Abstract
Changes in dynamic properties of mitochondria are increasingly implicated in neurodegenerative diseases, particularly Parkinson’s disease (PD). Static changes in mitochondrial morphology, often under acutely toxic conditions, are commonly utilized as indicators of changes in mitochondrial fission and fusion. However, in neurons, mitochondrial fission and fusion occur in a dynamic system of axonal/dendritic transport, biogenesis and degradation, and thus, likely interact and change over time. We sought to explore this using a chronic neuronal model (nonlethal low-concentration rotenone over several weeks), examining distal neurites, which may give insight into the earliest changes occurring in PD. Using this model, in live primary neurons, we directly quantified mitochondrial fission, fusion, and transport over time and integrated multiple aspects of mitochondrial dynamics, including morphology and growth/mitophagy. We found that rates of mitochondrial fission and fusion change as neurons age. In addition, we found that chronic rotenone exposure initially increased the ratio of fusion to fission, but later, this was reversed. Surprisingly, despite changes in rates of fission and fusion, mitochondrial morphology was minimally affected, demonstrating that morphology can be an inaccurate indicator of fission/fusion changes. In addition, we found evidence of subcellular compartmentalization of compensatory changes, as mitochondrial density increased in distal neurites first, which may be important in PD, where pathology may begin distally. We propose that rotenone-induced early changes such as in mitochondrial fusion are compensatory, accompanied later by detrimental fission. As evidence, in a dopaminergic neuronal model, in which chronic rotenone caused loss of neurites before cell death (like PD pathology), inhibiting fission protected against the neurite loss. This suggests that aberrant mitochondrial dynamics may contribute to the earliest neuropathologic mechanisms in PD. These data also emphasize that mitochondrial fission and fusion do not occur in isolation, and highlight the importance of analysis and integration of multiple mitochondrial dynamic functions in neurons.
Keywords: mitochondria, mitochondrial, fission, fusion, transport, Parkinson’s disease, dynamics, mitophagy, neurodegenerative, neuron, neurodegeneration
INTRODUCTION
Mitochondrial dysfunction has long been linked to neurodegenerative diseases, particularly Parkinson’s disease (PD) (Abou-Sleiman et al., 2006). More recently, the dynamic properties of mitochondria, which include division (fission), fusion, transport along axons and dendrites, biogenesis, and degradation, are being recognized as playing a critical role in neurodegeneration. Mitochondrial dynamics have a multitude of important functions that may be especially critical to neurons, including maintenance of mitochondrial DNA (Parone et al., 2008; Westermann, 2002), involvement in apoptosis (Suen et al., 2008), formation and function of synapses and dendritic spines, and proper distribution of mitochondria (Chen et al., 2007; Li et al., 2004; Liu and Shio, 2008; Stowers et al., 2002; Verstreken et al., 2005).
Mitochondrial dynamics may be particularly important in the neurodegeneration of PD (see (Van Laar and Berman, 2009). The selectively vulnerable neurons in PD all share poorly-myelinated, long, thin axons (Braak et al., 2004), which could be hypothesized to be especially dependent on proper mitochondrial dynamics. In addition, evidence from both postmortem human tissue and animal models suggest that the degenerative process in PD may begin at the terminals (e.g., see (Betarbet et al., 2000; Braak et al., 2004), pointing to a potential role for mitochondrial dynamics early in the disease process. Most recently, it has been shown that dysregulation of mitochondrial fission/fusion or mitochondrial homeostasis may be responsible for mitochondrial abnormalities caused by defects in two gene products associated with familial PD, PTEN-induced putative kinase 1 (PINK1) and parkin. These have been linked both to regulation of mitochondrial fission and fusion, and to mitochondrial homeostasis through regulation of autophagic degradation (mitophagy)(Cui et al., 2010; Dagda et al., 2009; Deng et al., 2008; Exner et al., 2007; Geisler et al., 2010; Kawajiri et al., 2010; Lutz et al., 2009; Narendra et al., 2008; Narendra et al., 2010; Park et al., 2009; Poole et al., 2008; Vives-Bauza et al., 2010; Yang et al., 2008).
Studying mitochondrial dynamics in a living system is difficult, particularly in neurons, where mitochondria are being transported and distributed along axons/dendrites. Many studies evaluating mitochondrial dynamics assess mitochondrial fission and fusion via evaluation of static mitochondrial morphologic changes. However, previous studies suggested that this may not always accurately predict underlying changes in fission and fusion (Berman et al., 2009). In addition, it does not allow for evaluation of other, related dynamic functions of mitochondria, namely transport, growth, and degradation. Given that fission/fusion, transport, biogenesis and degradation likely interact and influence each other, it is important to begin to integrate analysis of these components of mitochondrial dynamics in neurons over time. In addition, static studies often focus on cell body changes, yet neuropathology of neurodegenerative diseases may originate in dendrites/axons, where mitochondrial fission/fusion is not well-characterized. Therefore, we wanted to develop the means to more directly evaluate multiple aspects of mitochondrial dynamics in living neurons, particularly in areas distal to the cell body.
We focused on a PD-related chronic model, given the strong evidence implicating mitochondrial dynamics in the neurodegeneration of PD. In addition, mitochondrial fission was associated with cell death in an acute toxicity model of PD (Barsoum et al., 2006) but chronic models of PD in neurons have not been well-studied. Also, as noted, axonal/dendritic changes may be important in PD-associated neurodegeneration. We therefore utilized a chronic, PD-relevant model, and directly and quantitatively evaluated changes over time in measures of mitochondrial dynamics in living neurons. We found that complex, interrelated changes in mitochondrial dynamics occur early in neurons and change over time, and that morphology is not necessarily an accurate predictor of changes in fission and fusion. In addition, in a chronic PD-relevant model, we found that early pathologic changes, prior to neuronal cell death, can be ameliorated by manipulating mitochondrial dynamics.
MATERIALS AND METHODS
Cell culture
Primary cortical neurons were derived from E18 rats utilizing previously described methods (Ghosh and Greenberg, 1995) with minor modifications. Briefly, pooled cortices from E18 rats were dissected on ice in HBSS, treated with papain enzyme solution (<200 U), then HBSS/trypsin inhibitor. Cells were washed, triturated, and plated overnight in Neurobasal medium (NBM) containing Pen/Strep, 2% glutamax, B27 supplement, and 5% fetal calf serum, at a density of 3 × 105/ml on glass-bottomed dishes (MatTek) and glass coverslips coated with poly-D-lysine and mouse laminin. Media was replaced with serum-free NBM with ½ media changes every 3 d. PC12 cell subline, PC6-3 cells, generously provided by Dr. Bruce Pittman (Pittman et al., 1993) were grown in DMEM with 10% HI-horse serum, 5% FBS, 4.5 mg/ml glucose, 4 mM L-glutamine, Pen/Strep and 10 mM HEPES. For differentiation, cells were plated at 2 × 104 cells/ml on poly-D-lysine-laminin/Collagen-I coated glass-bottom dishes and cultured in 1% heat-inactivated horse serum and 0.5% FBS, supplemented with 5.0 μg/ml insulin, 5.0 μg/ml transferrin, 5.0 ng/ml sodium selenite, and 40 ng/ml NGF (BD Biosciences). Cell culture media was replenished by ½ every 2 d. Cell death was determined by Trypan blue exclusion. Cell process morphology was determined by staining fixed cells with Alexa 488-phalloidin (Invitrogen). For each 6-well dish, cells in 50 fields (at 200x magnification) across each entire well were manually scored as having neuronal processes > 3x cell body length, < 3x cell body length, or no processes.
