Abstract
The biosynthesis of the recently identified novel class of plant hormones, strigolactones, is up-regulated upon phosphate deficiency in many plant species. It is generally accepted that the evolutionary origin of strigolactone up-regulation is their function as a rhizosphere signal that stimulates hyphal branching of arbuscular mycorrhizal fungi. In this work, we demonstrate that this induction is conserved in Arabidopsis (Arabidopsis thaliana), although Arabidopsis is not a host for arbuscular mycorrhizal fungi. We demonstrate that the increase in strigolactone production contributes to the changes in shoot architecture observed in response to phosphate deficiency. Using high-performance liquid chromatography, column chromatography, and multiple reaction monitoring-liquid chromatography-tandem mass spectrometry analysis, we identified two strigolactones (orobanchol and orobanchyl acetate) in Arabidopsis and have evidence of the presence of a third (5-deoxystrigol). We show that at least one of them (orobanchol) is strongly reduced in the putative strigolactone biosynthetic mutants more axillary growth1 (max1) and max4 but not in the signal transduction mutant max2. Orobanchol was also detected in xylem sap and up-regulated under phosphate deficiency, which is consistent with the idea that root-derived strigolactones are transported to the shoot, where they regulate branching. Moreover, two additional putative strigolactone-like compounds were detected in xylem sap, one of which was not detected in root exudates. Together, these results show that xylem-transported strigolactones contribute to the regulation of shoot architectural response to phosphate-limiting conditions.
For many years, strigolactones have been known as rhizosphere signaling molecules. They were first described as germination stimulants for the seeds of parasitic plant species of the genera Striga, Orobanche, and Phelipanche (Cook et al., 1972; Bouwmeester et al., 2003). A relationship between phosphorus starvation and root parasitism became apparent when it was shown that red clover (Trifolium pratense) exudates, collected from phosphate-starved plants, induced higher germination of Orobanche minor seeds (Yoneyama et al., 2001) and that the increase in germination was due to increased secretion of orobanchol (Yoneyama et al., 2007). Recently, it was proven that phosphate starvation increased not only the secretion but also the production of orobanchol, solanacol, and the didehydro-orobanchol isomers 1 and 2 in the roots of tomato (Solanum lycopersicum; López-Ráez et al., 2008a) and orobanchol and 5-deoxystrigol in rice (Oryza sativa; Jamil et al., 2010).
Low-phosphate conditions have also been shown to improve the colonization rate by arbuscular mycorrhizal (AM) fungi in carrot (Daucus carota) and tomato (Nagahashi and Douds, 2000) through the stimulation of hyphal branching of the symbiotic AM fungi (Akiyama et al., 2005). This led to the hypothesis that the production and secretion of strigolactones in soils with a limited availability of free phosphate functions to improve the establishment of AM symbiosis (Bouwmeester et al., 2007). Hence, this shows that the strigolactones have a dual role in the rhizosphere, as host detection signals for both AM fungi and root parasitic plants (Akiyama et al., 2005; Harrison, 2005; Paszkowski, 2006; Bouwmeester et al., 2007; Yoneyama et al., 2008).
Several strigolactones have been detected in the root exudates and extracts of a wide range of monocotyledonous and dicotyledonous plant species, including the non-AM fungi host Arabidopsis (Arabidopsis thaliana; Goldwasser et al., 2008). It has been postulated that all strigolactones are derived from a single carotenoid substrate through oxidative cleavage performed by a carotenoid cleavage dioxygenase (CCD), the product of which is subsequently oxidized by cytochrome P450s (Matusova et al., 2005). Indeed, the involvement of CCD enzymes in strigolactone biosynthesis was recently confirmed. The ramosus5 (rms5) and rms1 mutants in pea (Pisum sativum) and high-tillering dwarf1 (htd1) or dwarf17 (d17) and d10 mutants in rice, which are compromised in CCD7 and CCD8 activity, respectively, produce no strigolactones, whereas their corresponding wild types do (Gomez-Roldan et al., 2008; Umehara et al., 2008). Earlier, CCD7 and CCD8 were also reported to be responsible for the biosynthesis of the elusive shoot-branching-inhibiting signal (Sorefan et al., 2003; Booker et al., 2004). Application of the synthetic strigolactone analog GR24 indeed rescued the bushy phenotype of these mutants and their Arabidopsis orthologous more axillary growth (max) mutants max3 and max4 but not in the putative signaling mutants max2 (Stirnberg et al., 2002, 2007), rms4 in pea, and d3 in rice, providing evidence that strigolactones, or strigolactone derivatives, are responsible for branching inhibition (Gomez-Roldan et al., 2008; Umehara et al., 2008). A third putative strigolactone biosynthetic mutant in Arabidopsis, max1 (with a mutation in the cytochrome P450-encoding AtCyp711A; Stirnberg et al., 2002; Booker et al., 2005), shows the same branching phenotype as the other max mutants, and also in this mutant, GR24 application reduced branching to wild-type levels (Gomez-Roldan et al., 2008; Crawford et al., 2010). MAX1 is believed to act downstream of CCD7 and CCD8 in the biosynthesis of the branching-inhibiting signal (Booker et al., 2005). However, proof of the involvement of MAX1 in strigolactone biosynthesis through analytical means is lacking, as an orthologous mutant is described neither in pea nor rice.
Interestingly, phosphate starvation in plants leads to a reduced number of shoot branches (Troughton 1977; Cline, 1997) and changes in the root system (López-Bucio et al., 2002; Al-Ghazi et al., 2003; Ma et al., 2003; Nacry et al., 2005; Sánchez-Calderón et al., 2005). In Arabidopsis, it has been demonstrated that upon phosphate starvation, auxin activity in the root system is altered, leading to a drastic modification of the root morphology (Williamson et al., 2001; López-Bucio et al., 2002; Ma et al., 2003; Nacry et al., 2005; Sánchez-Calderón et al., 2005). The growth of the primary root is reduced and the outgrowth of the lateral roots near the soil surface is stimulated such that phosphate-rich areas that are usually found in the top layers of the soil can be explored (Al-Ghazi et al., 2003). In addition to these changes in root system architecture, resources are redirected from the shoot to the root (López-Bucio et al., 2002), contributing to the change in the root-to-shoot ratio, enabling the plant to better cope with its environment (Bonser et al., 1996).
Grafting studies performed in several species showed that a wild-type rootstock grafted to either a ccd7 or ccd8 mutant scion was able to restore wild-type branching patterns, indicating that a transmissible signal (as we now know likely strigolactone or strigolactone derived) is produced in the roots (Beveridge et al., 1994; Napoli, 1996; Turnbull et al., 2002). However, wild-type shoots on mutant roots also have near-wild-type branching patterns (Beveridge et al., 1994, 1997b; Napoli, 1996; Sorefan et al., 2003). In addition, wild-type epicotyl interstock grafts into rms1 and hypocotyl grafts into Arabidopsis max3 are also able to reduce branching (Foo et al., 2001), indicating that biosynthesis is not limited to the root system. At present, therefore, the exact origin of strigolactones in the shoot is unknown. Nevertheless, it is likely that they must be transported to the shoot, where they exert their shoot-branching-inhibiting effect (Bennett et al., 2006). It has been shown that other phytohormones that are also produced in the roots, such as abscisic acid and cytokinins, are transported through the xylem (Hartung et al., 2002; Sakakibara, 2006), making the xylem a good candidate for strigolactone transport.
In this study, strigolactones in Arabidopsis were analyzed using multiple reaction monitoring-liquid chromatography-tandem mass spectrometry (MRM-LC-MS/MS) and germination bioassays with seeds of Phelipanche (Orobanche) ramosa (Joel, 2009). The regulation of strigolactone levels by phosphate starvation was investigated, and the involvement of MAX1 and MAX4 in strigolactone biosynthesis was examined. Furthermore, the role of strigolactones or strigolactone-like compounds in phosphate starvation-induced shoot architectural changes was investigated using strigolactone biosynthesis and signal transduction mutants max1, max2, and max4. Finally, the involvement of the xylem in the transport of strigolactones and or strigolactone-like compounds from the root to the shoot was explored.
