Abstract
Skeletal muscle is the primary site of dietary glucose disposal, a function that depends on insulin-mediated exocytosis of GLUT4 vesicles to its cell surface. In skeletal muscle and adipocytes, this response involves Akt signaling to the Rab-GAP (GTPase-activating protein) AS160/TBC1D4. Intriguingly, the AS160-targeted Rabs appear to differ, with Rab8A participating in GLUT4 exocytosis in muscle cells and Rab10 in adipocytes, and their activation by insulin is unknown. Rabs 8A, 10, and 13 belong to the same subfamily of Rab-GTPases. Here we show that insulin promotes GTP loading of Rab13 and Rab8A but not Rab10 in rat L6 muscle cells, Rab8A activation preceding that of Rab13. siRNA-mediated Rab13 knockdown blocked the insulin-induced increase of GLUT4 at the muscle cell surface that was rescued by a Rab13 ortholog but not by Rab8A. Constitutively active AS160 lowered basal and insulin-stimulated levels of surface GLUT4, effects that were reversed by overexpressing Rab8A or Rab13, suggesting that both Rabs are targets of AS160-GAP activity in the context of GLUT4 traffic. Rab13 had a broader intracellular distribution compared with the perinuclear restriction of Rab8A, and insulin promoted Rab13 colocalization with GLUT4 at the cell periphery. We conclude that Rab13 and Rab8A are Rab-GTPases activated by insulin, and that downstream of AS160 they regulate traffic of GLUT4 vesicles, possibly acting at distinct steps and sites. These findings close in on the series of events regulating muscle GLUT4 traffic in response to insulin, crucial for whole-body glucose homeostasis.
Keywords: vesicle traffic, glucose uptake, Rab activation, TBC1D4
Insulin stimulates glucose uptake by rapidly increasing GLUT4 glucose transporters at the plasma membrane of muscle cells and adipocytes (1). In both cell types, IRS-1, class I PI-3 kinase, and Akt are required for the stimulation of exocytosis of GLUT4-containing membrane vesicles from intracellular storage compartments (2, 3). GLUT4 partially segregates away from the recycling endosomes into GLUT4-storage or GLUT4-specialized vesicular compartments (2, 3), but how these vesicles are mobilized to the cell surface and under which specific signals this occurs remain ill-defined.
Rab-GTPases are molecular switches controlling diverse stages of vesicle traffic, from unicellular yeast to mammals (4). Rab-GTPases cycle between active GTP-bound and inactive GDP-bound states, controlled by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) (5). The Rab-GAP AS160 (Akt substrate of 160 kDa)/TBC1D4 is a target of Akt, recently found to participate in the insulin signaling relay that regulates GLUT4 traffic in adipose and muscle cells and tissues (6–8). AS160 phosphorylation by Akt is predicted to inhibit its GAP activity, allowing the GTP-bound form of its target Rabs to prevail (6). A mutant AS160 with four Akt serine/threonine phosphorylation sites replaced by alanine (AS160-4A, also known as AS160-4P) reduces basal and insulin-stimulated glucose uptake and surface GLUT4 levels when expressed in fat cells (6, 9) and skeletal muscle cells and tissues (8, 10). Not being able to be phosphorylated at key sites, it is postulated that AS160-4A remains constitutively active as a Rab-GAP upon insulin stimulation, because an additional mutation in the GAP domain of AS160-4A renders it unable to affect GLUT4 cell-surface density (6).
The recombinant GAP domain of AS160 displays activity toward a number of Rab-GTPases in vitro, among which Rabs 8A, 10, and 14 have been detected in GLUT4-containing compartments from 3T3-L1 adipocytes (11, 12). We recently demonstrated that overexpression of Rab8A partially rescues the block in insulin-stimulated GLUT4 translocation exerted by AS160-4A in muscle cells (13). Further, siRNA-mediated knockdown of Rab8A reduced the insulin-dependent gain in surface GLUT4 at the muscle cell membrane (14). Similar approaches were ineffective in demonstrating a role for Rab10 in muscle cells, although silencing this AS160 target reduces GLUT4 translocation in adipocytes (15). Therefore, Rab8A appears to be a muscle cell-specific regulator of GLUT4 traffic. Surprisingly, it has not been possible to demonstrate insulin-dependent activation of Rab10 in adipocytes (15), and it remains untested whether Rab8A is activated by the hormone. Demonstrating insulin-dependent Rab activation is crucial to position Rabs in the insulin signaling relay. Further, additional AS160 targets may contribute to GLUT4 traffic, given that AS160-4A affects steps as distinct as perinuclear GLUT4 retention, GLUT4 vesicle tethering, and/or vesicle fusion with the membrane (1, 16).
