Abstract
Context: Differentiation (decidualization) of endometrial stromal cells (ESC) is an essential prerequisite for successful implantation and establishment of pregnancy.
Objective: The aim was to determine whether the orphan nuclear receptor estrogen-related receptor α (ERRα, NR3B1), and its target genes, medium chain specific acyl-CoA dehydrogenase (MCAD, ACADM), pyruvate dehydrogenase kinase 4 (PDK4), and phosphoenolpyruvate carboxykinase 2 (PEPCK, PCK2), play a role in the decidualization process.
Setting: We conducted the study at a University Research Institute.
Patients and Methods: Endometrial tissues were collected from women with regular menstrual cycles; tissues were used for recovery of primary ESC or RNA extraction or were fixed for immunohistochemistry. Primary ESC were decidualized in vitro; some cells were treated with XCT790 (ERRα inverse agonist).
Results: Decidualization of ESC in vitro was associated with a significant increase in expression of transcripts encoding ERRα and its coactivator peroxisome proliferator-activated receptor γ coactivator-1 α. Expression of ERRα target genes was altered with increased expression of MCAD and PDK4 and reduced expression of PEPCK. Incubation of decidualized ESC with XCT790 reduced expression of ERRα and markers of decidualization such as IGFBP-1.
Conclusion: Increased expression of ERRα may play a role in altering the bioenergetics of decidualized ESC in preparation for implantation of the embryo and successful establishment of early pregnancy.
Expression of the orphan receptor estrogen-related receptor α and its target genes at the time of stromal decidualization may alter cell metabolism ready for implantation.
The human endometrium is a sex-steroid target tissue that differentiates (decidualizes) in preparation for embryo implantation during the mid-late secretory phases of the menstrual cycle. Decidualization is a progressive process initiated in the perivascular stromal cells that spreads “wave-like” throughout the stromal region. It is associated with differentiation of fibroblast-like cells into rounded cells characterized by release of prolactin and IGF binding protein-1 (IGFBP-1) (1) and can be modeled in vitro using primary endometrial stromal cells (ESC) (2). Deficits in decidualization can have consequences for the degree of trophoblast invasion at the time of implantation, and inadequate invasion has been implicated in the pathophysiology of conditions such as preeclampsia, premature rupture of membranes, preterm labor, and intrauterine growth restriction (reviewed in Ref. 3).
The steroid hormone receptor superfamily includes ligand-activated transcription factors including the estrogen receptors, which integrate the effects of hormones by regulating gene expression. Genome-wide profiling of human endometrium has identified gene signatures for tissue recovered from different phases of the normal cycle with a distinct profile of gene expression during the midsecretory (MS) phase (implantation window) (4,5,6). Estrogen-related receptor α (ERRα) is an orphan member of the steroid hormone receptor superfamily implicated in the regulation of energy homeostasis (7). ERRα, together with its transcriptional coactivator, the peroxisome proliferator-activated receptor γ coactivator-1 α (PGC1α), are considered key players in the regulation of genes coding for components of metabolic pathways responsible for metabolism of fatty acids or glucose, and for regulating mitochondrial biogenesis and oxidative capacity (7). ERRα is expressed in endometrial cancers, and overexpression of ERRα in Ishikawa cells blunts estradiol-induced estrogen response element-dependent reporter gene activation (8).
We have investigated expression of ERRα and three ERRα target genes in primary ESC decidualized in vitro and the impact of a pharmacological inhibitor of ERRα on markers of phenotypic decidualization.
Patients and Methods
Tissue collection
Endometrial tissues were collected from women with regular menstrual cycles; written informed consent was obtained from all subjects, and ethical approval was granted by the Lothian research ethics committee. Samples (n = 41) included in our analyses were characterized using three independent parameters: histological stage (9), the patient’s reported last menstrual period, and circulating sex steroid levels at time of collection. Tissues were fixed in 4% neutral buffered formalin, used for RNA extraction or for preparation of ESC cultures.