RESULTS
Germination Stimulatory Activity of Arabidopsis Root Exudates
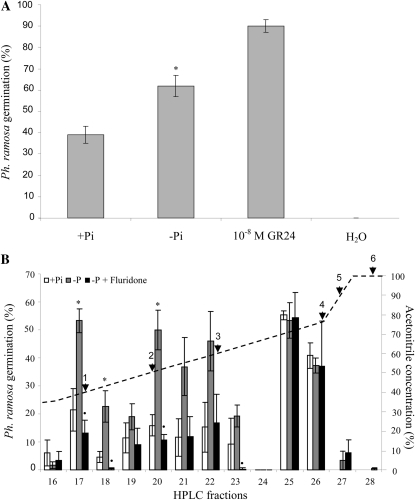
To assess if the induction by Arabidopsis root exudates of germination of P. ramosa seed is increased by phosphate deficiency, 4-week-old Arabidopsis plants were subjected to phosphate starvation for 2 weeks, in parallel with fully fertilized plants. Ten-fold-concentrated Arabidopsis root exudates were applied to P. ramosa seeds, and germination was scored after 6 d. The exudates of fully fertilized plants induced 38% germination, whereas exudates of phosphate-starved plants induced 63% germination of P. ramosa seeds. The synthetic strigolactone GR24 (10−8 μm) was used as a positive control and induced 90% germination, whereas water (negative control) induced no germination (Fig. 1A). When these exudates were tested for strigolactone content using MRM-LC-MS/MS, no strigolactones were detectable.
Figure 1.
Germination of P. ramosa seeds induced by root exudates of Arabidopsis (Col-0). A, Effect of phosphate starvation on the germination stimulatory capacity of 20-fold-concentrated Arabidopsis root exudates. Bars represent the average of three independent biological replicates ± se. * Significant difference between limiting phosphate (–Pi) and sufficient phosphate (+Pi) treatment (P < 0.05). B, Effect of treatments, sufficient phosphate (+Pi), limiting phosphate (–Pi), and limiting phosphate plus fluridone (–Pi + fluridone), on the germination stimulatory capacity of HPLC fractions of Arabidopsis root exudates. Bars represent the average of five independent biological replicates ± se. * Significant difference between –Pi and +Pi treatment; • significant difference between –Pi + fluridone treatment and –Pi treatment (P < 0.05). The dashed line indicates the HPLC gradient of acetonitrile concentration, and arrowheads point to fractions in which strigolactone standards elute: 1, solanacol and 7-hydroxyorobanchyl acetate; 2, 2′-epiorobanchol, orobanchol, strigol, and sorgomol; 3, GR24; 4, orobanchyl acetate; 5, sorgolactone; 6, 5-deoxystrigol.
HPLC Fractionation for Germination Stimulant Profiling
To investigate whether strigolactones are responsible for the increase in germination stimulatory activity under phosphate starvation, HPLC purification was used to fractionate Arabidopsis root exudates. To identify the strigolactone-containing fractions, a mix of strigolactone standards was also fractioned using the same protocol and analyzed using MRM-LC-MS/MS. The first strigolactone from the standard mix to elute from the column was detected in fractions 11 and 12, which both contained 7-hydroxyorobanchol. 7-Oxoorobanchol eluted in fraction 15, solanacol and 7-hydroxyorobanchyl acetate in fraction 17, and 7-oxoorobanchyl acetate in fraction 19. Orobanchol, strigol, and sorgomol coeluted in fraction 20, and orobanchyl acetate, sorgolactone, and 5-deoxystrigol eluted in fractions 26, 27, and 28, respectively (Fig. 1B; Supplemental Table S1).
Exudates of Arabidopsis plants grown on sufficient phosphate, without phosphate (for 2 weeks), and without phosphate + 0.01 μm fluridone, an inhibitor of carotenoid biosynthesis that also blocks strigolactone biosynthesis, were fractioned and assayed for their germination stimulatory activity on P. ramosa seeds (Fig. 1B). No germination was induced by HPLC fractions eluting before fraction 15 in any of the treatments (data not shown). Fraction 16 induced a small percentage of germination in all treatments. Fractions 17, 20, 21, and 22 from plants grown on sufficient phosphate induced some germination (22%, 16%, 12%, and 14%, respectively), while phosphate starvation strongly increased germination stimulatory activity of these fractions (2.5-, 3.1-, 3.2-, and 3.3-fold, respectively). When plants were grown in the presence of fluridone, the germination induced by these fractions was reduced by 71%, 78%, 70%, and 65%, respectively. High germination was also observed in fractions 25 (54%) and 26 (40%) of plants grown under sufficient phosphate levels, but no effect of phosphate starvation or fluridone was observed in these fractions. Fractions 27 and higher induced only little germination, and this was not affected by phosphate starvation or fluridone (Fig. 1B).
Germination Stimulatory Activity of HPLC-Fractioned Root Exudates of max Mutants
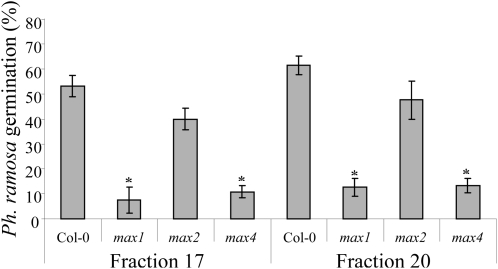
Root exudates of phosphate-starved Arabidopsis ecotype Columbia (Col-0), max1-1, max2-1, and max4-1 genotypes were HPLC fractioned. Fractions 17 and 20 were analyzed with the P. ramosa germination bioassay. As described above, these fractions responded both to phosphate starvation and fluridone treatment, suggesting that they may contain strigolactone(-like) compounds. Moreover, known strigolactones were shown to coelute with these fractions (Fig. 1B). The germination bioassay showed a high percentage of germination in both fractions in the Col-0 wild type (52% and 61%, respectively), and this was strongly reduced in the putative strigolactone biosynthetic mutants max1-1 and max4-1 (fraction 17, 85% and 79% lower germination; fraction 20, 81% and 77% lower germination). Fractions of the exudates collected from the putative strigolactone signal transduction mutant max2-1 did not differ significantly in their bioactivity from the wild type (Fig. 2). As described above, neither fraction 25 nor 26 responded to phosphate deficiency or fluridone treatment, even though the HPLC fractionation of strigolactone standards shows that germination in the latter fraction may be (partially) due to orobanchyl acetate. However, when fractions 25 and 26 were analyzed for germination-inducing activity in the max mutants, no differences were found compared with the wild type (Supplemental Fig. S1).
Figure 2.
Germination of P. ramosa seeds induced by HPLC fractions 17 and 20 of Arabidopsis (Col-0, max1-1, max2-1, and max4-1) root exudates. Bars represent the average of three independent biological replicates ± se. * Significant difference from Col-0 (P < 0.05).
MRM-LC-MS/MS Analysis of Strigolactones
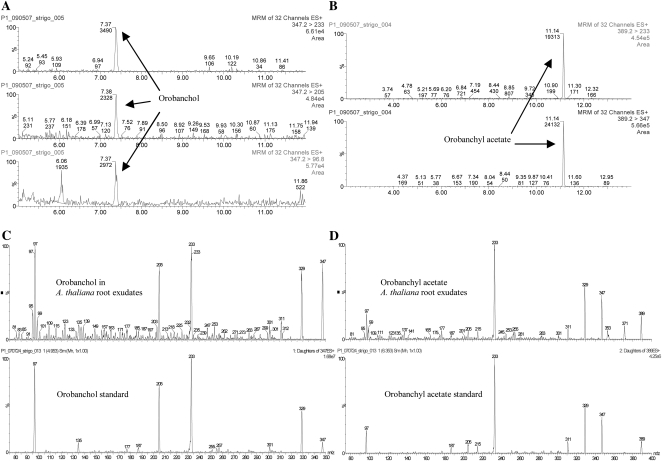
For strigolactone analysis in root exudates, approximately 800 Arabidopsis plants were grown for 4 weeks under phosphate-sufficient conditions. Because our bioassays indicated an up-regulation of strigolactone secretion upon phosphate deficiency, plants were grown under phosphate-limiting conditions for 2 weeks prior to root exudate collection. The exudates were purified using C18 and silica column chromatography. Orobanchol, orobanchyl acetate, and 5-deoxystrigol were detected in the exudates of phosphate-starved plants (Fig. 3, A and B; Supplemental Fig. S2). To verify the presence of these strigolactones, the MS/MS spectra of the putative orobanchol (Fig. 3C) and orobanchyl acetate peaks were compared with the MS/MS spectra of standards (Fig. 3D) and the samples were spiked with these standards (data not shown). No MS/MS spectrum could be obtained from 5-deoxystrigol; however, spiking (data not shown) and ratio analysis of the MRM transitions suggested the compound to be 5-deoxystrigol. Orobanchol and orobanchyl acetate were also detected in root extracts of Col-0 (Supplemental Fig. S3).