Transfection and treatment of cortical neurons
Transfection was performed at DIV6 using Lipofectamine 2000 per manufacturer’s instructions with modifications. Briefly, a total of 4 μg DNA pre-incubated in 250 ml Optimem was combined with 5 μl Lipofectamine pre-incubated in Optimem prior to placement on the cell cultures placed in transfection media (MEM pH 7.4, supplemented with 2% glutamax, 20 mM HEPES, 33 mM glucose, 1mM Na-pyruvate). Cells were incubated at 37°C in a non-CO2 incubator for 2-2.5 h, then media was replaced with a mixture of ½ conditioned neuronal media and ½ fresh supplemented SF-NBM. Cells were transfected with plasmids expressing mtDsRed2 (Clontech), mitochondrially-targeted, photoactivatable GFP containing the mitochondrial targeting sequence from cytochrome c oxidase subunit VIII (PA-mtGFP, provided by R.J. Youle, National Institute of Neurological Disease and Stroke at the National Institutes of Health, Bethesda, MD) along with control vector (pSG5), fission protein Drp1, or dominant-interfering mutant dnDrp1K38A (Labrousse et al., 1999; Smirnova et al., 1998) (provided by J.M. Hardwick, Johns Hopkins University, Department of Molecular Microbiology and Immunology) as specified. Transfection rates were 2-10%, and in 100% of cases, cells transfected with mtDsRed2 were co-transfected with PA-mtGFP. Viability was determined by Trypan blue exclusion. For chronic rotenone experiments, rotenone or control treatments were started 24 h following transfection, at 7 DIV. Cells were treated with concentrations of rotenone (Sigma, cat #R8875) as described in results from 5 mM stock solutions dissolved in DMSO (Sigma, cat # D2438). Cells received ½ media changes with 2X concentrations of rotenone or equivalent DMSO vehicle, followed by ½ media changes every 3d with 1X concentrations of rotenone or DMSO.
ATP measurements
Intracellular ATP levels were estimated using an ATP Determination Kit (Invitrogen) based on the reaction of luciferase with luciferin, using recommended manufacturer procedures. Briefly, treated cells were washed and harvested into cold PBS. The cell pellets were collected by centrifugation at 2,000 x g for 3 min. at 4°C, and resuspended in RIPA Lysis Buffer (50 mM Tris HCl, 150 mM NaCl, 10 mM EDTA, 0.1% SDS, 1% Triton X-100, and 1% Sodium Deoxycholate) in the presence of 1 mM dithiothreitol and a protease inhibitor cocktail (BD Biosciences). After incubating for 20 min on ice, cell extracts were sonicated, and centrifuged at 13,000 x g for 15 min at 4°C. Samples were stored at −80°C until analysis. ATP standards or diluted sample was added to wells of a 96 well plate, followed by the addition of reaction buffer (25 mM Tricine, pH 7.8, 5 mM MgSO4, 1 mM Dithiothreitol, 0.1 mM EDTA, 0.1 mM azide, 150 μg/ml luciferin and. 1.25 μg/ml luciferase). Luminometer measurements (560 nm) were quantified (L Max II Luminometer, Molecular Devices), and data were normalized to protein concentration determined with the BCA Protein Assay Kit (Pierce; experiments run in triplicate).
Direct observation of mitochondrial fission and fusion (DrOF)
Quantification of mitochondrial fission and fusion using DrOF was performed as described previously (Berman et al., 2009) with minor modifications. Briefly, all live-cell imaging was performed using an Olympus Fluoview 1000 confocal microscope (60x oil immersion). Neurons grown on glass-bottom MatTek dishes were placed in a 37°C chamber attached to the confocal stage, after media was replaced with transfection media as described above. For all studies, 3-6 neurites (each from individual cells expressing mtDsRed2) per condition per coverslip were evaluated for each experiment, randomly selected and at distances greater than 300 μm from the cell body. Individual mitochondria were randomly selected and PA-mtGFP photoactivated (405 nm). Time lapse imaging was then performed by acquiring images (excitation at 488 and 543 nm) taken every 10 s for a total of 15 min (90 images). Mitochondrial fission and fusion were later quantified by manual, blinded analysis. For this analysis, fusion events are defined by diffusion of PA-mtGFP from one mitochondrion to another non-actiated one. Fission events are defined as division of an individual mitochondrion; apparent division occurring within the first two images are not counted as fission events in order to reduce false events from passing mitochondria. Neurons in which no mitochondrial movement occurs were not analyzed, as they were considered to be unhealthy. There were no differences among conditions in terms of the proportion of immobile processes. For both this and the transport analysis, axons and dendrites were not able to be reliably distinguished, and thus all neurites were grouped together.
Analysis of mitochondrial transport
Neuronal cells were prepared and imaged as described above. NIH ImageJ software (Rasband, 1997-2009) with the Manual Tracking plug-in (F. Cordelieres; fabrice.cordelieres@curie.u-psud.fr) was utilized to identify and track individual mitochondria throughout the series and to calculate mitochondrial length (μm). Excel Visual Basic macros were designed to calculate direction, instantaneous and average velocities, and number of fast (> 0.5 μm/s), slow and immobile mitochondria. Although differences have been reported between axonal and dendritic transport of mitochondria, no net differences in directional transport have been found (Ligon and Steward, 2000; Overly et al., 1996), and we, as noted, were unable to reliably and consistently distinguish between these in our longterm cultures during live imaging.
Mitochondrial morphology and density measurements
To determine mitochondrial density in neuronal processes, cortical neurons were treated as described after transfection with mtDsRed2 and soluble GFP (generously provided by J. Marie Hardwick). Confocal z-stack images of neuronal processes were taken at distances of approximately 300 and 1,200 μm from the cell body at a thickness of 1 μm per slice. Processes were scanned for both mitochondrial RFP (dsRed2) and diffusible GFP at intensities below saturation. Using ImageJ (National Institutes of Health, Bethesda, MD), individual mitochondria were thresholded from background and analyzed using the program’s built-in particle detection feature. Feret’s diameter, measuring the longest diameter for each mitochondrion, was obtained and totaled. Using the NeuronJ plug-in for ImageJ (Meijering et al., 2004), the neuronal processes were traced with computer assistance and measured for length. The summated total length of all mitochondria within the process was divided by that process’ total length in order to obtain a ratio for each image. Three-dimensional cell body mitochondrial volume density was calculated by performing confocal z-stack images of neuron cell bodies, taken with thicknesses of 0.25 μm per slice. Using the Yawi3D Plugin for ImageJ (Mario Guaraccino, ICAR-CNR), the area of the cell body was detected automatically at each slice, measured and multiplied by the slice thickness to obtain a rough estimate of cell body volume. Using Metamorph 7.3 (MDS Analytical Technologies, Inc., Mississauga, Ontario), individual mitochondria within the 3D image of the cell body were isolated using the isosurface feature and measured for volume. As above, a ratio was obtained by dividing the total volume of the mitochondria by the total volume of the cell body.
Measures of autophagy/mitophagy
For immunocytochemistry, briefly, coverslips of cultured neurons were fixed with 4% PFA after one and two weeks of treatment with rotenone or vehicle control as above. Coverslips were permeabilized using 0.2% Triton in PBS, washed, blocked for non-specific binding with goat serum and labeled overnight with 1:250 rabbit anti-LC3 (Abgent BioTechnology, San Diego, CA). Cells were then labeled with 1:300 Alexa Fluor 488 goat anti-rabbit antibody (Invitrogen, Eugene, OR) and mounted on slides with Fluoromount-G (Southern Biotech, Birmingham, AL). For image analysis, slides were captured as z-stacks using an Olympus FV1000 confocal microscope using a 60x oil immersion lens at 2x zoom. Open-field fluorescent images of neurons were collected and blinded to condition for subsequent analysis. Images were viewed using the Olympus FV1000 Fluoview Viewer (Olympus America, Center Valley, PA). Neurons with five or more bright LC3-containing puncta were recorded by the analyzer as undergoing constitutive autophagy. After image analysis, the total number of neurons undergoing constitutive autophagy was ascertained as a percentage of the total neurons in each condition. Transfection did not affect the proportion of ‘autophagic’ neurons. For determination of mitochondrial colocalization with autophagosomes, cells were transfected with mtDsRed 2 prior to treatment and immunocytochemistry for LC3 was performed as above. Confocal z-stack images were acquired as above, using sequential imaging, and Olympus Fluoview colocalization software was utilized to determine Pearson’s coefficients in each condition.