Immunohistochemistry
Immunostaining was carried out according to established protocols (10); antigen retrieval was at pH 6, endogenous biotin activity was blocked, and washes between each step were carried out in Tris-buffered saline. Briefly, rabbit anti-ERRα (Abcam, Inc., Cambridge, UK) was diluted 1:500 and incubated on sections overnight at 4 C (negative controls were incubated in buffer alone). Sections were incubated with biotinylated goat antirabbit antibody diluted 1:500 for 30 min and in Streptavidin-horseradish peroxidase for 30 min, and bound antibodies were visualized by incubation with 3,3′-diaminobenzidine tetra-hydrochloride (liquid DAB+; Dako, Glostrup, Denmark).
In vitro decidualization of primary human ESC
Primary ESC were purified from endometrial specimens as previously described (2). Purity was assessed by fluorescence-activated cell sorting (CD90+); in this and previous (2) studies, it was routinely greater than 95% (data not shown). Cells were maintained at 37 C in RPMI medium with 10% fetal calf serum, penicillin (50 μg/ml), streptomycin (50 μg/ml), and gentamycin (5 μg/ml) and passaged up to four times. For experiments, ESC were seeded in six-well plates (2.5 × 105 per well) and allowed to reach 90% confluence; decidualization was induced by addition of decidualization media (DM; RPMI 1640, 2% fetal calf serum, 0.1 mg/ml 8-Br-cAMP, and 1 μm 6α-methyl-17α-acetoxyprogesterone) for 4 d. The impact of ERRα on decidualization of ESC was investigated by incubating cells with 1, 5, or 10 μm of XCT790 (Sigma, St. Louis, MO; catalog no. X4753), a potent inverse agonist specific to ERRα that reverses constitutive activity of the protein in both biochemical and cell-based assays; control cultures contained dimethylsulfoxide (DMSO) alone. A second set of cultures that had been incubated in DM for 4 d was maintained in DM in the presence or absence of XCT790 (as above) for a further 4 d (see Fig. 2A). Stocks of hormones and antagonist were prepared in DMSO and diluted in PBS before use, so the final concentration of DMSO was 0.2% or less. Media were recovered for ELISA (stored at −20 C), and RNA was extracted from cells for quantitative real-time PCR (qRTPCR).
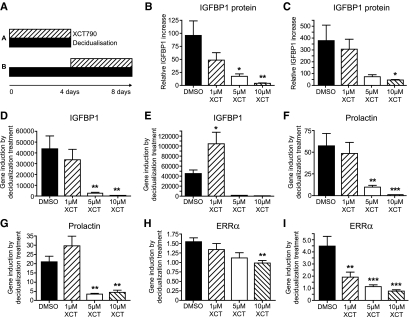
Figure 2.
Pharmacological inhibition of ERRα with the inverse agonist XCT790 impairs the induction and maintenance of a decidualized phenotype. A, Experimental protocol. ESC were either decidualized for 4 d in the presence of XCT790 (protocol A) or decidualized for 4 d, then incubated with DM containing XCT790 (1, 5, or 10 μm; protocol B). B and C, IGFBP-1 protein detected in medium by ELISA after protocols A or B. D–I, mRNA concentrations of IGFBP-1 (D and E), prolactin (F and G), or ERRα (H and I) for protocols A and B, respectively, in each case. Note that incubation of cells with XCT790 had a significant impact on gene expression (*, P < 0.05; **, P < 0.01; ***, P < 0.001) compared with DMSO controls (n = 4).
Measurement of IGFBP-1 by ELISA
The IGFBP-1 assay used matched antibody pairs (R&D Systems, Abingdon, UK) according to the manufacturer’s protocol. Media were assayed in duplicate; the concentration of IGFBP-1 was determined using a standard curve of IGFBP-1 standards. Intraassay coefficient of variation was 2.4–10.2%; interassay coefficient of variation was 5.5–8.7%.