Figure 3.
MRM-LC-MS/MS analysis of Arabidopsis root exudates of plants grown under phosphate starvation. A, Transitions 347 > 233, 347 > 205, and 347 > 96.8 for orobanchol. B, Transitions 389.2 > 233 and 389.2 > 347 for orobanchyl acetate. C, Full daughter ion scan MS/MS spectrum of orobanchol in Arabidopsis exudate and orobanchol standard. D, Full daughter ion scan MS/MS spectrum of orobanchyl acetate in Arabidopsis exudate and orobanchyl acetate standard.
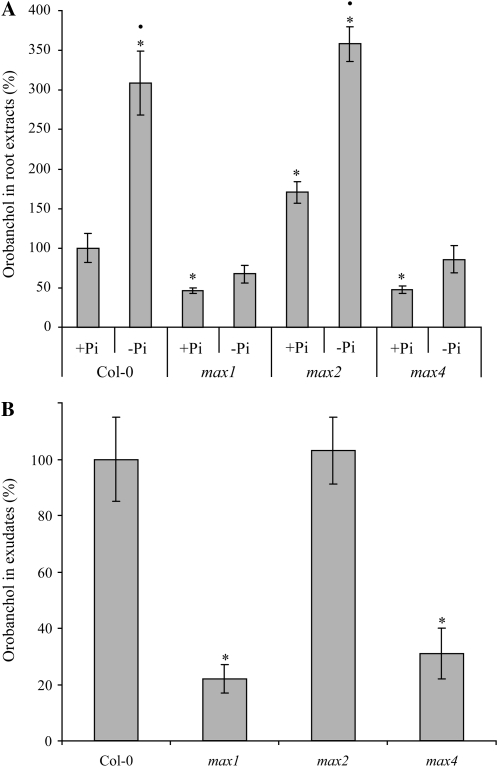
When root extracts of Col-0, max1-1, max2-1, and max4-1 were analyzed, an up-regulation of orobanchol production under phosphate-limiting conditions was observed in Col-0 and max2 (3.1- and 2.1-fold, respectively) but not in max1 and max4 (Fig. 4A). Moreover, a strong reduction in orobanchol content was found in max1-1 and max4-1 under phosphate-sufficient conditions (54% and 52% lower than the wild type, respectively) as well as under phosphate starvation (78% and 72% lower than the wild type, respectively; Fig. 4A). In line with these findings, orobanchol was also reduced in the exudates of both biosynthetic mutants under phosphate starvation (78% and 69% lower than the wild type, respectively; Fig. 4B). Interestingly, orobanchol biosynthesis in max2 grown under phosphate-sufficient conditions was 1.7-fold higher than in Col-0 (Fig. 4A). In these experiments, the concentration of orobanchyl acetate and 5-deoxystrigol in both Col-0 and the max mutants was below the detection level required for accurate quantification.
Figure 4.
Analysis of orobanchol content in Arabidopsis Col-0, max1-1, max2-1, and max4-1. A, Effect of treatments with sufficient phosphate (+Pi) and limiting phosphate (–Pi) on root extracts (mean value for orobanchol level in Col-0 +Pi root extract was set to 100%). Bars represent the average of three independent biological replicates. • Significant –Pi up-regulation within genotypes (P < 0.05); * significant difference compared with Col-0 +Pi (P < 0.05). B, Root exudate analysis of –Pi (mean value for orobanchol level in Col-0 –Pi [P < 0.05] root exudate was set to 100%). Bars represent the average of three independent biological replicates each consisting of about 800 plants ± se. * Significant difference compared with Col-0 (P < 0.05).
Effect of Phosphate Deficiency on Shoot Architecture
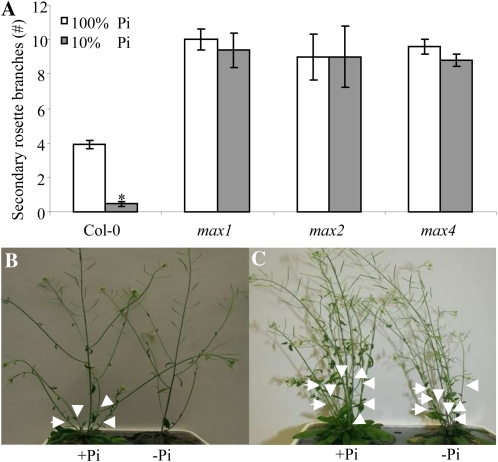
To assess whether strigolactones are involved in the shoot architectural response to phosphate stress, the branching phenotype of max mutants under phosphate starvation was assessed. Arabidopsis Col-0, max1-1, max2-1, and max4-1 plants were grown for 4 weeks under phosphate-sufficient conditions. Phosphate starvation was induced in half of the plants 3 d prior to bolting by reducing the phosphate concentration in the medium to 10% of the control. After 2 weeks, plants were analyzed and the number of secondary rosette branches was assessed. Under phosphate-sufficient conditions, Col-0 had on average four secondary rosette branches per plant (Fig. 5, A and B). All three max mutants (max1-1, max2-1, and max4-1) had a higher number of secondary rosette branches (10, 9, and 9.5, respectively; Fig. 5, A and C). Under phosphate deficiency, the number of secondary rosette branches in Col-0 was strongly reduced (P < 0.05; Fig. 5, A and B), but the max mutants showed no significant reduction (Fig. 5, A and C).
Figure 5.
The effect of phosphate levels on Arabidopsis axillary shoot branching. Measurements were done 2 weeks after initiation of the treatments in 7-week-old plants grown under long-day conditions. Bars indicate average of 10 independent replicates ± se. A, Effect of phosphate starvation on the number of secondary rosette branches. * Significant difference of low-phosphate (10% Pi) from high-phosphate (100% Pi) treatment (P < 0.05). B, Col-0 grown under phosphate-sufficient conditions (left) and under low phosphate (right). C, max4-1 grown under phosphate-sufficient conditions (left) and under low phosphate (right). Arrowheads point to secondary rosette branches. [See online article for color version of this figure.]
