Abstract
There is near consensus that Wnt family molecules establish important gradients within niches where hematopoietic stem cells (HSC) reside. We review recent papers suggesting that a delicate balance is required between competing Wnt ligands and corresponding signaling pathways to maintain HSC integrity. Some steps in the transitions from HSC to lymphoid progenitor seem to be partially reversible and under the influence of Wnts. In addition, it has been recently suggested that HSC can oscillate between dormant versus active or lineage-biased states. We speculate that Wnts control a reflux process that may sustain stem cell self-renewal and differentiation potential.
Secreted Wnt glycoproteins are involved in multiple aspects of embryonic development, governing cell fates, polarity, and proliferation (Nusse, 2005). Consequently, Wnt associated abnormalities contribute to embryonic lethality and underlie such disorders as cancer, bone density defects, and polycystic kidney disease. Many Wnt family proteins are expressed in hematopoietic tissues, and a series of reports suggest there must be important roles in blood cell formation. However, few topics have been more controversial, and generalization of Wnt regulation of hematopoietic stem cells (HSC) has been difficult. The daunting complexity and functional redundancy of Wnt-related molecules accounts in part for this failure to reach consensus. Also contributing to the wide range of conclusions drawn with respect to the function of Wnt signaling, investigators have used Wnt-related proteins that vary in structure, potency, and mode of presentation to influence different types of target cells. Moreover, experimental depletion of Wnt signaling may have been incomplete in some cases, and overexpression paradigms that result in very high levels could have given misleading results.
A series of recent reports provide new perspectives on these important issues and will provide the basis for discussion throughout this review. Contrary to original expectations, it remains unclear if any Wnt ligands can be regarded as typical growth and differentiation factors for hematopoietic cells. Indeed, some of these ligands impede the expansion and/or differentiation of particular cell types, and such Wnt-dependent responses may be critical to maintaining stem cell integrity under normal circumstances. Other results indicate that early events in hematopoiesis may not be unidirectional and can be experimentally reversed with Wnt signaling. Thus, further study could reveal new ways to exploit Wnt family molecules for protecting, expanding, and even reprogramming HSC. We will focus here on the role of Wnt signaling on the regulation of HSC and their progression toward cells of the humoral immune system. An excellent review concerning the role of Wnts in T lymphocyte development has just appeared (Staal et al., 2008).
Complexity of Wnt Signaling Components and Pathways
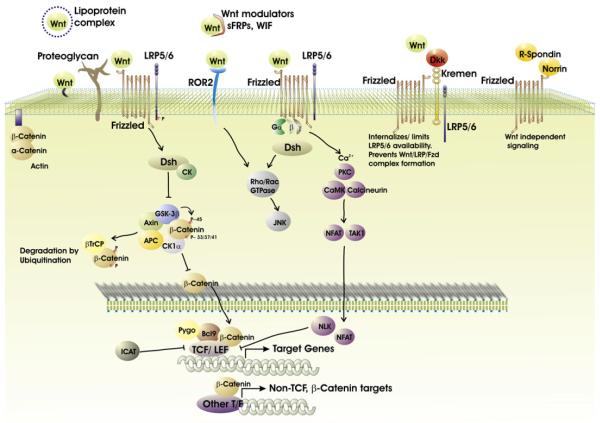
Some 19 Wnt ligands interact with both secreted and membrane-associated proteins to initiate signaling processes (detailed in Staal et al. [2008]; references in Table 1; summarized in Figure 1). The list of Wnt binding proteins include at least ten seven-pass transmembrane Frizzled (Fzd) receptors, two low-density lipoprotein receptor-related proteins (LRP), and a number of extracellular Wnt-modulating proteins such as Kremlin, Dickkopf (Dkk), Wnt-inhibitory factor (WIF), secreted Fzds (SFRP), and Norrin. Depending on the type of ligand-receptor interaction, the availability of intracellular signaling components and the specific target cell, an astonishing ten Wnt signaling pathways have been identified (Semenov et al., 2007; Macdonald et al., 2007). The canonical pathway, arguably the most studied and best understood, results in stabilization and nuclear translocation of β-catenin (Figure 1). This molecule is normally targeted for degradation by a GSK protein complex, but canonical signaling stabilizes β-catenin by destruction of the degradation complex. Stabilized β-catenin then enters the nucleus and acts as a coactivator for transcription factors such as LEF and TCF. A related molecule, γ-catenin (plakogloblin), is at least partially redundant to β-catenin and can bind TCF/LEF. Simultaneous targeting of both catenin genes results in embryonic lethality (Haegel et al., 1995; Ruiz et al., 1996). Further, Wnt signaling continued in the combined absence of β/γ catenin after inducible deletions, suggesting involvement of unknown redundant proteins (Jeannet et al., 2007). Adenomatous polyposis coli (APC) is a multidomain protein that can bind GSK3b, Axin, and β-catenin to prevent nuclear localization of catenin, thus decreasing canonical Wnt signaling (Aoki and Taketo, 2007). Independently of Wnt signaling, APC is involved in cellular adhesion, microtubule stabilization, chromosomal segregation, and proliferation events.