Statistics
Binomial tests of significance of the difference between two independent proportions, T-tests of unpaired samples or ANOVA with Bonferroni post-hoc corrections for multiple analyses were utilized as indicated.
RESULTS
Quantified, direct observation of mitochondrial fission and fusion (DrOF) in neurons can be used in chronic neuronal models
We first established long-term primary neuronal cultures and utilized our previously established methodology for quantitatively assessing mitochondrial fission and fusion using fluorescent and photoactivatable mitochondrially targeted proteins, direct evaluation of mitochondrial fission and fusion (DrOF) (Berman et al., 2009), as well as methodology for evaluating mitochondrial transport in primary neurons. Earlier work utilized primary rat cortical neurons that were DIV 8 (Berman et al., 2009), and we found that we were able to utilize DrOF to analyze mitochondrial fission and fusion in neurons even after four weeks (28 DIV; 22 days after transfection). An example in a single neuronal process, in which a photoactivated (yellow) mitochondrion undergoes fusion with a non-photoactivated mitochondrion containing mtDsRed2, followed by a fission event is shown in Figure 1A and Supplemental Movie 1. Using this methodology, we are able to distinguish true fusion events from passing mitochondria, which occurs much more frequently than fusion (Berman et al., 2009). One caveat to these studies is that we could not distinguish between axons and dendrites, which could be an important distinction if presynaptic versus postsynaptic mechanisms are involved in the neuropathologic process. However, although there is some evidence that mitochondrial morphology and distribution can differ between axons and dendrites (Chang et al., 2006), differences in rates of fission and fusion have not been studied, and mitochondrial transport has been reported to be similar between axons and dendrites (Ligon and Steward, 2000).
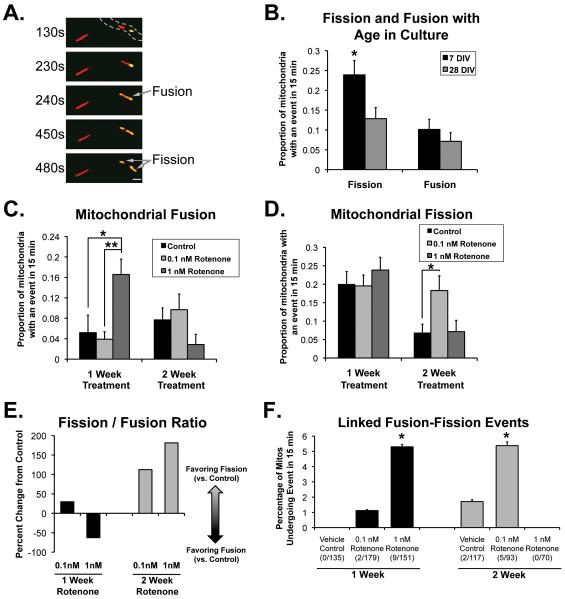
Figure 1.
Chronic rotenone alters mitochondrial fission and fusion. Primary rat cortical neurons were transfected with mtDsRed2 and PAmtGFP, then imaged under normal culturing conditions at times indicated (B) or treated beginning at DIV7 for 1or 2 weeks with media containing rotenone at listed concentrations or DMSO vehicle control (C-F). Live images were recorded for 15 min as described in Methods, and fission and fusion events were quantified blindly as described (n = at least 7 independent experiments, with 3-6 neuronal processes imaged per condition in each experiment. A, Sequential images showing example of mitochondrial fusion (2nd and 3rd panel: mitochondrion with mtDsRed2 and photoactivated PAmtGFP (yellow) fuses with neighboring mitochondrion (red) with resulting diffusion of GFP into newly fused side) and fission (4th panel: yellow mitochondrion now divides into two) (Also see Supplemental Movie 1). Gray hashed lines outline neuronal process. Scale bar is 2 μm. B, Probability of fission and fusion events occurring in photoactivated mitochondria over 15 min at DIV7 and DIV28 in culture (*Significantly different from DIV28 neurons, p=0.0173, two-tailed binomial test of differences between proportions). C, Probability of fusion events under conditions as noted. *Control vs 1 nM p = 0.0023 two tailed; **0.1 nM vs 1 nM p < 0.002. D, Probability of fission events under conditions noted. *p = 0.011 (binomial test of differences between proportions with Bonferroni correction). E, Change in the ratio of fission events to fusion events after one or two weeks exposure to rotenone (0.1 and 1 nM) compared to DMSO control. F, Percentage of events in which an individual mitochondrion underwent a fusion event followed by at least one fission event in a 15 min observation, after one or two weeks of rotenone or vehicle control (+/− SE). *95% confidence interval of proportions different from control.
Age-related changes in mitochondrial fission and fusion
We found that mitochondrial fission and fusion rates change as neurons mature/age in culture. Fission rates were higher in neurites of DIV 7 neurons than in older neurons (DIV 28; Figure 1B). Fusion rates, however, remained unchanged. This suggests that higher rates of mitochondrial fission occur at the time period while synaptic connections are being formed (Chang and Reynolds, 2006), and a lower rate as neurons age in culture, leading to an overall decrease in the ratio of fission to fusion. Although this is clearly not an ideal model of ‘aging’, there is evidence suggesting that primary neurons in culture do show signs suggestive of senescence by this age (Blalock et al., 1999; Chernova et al., 2006; Xiong et al., 2004). Thus, this could reflect physiologic aging-associated changes in mitochondrial fission and fusion.
Chronic rotenone-induced effects on mitochondrial fission and fusion change over time
We then developed a chronic neuronal model to look at changes in mitochondrial dynamics over time, prior to cell death. In Parkinson’s disease (PD), mitochondrial fission had been implicated in high-concentration, acutely lethal rotenone toxicity, but whether this is relevant to the chronic, slow changes in neurodegenerative disease is unclear. Therefore, we modified the well-established model of chronic rotenone, in which chronic, systemic rotenone in rats results in specific pathology similar to that seen in human PD (Betarbet et al., 2000), in order to evaluate a potential role for mitochondrial dynamics in early phases of the neurodegeneration of Parkinson’s disease (PD). We first utilized a low-concentration, chronic rotenone treatment model in cell culture and used primary rat neurons to look directly at mitochondrial dynamics after chronic rotenone treatment. We utilized DrOF and direct manual tracking to provide direct evaluation of the effect of chronic low-dose rotenone on mitochondrial dynamics in living neurons.