Rab8 and Rab10 along with Rab13 comprise a subfamily of Rab proteins displaying over 60% amino acid identity. Rab13 was not reported in proteomic screens of GLUT4-containing vesicles from adipocytes and hence was not tested as a target of AS160-GAP activity (11). However, Rab13 is expressed in skeletal muscle cells, and we hypothesize that it may participate in insulin-stimulated GLUT4 translocation in muscle. Here we report that insulin stimulates GTP loading of Rab13 and Rab8A, that these Rabs are found within overlapping and distinct cellular locations, and that each contributes independently to GLUT4 exocytosis in response to insulin in muscle cells, acting downstream to AS160. These results position Rab proteins as elements within the insulin signaling cascade and suggest mechanistic links between insulin signal transduction and vesicle traffic.
Results
Rab13 Is Expressed in Muscle and Fat.
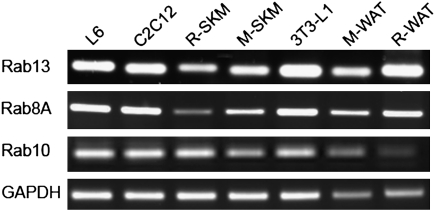
Rab13, together with Rab10, Rab8A, and Rab8B, compose a subfamily of closely related Rab proteins (5). Rab13 and Rab8A display 63% amino acid identity and an additional 29% similarity (Fig. S1). The highest conservation is found in the first 100 N-terminal amino acids of the Rabs that encompass the GAP and GEF interaction (switch I and II) regions. In addition, amino acid determinants of specific effector regions in the C termini are divergent. By RT-PCR, Rab13 and Rab8A expression was detected in both myoblast and myotube stages of differentiation of L6-GLUT4myc muscle cells, in mature rat and mouse skeletal muscle, and in adipose tissues and 3T3-L1 adipocytes (Fig. 1).
Fig. 1.
Rab13 mRNA is expressed in muscle and fat. RT-PCR detection of Rab13, Rab8A, and Rab10 in total RNA from rat L6GLUT4myc (L6) and mouse C2C12 muscle cells; rat (R) and mouse (M) skeletal muscle (SKM); mouse 3T3-L1 adipocytes (3T3-L1); rat and mouse perigonadal white adipose tissue (WAT); and analysis by agarose gel electrophoresis. Rab13 detection was confirmed by sequencing the PCR products from L6.
Rab13 and Rab8A GTPases Are Activated in Response to Insulin.
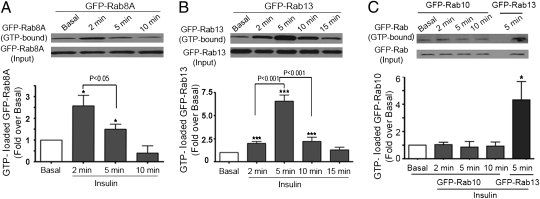
Rab8A and Rab10 are in vitro substrates of the GAP domain of AS160, and recent studies implicate them in insulin-dependent GLUT4 translocation in muscle and adipose cells, respectively. However, no putative AS160-target Rab, Rab8A and Rab10 included, has been shown to be activated in response to insulin, an essential requirement to position these Rabs in the signal relay toward GLUT4 mobilization. To explore whether Rab13, Rab8A, and/or Rab10 are activated by insulin, we implemented a GTP-photoaffinity labeling assay (17). L6-GLUT4myc-IR myoblasts were transiently transfected with GFP-Rab8A, GFP-Rab13, or GFP-Rab10, then permeabilized with digitonin to introduce a biotinylated GTP-photoprobe (Methods) before insulin stimulation, followed by photoactivation and precipitation of biotin-containing proteins. In this permeabilized cell system, insulin signaling to Akt and AS160 proceeded with a normal time course, phosphorylation increasing already at 2 min and remaining elevated for the duration of the assay (Fig. S2 A and B). Importantly, Rab8A was activated by insulin, and this response was transient, with peak activation of 2.6 ± 0.5 by 2 min from insulin administration (Fig. 2A). Rab13 was also transiently activated, with a peak of 6.6 ± 0.7-fold by 5 min (Fig. 2B). Because Rab8A activation peaked earlier than Rab13 activation, insulin signaling may sequentially activate Rab8A and Rab13, revealing a possible relay in the regulation of GLUT4 translocation by these two Rab proteins in muscle cells. In contrast, Rab10 was not activated in response to insulin under identical experimental conditions (Fig. 2C).
