated after 48 h H2O2 (Figure 4f). This may represent a late damage response.
TGF-β induces Tenascin-C protein and mRNA expression but decreases SHH protein secretion
To determine its effects on SHH and tenascin-C, A549 cells were cultured with TGF-β. Real-time RT-PCR (normalized to a value of 1 for untreated cells in parallel wells) demonstrated significantly increased tenascin-C mRNA after 48 h (Figure 5a). In 72 h lysates, Western blotting (Figure 5b) showed constitutive expression (lane 2) of the two tenascin-C isoforms seen previously (Figure 4e). TGF-β (lane 1) additionally induced a band of approximately 200 kDa. This band was also found in supernatants of TGF-β treated (lane 3) but not untreated (lane 4) cells. Thus TGF-β induced both tenascin-C gene expression and protein release. In contrast to its effects on tenascin-C and to oxidative damage, TGF-β exposure downregulated both constitutive release (Figure 5c) and intracellular SHH protein (Figure 5d). There was no significant change in SHH mRNA (Figure 5e).
Figure 5.
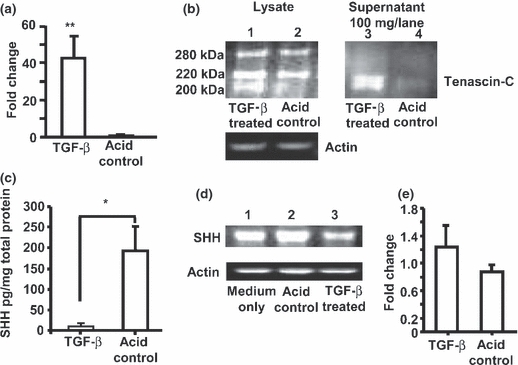
Effect of TGF-β exposure on tenascin-C and SHH. (a) Mean ± SEM fold increase (normalized to a value of 1 for untreated cells) in tenascin-C mRNA in A549 extracts after 48 h exposure to 10 ng/ml acid-activated TGF-β compared to cells in acid control medium only. All samples were normalized to a value of 1 for untreated cells in medium only; data pooled from four separate experiments, normalized to 18s (similar results observed with GAPDH normalization). **P =0.0043, unpaired t-test with Welch’s correction. (b) Western blot (representative of four separate experiments) for tenascin-C protein and β-actin loading controls in cell lysates (25 μg/lane) and tenascin-C protein in supernatants (100 μg/lane) of A549 cells cultured for 72 h in TGF-β (lanes 1 and 3) or control medium (lanes 2 and 4) showing induction of a lower molecular weight isoform released from TGF-β treated cells. (c) SHH protein in supernatants of A549 cells (data pooled from four separate experiments) after 72 h culture with TGF-β. Results are normalized to the total amount of protein in the supernatants. *P =0.0253, unpaired t-test with Welch’s correction. (d) Western blot (representative of four separate experiments) of SHH protein and β-actin loading controls in A549 cell lysates after 72 h culture in medium only (lane 1), in medium with acid diluent control (lane 2) or with TGF-β (lane 3). (e) Mean ± SEM fold increase (normalized to a value of 1 for untreated cells) in SHH mRNA after 48 h exposure to 10 ng/ml acid-activated TGF-β compared to cells in acid control medium only.
Discussion
TGF-β (Chen et al. 2005; Warburton et al. 2005), SHH (Pepicelli et al. 1998; Warburton et al. 2005) and tenascin-C (Roth-Kleiner et al. 2004) are all involved in lung development. TGF-β causes experimental lung fibrosis (Decologne et al. 2007), is involved in epithelial/mesenchymal transition (de Longh et al. 2005; Forino et al. 2006a) and is present in iILD tissue (Khalil et al. 2001; Stewart et al. 2003). SHH signalling has been associated with epithelial injury and repair (Watkins et al. 2003) and was previously reported in Type-II AECs of UIP (Stewart et al. 2003; Coon et al. 2006) and NSIP (Coon et al. 2006). Here SHH protein was also found in the same cells in COP suggesting that SHH expression is a common feature. We further show SHH signalling is functional in iILDs as GLI1 mRNA was present in UIP and NSIP and there was nuclear translocation of this transcription factor in Type-II AECs of UIP, NSIP and COP. Tenascin-C is a damage associated extracellular matrix protein (Hsia & Schwarzbauer 2005) known to mobilize fibroblasts (Trebaul et al. 2007) and contribute to epithelial/mesenchymal transition (Forino et al. 2006b; Ishikawa et al. 2008). Tenascin-C is secreted by Type-II AECs in response to damage (Wallace & Howie 2001), was previously shown in UIP (Wallace et al. 1995) and COP (Kuhn & Mason 1995) and here also demonstrated in NSIP. Thus, tenascin-C, like SHH, is a common feature.