We wanted to develop a model in which the concentration of chronic rotenone did not lead acutely to a large amount of cell death, but rather was able to be provided over several weeks, in order to more closely model the chronic condition of PD. In these studies, then, we utilized primary rat cortical neurons, began treatment with 0.1 nM or 1 nM rotenone or vehicle control at 7 DIV, and treated for one and two weeks duration. At these low concentrations of rotenone, there was little increase in cell death, whereas higher concentrations of rotenone did cause significant neuronal cell death (Supplemental Figure 1A). In addition, these neurons were able to maintain normal ATP levels (Supplemental Figure 1B). This is consistent with previous data in cell culture that show that concentrations of rotenone less than 15 nM had minimal effect on mitochondrial respiration (Sherer et al., 2002)
Although up to two weeks of exposure to low concentrations of rotenone caused little cell death, we discovered effects on mitochondrial dynamics, resulting in quantitative changes in fission and fusion. We observed that one week of exposure to our higher concentration of rotenone (1 nM) resulted in a significant increase in mitochondrial fusion (Figures 1C), an effect that was gone by two weeks of exposure. The lower concentration of rotenone did not have significant effects on fusion itself, though analysis is limited by the rarity of fusion events. Conversely, after two weeks of exposure to low-dose rotenone (0.1 nM), mitochondrial fission increased significantly (Fig 1D). Based on these data, one can estimate the overall ratio of fission events to fusion events. This reveals that after one week of exposure to 1 nM chronic rotenone, the ratio of mitochondrial fission to fusion in neurons decreases, secondary to the increase in fusion (Figure 1E). However, after two weeks, the overall ratio of mitochondrial fission to fusion increases in both rotenone conditions compared to vehicle control (Figure 1E).
Chronic rotenone increases the frequency of linked fusion/fission events
Additionally, we observed an interesting phenomenon: chronic exposure to rotenone also increased the number of mitochondria undergoing fusion (with mixing of matrix contents) followed by linked fission (Figures 1F), defined as a fusion event followed by a fission event in the resulting mitochondrion within the 15 minute observation period. These events were rare in general in neuronal processes, but when occurring, the fission events occurred from 60 to 260 seconds following the initial fusion event, with an average duration of 151 seconds between the fusion and fission. This is similar to the time course of linked fusion/fission events noted in non-neuronal cells that was proposed to be involved in autophagic degradation of damaged mitochondria (mitophagy) (Twig et al., 2008).
Chronic rotenone alters mitochondrial trafficking
To further evaluate possible interrelated changes in mitochondrial dynamics, we then quantitatively evaluated the effects of low-dose, chronic rotenone on neuronal mitochondrial anterograde and retrograde transport. Using live imaging of primary cortical neurons transfected with mtDsRed2, treated under control or chronic rotenone conditions, images were taken every 10 s at 37° for 15 min as described above for fusion/fission analysis. Using manual object tracking software (as described in Methods), we were able to individually track each fluorescent mitochondrion in a given neurite at each 10s imaged timepoint, as well as measure its starting length. Excel macros were developed to calculate average and maximal velocities, as well as direction from the neuron cell body (anterograde or retrograde) (Figure 2A-C and Supplemental Movie 2). While we could not reliably distinguish between axons and dendrites in this living system, there is evidence that mitochondrial transport is similar between them (Ligon and Steward, 2000).
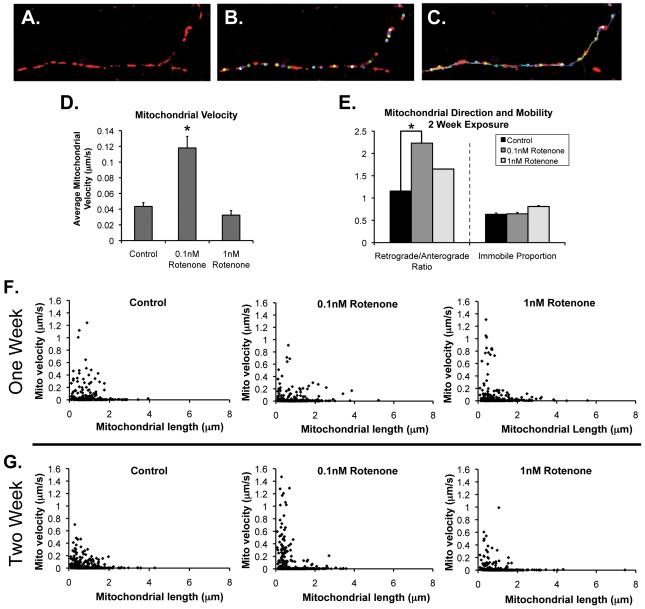
Figure 2.
Mitochondrial transport after chronic rotenone. Cortical neurons transfected and treated with chronic rotenone as in Figure 1 were imaged every 10 s for 15 min. A-C, Example of manual tracking using Image J with Manual Tracking plug-in as described. A, Fluorescent mitochondria in neurite. B, Mitochondria are identified, and as shown in (C), are tracked throughout the image series. D, Overall average individual mitochondrial transport velocity. E, Ratio of retrograde-moving mitochondria to anterograde-moving mitochondria in each condition. *Total proportion of retrograde mitochondria in motile population (59/110 in control vs. 58/84 in 0.1 nM rotenone different than control by two-tailed test of proportions as described) and the total proportion of non-moving mitochondria in each condition (no differences). F-G, Relationship of mitochondrial length and transport velocity. Distribution of mitochondrial velocity by individual mitochondrial length under conditions as listed.
After two weeks of exposure to 0.1 nM rotenone, changes in mitochondrial transport occurred. Surprisingly, we found that overall average velocity increased when neurons were exposed to a very low concentration of rotenone (0.1 nM; Figure 2D), but not 1 nM rotenone. Both concentrations of rotenone resulted in a higher proportion of retrograde mitochondrial transport (Figure 2E). Using this methodology, we were also able to evaluate the mitochondrial length/velocity distribution of the mitochondrial in the neuronal processes (Figures 2F and G). This analysis suggests that an increase in the population of very small, fast moving mitochondria contribute to the higher average velocity of the rotenone-treated neurons as compared to controls. One can observe that this increase in fast-moving, small mitochondria appears to start earlier in neurons exposed to the higher concentration of rotenone (2F) and takes longer to develop in the lower concentration of chronic rotenone (2G). Earlier in exposure (1 week), neuronal mitochondria did not show a significant difference in mitochondrial velocity (not shown) but begin to show a similar distribution of smaller, fast-moving mitochondria at higher concentrations of rotenone. Although we did not directly quantify this, our observations suggest that the increased velocity is due to a higher proportion of mitochondria undergoing fast transport with minimal ‘stopping’ than to an increase in speed of transport.
Chronic rotenone leads only to minor changes in mitochondrial morphology in neurons
In order to further explore the implications of the changes in mitochondrial fission and fusion observed after chronic rotenone in neurons, we evaluated mitochondrial morphology in the neuritic processes. In contrast to what might be predicted by a simple model of mitochondrial morphology being related to the simple balance of fission and fusion, where we might expect the increased mitochondrial fission and fission/fusion ratio after two weeks of chronic rotenone (Figure 1E) to result in smaller, more fragmented mitochondria, we found that we did not see fragmentation of mitochondria. Instead, when mitochondrial fission was greatest (2 week, 0.1 nM rotenone, Figure 1D), mitochondrial length was unchanged (Figure 3B). Although the fission/fusion ratio increased also after 2 weeks of 1 nM rotenone exposure (Figure 1E), average mitochondrial length actually increased (Figure 3B). We thus began to explore possible mechanisms for this apparent conundrum.
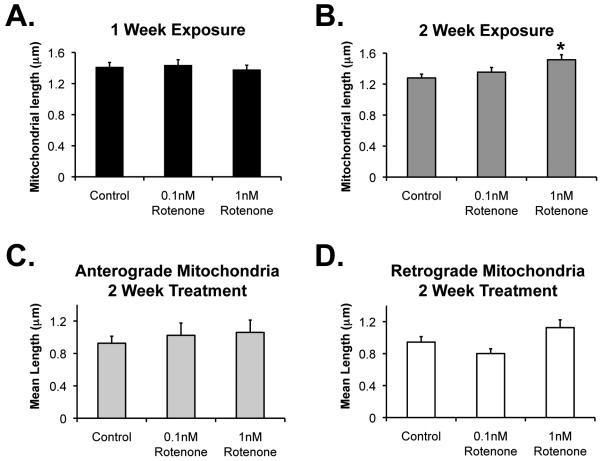
Figure 3.