Germination Stimulatory Activity of Arabidopsis Xylem Sap
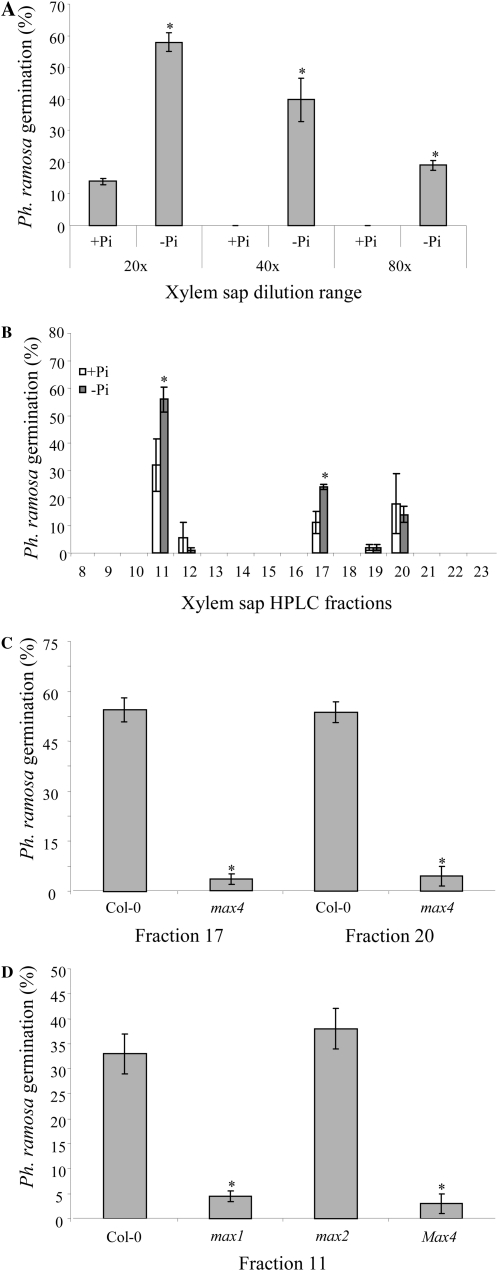
To investigate whether a signal responsible for the change in branching phenotype under phosphate starvation is transported from the root to the shoot through the vascular tissue, xylem sap was collected from Col-0 plants. The strigolactone-like nature of putative compounds in the xylem sap was assessed using the P. ramosa germination assay. Twenty-fold-diluted xylem sap from plants grown under phosphate-sufficient conditions induced about 13% germination, whereas phosphate-starved plants induced much higher germination (58%; Fig. 6A). Upon 40-fold and 80-fold dilution, xylem sap from phosphate-starved plants still induced 40% and 19% germination, respectively, whereas xylem sap collected from control plants did not induce any germination at these dilutions (Fig. 6A). Subsequently, xylem sap was fractioned on HPLC using the same gradient as used for the exudates, and the fractions were analyzed for their induction of P. ramosa germination. Fraction 11 of xylem sap collected from plants grown under phosphate-sufficient conditions induced germination (32%), whereas above it was shown that fraction 11 of the root exudates was inactive. Upon phosphate starvation, germination stimulated by this fraction increased 1.8-fold (Fig. 6B). Fractions 17 and 20 induced 11% and 18% germination, respectively. Upon phosphate starvation, germination induced by fraction 17 increased 2.2-fold compared with the control. This increase was not observed in fraction 20 (Fig. 6B). For both these xylem sap fractions in max4-1 plants, there was a 90% reduction (P < 0.05) in germination stimulation (Fig. 6C). No germination was detected in response to fractions higher than fraction 20. Xylem sap fractions 11 of max1-1 and max4-1 induced much lower germination (90% and 91% lower than the wild type, respectively; Fig. 6D). Xylem sap fraction 11 of max2-1 showed no significant change in germination stimulation compared with wild-type plants (Fig. 6D).
Figure 6.
Germination of P. ramosa seeds induced by xylem sap collected from Arabidopsis. A, Effect of several concentrations of xylem sap of Col-0, grown under sufficient phosphate (+Pi) and limiting phosphate (–Pi) levels, on germination of P. ramosa. Bars represent the average of three independent biological replicates ± se. * Significant difference of low-phosphate from high-phosphate treatment (P < 0.05). B, Effect of treatments with sufficient phosphate and limiting phosphate on germination stimulatory capacity of HPLC-fractioned xylem sap. Bars represent the average of three independent biological replicates ± se. * Significant difference of low-phosphate from high-phosphate treatment (P < 0.05). C, Germination induced by HPLC fractions 17 and 20 of Arabidopsis (Col-0 and max4-1) xylem sap. Bars represent the average of three independent biological replicates ± se. * Significant difference compared with Col-0 (P < 0.05). D, Germination induced by HPLC fraction 11 of Arabidopsis (Col-0, max1-1, max2-1, and max4-1) xylem sap. Bars represent the average of three independent biological replicates ± se. * Significant difference compared with Col-0 (P < 0.05).
Strigolactone Detection in Xylem Sap
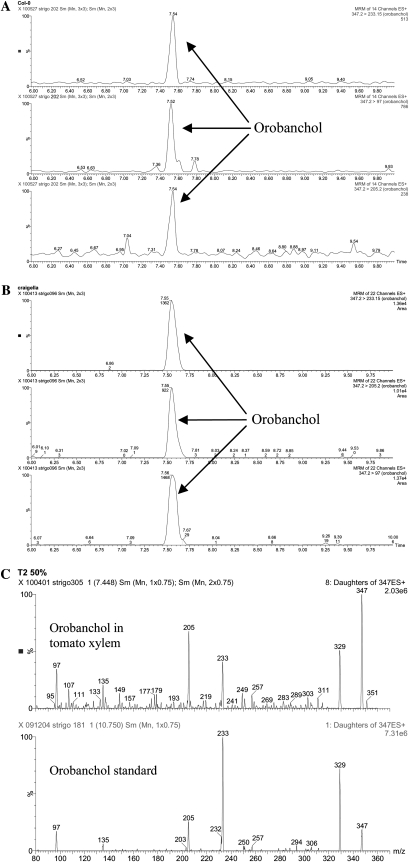
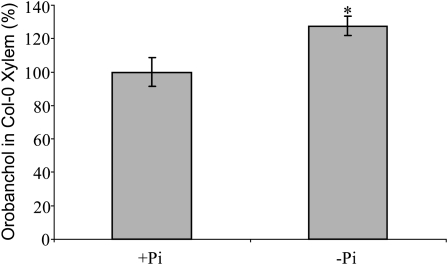
Xylem sap from Col-0 was collected from 10 phosphate-starved plants, divided over two experiments. These samples were individually purified and analyzed by MRM-LC-MS/MS. A clear peak of orobanchol (Fig. 7A) was detected in all samples, although the concentration was below the level required for MS/MS analysis. The concentration of orobanchol in xylem sap of plants grown under limited phosphate was 27% higher than in plants grown under sufficient phosphate (Fig. 8). To confirm these findings, tomato xylem sap was analyzed for the presence of strigolactones. Also, tomato xylem sap contained orobanchol, which could be unambiguously confirmed by MS/MS analysis and comparison with an authentic standard (Fig. 7, B and C).
Figure 7.
A, MRM-LC-MS/MS analysis of Arabidopsis xylem sap (Col-0) showing transitions 347 > 233, 347 > 205, and 347 > 96.8 for orobanchol. B, MRM-LC-MS/MS analysis of tomato xylem sap (cv Craigella) showing transitions 347 > 233, 347 > 205, and 347 > 96.8 for orobanchol. C, Full daughter ion scan MS/MS spectrum of orobanchol in tomato xylem sap and orobanchyl acetate standard.
Figure 8.
Analysis of orobanchol content of Arabidopsis Col-0 xylem sap. * Significant difference compared with sufficient phosphate conditions (+Pi; P < 0.05).
To investigate further whether strigolactones can be transported from the root to the shoot, the synthetic strigolactone analog GR24 was applied to the roots of hydroponically grown Arabidopsis. Extracts from the aerial parts were made, and GR24 was detected using MRM-LC-MS/MS in tissues that had not been in contact with the medium (Supplemental Fig. S4).
DISCUSSION
Arabidopsis Produces Strigolactones
In the work reported here, two (orobanchol and orobanchyl acetate) and possibly three (5-deoxystrigol) strigolactones were identified in Arabidopsis exudates using MRM-LC-MS/MS. It was shown that strigolactone production in Arabidopsis is up-regulated by phosphate deficiency and that MAX1 and MAX4 activity is required for the biosynthesis of orobanchol and the germination stimulatory compounds eluting in HPLC fractions 11 and 17. Furthermore, it was demonstrated that strigolactone(-like) compounds are involved in the regulation of shoot branching under phosphate starvation and that they are transported from the root to the shoot through the xylem.
The increase in P. ramosa germination induced by Arabidopsis root exudates of plants grown under phosphate starvation shows that phosphate limitation increases the secretion of germination stimulatory compounds by this non-AM host (Fig. 1A). HPLC fractionation of Arabidopsis root exudates resulted in a number of bioactive fractions, which are only partially explained by our MRM-LC-MS/MS data (Fig. 1B). The germination stimulatory activity in fractions 17 and 20 was shown to be carotenoid derived (inhibited by fluridone; Fig. 1B) and dependent on MAX1 and MAX4 (Fig. 2) and hence is very likely strigolactone like in nature, whereas fractions 25 and 26 were not (Fig. 1B). MRM-LC-MS/MS analysis of HPLC-fractioned strigolactone standards showed that the strigolactones 7-hydroxyorobanchyl acetate and solanacol elute in fraction 17 and orobanchol elutes in fraction 20 (Fig. 1B; Supplemental Table S1), with traces of these strigolactone standards also present in the next fractions (18 and 21, respectively), which probably explains the residual germination observed in these fractions (Fig. 1B). When fractions 17 and 20 were analyzed in three of the max mutants (max1, max2, and max4), there was a significant reduction in germination-inducing activity for these fractions in the putative strigolactone biosynthetic mutants max1-1 and max4-1 but not in the putative signaling mutant max2-1 (Fig. 2). Together, these results indicate that the active compounds responsible for the induction of P. ramosa seed germination in these two fractions are strigolactone like.