Fig. 2.
Rab8A and Rab13, but not Rab10 are GTP-loaded in response to insulin. L6-GLUT4myc-IR myoblasts were transiently transfected with GFP-Rab8A, GFP-Rab13, or GFP-Rab10 for 48 h. Serum-deprived cells (3 h) were permeabilized to introduce the biotinylated GTP-photoprobe before incubation with insulin (100 nM) for the indicated times, followed by UV illumination. Cells were immediately processed for pull-down with streptavidin resin, SDS/PAGE, and immunoblotting with anti-GFP antibody to detect GFP-Rab as detailed in Methods. Representative gels and the fold change of GTP-loaded GFP-Rab relative to basal are illustrated for (A) GFP-Rab8A, (B) GFP-Rab13, and (C) GFP-Rab10. *P < 0.05 and ***P < 0.001 versus basal. Other statistical comparisons are shown by brackets.
Rab13 Knockdown Inhibits the Insulin-Stimulated Increase in Surface GLUT4myc.
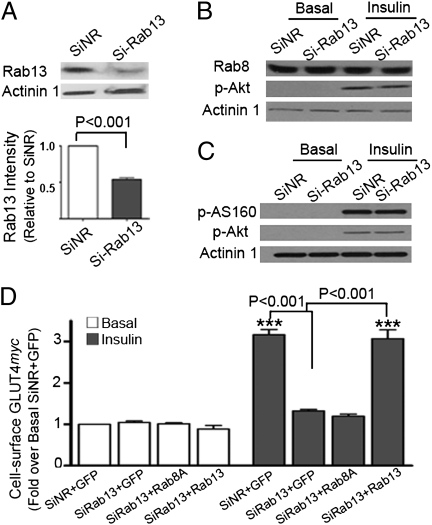
The effects of depleting Rab13 were next tested. Reducing Rab13 protein content by 54 ± 3% (Fig. 3A) via siRNA to Rab13 (siRab13) did not affect Rab8 protein expression, insulin-stimulated phosphorylation of Akt, or AS160 phosphorylation (Fig. 3 B and C). However, such Rab13 knockdown markedly prevented insulin-dependent GLUT4 gain in surface GLUT4 (by 81 ± 4%) compared with cells transfected with unrelated siRNA (siNR) (Fig. 3D). Notably, the inhibition of insulin-dependent GLUT4 translocation was rescued to 96 ± 22% by re-expressing a GFP-Rab13 ortholog, but not GFP-Rab8A (which led to only a 9 ± 5% recovery of siRab13-inhibited GLUT4myc translocation; Fig. 3D). These results suggest that Rab13 participates in the regulation of insulin-stimulated GLUT4 exocytic traffic in a manner not functionally redundant with Rab8A. Therefore, these two small GTPases may participate in different steps of insulin-regulated GLUT4 exocytosis.
Fig. 3.
Rab13 knockdown prevents insulin-induced GLUT4myc translocation to the plasma membrane without affecting basal levels of surface GLUT4myc. L6-GLUT4myc (A and B) or L6-GLUT4myc-AS160 (C) myoblasts were transfected with the indicated siRNA to rat Rab13 (siRab13) or nonrelated (nontargeting) siRNA (siNR) for 72 h. Cells were serum-deprived for 3 h before treatment without (basal, open bars) or with insulin (100 nM, 20 min, filled bars) and lysed to detect (A) Rab13 and loading control actinin1; (B) Rab8, phospho-Akt S473 (p-Akt), and actinin1; and (C) phospho-T642-AS160 (p-AS160), p-Akt, and actinin1. (D) L6-GLUT4myc myoblasts were transfected with siRNA for 24 h and then retransfected with GFP-Rab8A (canine), GFP-Rab13 (human), or GFP cDNA for 24 h and processed for cell-surface GLUT4myc levels as described in Methods. Shown are mean ± SEM fold changes from four experiments. ***P < 0.001 versus basal in siNR+GFP-transfected myoblasts. Other statistical comparisons are shown by brackets.
Rab13 Rescues the Inhibition of GLUT4myc Translocation Caused by AS160-4A.