Effect of chronic rotenone on mitochondrial morphology. Cortical neurons were transfected and treated with rotenone or DMSO vehicle control as described in previous figures. Randomly identified neuronal processes were imaged as described above. Image J was utilized to identify fluorescent mitochondria, and length was measured. A, One week rotenone or control exposure. B, Two week exposure. *Significantly different than control (p = 0.003). C-D, Using the manual tracking procedure as in Figure 2, mitochondria were separated into those traveling anterograde or retrograde (non-moving mitochondria not included in this analysis), and average length calculated after 1 (C) or 2 (D) week exposure to rotenone or vehicle control. There are no significant differences between groups.
Chronic rotenone does not cause preferential directional transport of differently sized mitochondria
One explanation for longer or unchanged morphology in mitochondria in neuritic processes, despite increases in mitochondrial fission in processes, would be that longer cell body mitochondria are transported away from the cell body to replace the smaller mitochondria in neurites. Alternatively, smaller mitochondria in processes could be transported back to the cell body. This was of particular interest, given the potential role for targeting damaged mitochondria for mitophagy and the role that linked fusion/fission events might play. One might suspect that fission results in division into damaged, smaller mitochondrial components that could be transported back towards the cell body for degradation, especially since we had observed an increase in smaller, faster-moving mitochondria and in retrograde movement after rotenone (Figure 2). Therefore, we evaluated the size of mitochondria being transported away from and towards the cell body. However, we found no evidence for preferential transport based on mitochondrial size. We found no significant difference in the size of the anterogradely or retrogradely moving mitochondria in any conditions (Figures 3C and D). The slightly longer mitochondria noted in the processes after two weeks of the higher concentration of rotenone cannot, then, be explained by preferential directional transport of certain-sized mitochondria.
Chronic rotenone increases mitochondrial density in distal neuronal processes prior to changes in cell bodies
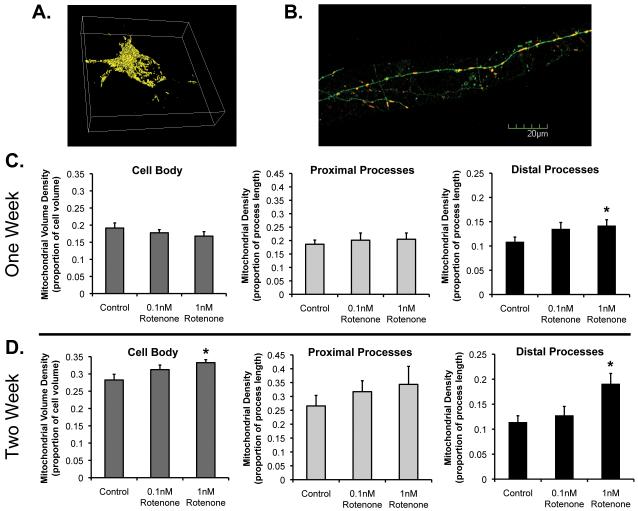
We also asked whether one possible mechanism for longer mitochondria in neuronal processes might be increased mitochondrial growth or biogenesis. If mitochondria were lengthened out in the distal process, followed by mitochondrial fission to make two daughter mitochondria, this might explain the greater rate of fission along with longer mitochondria. Alternatively, if the increase in fusion/fission events were linked to mitochondrial degradation through mitophagy, we might expect to see less mitochondrial density in neuritic processes. As a first pass to begin to look at these factors in compartmentalized regions of neurons, we evaluated mitochondrial density in neuronal cell bodies, proximal processes (approximately 300 uM from cell body) and distal processes (approximately 1200 um from cell body) (Figure 4). Example neurons are shown in figures 4A and B. We noted that as neurons age in vitro, mitochondrial density increases under DMSO vehicle control conditions (Figs. 4C-D). In addition, we found evidence of differential changes in different regions of the neurons in response to chronic toxicity. After one week of rotenone treatment, we found no change in overall mitochondrial density in neuronal cell bodies compared to control conditions (as estimated by total mitochondrial volume as a proportion of total cell body volume; Figure 4C). However, out in neuronal processes, changes do occur. We found greater mitochondrial density in the distal processes after one week of rotenone treatment, but no significant change in the proximal processes. After two weeks, we see even greater increases in mitochondrial density in the distal processes after chronic rotenone (1 nM) treatment compared to control (Figure 4D). Interestingly, after two weeks of rotenone treatment, we now observe a significant increase in mitochondrial volume in the cell body as well. This is the same time point when longer mitochondria in processes are noted. This suggests the possibility that chronic, compensatory changes occur, possibly increased biogenesis or growth, in an attempt to compensate for accumulating mitochondrial toxicity over time. On the other hand, there is no evidence of loss of mitochondria after chronic rotenone treatment. This also supports that mitochondrial dynamics can be differentially affected over time in specific subcellular regions.
Figure 4.
Mitochondrial density after chronic rotenone exposure. Cortical neurons were treated as described previously, and images taken as described in Methods. A, Example of 3D Metamorph reconstruction of fluorescent mitochondria in neuronal cell body for volume measurements. B, Example of soluble GFP-expressing neurites with mtDsRed2 in distal process. C-D, Mitochondrial density in cell body, proximal neurites, or distal neurites after one or two weeks of rotenone exposure or vehicle control. *Rotenone treatment conditions significantly different than vehicle control. In addition, there are age-related changes, unmarked in graph: control-treated neuronal cell bodies have greater mitochondrial density at the two week time point (DIV21) than at the one week time point (DIV14; p=0.005), but are not statistically significantly different in the other regions at those time points.
Chronic low-concentration rotenone does not alter autophagy nor affect mitophagy in neurons
To corroborate our findings with regard to mitochondrial density, we more directly evaluated autophagy and specific mitochondrial autophagy (or mitophagy), which has been hypothesized to be an important mechanism of clearing dysfunctional mitochondria (Tolkovsky, 2009), and which has been linked to the function of the PD-linked gene products parkin and PINK1(Dagda et al., 2009; Geisler et al., 2010; Kawajiri et al., 2010; Narendra et al., 2008; Narendra et al., 2010; Vives-Bauza et al., 2010). We wondered whether autophagic clearance was altered and were specifically interested in whether autophagy of mitochondria might be altered, and whether this might contribute to the density changes noted. Utilizing immunocytochemistry to assess whether accumulations of the autophagic vesicle marker LC3 were present under our conditions, we found that approximately 20-25% of neurons contained more than four autophagic puncta under control conditions, and this was unchanged by chronic rotenone (Figure 5). We only rarely detected autophagic puncta in distal neurites under any conditions (not shown). Using endogenous LC3 immunostaining along with mtDsRed2 to mark mitochondria, we also attempted to evaluate whether specific mitophagy in neurons was altered after chronic rotenone. Although we could occasionally detect LC3-containing vesicles surrounding mitochondria in neurites and cell bodies, and when coexpressing GFP-tagged LC3 in order to perform live imaging, we could occasionally detect these mitochondria-containing vesicles in neurites transported towards cell bodies (not shown), these were detected very rarely, and the vast majority of neurons under any conditions did not reveal significant colocalization with LC3 and mitochondrial fluorescence. This was true equally in control and rotenone-treated conditions (Figure 5C), suggesting that at least in our model system, we could not detect a role for mitophagy, though low-level changes cannot be ruled out.
Figure 5.