No known strigolactone from the standard mix eluted in fraction 22, which could indicate the presence of a so far unidentified strigolactone in Arabidopsis. No effect of fluridone, phosphate starvation (Fig. 1B), or max mutation (Supplemental Fig. S1) was observed on the germination-inducing activity of fractions 25 and 26, indicating that the activity in these fractions is not carotenoid derived and not strigolactone like. It has been reported that the germination of several Orobanche and Phelipanche species is induced by compounds other than strigolactones. For example, the germination of Orobanche foetida is induced by polyphenolic compounds isolated from pea (Evidente et al., 2010). It is possible that Arabidopsis root exudates contain similar non-strigolactone-like germination stimulants. The secretion of these unknown stimulants under all conditions (Fig. 1B) indicates that they could be responsible for the relatively high germination-inducing activity of crude root exudates of plants grown under phosphate-sufficient conditions (Fig. 1A). In addition, these compounds could be masking the germination stimulatory activity of orobanchyl acetate, which we detected in purified crude exudates and the standard of which elutes in fraction 26 (Fig. 1B). Additional purification or a different HPLC gradient could possibly resolve this.
Detection of Strigolactones in Arabidopsis Root Exudates and Extracts
Analysis of an orobanchol standard in an Arabidopsis root exudate matrix using MRM-LC-MS/MS resulted in an 80% reduction in the recovery of orobanchol (data not shown). This suggests that in-source ion suppression is interfering with strigolactone (orobanchol) detection in Arabidopsis samples. Ion suppression occurs when easily ionizable molecules coelute from the liquid chromatograph and are coinjected into the ionization chamber, preventing optimal ionization and thus detection of the target compound. Purification and concentration of crude Arabidopsis root exudates using silica column chromatography resulted in a much better recovery of strigolactones and allowed us to identify two known strigolactones, orobanchol and orobanchyl acetate, and to have evidence of the presence of a third one, 5-deoxystrigol, under phosphate starvation. The fact that orobanchol could now be detected suggests that the additional purification eliminated interfering molecules and effectively reduced ion suppression. Orobanchol standard addition to the purified sample confirmed this (data not shown). MS/MS spectra (Fig. 3, C and D) and spiking (data not shown) with standards of the first two compounds proved that they were indeed orobanchol and orobanchyl acetate. The concentration of 5-deoxystrigol proved to be insufficient for MS/MS analysis (Supplemental Fig. S2). However, spiking of the sample with a 5-deoxystrigol standard (data not shown) and comparison of the ratio between MRM transitions of the sample and that of the 5-deoxystrigol standard indicate that 5-deoxystrigol is likely present in Arabidopsis. The germination bioassay on fraction 28, where a 5-deoxystrigol standard elutes, only revealed a low percentage of germination (less than 1%), an indication that the concentration of 5-deoxystrigol in Arabidopsis root exudates is very low. No strigolactones were detected in exudates of plants grown under phosphate-sufficient conditions, again confirming their induction upon phosphate deficiency. Orobanchol has been reported in Arabidopsis before, although no MS/MS spectrum was obtained (Goldwasser et al., 2008). Based on germination assays using HPLC-fractioned Arabidopsis root exudates, Goldwasser et al. (2008) postulated that orobanchyl acetate may also be present in Arabidopsis exudate. However, it was not detected by LC-MS/MS analysis at that time, nor was 5-deoxystrigol (Goldwasser et al., 2008), but in our work here, the presence of both in Arabidopsis was analytically confirmed. The detection of orobanchol by MRM-LC-MS/MS makes it likely that this strigolactone is responsible for the germination-inducing activity of fraction 20.
Interestingly, all three strigolactones detected in this study were postulated to be biosynthetically linked to each other (Supplemental Fig. S5; Rani et al., 2008). In the biosynthetic scheme proposed by Rani et al. (2008), 5-deoxystrigol is the first real strigolactone in the pathway and serves as the common substrate for all known strigolactones, which could be an explanation for the low 5-deoxystrigol concentration. In one branch of the proposed pathway, 5-deoxystrigol is hydroxylated at position 4 to form orobanchol (Supplemental Fig. S5). Acetylation, a common reaction in the strigolactone pathway (Rani et al., 2008), of orobanchol would then yield orobanchyl acetate. In line with this, a reasonable hypothesis is that the activity detected in fraction 17, which shows the typical strigolactone-like pattern (Figs. 1B and 2), is due to another related strigolactone, the recently discovered 7-hydroxyorobanchyl acetate (Koichi Yoneyama, personal communication; Supplemental Table S1), which is proposed to be two putative biosynthetic steps away from orobanchol (Xie et al., 2010). However, 7-hydroxyorobanchyl acetate was not detected by MRM-LC-MS/MS analysis in any of the Arabidopsis samples.
Strigolactones in max Mutants
MRM-LC-MS/MS analysis revealed a strong reduction in orobanchol in root extracts and exudates of both putative biosynthetic max mutants, max1-1 and max4-1, but not in the signal transduction mutant, max2-1 (Fig. 4, A and B), providing evidence for a role in strigolactone biosynthesis for both MAX1 and MAX4 and hence analytically confirming the complementation studies with GR24 carried out with these mutants (Gomez-Roldan et al., 2008; Umehara et al., 2008; Crawford et al., 2010). Similar to the rice strigolactone response mutant d3 (Umehara et al., 2008), extracts of max2 grown under phosphate-sufficient conditions displayed an elevated orobanchol concentration compared with Col-0. This could be explained by impaired feedback regulation on strigolactone biosynthesis in this mutant (Mashiguchi et al., 2009). Our germination bioassays show that MAX1 and MAX4 activity is needed for the formation of the P. ramosa germination-inducing compound eluting in fraction 20 (probably orobanchol), which further confirms our analytical data.
It was previously postulated that one or more cytochrome P450 enzymes must be involved in strigolactone biosynthesis (Matusova et al., 2005; Humphrey et al., 2006; Rani et al., 2008). The reduction of orobanchol in max1-1 (Fig. 4) indicates that, indeed, MAX1, which was shown to encode a cytochrome P450 (Booker et al., 2005), is essential for the biosynthesis of this strigolactone. The biosynthesis of the unknown, putative strigolactones eluting in exudate fraction 17 and xylem sap fraction 11 also requires MAX1 (Figs. 2 and 6D). We cannot conclude whether MAX1 catalyzes an early step in strigolactone biosynthesis or whether it is involved in the conversion of one strigolactone or group of strigolactones into another. However, the facts that all strigolactone-like activities require MAX1 and that the predicted catalytic activity of MAX1 is that of intramolecular rearrangement (Booker et al., 2005) make it likely that MAX1 involvement in strigolactone biosynthesis is located upstream of 5-deoxystrigol. In the comparison of root exudates and root extracts of the max mutants with Col-0, 5-deoxystrigol was below the detection level, making it impossible to confirm MAX1 involvement in its biosynthesis.
The fact that the max biosynthetic mutants max1-1 and max4-1 still produce some orobanchol (Fig. 4) indicates that either both mutants are leaky or that there is a second, less active, pathway leading to strigolactone biosynthesis, separate from the MAX1/3/4 pathway. It has been reported that the bushy phenotype of max4-5 is more severe than that of max1-1 or max4-1 (Bennett, 2006), suggesting that the mutants used in this study retain some catalytic activity. The possibility of alternative biosynthetic pathways is also plausible. An alternative low-level pathway would explain why overexpression of MAX2 in max biosynthetic mutant backgrounds can partially suppress their increased branching phenotypes (Stirnberg et al., 2007). Multiple biosynthetic pathways have been described for auxin (Zhao, 2010) and cytokinins (Sakakibara, 2006). In addition, two so-called bypasses in the conversion of xanthoxin to abscisic acid have been postulated for abscisic acid biosynthesis (Cutler and Krochko, 1999; Seo and Koshiba, 2002).