It has also been postulated that oxidative injury might also be a common feature within these iILDs, thus we aimed to determine the relationship between tenascin, TGF-β, oxidative injury and the SHH signalling pathway in activated Type-II AECs.
Due to the inherent difficulties in obtaining and maintaining high purity primary human Type-II AECs in culture where rapid differentiation into Type-I AECs occurs, and the difficulty in obtaining primary material from patients with ILD, we utilized the well-characterized A549 human cell line as a model and accept that findings may need further clarification with primary cultures. TGF-β and oxidative damage had differential effects on gene expression and release of SHH and tenascin-C. A549 cells constitutively secreted SHH but not tenascin-C although both were present in cell lysates. Oxidative damage rapidly increased SHH protein release without mRNA upregulation; TGF-β had no effect on SHH mRNA but abrogated the constitutive secretion of SHH protein. In contrast, oxidative damage upregulated tenascin-C mRNA after 48 h, but did not induce tenascin-C secretion. Since intracellular tenascin-C was not released by H2O2 treatment, SHH release is a specific response to oxidative damage. Similarly, TGF-β but not oxidative damage induced tenascin-C secretion. Thus tenascin-C release by activated/injured type-II AECs also represents a response to specific stimuli.
Tenascin-C has a number of isoforms with molecular weights between 200 and 300 kDa but their significance is unclear (Hsia & Schwarzbauer 2005). The tenascin-C gene promoter has a SMAD binding site (Jinnin et al. 2004), the protein can be upregulated by TGF-β (Jinnin et al. 2004; Hau et al. 2006) and TGF-β induced epithelial/mesenchymal transition is associated with tenascin-C production (Forino et al. 2006; Ishikawa et al. 2008). Expression of tenascin-C in the three iILDs studied here may be indicative of increased TGF-β signalling in Type-II AEC in areas of developing fibrosis. In support of this we previously showed that cross-linking a UIP autoantigen on A549 cells increased both tenascin-C and TGF-β secretion (Wallace & Howie 2001). It is tempting to speculate that similar processes may happen in vivo as autoantibodies and complement are fixed on to hyperplastic Type-II cells in UIP (Wallace et al. 1994).
That SHH but not tenascin-C was released from A549 cells after oxidative damage may reflect different cellular localization or stability. However, it is tempting to hypothesize from our in vitro data that release of SHH, an AEC proliferative factor (Watkins et al. 2003), represents a positive feed-back mechanism which might promote re-epithelialization after injury. Simultaneous down regulation of SHH release and upregulation of tenascin-C by TGF-β suggests that TGF-β may alter the balance from re-epithialization to a more fibrotic process. The occurrence of active SHH signalling at the sites of active fibrosis and TGF-β expression, the fibroblastic foci, may be attributable to the presence of oxidative stimuli. Further prospective study with large numbers of biopsy samples with full patient history regards lung function and lifestyle factors such as smoking and drug treatments would be required to formally test these hypothesize.