Measures of autophagy and mitophagy in neurons after chronic rotenone. Cortical neurons were transfected with mtDsRed2, treated at DIV7 with rotenone (1 nM) or vehicle control for one or two weeks as described in Methods, then fixed. Immunocytochemistry was performed to stain the autophagic vesicle marker LC3. A, Representative neuronal cell body image (compressed z-stack). B, Percentage of neurons in listed conditions containing greater than 4 autophagic puncta (no significant differences; 5 individual experiments, 64-99 neurons/condition). C, Colocalization of mitochondria with LC3-containing puncta (Pearson’s colocalization coefficients of red and green fluorescence, calculated from each individual z-slice; 3 individual experiments with 5 neurons per condition per experiment; no significant differences).
Chronic low-concentration rotenone results in loss of neuronal processes without cell death in dopaminergic neuronal cells
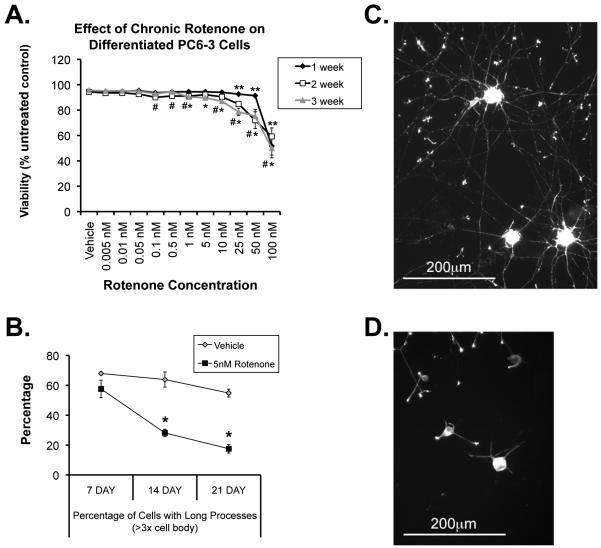
We now wanted to know whether these early changes in mitochondrial dynamics could be important in neuropathology. To begin to examine this, we wanted to utilize a dopaminergic neuronal model, so we modified the chronic rotenone model to nerve growth factor (NGF)-differentiated, dopamine-producing PC6-3 cells. After seven days of differentiation, standard media was changed to media containing either DMSO vehicle control or concentrations of rotenone ranging from 1 nM to 100 nM, for one, two, or three additional weeks (Figure 6). We found that at concentrations under 25 nM, little cell death occurred, even after 3 weeks of exposure (Figure 6A). Interestingly, however, we found that in these dopaminergic cells, the low concentrations of chronic rotenone did result in clear neuropathologic changes that have been observed both in PD and in the systemic chronic rotenone animal model of PD (Betarbet et al., 2000)), specifically that these differentiated cells began to lose their neuritic processes, although they do not die (Figure 6B-D), similar to that observed in SH-SY5Y cells (Borland et al., 2008). We took this loss of neurites to be indicative of early pathology that could be similar in nature to that seen in PD and other PD models. Mitochondria from processes after chronic low-dose rotenone, however, were indistinguishable in general morphology from vehicle-treated controls (not shown). This is in contrast to the dramatic fragmentation reported after acute rotenone treatment in other cell types (Barsoum et al., 2006), suggesting again significant mechanistic differences between acute high-dose and lower-dose chronic models.
Figure 6.
Effect of chronic rotenone on differentiated dopaminergic PC6-3 cells. Cells were differentiated for 7d with 40 ng/ml NGF, and then treated with rotenone or DMSO vehicle for an additional 7,14, or 21 days. A, Effect of rotenone concentration on cell viability over time. **1 week, #2 week, or *3 week significantly different from control (ANOVA with Bonferroni corrections to p < 0.005; n= 4 individual experiments in triplicate). B, Percentage of long processes in cells after chronic rotenone over time (assessed as those measuring greater than 3x the width of the cell body). n=3 individual experiments in triplicate. Means +/−SEM. C-D, Representative cells after 3 weeks of DMSO vehicle control (C) or 5 nM rotenone (D).
Reducing balance of mitochondrial fission protects against loss of neurites
We then asked whether the altered mitochondrial dynamics might be influencing toxicity at this early stage. One could hypothesize that reducing the overall balance of fission could be either protective or harmful to neurons. If excessive fission is promoting cell death, or if fusion is critical for maintaining healthy mitochondria exposed to chronic rotenone, reducing fission could be protective. However, if the role of fission in maintenance of synapses and mitochondrial distribution is important, then promoting fission could be protective to early neuropathology.
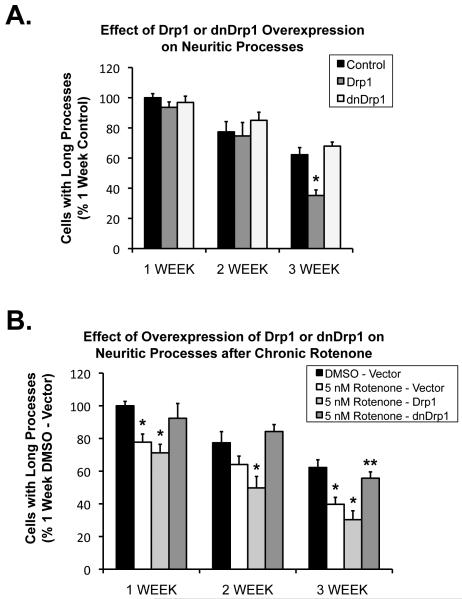
To begin to evaluate how mitochondrial dynamics contribute to the early neuropathology of neuritic processes, we thus examined the effects of overexpression of the mitochondrial fission protein Drp1 or its dominant negative form, mutant Drp1K38A (Labrousse et al., 1999; Smirnova et al., 1998) on these early changes in neuronal process morphology. We have previously shown directly in neurons that overexpression of the dominant-negative Drp1K38A resulted in a shift towards increased mitochondrial fusion in neurons (Berman et al., 2009). In neuronally-differentiated dopaminergic PC6-3 cells treated for three weeks with low-concentration rotenone, we found that Drp1K38A had no effect on neuritic processes under control conditions (Figure 7A), although unexpectedly, overexpression of Drp1 itself resulted in a loss of neuronal processes, regardless of whether rotenone was present or not. However, we also found that overexpression of Drp1K38A resulted in significant protection against the rotenone-induced loss of neuritic processes compared with vector-transfected control cells (Figure 7B). The effect was greatest after 3 weeks of chronic rotenone treatment.
Figure 7.
Differentiated PC6-3 cells were treated with rotenone (5 nM) or DMSO vehicle for 7,14, or 21 days. A, Control results testing the effect of overexpression of fission protein Drp1 or the dominant-negative form (dnDrp1) alone on neuritic processes. Overexpression of dnDrp1 alone does not affect neurite length after 3wk. *Drp1 overexpression significantly different than control (p=0.0007) at 3 weeks. B, Overexpression of fission inhibitor dnDrp1 protects against the rotenone-induced loss of processes. n=3 individual experiments, +/−SEM; *Significantly different from respective DMSO control, (ANOVA with Bonferroni post-hoc correction); **Not different than DMSO-vector control; p=0.00167 compared to 5 nM rotenone-vector and p=0.0013 compared to 5 nM-Drp1. Note (A) and (B) from same experimental sets with same controls, grouped separately for ease of visualization.