Strigolactone-Mediated Regulation of Shoot Branching under Low-Phosphorus Conditions
When plants were grown under phosphate deficiency, the outgrowth of secondary rosette branches in Col-0 was strongly suppressed, but no such suppression was observed in the max mutants (max1-1, max2-1, and max4-1; Fig. 5A). This confirms results with rice, where inhibition of tiller outgrowth under phosphate starvation was observed in wild-type rice but not in the strigolactone mutants d3 and d10 (Umehara et al., 2010). However, here, we show that this effect is also present in Arabidopsis, a plant species that is not an AM host. The fact that the reduction in secondary rosette branches was not observed in any of the analyzed max mutants (max1-1, max2-1, and max4-1) indicates that the architectural response to low phosphate levels in Arabidopsis is likely mediated by strigolactones and transduced through MAX2.
Interestingly, strigolactone secretion was reported to be unaffected by phosphate deficiency in another non-AM host, Lupinus albus (Yoneyama et al., 2008), indicating that the response to phosphate deficiency is not conserved in the whole plant kingdom. However, L. albus has a different strategy to ensure phosphate uptake by forming cluster roots (Lambers et al., 2006). Therefore, it is possible that strigolactone biosynthesis in that species is up-regulated without increased exudation. Unfortunately, analysis of root extracts in L. albus to investigate the effect on strigolactone biosynthesis was not performed.
Strigolactones Are Transported through the Xylem
In xylem sap, three germination-stimulating fractions (fractions 11, 17, and 20) were identified. When tested in max4-1, fractions 17 and 20 showed a 90% reduction in germination compared with the wild type (Fig. 6C). A similar reduction in fractions 17 and 20 was observed in root exudates of max1-1 and max4-1 (Fig. 2). This suggests that the compounds responsible for germination in both fractions are the same for root exudates and xylem sap. Interestingly, no activity was detected in fraction 11 of root exudates in any of the experiments, whereas the germination activity in xylem sap fraction 11 was up-regulated upon phosphate deficiency, reduced in max1-1 and max4-1, and present in max2-1 xylem sap (Fig. 6D). This suggests that this xylem sap fraction contains a strigolactone-like compound. However, it is much more polar than any of the known Arabidopsis strigolactones. It is possible that this shift in polarity is caused by minor modifications such as hydroxylation. In line with the hypothesis about fraction 17 (possibly 7-hydroxyorobanchyl acetate), the compound in fraction 11 could be 7-hydroxyorobanchol (Supplemental Table S1). The fact that germination activity was detected in xylem sap fraction 11 but not in the same fraction of root exudates suggests that the compound responsible for the P. ramosa germination in this fraction is either not synthesized in the root (but, for example, in the hypocotyl) or is produced in the root and by selective mechanisms of strigolactone secretion and/or transport is only secreted into the xylem. More research is needed to identify the active compound present in fraction 11 and to study its biological function.
When xylem sap collected from Col-0 was purified by silica column chromatography and analyzed by MRM-LC-MS/MS, orobanchol was detected (Fig. 7A), providing, to our knowledge for the first time, evidence that this strigolactone is transported through the xylem. The transport rate of orobanchol in xylem sap was estimated to be 50 pg plant−1 h−1. The orobanchol concentration in Arabidopsis xylem sap samples proved to be insufficient for MS/MS analysis. To confirm the presence of strigolactones in xylem sap, xylem sap of tomato, a relatively high strigolactone producer, was analyzed. Again, a clear peak was detected (Fig. 7B), and MS/MS analysis identified the compound as orobanchol (Fig. 7C). Finally, GR24 applied to the root system of Arabidopsis was detected in the hypocotyl and stem (Supplemental Fig. S4), indicating also that this synthetic strigolactone is transported. Taken together, these findings provide compelling evidence that strigolactones themselves are transported through the xylem.
When germination-inducing activity of these three xylem sap fractions (11, 17, and 20) was analyzed under phosphate limitation, a clear increase in germination was detected in fractions 11 and 17 but not in fraction 20. This suggests that only the active compounds in the first two fractions, assuming that they are strigolactone(-like), would be involved in the regulation of shoot branching under phosphate deficiency. However, fraction 20 induced only minor germination, and a large variation was observed within the replicates. When concentrated Col-0 xylem sap was analyzed by MRM-LC-MS/MS (n = 5), a significant 27% increase (P < 0.05) of orobanchol was detected under phosphate depletion compared with plants grown under phosphate-sufficient levels (Fig. 8), indicating that orobanchol, likely fraction 20, is also involved in the regulation of the shoot architectural response to phosphate starvation.
Are Arabidopsis Strigolactones Involved in Rhizosphere Signaling?
It has been postulated that the evolution of plant-AM fungal symbiosis was a key step in the evolution of plants, enabling them to leave the oceans and colonize the land (Pirozynski and Malloch, 1975; Bonfante and Genre, 2008). In addition, it is believed that the main selective driving force for the up-regulation of strigolactone secretion upon phosphate deficiency in plants is to function as a signal in the rhizosphere to stimulate hyphal branching of AM fungi (Bouwmeester et al., 2007). However, Arabidopsis does not engage in symbiosis with AM fungi and seems to have no need for strigolactone secretion into the rhizosphere. Given that most land plants are mycorrhizal, this is almost certainly the ancestral condition; hence, lack of AM symbiosis in Arabidopsis could be a derived trait. It is possible, therefore, that strigolactone exudation from roots is a relic of this ancestral trait, which was lost by Arabidopsis, for example, because orthologs of genes such as DMI3 (Zhu et al., 2006) and DXS-2 (Walter et al., 2007) were lost. However, in this study, we demonstrate that up-regulation of strigolactone biosynthesis during phosphate starvation likely plays a role in the regulation of processes resulting in a reduced shoot-branching phenotype. This response allows the plant to ensure production of some high-quality seed as well as to invest energy in enhanced lateral root formation, a process shown to occur under phosphate-limiting conditions (Bates and Lynch, 1996; Williamson et al., 2001; López-Bucio et al., 2002; Sánchez-Calderón et al., 2005). This enables the plant to focus and intensify root growth in topsoil, where phosphorus is usually at higher concentrations. We assume that these responses also represent an evolutionary advantage, which would be the driving force for the preservation of low-phosphate-induced strigolactone biosynthesis in Arabidopsis.
MATERIALS AND METHODS
Plant Material and Chemicals
Arabidopsis (Arabidopsis thaliana) Col-0, max1 introduced into the Col-0 genetic background by seven backcrosses (Stirnberg et al., 2002), max2-1, and max4-1 plants were used for all experiments. The carotenoid pathway inhibitor fluridone was obtained from Duchefa Biochemie. The synthetic germination stimulant, strigolactone analog GR24, was kindly provided by Binne Zwanenburg (Department of Organic Chemistry, Radboud University). The natural germination stimulants 5-deoxystrigol, 7-hydroxyorobanchol, 7-hydroxyorobanchyl acetate, 7-oxoorobanchol, 7-oxoorobanchyl acetate, orobanchol, orobanchyl acetate, sorgolactone, solanacol, sorgomol, and strigol were kindly provided by Koichi Yoneyama (Weed Science Center, Utsunomiya University).