This study suggests that prevention of iILD progression will need to account for the sum of and the balance between signalling events triggered by multiple stimuli involved in the disease processes. Our studies suggest that whereas oxidative damage of AECs might promote proliferative epithelial reparative mechanisms (SHH secretion), TGF-β promotes repair by remodelling (tenascin-C secretion) which can lead to fibrosis, with both processes disrupted by oxidative injury. Thus, whilst prevention of TGF-β signalling may be beneficial in ameliorating progressive fibrosis in iILD, simultaneous stimulation of SHH signalling may be advantageous in promoting repair of the epithelial surface, processes which may become deregulated by oxidative damage at the respiratory surfaces.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Su Haley, Anne Grant, Robert Morris and Susan Harvey. We thank Professor J. Schwarze for critically reading the manuscript.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am. J. Respir. Crit. Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- Ask K, Martin GE, Kolb M, Gauldie J. Targeting genes for treatment in idiopathic pulmonary fibrosis: challenges and opportunities, promises and pitfalls. Proc. Am. Thorac. Soc. 2006;3:389–393. doi: 10.1513/pats.200602-021TK. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Chambers RC. Gene expression profiling: good housekeeping and a clean message. Thorax. 2002;57:754–756. doi: 10.1136/thorax.57.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sun J, Buckley S, et al. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L683–L691. doi: 10.1152/ajplung.00298.2004. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J. Pathol. 2003;200:488–499. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am. J. Respir. Cell Mol. Biol. 2005;33:9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- Coon DR, Roberts DJ, Loscertales M, Kradin R. Differential epithelial expression of SHH and FOXF1 in usual and nonspecific interstitial pneumonia. Exp. Mol. Pathol. 2006;80:119–123. doi: 10.1016/j.yexmp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Daniil ZD, Gilchrist FC, Nicholson AG, et al. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 1999;160:899–905. doi: 10.1164/ajrccm.160.3.9903021. [DOI] [PubMed] [Google Scholar]
- Decologne N, Kolb M, Margetts PJ, et al. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J. Immunol. 2007;179:6043–6051. doi: 10.4049/jimmunol.179.9.6043. [DOI] [PubMed] [Google Scholar]
- Forino M, Torregrossa R, Ceol M, et al. TGFbeta1 induces epithelial-mesenchymal transition, but not myofibroblast transdifferentiation of human kidney tubular epithelial cells in primary culture. Int. J. Exp. Pathol. 2006a;87:197–208. doi: 10.1111/j.1365-2613.2006.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Kinnula VL, Myllarniemi M, Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid. Redox Signal. 2008;10:343–354. doi: 10.1089/ars.2007.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau P, Kunz-Schughart LA, Rümmele P, et al. Tenascin-C protein is induced by transforming growth factor-beta1 but does not correlate with time to tumor progression in high-grade gliomas. J. Neurooncol. 2006;77:1–7. doi: 10.1007/s11060-005-9000-5. [DOI] [PubMed] [Google Scholar]
- Honda T, Ota H, Arai K, et al. Three-dimensional analysis of alveolar structure in usual interstitial pneumonia. Virchows Arch. 2002;441:47–52. doi: 10.1007/s00428-001-0567-8. [DOI] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious: J. Biol. Chem. 2005;280:26641–26644. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- Ide M, Ishii H, Mukae H, et al. High serum levels of thrombospondin-1 in patients with idiopathic interstitial pneumonia. Respir. Med. 2008;102:1625–1630. doi: 10.1016/j.rmed.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Nose K, Shibanuma M. Downregulation of hepatocyte nuclear factor-4alpha and its role in regulation of gene expression by TGF-beta in mammary epithelial cells. Exp. Cell Res. 2008;314:2131–2140. doi: 10.1016/j.yexcr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Tenascin-C upregulation by transforming growth factor-beta in human dermal fibroblasts involves Smad3, Sp1, and Ets1. Oncogene. 2004;23:1656–1667. doi: 10.1038/sj.onc.1207064. [DOI] [PubMed] [Google Scholar]
- Kanoh S, Kobayashi H, Motoyoshi K. Exhaled ethane: an in vivo biomarker of lipid peroxidation in interstitial lung diseases. Chest. 2005;128:2387–2392. doi: 10.1378/chest.128.4.2387. [DOI] [PubMed] [Google Scholar]
- Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am. J. Surg. Pathol. 1994;18:136–147. [PubMed] [Google Scholar]