DISCUSSION
This is the first in-depth, direct, quantitative evaluation over time of changes in multiple aspects of mitochondrial dynamics in a living neuronal model. We found that changes in individual aspects of mitochondrial dynamics, such as fission and fusion, do not occur in a vacuum, but that other interrelated aspects of mitochondrial dynamics are also changing as well. In addition, in a chronic rotenone model, which may more closely represent chronically-developing PD than acute toxicity models, we provide the first evidence suggesting that chronically developing pathology can be altered by manipulating mitochondrial dynamics. Importantly, regulation of mitochondrial dynamics may be involved in early changes, rather than as a late component of the cell death pathway, and thus may provide therapeutic opportunities prior to irreversible neuronal death. In addition, we have identified changes that may occur in distal neurites prior to changes in cell bodies, that may in turn influence later neuropathology and cell loss, and which may provide targets for neuroprotective strategies.
A complex mitochondrial homeostatic system: mitochondrial morphologic changes do not always predict changes in mitochondrial dynamics
We found that alterations in mitochondrial fission and fusion in neurites do not necessarily lead to widescale changes in mitochondrial morphology, as has been presumed. In fact, under conditions of chronic rotenone exposure where mitochondrial fission increases in neuritic processes, mitochondria were unchanged in length, suggesting other interrelated aspects of mitochondrial dynamics are contributing. What other factors could explain this? Since mitochondrial maintenance is not only a function of fission and fusion, but also of degradation, biogenesis, and transport, there are many possible explanations. In fact, earlier work has examined how some of these dynamic properties interact (Berman et al., 2009). For example, increasing fission without concomitant decrease in mitochondrial length could be explained by preferential transport and compartmentalization of longer or smaller mitochondria, increased growth of mitochondria, preferential degradation of smaller mitochondria, or combinations of these types of interactions. These could have different implications for cell physiology and thus pathophysiology. Our studies examined some of these possibilities and suggest that in the case of chronic rotenone exposure, increased mitochondrial growth in neurites may be involved. We found no evidence for preferential directional transport of smaller mitochondria, and we observed no evidence for changes in mitochondrial degradation. These findings are consistent with previous findings that the anti-apoptotic protein Bcl-xL increased both mitochondrial fission and biogenesis (Berman et al., 2009). These results emphasize the importance of moving towards more complex analysis of mitochondrial dynamics than morphologic analysis.
In addition, we observed that effects of chronic exposure to rotenone on mitochondrial dynamics varied depending on the concentration of rotenone. This might also suggest that the severity of the insult might influence the way in which mitochondrial dynamics are altered, perhaps contributing to variability in observations of mitochondrial dynamic changes and potentially important to the neurodegenerative process.
Mitochondrial dynamics change as neurons mature in culture
We also found that in axons/dendrites, rates of mitochondrial fission and fusion change as neurons mature in culture. We found that fission rates are higher when neurons are 7 days of age in vitro than at 28 days, whereas the rate of fusion stays relatively constant. This might be due to the fact that neuronal axons and dendrites, as well as synapses, are forming during this early time in culture (Chang and Reynolds, 2006). Since mitochondrial fission is important in proper mitochondrial distribution to synapses and development of functional synapses (Li et al., 2004), the higher rate of mitochondrial fission is likely developmentally beneficial, rather than detrimental. This is interesting, however, in light of the fact that the levels of expression of the mitochondrial fission protein, Drp1, have been shown to be higher in cortical neurons at 14 DIV than at 5 DIV (Chang and Reynolds, 2006), though levels of expression at older ages are not known. This might suggest, though, that since Drp1 is largely cytosolic and recruited to mitochondria under specific conditions (Smirnova et al., 2001), either there may be less recruitment of Drp1 to mitochondria as neurons age in culture, or that other regulating factors are implicated in the age-related decrease in mitochondrial fission rate. Overall, the balance of mitochondrial fusion to fission increases as the neurons age in our cultured neuronal system. This might be expected to be protective, since mitochondrial fusion is thought to help to protect from accumulation of damaged mtDNA and proteins (Parone et al., 2008).
Regulation of mitochondrial dynamics may be compartmentalized in neurons
We also provide the first evidence that effects on mitochondrial dynamics can be compartmentalized subcellularly. Specifically, we found that, in response to chronic rotenone, mitochondrial density increases first in distal neurites and later in proximal neurites and cell bodies. Because the neuropathology of neurodegenerative diseases like PD might start distally in neurons, this suggests that focus away from the cell body processes might provide more specific information on early pathologic processes of chronic neurodegenerative diseases. Although we do not examine neuronal cell body fission and fusion, we suspect the same is true for mitochondrial fission and fusion – we know that mitochondrial fission and fusion appear to be more tightly regulated in distal neurites, compared to what is typically observed and reported in cell bodies (Berman et al., 2009). In fact, the protection we found against early pathologic changes in neuritic processes by altering mitochondrial dynamics supports the pathopysiologic relevance of this subcellular compartmentalization.
We cannot say whether the increased mitochondrial density is due to actual increased biogenesis in distal neurites after chronic rotenone. At first, after chronic rotenone treatment, overall mitochondrial density in the cell bodies remains unchanged, while it increases in distal neurites. This, in conjunction with increased retrograde transport and fission late in chronic rotenone exposure, suggests that biogenesis might be taking place to compensate for the damaged mitochondria. It is interesting that this seems to be compartmentalized – little is known about mitochondrial biogenesis in distal neurites; however, there is evidence that it can and does occur distal from the neuronal cell body (Amiri and Hollenbeck, 2008; Lentz et al., 2009).
Mitochondrial fusion may be an early compensatory response to chronic toxicity, prior to neurodegeneration, with increasing mitochondrial fission being detrimental
Chronic models may better represent neuropathologic mechanisms related to chronic neurodegenerative diseases than acute toxicity models. We observed that changes in the regulation of mitochondrial dynamics in our chronic rotenone model were quite different than what has been seen in acute rotenone toxicity, where diffuse mitochondrial fragmentation occurs in the setting of significant cell death (Barsoum et al., 2006). Instead, in our chronic model, without significant cell death, there appears to be much more regulated control of mitochondrial fusion and fission, with fusion actually increasing early and fission increasing later, with no evidence of overt mitochondrial fragmentation.
Our data show that chronic rotenone in neurons leads first to an increase in mitochondrial fusion in neurites. Although we have not delineated the mechanistic pathway, we favor the hypothesis that this early increase in fusion is a compensatory response to low-level chronic toxicity. Mitochondrial fusion has been suggested to be a means for damaged mitochondria to combine their contents with functioning mitochondria, eventually leading to replacement of damaged proteins or mtDNA from their healthier counterparts (Nakada et al., 2001; Ono et al., 2001). We also see an increase in linked fusion-fission events in individual mitochondria, which also may be part of the mechanism for restoring components of damaged mitochondria. Although these linked fusion-fission events have also been hypothesized to be related to mitophagic degradation (Mouli et al., 2009), we did not find evidence of this in our neuron model. However, this still could signify the compensatory response to partially damaged mitochondria.
As the chronic toxicity continues, we observed that eventually mitochondrial fission increases, along with more retrograde transport to the cell bodies of neurons. This could represent a dysfunctional response, which is supported by the fact that inhibition of fission partially prevented the loss of neurites caused by chronic rotenone in differentiated dopaminergic cells. We have also observed that mitochondrial fission often precedes rapid transport, and thus, it is possible that inhibition of mitochondrial fission in neuritic processes also forces more mitochondria to remain in distal processes, providing at least some support for maintenance of neurites early in this chronic neurodegeneration model, and consistent with our findings that there is an increase in mitochondrial density in distal neurites after chronic rotenone.