Plant Material, Growth Conditions, and Experiments
Arabidopsis seeds were sterilized in 4% sodium hypochlorite containing 0.02% (v/v) Tween 20, rinsed thoroughly with sterile water, and stratified for 48 h on moistened filter paper at 4°C in darkness. For hydroponics, seeds were sown on rockwool-filled Eppendorf vials of which the bottom was removed to enable the root to access the liquid medium. Plants were grown under controlled conditions in a climate chamber at 16 h of light/8 h of dark, 20°C/18°C, 60% relative humidity, and a light intensity of 150 μmol m−2 s−1 using either one-third-strength Hoagland solution or Tocquin Arabidopsis medium (Tocquin et al., 2003). Phosphate starvation was induced by reducing the level of NH4H2PO4 to 10% (0.013 mm) of the control nutrient solution. Root exudates were collected for 24 h in 50-mL tubes. For LC-MS/MS analysis of strigolactones, Arabidopsis was grown in pots filled with 500 mL of a sand:vermiculite mixture (1:1). Seeds were sown on a small block of washed rockwool. Each pot contained 24 plants, and each sample consisted of 40 pots. The plants were grown in a greenhouse from March until May at 16 h of light/8 h of dark, 20°C/18°C, and 60% relative humidity. Extra light was provided to achieve a relatively high light intensity of 300 μmol m−2 s−1. Plants were watered using one-third-strength Hoagland solution. Two weeks before root exudate collection, pots were rinsed with 1.5 L (three times the pot volume) of tap water to remove phosphate. Plants were then watered (three times per week) with 50 mL per pot of one-third-strength Hoagland nutrient solution without phosphate. For root exudate collection, 1 L of tap water was added to the pots and the flow-through was collected. Root material was collected, quick frozen using liquid nitrogen, and stored at −80°C until further use.
HPLC Fractionation
Fifty milliliters of root exudate was loaded onto the preequilibrated column (Grace Pure C18-Fast 500 mg/3 mL SPE). Subsequently, the columns were washed with 5 mL of demineralized water and eluted with 5 mL of acetone. Five hundred microliters of C18 purified sample was concentrated to 100 μL and injected into an XBridge C18 column (4.6 × 150 mm; Waters) using the following gradient: 1 min of 100% water, 2 min of 27% (v/v) acetonitrile in water, 15 min of 45% (v/v) acetonitrile, 24 min of 80% (v/v) acetonitrile, and 24.2 min of 100% acetonitrile, which was maintained for 4 min to clean the column. The flow rate was 1 mL min −1, and the column temperature was set at 25°C. One-minute fractions were collected using a Biofrac fraction collector (Bio-Rad).
Germination Bioassay
Germination assays with Phelipanche ramosa seeds were conducted as reported previously (Matusova et al., 2005). Seeds of P. ramosa were cleaned using a Suc gradient (Hartman and Tanimonure, 1991) and preconditioned (“warm stratification”) for 10 to 12 d at 21°C. Aliquots (50 μL) of root exudates or xylem sap (without organic solvent) were added to triplicate 1-cm discs containing approximately 50 preconditioned seeds each. The synthetic germination stimulant GR24 at 3.3 × 10−9 m (inducing about 50% germination) and demineralized water were included as positive and negative controls, respectively, in each bioassay. After 6 d, the germinated and nongerminated seeds were counted using a stereomicroscope. Seeds were considered germinated when the radicle had protruded through the seed coat. Root exudates were concentrated 10-fold before germination assays, and xylem sap was diluted 20-fold.
Xylem Extraction
Xylem sap was collected from Arabidopsis plants by the syringe-suction method as described by Beveridge et al. (1997a) with minor modifications. Plants were decapitated above the hypocotyl. A flexible silicon tube (length, 30 mm; internal diameter, 1 mm) attached to a 5-mL syringe was placed about 5 to 10 mm over the stump and tied tightly in place. The plunger of the syringe was pulled and held at that position to create a vacuum within the syringe. About 100 μL of xylem sap was collected per plant. For tomato (Solanum lycopersicum) xylem sap collection, tomato plants were grown in a hydroponic system on one-half-strength Hoagland solution. Four-week-old plants were decapitated above the hypocotyl, and a plastic ring was placed around the stem. The accumulating xylem sap was collected every 30 min.
All xylem sap was collected for 90 min after decapitation, and the first and last plant were decapitated within 30 min of each other. In order to remove any debris, xylem sap was centrifuged at 13,500 rpm (germination assay) or hand filtered (MRM-LC-MS/MS analysis) with a Minisart SRP4 0.45-μm filter (Sartorius Stedim Biotech), after which the xylem sap was frozen in liquid nitrogen and stored at −80°C before further use.
Silica Column Chromatography
To further purify strigolactones, silica column chromatography was performed. Silica columns were custom made using 200 mg of silica gel 60 (Merck), particle size 0.063 to 0.200 mm (70–230 mesh ASTM), for each 1 mg of residue. The residue was dissolved in chloroform, after which 100 mg of silica was added. The solvent was evaporated, and the silica-bound sample was added to the silica column. A mixture of n-hexane and an increasing percentage of ethyl acetate was used to elute compounds. In total, 10 silica fractions were collected from 0% to 100% in 10% increments of ethyl acetate. Solvents of these fractions were evaporated under vacuum, and the residue was dissolved in 1 mL of acetone and stored at −20°C until further use. Before MRM-LC-MS/MS measurement, silica fractions were concentrated 4-fold.
Strigolactone Purification from Root Exudates
For MRM-LC-MS/MS analysis, 5 L of root exudates was loaded onto a preequilibrated column (Grace Pure C18-Fast 5000 mg/20 mL SPE). Subsequently, the columns were washed with 50 mL of 30% acetone in water and strigolactones were eluted with 50 mL of 60% acetone in water, creating a C18 fraction in which all known strigolactones elute. Samples were evaporated to dryness, and the residue was dissolved in chloroform and purified by silica column chromatography, as described above.
Strigolactone Extraction from Roots
For strigolactone analysis, 0.5 g of roots was ground with a mortar and pestle in liquid nitrogen. The samples were extracted with 2 mL of cold ethyl acetate containing [13C3]strigol as an internal standard in a 10-mL glass vial. The vials were vortexed and sonicated for 10 min in a Branson 3510 ultrasonic bath (Branson Ultrasonics). Samples were centrifuged for 10 min at 2,000 rpm in an MSE Mistral 2000 centrifuge (Mistral Instruments), after which the organic phase was transferred to a 4-mL glass vial. The pellets were reextracted with another 2 mL of ethyl acetate. The combined ethyl acetate supernatants were dried under a flow of nitrogen gas, and the residue was purified by silica column chromatography as described above.
Strigolactone Extraction from Xylem Sap
For Arabidopsis, xylem sap from three individual plants was pooled (n = 5) and loaded onto the preequilibrated column (Grace Pure C18-Fast 50 mg/1.5 mL SPE). Subsequently, the columns were washed with 0.5 mL of demineralized water and eluted with 0.5 mL of acetone. Samples were evaporated to dryness, and the residue was purified by silica column chromatography as described above. For tomato strigolactone analysis, 1 mL of xylem sap pooled from three individual plants was used (n = 5).
GR24 Feeding Experiment
For GR24 uptake and transport analysis, Arabidopsis plants were hydroponically grown on one-half-strength Hoagland solution. Six-week-old flowering Arabidopsis plants were transferred to individual 50-mL tubes and provided with nutrient solution containing either 2.5 μm or 0 μm GR24 (n = 3). Root, hypocotyl, rosette stem, lower stem, and higher stem (Supplemental Fig. S4) were individually sampled, extracted, and analyzed for GR24 content using MRM transitions 299 > 97, 299 > 157, and 299 > 185.
Strigolactone Detection and Quantification by LC-MS/MS
Analysis of strigolactones in Arabidopsis root exudates was performed by comparing retention times and mass transitions with those of available strigolactone standards (sorgolactone, strigol, 2′-epistrigol, orobanchol, 2′-epiorobanchol, 5-deoxystrigol, 2′-epi-5-deoxystrigol, solanacol, orobanchyl acetate, sorgomol, 7-oxoorobanchol, and 7-oxoorobanchyl acetate) using ultraperformance liquid chromatography (UPLC) coupled to MS/MS essentially as described by López-Ráez et al. (2008b) with some modifications. Analyses were performed using a Waters Micromass Quattro Premier XE tandem mass spectrometer and Waters Xevo tandem quadruple mass spectrometer equipped with an electrospray ionization source and coupled to an Acquity UPLC system (Waters). Chromatographic separation was obtained on an Acquity UPLC BEH C18 column (150 × 2.1 mm, 1.7 μm; Waters) by applying a water/acetonitrile gradient to the column, starting from 5% acetonitrile for 2.0 min and rising to 50% (v/v) acetonitrile in 8.0 min, followed by a 1.0-min gradient to 90% (v/v) acetonitrile, which was maintained for 0.1 min before going back to 5% acetonitrile using a 0.2-min gradient, prior to the next run. Finally, the column was equilibrated for 2.8 min using this solvent composition. Operation temperature and flow rate of the column were 50°C and 0.4 mL min−1, respectively. Sample injection volume was 15 μL. The mass spectrometer was operated in positive electrospray ionization mode. Cone and desolvation gas flows were set to 50 and 1,000 L h−1, respectively. The capillary voltage was set at 3.0 kV, the source temperature at 150°C, and the desolvation temperature at 650°C. The cone voltage was optimized for each standard compound using the Waters IntelliStart MS Console. Argon was used for fragmentation by collision-induced dissociation in the ScanWave collision cell. MRM was used for identification of strigolactones in root exudates and extracts by comparing retention times and MRM mass transitions with those of the strigolactone standards. MRM transitions were optimized for each standard compound using the Waters IntelliStart MS Console. Data acquisition and analysis were performed using MassLynx 4.1 (TargetLynx) software (Waters).