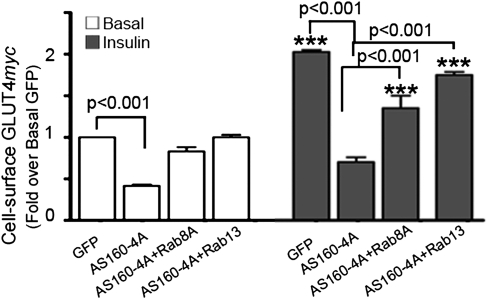
The constitutively active GAP AS160-4A reduces basal and insulin-stimulated levels of GLUT4myc at the surface of L6 muscle cells (8). Here we show that coexpression of GFP-Rab13 reversed these suppressive effects of AS160-4A and was somewhat more effective than GFP-Rab8A in this context (Fig. 4). These results suggest that both Rabs function downstream of AS160, and moreover that they may each separately annul the negative effects of AS160-4A mutant. This may potentially arise from GFP-Rab overwhelming AS160-4A, allowing the endogenous AS160 targets to stimulate GLUT4 exocytosis. In contrast, overexpression of GFP-Rab8A did not change the basal levels of surface GLUT4myc (13), and likewise overexpression of GFP-Rab13 alone did not affect the basal levels of GLUT4myc at the muscle cell surface (Fig. S3). These results suggest that overexpression of these Rabs per se does not mimic insulin action.
Fig. 4.
Rab8A and Rab13 relieve the repression of basal and insulin-stimulated cell-surface GLUT4myc levels caused by AS160-4A. L6-GLUT4myc myoblasts were cotransfected with Flag-tagged AS160-4A plus GFP-Rab8A, GFP-Rab13, or GFP for 24 h, and then incubated without (open bars) or with insulin (filled bars) and processed for cell-surface GLUT4myc levels as described in Methods. Results are the mean ± SEM fold change over basal in cells expressing AS160-4A and indicated GFP-Rab from four experiments. ***P < 0.001 versus basal in GFP-transfected control myoblasts. Other statistical comparisons are shown by brackets.
Only Rab13 Colocalizes with GLUT4myc near the Cell Surface upon Insulin Stimulation.
To examine their localization with respect to GLUT4myc, GFP-Rab13 and GFP-Rab8A were separately expressed in L6-GLUT4myc myoblasts that were then permeabilized to label GLUT4myc. In the basal state, Rab13 was abundant in the perinuclear region (Fig. 5), but was also found at the cell periphery. Also in the basal state, Rab13 and GLUT4 partly colocalized in the perinuclear region and, importantly, Rab13 also partly colocalized with the GLUT4 that translocated to the cell periphery in response to insulin (Fig. 5). At the dorsal cell surface, these proteins colocalized in ridges (Fig. 5) reminiscent of the ruffles formed by insulin-dependent actin remodeling (18). This behavior is illustrated at 5 and 15 min into insulin stimulation (Fig. 5 and Fig. S4A), and further confirmed by the colocalization of Rab13 with GLUT4 fluorescently decorated at the surface of nonpermeabilized cells, that is, GLUT4 inserted into the membrane (Fig. S4B). On the other hand, Rab8A mostly distributed in the perinuclear region where it also colocalized with GLUT4 but, in sharp contrast to Rab13, Rab8A did not colocalize with the translocated GLUT4 at/near the cell periphery in insulin-stimulated cells (Fig. 5). These observations suggest the possibility that only Rab13 acts on GLUT4 near or at the plasma membrane of the muscle cell.
Fig. 5.
Rab13 colocalizes with GLUT4myc in perinuclear regions in basal conditions and at the cell periphery upon insulin stimulation. L6-GLUT4myc myoblasts were transfected with (A) GFP-Rab8A or (B) GFP-Rab13 for 24 h. Basal or insulin-stimulated (100 nM, 5 and 15 min) cells were permeabilized and processed for confocal fluorescence microscopy as described in Methods: GFP-Rab (green) or GLUT4myc (red). Shown are representative images of merged signals at the middle and dorsal optical planes from basal and insulin-treated cells from three experiments. Insets show magnifications of the boxed areas of interest. (Scale bars, 10 μm.) The pixel coincidence analysis of GLUT4myc with each GFP-Rab at the dorsal plane in insulin-stimulated cells is shown in grayscale.
Discussion
Insulin Sequentially Activates Rab8A and Rab13.