The protection by inhibition of mitochondrial fission is also interesting in light of recent studies of the PD genes PINK1 and parkin, where they have been suggested to serve a pro-fusion purpose in some mammalian cells, and, when mutated, lead to dysfunctional mitochondrial fusion (Cui et al., 2010; Exner et al., 2007; Lutz et al., 2009). On the other hand, one could hypothesize that increasing, rather than decreasing, mitochondrial fission might be protective, given the important role of mitochondrial fission in maintenance of synapses and mitochondrial distribution (Li et al., 2004; Verstreken et al., 2005) and the suggestion in Drosophila and other cell types that PINK1and parkin may instead be pro-fission (Deng et al., 2008; Park et al., 2009; Poole et al., 2008; Yang et al., 2008). However, we did not find this to be the case, supporting a more protective role for mitochondrial fusion.
We also did not see the significant loss of mitochondrial motility after rotenone exposure as reported in SH-SY5Y cells (Borland et al., 2008). Rather, in neurons at low doses, chronic rotenone did not decrease mitochondrial motility, but appeared to increase the retrograde transport of mitochondria. These results are interestingly similar to that reported for another Complex I inhibitor, annonacin (Escobar-Khondiker et al., 2007).
The mechanism for the effects on mitochondrial dynamics of low-dose rotenone is not yet known. Low-dose rotenone has been shown previously to transcriptionally activate both pro-cell death and survival genes, including the mitochondrial fusion gene OPA1 (Meurers et al., 2009), can alter calcium signaling (Sherer et al., 2001), and can induce oxidative stress, even at low levels (Testa et al., 2005). Calcium is known to regulate mitochondrial transport and mitochondrial fission (Cereghetti et al., 2008; Cribbs and Strack, 2007; Macaskill et al., 2009; Wang and Schwarz, 2009), and oxidative stress may alter mitochondrial dynamics as well (Knott et al., 2008). In addition, rotenone has effects on microtubules and tau phosphorylation (Hoglinger et al., 2005; Ren et al., 2005), which could influence mitochondrial transport and secondary dynamics. Thus, these could provide possible mechanisms by which low-level chronic exposure could alter mitochondrial dynamics over time, mechanisms which remain to be explored.
Conclusions
It is clear that the mitochondrial homeostatic system is complex, especially in neurons, that there are more contributing factors than the simple balance of fission and fusion, and that fission, fusion, transport, biogenesis, and degradation must all be considered. Changes in mitochondrial dynamics can occur without significant changes in morphology, but which can still have physiologic significance, particularly in neurites. In addition, we have found that changes can be compartmentalized to different subcellular regions. While the complexities inherent in this complicated system make it difficult to fully integrate models, we support moving beyond a model of morphology/structure as a simple balance of fission versus fusion.
In addition, in a chronic, PD-relevant model, that may be more likely to approximate neurodegenerative diseases than acute models, we have found that mitochondrial dynamics are altered early, and that multiple aspects of mitochondrial dynamics, including fission, fusion, transport, and growth likely interact in a regulated manner to control mitochondrial homeostasis. This regulation changes over time in our chronic model, emphasizing the dynamic nature of these processes. Overall, our findings suggest that very early changes in mitochondrial dynamics can precede neuronal cell death, and that manipulating mitochondrial dynamics early can protect against chronically developing neuropathology.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by NIH NINDS K08NS059576 (S.B.B) and American Parkinson Disease Association (APDA) Research Grants (S.B.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abou-Sleiman PM, et al. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–19. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Amiri M, Hollenbeck PJ. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neurobiol. 2008;68:1348–61. doi: 10.1002/dneu.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–11. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SB, et al. Bcl-xL increases mitochondrial fission, fusion, and biomass in neurons. Journal of Cell Biology. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Blalock EM, et al. Decreased G-protein-mediated regulation and shift in calcium channel types with age in hippocampal cultures. J Neurosci. 1999;19:8674–84. doi: 10.1523/JNEUROSCI.19-19-08674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland MK, et al. Chronic, low-dose rotenone reproduces Lewy neurites found in early stages of Parkinson’s disease, reduces mitochondrial movement and slowly kills differentiated SH-SY5Y neural cells. Mol Neurodegener. 2008;3:21. doi: 10.1186/1750-1326-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, et al. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Cereghetti GM, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–8. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, et al. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–45. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Differences in mitochondrial movement and morphology in young and mature primary cortical neurons in culture. Neuroscience. 2006;141:727–36. doi: 10.1016/j.neuroscience.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–62. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Chernova T, et al. Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons. Mol Pharmacol. 2006;69:697–705. doi: 10.1124/mol.105.016675. [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–44. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, et al. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem. 2010;285:11740–52. doi: 10.1074/jbc.M109.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, et al. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–55. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, et al. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–8. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Khondiker M, et al. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci. 2007;27:7827–37. doi: 10.1523/JNEUROSCI.1644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–8. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–47. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, et al. The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. J Neurochem. 2005;95:930–9. doi: 10.1111/j.1471-4159.2005.03493.x. [DOI] [PubMed] [Google Scholar]
- Kawajiri S, et al. PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. FEBS Lett. 2010;584:1073–9. doi: 10.1016/j.febslet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Knott AB, et al. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–18. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse AM, et al. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–26. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Lentz SI, et al. Mitochondrial DNA (mtDNA) Biogenesis: Visualization and Duel Incorporation of BrdU and EdU Into Newly Synthesized mtDNA In Vitro. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.954701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–50. doi: 10.1002/1096-9861(20001120)427:3<340::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Liu QA, Shio H. Mitochondrial morphogenesis, dendrite development, and synapse formation in cerebellum require both Bcl-w and the glutamate receptor delta2. PLoS Genet. 2008;4:e1000097. doi: 10.1371/journal.pgen.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz AK, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–51. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill AF, et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–55. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijering E, et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–76. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- Meurers BH, et al. Low dose rotenone treatment causes selective transcriptional activation of cell death related pathways in dopaminergic neurons in vivo. Neurobiol Dis. 2009;33:182–92. doi: 10.1016/j.nbd.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouli PK, et al. Frequency and selectivity of mitochondrial fusion are key to its quality maintenance function. Biophys J. 2009;96:3509–18. doi: 10.1016/j.bpj.2008.12.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada K, et al. Inter-mitochondrial complementation: Mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med. 2001;7:934–40. doi: 10.1038/90976. [DOI] [PubMed] [Google Scholar]
- Narendra D, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, et al. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–5. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]
- Overly CC, et al. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J Cell Sci. 1996;109(Pt 5):971–80. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- Park J, et al. The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem Biophys Res Commun. 2009;378:518–23. doi: 10.1016/j.bbrc.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Parone PA, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman RN, et al. A system for characterizing cellular and molecular events in programmed neuronal cell death. J Neurosci. 1993;13:3669–80. doi: 10.1523/JNEUROSCI.13-09-03669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997-2009. http://rsb.info.nih.gov/ij/. [Google Scholar]
- Ren Y, et al. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem. 2005;280:34105–12. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- Sherer TB, et al. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–15. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, et al. Chronic reduction in complex I function alters calcium signaling in SH-SY5Y neuroblastoma cells. Brain Res. 2001;891:94–105. doi: 10.1016/s0006-8993(00)03203-0. [DOI] [PubMed] [Google Scholar]
- Smirnova E, et al. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–56. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, et al. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–8. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, et al. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–77. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Suen DF, et al. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, et al. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–18. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Tolkovsky AM. Mitophagy. Biochim Biophys Acta. 2009;1793:1508–15. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Twig G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson’s disease. Exp Neurol. 2009;218:247–56. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–78. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–83. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–74. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B. Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 2002;3:527–31. doi: 10.1093/embo-reports/kvf113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, et al. Mitochondrial polarisation status and [Ca2+]i signalling in rat cerebellar granule neurones aged in vitro. Neurobiol Aging. 2004;25:349–59. doi: 10.1016/S0197-4580(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–5. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.