Statistical Analysis
Student’s t tests were performed when appropriate.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. P. ramosa germination of HPLC fractions 25 and 26.
Supplemental Figure S2. 5-Deoxystrigol in Arabidopsis exudates.
Supplemental Figure S3. Arabidopsis strigolactone root extracts.
Supplemental Figure S4. GR24 uptake assay.
Supplemental Figure S5. Postulated strigolactone conversion scheme.
Supplemental Table S1. Standard mix HPLC fractions.
Supplementary Material
Acknowledgments
We thank Binne Zwanenburg for supplying GR24, Thierry Levgeux for provided P. ramosa seeds, Koichi Yoneyama for advice and strigolactone standards, Juan Antonio Lopéz-Ráez, Peter Toth, James H. Westwood, and Wim Vriezen for their intellectual input, and Padraic Flood for critical reading of the manuscript.
References
- Akiyama K, Matsuzaki K, Hayashi H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P. (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ 26: 1053–1066 [Google Scholar]
- Bates TR, Lynch JP. (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19: 529–538 [Google Scholar]
- Bennett T. (2006) The regulation of shoot branching in Arabidopsis thaliana. PhD thesis. University of York, York, UK [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C. (1997a) The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J 11: 339–345 [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC. (1994) Branching mutant rms-2 in Pisum sativum (grafting studies and endogenous indole-3-acetic acid levels). Plant Physiol 104: 953–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C. (1997b) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol 115: 1251–1258 [Google Scholar]
- Bonfante P, Genre A. (2008) Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sci 13: 492–498 [DOI] [PubMed] [Google Scholar]
- Bonser AM, Lynch J, Snapp S. (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132: 281–288 [DOI] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8: 443–449 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. (2003) Secondary metabolite signalling in host-parasitic plant interactions. Curr Opin Plant Biol 6: 358–364 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G. (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12: 224–230 [DOI] [PubMed] [Google Scholar]
- Cline M. (1997) Concepts and terminology of apical dominance. Am J Bot 84: 1064–1069 [PubMed] [Google Scholar]
- Cook CE WL, Wall ME, Egley GH, Coggon P, Luhan PA, McPhail AT. (1972) The structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea Lour.). J Am Chem Soc 94: 6198–6199 [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O. (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE. (1999) Formation and breakdown of ABA. Trends Plant Sci 4: 472–478 [DOI] [PubMed] [Google Scholar]
- Evidente A, Cimmino A, Fernández-Aparicio M, Andolfi A, Rubiales D, Motta A. (2010) Polyphenols, including the new pea polyphenols A-C, from pea root exudates stimulate Orobanche foetida seed germination. J Agric Food Chem 58: 2902–2907 [DOI] [PubMed] [Google Scholar]
- Foo E, Turnbull CGN, Beveridge CA. (2001) Long-distance signaling and the control of branching in the rms1 mutant of pea. Plant Physiol 126: 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser Y, Yoneyama K, Xie X, Yoneyama K. (2008) Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regul 55: 21–28 [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Harrison MJ. (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59: 19–42 [DOI] [PubMed] [Google Scholar]
- Hartman GL, Tanimonure OA. (1991) Seed populations of Striga species in Nigeria. Plant Dis 75: 494–496 [Google Scholar]
- Hartung W, Sauter A, Hose E. (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53: 27–32 [PubMed] [Google Scholar]
- Humphrey AJ, Galster AM, Beale MH. (2006) Strigolactones in chemical ecology: waste products or vital allelochemicals? Nat Prod Rep 23: 592–614 [DOI] [PubMed] [Google Scholar]
- Jamil M, Charnikhova T, Cardoso C, Jamil T, Ueno K, Verstappen F, Asami T, Bouwmeester H. (2010) Quantification of the relationship between strigolactones and Striga hermonthica in rice under varying levels of nitrogen and phosphorus. Weed Res (in press) [Google Scholar]
- Joel DM. (2009) The new nomenclature of Orobanche and Phelipanche. Weed Res 49: 6–7 [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot (Lond) 98: 693–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, Verstappen F, Puech-Pages V, Bécard G, Mulder P, et al. (2008a) Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 178: 863–874 [DOI] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Mulder P, Kohlen W, Bino R, Levin I, Bouwmeester H. (2008b) Susceptibility of the tomato mutant high pigment-2dg (hp-2dg) to Orobanche spp. infection. J Agric Food Chem 56: 6326–6332 [DOI] [PubMed] [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP. (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Sasaki E, Shimada Y, Nagae M, Ueno K, Nakano T, Yoneyama K, Suzuki Y, Asami T. (2009) Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci Biotechnol Biochem 73: 2460–2465 [DOI] [PubMed] [Google Scholar]
- Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ. (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139: 920–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P. (2005) A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138: 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi G, Douds DD. (2000) Partial separation of root exudate components and their effects upon the growth of germinated spores of AM fungi. Mycol Res 104: 1453–1464 [Google Scholar]
- Napoli C. (1996) Highly branched phenotype of the petunia dad1-1 mutant is reversed by grafting. Plant Physiol 111: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski U. (2006) Mutualism and parasitism: the yin and yang of plant symbioses. Curr Opin Plant Biol 9: 364–370 [DOI] [PubMed] [Google Scholar]
- Pirozynski KA, Malloch DW. (1975) The origin of land plants: a matter of mycotrophism. Biosystems 6: 153–164 [DOI] [PubMed] [Google Scholar]
- Rani K, Zwanenburg B, Sugimoto Y, Yoneyama K, Bouwmeester HJ. (2008) Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiol Biochem 46: 617–626 [DOI] [PubMed] [Google Scholar]
- Sakakibara H. (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57: 431–449 [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L. (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46: 174–184 [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T. (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7: 41–48 [DOI] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al. (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17: 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Leyser HMO. (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50: 80–94 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, van De Sande K, Leyser HMO. (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Tocquin P, Corbesier L, Havelange A, Pieltain A, Kurtem E, Bernier G, Périlleux C. (2003) A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troughton A. (1977) The effect of phosphorus nutrition upon the growth and morphology of young plants of Lolium perenne L. Ann Bot (Lond) 41: 85–92 [Google Scholar]
- Turnbull CGN, Booker JP, Leyser HMO. (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32: 255–262 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S. (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51: 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Walter MH, Floss DS, Hans J, Fester T, Strack D. (2007) Apocarotenoid biosynthesis in arbuscular mycorrhizal roots: contributions from methylerythritol phosphate pathway isogenes and tools for its manipulation. Phytochemistry 68: 130–138 [DOI] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO. (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126: 875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Yoneyama K. (2010) The strigolactone story. Annu Rev Phytopathol 48: 93–117 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Takeuchi Y, Yokota T. (2001) Production of clover broomrape seed germination stimulants by red clover root requires nitrate but is inhibited by phosphate and ammonium. Physiol Plant 112: 25–30 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, Hayashi H, Yoneyama K. (2008) Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol 179: 484–494 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. (2007) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225: 1031–1038 [DOI] [PubMed] [Google Scholar]
- Zhao Y. (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61: 49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Riely BK, Burns NJ, Ané JM. (2006) Tracing nonlegume orthologs of legume genes required for nodulation and arbuscular mycorrhizal symbioses. Genetics 172: 2491–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.