Taqman qRTPCR
RNA was extracted using a QIAGEN kit (QIAGEN, Valencia, CA) and reverse transcribed with Vilo Superscript (Invitrogen, San Diego, CA). Estrogen receptor α (ERα) and ERRα mRNAs were measured using primer/probe assays from Applied Biosystems (Foster City, CA; Hs00174860 and Hs00607062_gH, respectively). mRNAs encoding IGFBP-1, prolactin, PGC1α, medium chain specific acyl-CoA dehydrogenase (MCAD), dehydrogenase kinase 4 (PDK4), and phosphoenolpyruvate carboxykinase 2 (PEPCK) were quantified using probes from the Universal Probe Library (Roche Diagnostics, Indianapolis, IN) and primers (MWG Biotech Inc., High Point, NC) were designed using online software (http://qpcr.probefinder.com/organism.jsp). See Supplemental Table 1 (published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). PCR amplification efficiency was 95–105% for each primer-probe pair; samples were quantified using the comparative ΔΔct method.
Statistical analysis
Data were analyzed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). Normality was checked, parametric tests were used (t test or ANOVA), and the statistical significance level was set at P < 0.05.
Results
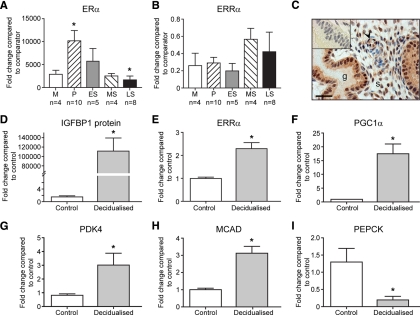
As expected, concentrations of ERα mRNA in endometrial tissue samples were significantly higher during the proliferative phase than during the late secretory (P = 0.03; Fig. 1A). ERRα transcripts were detected in the same samples; although there was a trend for higher concentrations during mid-late secretory phases, this was not statistically significant (P = 0.334; Fig. 1B). Immunoexpression of ERRα was detected in stromal, epithelial, and endothelial cells (Fig. 1C). Decidualization of ESC was confirmed by measuring IGFBP-1 secretion (P < 0.001; Fig. 1D). In the same cultures, we detected a statistically significant increase in mRNAs encoding ERRα (P < 0.001; Fig. 1E) and the coactivator PGC1α (P < 0.001; Fig. 1F) in response to DM. Concentrations of mRNAs encoded by three ERRα target genes was also altered in the decidualized ESC; PDK4 and MCAD were significantly increased, whereas PEPCK was reduced (Fig. 1, G–I, respectively).
Figure 1.
ERRα is expressed in human endometrium during the menstrual cycle and up-regulated upon in vitro stromal cell decidualization. Expression of mRNAs encoding ERα (A) and ERRα (B) were quantified in staged human endometrial biopsies using qRTPCR with 18S as a comparator. Data are expressed as mean ± sem (M, menstrual; P, proliferative; ES, early secretory; MS, midsecretory; LS, late secretory). The number of samples analyzed from each stage (n) is indicated on the x-axis. *, P = 0.03 between P and LS. C, ERRα protein was immunolocalized to epithelial cells lining glands (g), stromal fibroblasts (s), and endothelial cells (arrowhead) in human endometrium at all stages of the cycle. Illustrated is a section of endometrium from the MS phase; inset, negative control of same tissue sample. The scale bar represents 50 μm. D–I, Results of qRTPCR analysis of primary ESC incubated in the presence or absence of decidualization medium for 4 d. Decidualization was confirmed by a significant increase in expression of IGFBP-1 (D, P < 0.001). In the same cells, there was a significant increase in mRNAs encoding ERRα (E) and the coactivator PGC1α (F), and changes in expression of mRNAs for three known ERRα target genes: PDK4 (G), MCAD (H), and PEPCK (I). *, P < 0.001 decidualized ESC compared with control (E to I). Data are expressed as fold changes from the control; data represent the average of four fibroblast cultures (±sem), and the experiments were done in duplicate.