Whereas it is well established that insulin signaling toward GLUT4 translocation requires signals relayed from IRS-1, PI-3 kinase, and Akt to AS160, the downstream steps remain poorly defined. Moreover, given that AS160 has Rab-GTPase activity that is inactivated by insulin, the prediction that specific Rabs are activated upon insulin action requires experimental scrutiny. The Rab8 subfamily of Rab-GTPases regulates vesicle traffic between the trans-Golgi network (TGN) and the plasma membrane (5). We have found that only Rab8A, but not two other subfamily members, Rab8B and Rab10, participate in insulin-induced GLUT4 translocation in muscle cells, established by individual Rab knockdown (14). Rab13 is also a member of this subfamily of GTPases, but its function in insulin action has not been previously considered. Here we demonstrate that Rab13 is expressed in muscle and adipose cells in culture and tissues. Furthermore, insulin rapidly stimulates GTP loading of Rab8A and Rab13 in muscle cells (Fig. 2), demonstrating that these AS160-targeted Rabs are activated by insulin within a cellular context. Interestingly, Rab10 was not activated in parallel, suggesting that this GTPase is not in the insulin signaling relay, consistent with the lack of effect of Rab10 knockdown on insulin-regulated GLUT4 traffic in muscle cells (14). These results show a selective and differentially timed activation of Rab8A and Rab13 in response to insulin; however, the transient nature of their activation deserves comment. In the continuous presence of insulin, Akt remains fully activated in intact cells (19). Similarly, Akt and AS160 phosphorylation remained steady for the duration of the Rab activation assay; thus, it is unlikely that the transient response is due to an artificial rundown of this signaling axis in the permeabilized cell system used (Fig. S2). Instead, we surmise that this behavior may be related to the transient activation of the GEFs mediating GTP-photoprobe loading of the Rabs. It is plausible that these as-yet unidentified GEFs are activated by PI-3,4,P3, the levels of which rise transiently in response to insulin in intact muscle cells (20). Although this scenario will require future testing, it is noteworthy that the same GTP-photoprobe introduced into rat cardiomyocytes by electroporation also revealed a transient activation of Rab11 (17). Finally, it cannot be ruled out that the transient activation results from the nonhydrolyzable nature of GTP-γ-S, which prevents Rab cycling back to the GDP-bound conformation that is recognized by the GEF for a subsequent round of activation (21).
Rab8A and Rab13 Lie Functionally Downstream of AS160 in the Insulin Pathway Leading to GLUT4 Translocation.
A second important conclusion of this study is that both Rab8A and Rab13 act downstream of AS160, based on their individual ability to restore GLUT4 translocation when overexpressed in cells with insulin signaling blocked by the constitutively active AS160-4A (Fig. 4). Despite the common ability of Rabs 13 and 8A to reverse the inhibition of GLUT4 translocation by AS160-4A, Rab13 does this independently from Rab8A, because selective knockdown of Rab13 and the resultant impairment in GLUT4 translocation were not rescued by Rab8A overexpression (Fig. 3). The fact that Rab13 overexpression per se did not suffice to mobilize GLUT4 (Fig. S3) indicates that this signal alone cannot reproduce insulin action. This is consistent with the finding that two parallel signaling events are required to mobilize GLUT4 to the cell surface in muscle cells: one arm involving Akt, AS160, and Rabs, and the other Rac and actin remodeling (22, 23). These arms bifurcate downstream of PI-3 kinase and do not influence each other, such that Rac1 knockdown does not affect insulin-stimulated Akt phosphorylation but prevents GLUT4 translocation (22), and Akt dominant-negative mutants allow Rac1-dependent actin remodeling but similarly prevent GLUT4 translocation (24). The independence of the two arms is further supported by analysis of their downstream targets. Knockdown of Rac1 or its linker α-actinin4 prevents GLUT4 association with the cortically remodeled actin without affecting Akt or AS160 phosphorylation (25), and conversely neither the AS160-4A mutant (13) nor knockdown of Rab 8A (14) or Rab13 (this study) impedes actin remodeling, yet in each case GLUT4 translocation is averted. Accordingly, it is not expected that overexpression of a Rab protein alone will emulate the insulin-dependent gain in surface GLUT4, explaining the ineffectiveness of Rab13 overexpression seen here, or Rab8A overexpression.
The participation of both small G proteins in regulating GLUT4 traffic is underscored by differences in their time course of activation by insulin (peak activation of Rab8A at 2 min preceding Rab13 at 5 min) and differences in their subcellular localization, with only Rab13 being noticeably present at the cell periphery, where it partially colocalizes with GLUT4 within minutes of insulin addition (Fig. 5 and Fig. S4). Along with Fig. 2, these results suggest that Rab8A acts at a perinuclear (and early) step in the mobilization of GLUT4 insulin-sensitive vesicles, whereas Rab13 acts at a more peripheral (and subsequent) step in GLUT4 translocation.