- Khalil N, Parekh TV, O’Connor R, et al. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001;56:907–915. doi: 10.1136/thorax.56.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL, Hodgson UA, Lakari EK, et al. Extracellular superoxide dismutase has a highly specific localization in idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology. 2006;49:66–74. doi: 10.1111/j.1365-2559.2006.02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegova E, Arakelyan A, Fillerova R, et al. PSMB2 and RPL32 are suitable denominators to normalize gene expression profiles in bronchoalveolar cells. BMC Mol. Biol. 2008;9 doi: 10.1186/1471-2199-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Mason RJ. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am. J. Pathol. 1995;147:1759–1769. [PMC free article] [PubMed] [Google Scholar]
- Lamm ML, Catbagan WS, Laciak RJ, et al. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev. Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lu X, Wang H, Gao Q, Cui Y. The up-regulated expression of tenascin C in human nasal polyp tissues is related to eosinophil-derived transforming growth factor beta1. Am. J. Rhinol. 2006;20:629–633. doi: 10.2500/ajr.2006.20.2918. [DOI] [PubMed] [Google Scholar]
- de Longh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- Lowrey JA, Stewart GA, Lindey S, et al. Sonic hedgehog promotes cell cycle progression in activated peripheral CD4(+) T lymphocytes. J. Immunol. 2002;169:1869–1875. doi: 10.4049/jimmunol.169.4.1869. [DOI] [PubMed] [Google Scholar]
- Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma. Oncology. 2006;70:378–389. doi: 10.1159/000098111. [DOI] [PubMed] [Google Scholar]
- Murray LA, Argentieri RL, Farrell FX, et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int. J. Biochem. Cell Biol. 2008;40:2174–2182. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Proell V, Carmona-Cuenca I, Murillo MM, Huber H, Fabregat I, Mikulits W. TGF-beta dependent regulation of oxygen radicals during transdifferentiation of activated hepatic stellate cells to myofibroblastoid cells. Comp. Hepatol. 2007;6 doi: 10.1186/1476-5926-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo NA, Manning DR. Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem. J. 2007;403:369–379. doi: 10.1042/BJ20061723. [DOI] [PubMed] [Google Scholar]
- Roth-Kleiner M, Hirsch E, Schittny JC. Fetal lungs of tenascin-C-deficient mice grow well, but branch poorly in organ culture. Am. J. Respir. Cell Mol. Biol. 2004;30:360–366. doi: 10.1165/rcmb.2002-0266OC. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc. Am. Thorac. Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- Stewart GA, Lowrey JA, Wakelin SJ, et al. Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4 + T cells. J. Immunol. 2002;69:5451–5457. doi: 10.4049/jimmunol.169.10.5451. [DOI] [PubMed] [Google Scholar]
- Stewart GA, Hoyne GF, Ahmad SA, et al. Expression of the developmental Sonic hedgehog (Shh) signalling pathway is up-regulated in chronic lung fibrosis and the Shh receptor patched 1 is present in circulating T lymphocytes. J. Pathol. 2003;199:488–495. doi: 10.1002/path.1295. [DOI] [PubMed] [Google Scholar]
- Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem. Soc. Trans. 2007;35:695–697. doi: 10.1042/BST0350695. [DOI] [PubMed] [Google Scholar]
- Wallace WA, Howie SE. Upregulation of tenascin and TGFbeta production in a type II alveolar epithelial cell line by antibody against a pulmonary auto-antigen. J. Pathol. 2001;195:251–256. doi: 10.1002/path.916. [DOI] [PubMed] [Google Scholar]
- Wallace WA, Schofield JA, Lamb D, Howie SE. Localisation of a pulmonary autoantigen in cryptogenic fibrosing alveolitis. Thorax. 1994;49:1139–1145. doi: 10.1136/thx.49.11.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WA, Howie SE, Lamb D, Salter DM. Tenascin immunoreactivity in cryptogenic fibrosing alveolitis. J. Pathol. 1995;175:415–420. doi: 10.1002/path.1711750409. [DOI] [PubMed] [Google Scholar]
- Wallace WA, Fitch PM, Simpson AJ, Howie SE. Inflammation-associated remodelling and fibrosis in the lung – a process and an end point. Int. J. Exp. Pathol. 2007;88:103–110. doi: 10.1111/j.1365-2613.2006.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Bellusci S, De Langhe S, et al. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr. Res. 2005;57:26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J. Pathol. 2003;201:343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–1517. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- Yang IV, Burch LH, Steele MP, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2007;175:45–54. doi: 10.1164/rccm.200601-062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