To test the hypothesis that ERRα may be involved in either the decidualization process or maintenance of the decidual cell phenotype, we used an ERRα-specific inverse agonist (XCT790) to block the activity of the receptor. Two protocols were used: 1) ESC were incubated in DM in the presence of 1–10 μm XCT790 for 4 d; and 2) after ESC had been decidualized for 4 d in normal DM, they were incubated for an additional 4 d with or without the addition of XCT790 (Fig. 2A). The morphology of cells incubated with XCT790 was the same as cells incubated in DM alone (data not shown). When expressed as a ratio to the increase in IGFBP-1 secretion seen in cells maintained in DM alone, the addition of XCT790 had a significant impact on secretion of IGFBP-1 in both experimental paradigms (Fig. 2, B and C). Likewise, a significant reduction in concentrations of both IGFBP-1 (Fig. 2, D and E) and prolactin mRNAs (Fig. 2, F and G) was detected in cells treated with 5 or 10 μm XCT790 recovered after 4 or 8 d. Notably, treatment with XCT790 also resulted in a dose-dependent decrease in the decidualization-dependent up-regulation of ERRα mRNA using both protocols (Fig. 2, H and I).
Discussion
We believe that this is the first study to demonstrate that expression of ERRα, PGC1α, and ERRα target genes is altered during decidualization of human ESC in vitro. Expression of ERRα appears important for full decidualization because treatment with an ERRα-specific inverse agonist reduced the induction and maintenance of the decidualization phenotype as indicated by reduced expression of IGFBP-1 and prolactin mRNAs. It is widely accepted that ERRα/PGC1α can have an impact on mitochondrial number and function (7). In 1950, Noyes et al. (9) described the “postovulatory triad” in microscopic studies of endometrial morphology that included the presence of large mitochondria and changes in glycogen content. Milwidsky et al. (11) reported an increase in endometrial glycogen during the secretory phase. We propose that changes in ERRα-dependent gene expression may explain these features because our data suggest that the endometrial stroma undergoes transcriptional changes consistent with a shift in energy source (from glucose to fatty acids), possibly reflecting the preparation of the tissue for the challenges of embryo implantation.
The suggestion that energy metabolism is altered as a consequence of decidualization is also supported by studies using microarrays. For example, expression of mitochondrial genes including aldehyde dehydrogenase, acyl-CoA dehydrogenase, pyruvate carboxylase, and lactate dehydrogenase has been reported to change during the window of implantation (4). Expression of PDK4, a gene involved in energy metabolism that was up-regulated in our ESC upon decidualization in vitro, was also up-regulated in samples from MS (receptive) phase endometrium, compared with those from the early secretory phase (12). Recently, expression of the glucose transporter GLUT1 has been shown to be induced by decidualization of human ESC (13).
Decidualization of human ESC can be likened to a differentiation process, and it is notable that up-regulation in expression of ERRα has been implicated in the differentiation of osteoblasts (14) and adipocytes (15). ERRα/PGC1α have also been implicated in the regulation of vascular endothelial growth factor in blood vessels (16). Notably, expression of vascular endothelial growth factor in endometrium is highest during the MS phase and is up-regulated in ESC treated with estradiol and medroxyprogesterone acetate (17).
Finally, the question of what regulates expression of ERRα remains to be addressed. One candidate is mammalian target of rapamycin (mTOR), a kinase that regulates cell growth and survival; in skeletal muscle, the mTOR inhibitor rapamycin reduced expression of PGC1α and ERRα and decreased both mitochondrial gene expression and oxygen consumption (18). The serine/threonine kinase Akt is an upstream positive regulator of mTOR expressed in ESC (19). Because it has been reported that decidualization of ESC reduced the amount of phospho-Akt (19), alternative pathways, of which there are several, may be involved in modulating mTOR activity in this cell type.