Possible Steps Regulated by Rab8A and Rab13.
Rab proteins are regulators of organelle traffic, functioning in endocytic and exocytic events that display appreciable specificity in their sites of action. Rab8A was initially identified as a regulator of post-Golgi vesicle traffic from the TGN to basolateral membrane in MDCK cells (26). Rab8A colocalizes with Rab11 (27), and the latter activates the Rab8A GEF Rabin8 to coordinate delivery of structural proteins to the apical region of intestinal cells (28). Interestingly, insulin stimulates GTP loading of Rab11 in H9c-hIR2 cardiomyoblasts (beginning at 2 min) (17), and Rab11 mutations impair insulin-stimulated GLUT4 traffic in 3T3-L1 adipocytes, cardiomyoblasts, and human skeletal myotubes (29, 30) by preventing GLUT4 transit from recycling endosomes to insulin-sensitive compartments (30). These results raise the possible scenario that Rab11, Rabin8, and Rab8A may participate in early events of insulin-stimulated GLUT4 exocytosis in muscle cells.
In contrast to the participation of Rab8A in endosomal transit, Rab13 regulates peripheral events such as tight junction morphogenesis in epithelial cells by promoting occludin vesicle fusion mediated by the t-SNARE syntaxin4 (31). Strikingly, syntaxin4 also mediates GLUT4 vesicle fusion with the plasma membrane in adipocytes and muscle cells (32). Thus, it is tempting to speculate that Rab13 may regulate GLUT4 vesicles at the level of fusion with the plasma membrane. This scenario would explain why Rab13 was not detected in the proteomic analysis of adipocyte GLUT4-containing vesicles (11, 12).
Additional AS160-Targeted Rabs.
Rab14 is an in vitro target of the TBC domain (with GAP activity) of AS160 (11), but it does not belong to the Rab8 subfamily (5). Whereas Rab14 knockdown reduced GLUT4 translocation in muscle cells and partly restored the inhibition caused by AS160-4A (13), these effects were less robust compared with Rab8A or Rab13 (this study). An AS160-related protein, TBC1D1, also shows in vitro GAP activity toward the same cohort of Rabs as AS160 (33), and silencing either AS160 or TBC1D1 raises cell-surface levels of GLUT4 in the absence of insulin (14). Interestingly, concomitantly silencing the pair AS160 and Rab8A, or the pair TBC1D1 and Rab14, restored basal GLUT4 levels (14). In contrast, in 3T3-L1 adipocytes, restoration of basal GLUT4 levels was observed upon concomitantly silencing AS160 and Rab10 (34). We speculate that, in muscle cells, Rab14 might be a more direct in vivo target of TBC1D1 compared with Rab8A. In the present study, we focused on the Rab8 subfamily, and future work should explore whether Rab14 is activated in response to insulin or other stimuli, and its possible sites of action.
Muscle GLUT4 Traffic in the Context of Insulin Resistance.
Recent studies reveal that insulin resistance may not be confined to defects in proximal insulin receptor signaling steps such as IRS-1 and Akt (35, 36). Each step along the process of GLUT4 translocation including vesicle fission, mobilization, tethering, docking, and fusion may be differentially affected, contributing to insulin resistance. Future work should consider whether activation of individual Rab proteins downstream of AS160 is altered in insulin-resistant states, allowing for more targeted approaches to treat insulin resistance and the ensuing type 2 diabetes.
In summary, we have identified Rab13 as an essential component of insulin-stimulated traffic of GLUT4 in muscle cells. Rab13 is not functionally redundant with Rab8A, and based on the time course of their activation and the insulin-stimulated colocalization of Rab13 (but not Rab8A) with GLUT4 at the plasma membrane, Rab13 appears to be the latest-acting signal known to date in the insulin signaling cascade, predicted to regulate an insulin-regulated step near/at the cell surface of muscle cells.
Methods
Antibodies, Chemicals, Small Interfering RNAs, and Plasmids.
Antibodies: Rab8 (BD Biosciences), Rab13 (Abcam), myc epitope, monoclonal (Santa Cruz Biotechnology) and polyclonal (Sigma-Aldrich), GFP (Invitrogen). Predesigned rat siRab13 (5′-CAAGAACGATTCAAGACAATA-3′) and siNR were from Qiagen. GFP-Rab13 (human) and GFP-Rab8A (canine) were provided by J. Brumell (The Hospital for Sick Children, Toronto, Canada) and I. Mellman (Yale University School of Medicine, New Haven, CT). AS160 and AS160-4A (with Akt phosphorylation sites Ser318, Ser588, Thr642, and Ser751 mutated to Ala, also known as AS160-4P) were provided by G.E. Lienhard (Dartmouth Medical School, Hanover, NH).