In conclusion, in addition to having an impact on estrogen receptor-dependent gene expression (8) and in agreement with a large body of evidence (7), expression of ERRα correlates with altered energy production at the tissue level. We therefore propose that increased expression of ERRα plays an important role in decidualized stromal cells in preparation for the challenges of implantation and trophoblast invasion. This work provides a molecular target for the study of bioenergetics in the human endometrium and, with the development of pharmacological agents targeting ERRα, may provide the rationale for novel therapies aimed at supporting embryo implantation (e.g. during in vitro fertilization treatments) or as a local endometrial contraceptive agent.
Supplementary Material
Acknowledgments
We thank Dr. Alistair Williams for histological evaluations, Catherine Murray and Sharon McPherson for patient recruitment, and Professor Karen Chapman for helpful discussion.
Footnotes
This work was supported by Medical Research Council (MRC) Human Reproductive Sciences Unit funding (Grant U1276.00.002.00005.01, to P.T.K.S.), and an MRC program grant (G0500047, to H.O.D.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 28, 2010
Abbreviations: DM, Decidualization media; DMSO, dimethylsulfoxide; ERα, estrogen receptor α; ERRα, estrogen-related receptor α; ESC, endometrial stromal cell(s); IGFBP-1, IGF binding protein-1; MCAD, medium chain specific acyl-CoA dehydrogenase; MS, midsecretory; mTOR, mammalian target of rapamycin; PDK4, pyruvate dehydrogenase kinase 4; PEPCK, phosphoenolpyruvate carboxykinase 2; PGC1α, peroxisome proliferator-activated receptor γ coactivator-1 α; qRTPCR, quantitative real-time PCR.
References
- Dunn CL, Kelly RW, Critchley HO 2003 Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 7:151–161 [DOI] [PubMed] [Google Scholar]
- Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO 2008 Transforming growth factor-β1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol 22:716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER 2006 Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online 13:591–599 [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC 2003 Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144:2870–2881 [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC 2006 Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Martín J, Cervero A, Mosselman S, Pellicer A, Simón C 2004 Global gene expression profiling of human endometrial receptivity. J Reprod Immunol 63:41–49 [DOI] [PubMed] [Google Scholar]
- Giguère V 2008 Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29:677–696 [DOI] [PubMed] [Google Scholar]
- Watanabe A, Kinoshita Y, Hosokawa K, Mori T, Yamaguchi T, Honjo H 2006 Function of estrogen-related receptor α in human endometrial cancer. J Clin Endocrinol Metab 91:1573–1577 [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J 1950 Dating the endometrial biopsy. Fertil Steril 1:3–25 [DOI] [PubMed] [Google Scholar]
- Bombail V, MacPherson S, Critchley HO, Saunders PT 2008 Estrogen receptor related β is expressed in human endometrium throughout the normal menstrual cycle. Hum Reprod 23:2782– 2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milwidsky A, Palti Z, Gutman A 1980 Glycogen metabolism of the human endometrium. J Clin Endocrinol Metab 51:765–770 [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B 2002 Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 8:871–879 [DOI] [PubMed] [Google Scholar]
- Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH 2009 Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 150:1512–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelye E, Vanacker JM, Dittmar T, Begue A, Desbiens X, Denhardt DT, Aubin JE, Laudet V, Fournier B 1997 The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol Endocrinol 11:905–916 [DOI] [PubMed] [Google Scholar]
- Vega RB, Kelly DP 1997 A role for estrogen-related receptor α in the control of mitochondrial fatty acid β-oxidation during brown adipocyte differentiation. J Biol Chem 272:31693–31699 [DOI] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM 2008 HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451:1008–1012 [DOI] [PubMed] [Google Scholar]
- Sugino N, Kashida S, Karube-Harada A, Takiguchi S, Kato H 2002 Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction 123:379–387 [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P 2007 mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450:736–740 [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Yano T, Tsutsumi O, Taketani Y 2003 Akt as a possible intracellular mediator for decidualization in human endometrial stromal cells. Mol Hum Reprod 9:265–269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.