Cell Culture and Transfections.
L6-GLUT4myc myoblasts without or with stable expression of AS160 (L6-GLUT4myc-AS160) or insulin receptor (L6-GLUT4myc-IR) were cultured as described (37). Cells grown on coverslips were transfected with cDNA constructs using Lipofectamine 2000 (Invitrogen). siRNA (400 nM) transfections were performed with Lipofectamine RNAiMax (Invitrogen) for 24 h and then cultured for 48 h. To rescue knocked-down proteins, cells were transfected with siRNA and then replated on coverslips for transfection of cDNA constructs for 24 h.
Single-Cell Image-Based Fluorescent Quantification of Surface GLUT4myc and GFP-Rabs.
Fluorescence staining of cell-surface GLUT4myc in nonpermeabilized myoblasts was performed as described (14, 38). Following insulin stimulation, cells were fixed without permeabilization, and incubated with polyclonal anti-myc antibody and Alexa-555 anti-rabbit conjugate (Invitrogen). AS160-4A expression was detected by subsequent cell permeabilization (see below) and staining with the Flag antibody and Alexa-647 anti-mouse conjugate combination. Fluorescence images obtained with a Zeiss LSM 510 laser scanning confocal microscope along the z axis and collapsed XY projections were generated by an LSM 5 Image Browser. The pixel intensity of GLUT4myc signal was quantified by ImageJ software (National Institutes of Health). Fifteen to 20 cells were analyzed per condition in at least three independent experiments. Statistical analysis was performed by one-way ANOVA using Prism 4.0 software (GraphPad Software Inc.) and Tukey's post hoc analysis.
The cellular location of GLUT4myc and GFP-Rab13 or GFP-Rab8A was determined without or with cell permeabilization with 0.05% wt/vol saponin (present at 0.01% wt/vol in all subsequent solutions), as indicated, and reaction with monoclonal anti-myc antibody and Alexa-555 anti-mouse conjugate. Optical planes (0.25 μm) were selected with a Zeiss LSM 5 Image Browser deconvolved by Volocity 5.3.2 (Improvision) and processed with Adobe Photoshop 6.0. Pixel coincidence of GFP-Rab and GLUT4myc was determined by Volocity 5.3.2.
Rab13 and Rab8A GTP Loading.
L6-GLUT4myc-IR myoblasts were seeded in 10-cm–diameter dishes and transfected with 5 μg of GFP-Rab constructs or empty vector using Fugene HD (Roche). After 48 h, cells were serum-starved for 3 h followed by permeabilization with 50 μg/mL digitonin for 5 min at room temperature. Cells were then incubated 15 min with 40 μM biotinylated GTP-photoprobe: guanosine 5′-triphosphate[γ]4-azidoanilide 2′,3′-biotin-long-chain-hydrazone (Affinity Photoprobes) in the presence of an ATP-generating system (40 IU/mL creatine phosphokinase with 1 mM phosphocreatine) in culture media. Next, cells were treated with insulin (100 nM) for indicated times and then irradiated with 300-nm UV light for 2 min (Rayonet RPR-100 Photochemical Reactor, Southern New England Ultraviolet Company) and lysed with 1% Nonidet P-40 in 132 mM NaCl, 2 mM MgCl2, 2 mM Na3VO4, 10 mM NaF, 20 mM Tris·HCl (pH 7.4) and protease inhibitors. Lysates were mixed with 40 μL of streptavidin resin (50% vol/vol slurry; Thermo-Scientific) for 2 h at 4 °C. Bound proteins were eluted and immunoblotted with anti-GFP.
Supplementary Material
Acknowledgments
We thank Dr. Shuhei Ishikura for advice with the Rab-GTP–loading assay and establishing the L6-GLUT4myc-AS160 cell line, Tim Chiu for stimulating discussions, and Joanna Wong for participation in the development of the L6-GLUT4myc-IR cell line. Y.S. was supported by a postdoctoral fellowship from the Banting and Best Diabetes Centre of the University of Toronto. This work was supported by Grant MT 7307 from the Canadian Institutes of Health Research to A.K.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009523107/-/DCSupplemental.
References
- 1.Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413:201–215. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- 2.Karylowski O, Zeigerer A, Cohen A, McGraw TE. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol Biol Cell. 2004;15:870–882. doi: 10.1091/mbc.E03-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govers R, Coster AC, James DE. Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway. Mol Cell Biol. 2004;24:6456–6466. doi: 10.1128/MCB.24.14.6456-6466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol. 2004;16:451–457. doi: 10.1016/j.ceb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews3007. REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sano H, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 7.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- 8.Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes. 2007;56:414–423. doi: 10.2337/db06-0900. [DOI] [PubMed] [Google Scholar]
- 9.Eguez L, et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Kramer HF, et al. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 11.Mîinea CP, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larance M, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 13.Ishikura S, Bilan PJ, Klip A. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem Biophys Res Commun. 2007;353:1074–1079. doi: 10.1016/j.bbrc.2006.12.140. [DOI] [PubMed] [Google Scholar]
- 14.Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol. 2008;295:C1016–C1025. doi: 10.1152/ajpcell.00277.2008. [DOI] [PubMed] [Google Scholar]
- 15.Sano H, Roach WG, Peck GR, Fukuda M, Lienhard GE. Rab10 in insulin-stimulated GLUT4 translocation. Biochem J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwenk RW, Eckel J. A novel method to monitor insulin-stimulated GTP-loading of Rab11a in cardiomyocytes. Cell Signal. 2007;19:825–830. doi: 10.1016/j.cellsig.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Tong P, et al. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J Clin Invest. 2001;108:371–381. doi: 10.1172/JCI12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somwar R, Sumitani S, Taha C, Sweeney G, Klip A. Temporal activation of p70 S6 kinase and Akt1 by insulin: PI 3-kinase-dependent and -independent mechanisms. Am J Physiol. 1998;275:E618–E625. doi: 10.1152/ajpendo.1998.275.4.E618. [DOI] [PubMed] [Google Scholar]
- 20.Tsakiridis T, et al. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology. 1995;136:4315–4322. doi: 10.1210/endo.136.10.7664650. [DOI] [PubMed] [Google Scholar]
- 21.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010;22:461–470. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JeBailey L, et al. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes. 2007;56:394–403. doi: 10.2337/db06-0823. [DOI] [PubMed] [Google Scholar]
- 23.Ishikura S, Koshkina A, Klip A. Small G proteins in insulin action: Rab and Rho families at the crossroads of signal transduction and GLUT4 vesicle traffic. Acta Physiol (Oxf) 2008;192:61–74. doi: 10.1111/j.1748-1716.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talior-Volodarsky I, Randhawa VK, Zaid H, Klip A. α-Actinin-4 is selectively required for insulin-induced GLUT4 translocation. J Biol Chem. 2008;283:25115–25123. doi: 10.1074/jbc.M801750200. [DOI] [PubMed] [Google Scholar]
- 26.Huber LA, et al. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattula K, et al. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- 28.Knödler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uhlig M, Passlack W, Eckel J. Functional role of Rab11 in GLUT4 trafficking in cardiomyocytes. Mol Cell Endocrinol. 2005;235:1–9. doi: 10.1016/j.mce.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Zeigerer A, et al. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol Biol Cell. 2002;13:2421–2435. doi: 10.1091/mbc.E02-02-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köhler K, Zahraoui A. Tight junction: A co-ordinator of cell signalling and membrane trafficking. Biol Cell. 2005;97:659–665. doi: 10.1042/BC20040147. [DOI] [PubMed] [Google Scholar]
- 32.Foster LJ, Klip A. Mechanism and regulation of GLUT-4 vesicle fusion in muscle and fat cells. Am J Physiol Cell Physiol. 2000;279:C877–C890. doi: 10.1152/ajpcell.2000.279.4.C877. [DOI] [PubMed] [Google Scholar]
- 33.Roach WG, Chavez JA, Mîinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sano H, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Bouzakri K, Koistinen HA, Zierath JR. Molecular mechanisms of skeletal muscle insulin resistance in type 2 diabetes. Curr Diabetes Rev. 2005;1:167–174. doi: 10.2174/1573399054022785. [DOI] [PubMed] [Google Scholar]
- 36.Hoehn KL, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu W, et al. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem. 2003;278:17953–17962. doi: 10.1074/jbc.M211136200. [DOI] [PubMed] [Google Scholar]
- 38.Randhawa VK, et al. GLUT4 vesicle recruitment and fusion are differentially regulated by Rac, AS160, and Rab8A in muscle cells. J Biol Chem. 2008;283:27208–27219. doi: 10.1074/jbc.M804282200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.