Table 1.
Selected Wnt Family Molecules Studied in Connection with Hematopoiesis
| Ligand | Hematopoietic Tissue Expression | Significance in Hematopoietic System | Pathway |
|---|---|---|---|
| Wnt1 | thymus (Mulroy et al., 2002) | G.F. (growth factor) for F.L. (fetal liver) progenitors (Austin et al., 1997); contributes to thymocyte cellularity (Mulroy et al., 2002) |
canonical |
| Wnt2b | F.L. (Van Den Berg et al., 1998); A.B.M. (Døsen et al., 2006) |
G.F. for A.B.M. (adult bone marrow) progenitors (Van Den Berg et al., 1998; Austin et al., 1997) |
canonical |
| Wnt3 | thymus (Balciunaite et al., 2002) | possible thymic regulatory signals, G.F, and hematopoietic commitment of embryoid bodies (Lako et al., 2001) |
canonical |
| Wnt3a | A.B.M.(Willert et al., 2003); thymus(Mulroy et al., 2002) |
G.F. for HSC, and increased their transplantation potential (Willert et al., 2003; Reya et al., 2003); detrimental for HSC expansion (Nemeth et al., 2007; Malhotra et al., 2008); reduced B and myeloid cells with stimulation via stroma (Yamane et al., 2001; Døsen et al., 2006); defective stem cells self-renewal in Wnt3a−/−(Luis et al., 2008); reduced VCAM-1 on hematopoietic cells after stimulation (Malhotra and Kincade, 2008); induced reflux in early hematopoietic progenitor compartment (Malhotra et al., 2008) |
canonical and noncanonical (Tu et al., 2007) |
| Wnt4 | Thymus (Balciunaite et al., 2002; Mulroy et al., 2002) |
contributes to thymocyte cellularity (Mulroy et al., 2002); low LSK (Lin−,c-KitHi,Sca-1+) frequency in Wnt4a−/−; overexpression expands Flt3+ LSK (Louis et al., 2008) |
noncanonical and canonical (Lyons et al., 2004) |
| Wnt5a | CD34+Lin− progenitors (Van Den Berg et al., 1998); F.L. and A.B.M.(Austin et al., 1997; Liang et al., 2003); thymus(Mulroy et al., 2002) |
G.F. for A.B.M. and F.L. progenitors (Van Den Berg et al., 1998; Austin et al., 1997); increased HSC repopulation on transplantion (Murdoch et al., 2003); maintains HSC in G0 (Nemeth et al., 2007); increased ProB and PreB cells in Wnt5a null F.L.(Liang et al., 2003); expansion of transplantable stem cells (Nemeth et al., 2007); increased B lymphopoiesis from HSC on Wn5a-expressing stroma (Malhotra et al., 2008) |
noncanonical and canonical (Mikels and Nusse, 2006); can antagonize canonical in hematopoietic (Nemeth et al., 2007) |
| Wnt10b | thymus (Weerkamp et al., 2006; Balciunaite et al., 2002); A.B.M. (Van Den Berg et al., 1998); F.L. progenitors (Austin et al., 1997) |
growth factor for A.B.M. and F.L. progenitors(Van Den Berg et al., 1998); expression in CD34+ progenitors regulated by Hox (Ferrell et al., 2005); upregulated after hematopoietic injury in A.B.M. (Congdon et al., 2008). |
canonical (Bennett et al., 2005) |
| sFRP1 | suppresses early B lymphopoiesis (Yokota et al., 2008) | Wnt antagonistic; induced canonical in hematopoietic cells (Yokota et al., 2008) |
|
| Dkk1 | A.B.M. (Døsen et al., 2006) | osteoblast specific transgenic expression increased stem cell cycling, compromised reconstitution following transplantation (Fleming et al., 2008); upregulated expression in Sox17−/− with failed HSC development (Kim et al., 2007) |
Wnt antagonistic; blocks canonical |
| β-catenin | expanded HSC, increased transplantation efficiency (Reya et al., 2003); induced reflux in early hematopoietic progenitor compartment (Baba et al., 2005); levels of catenin decreased with HSC differentiation (Baba et al., 2006); marrow failure and abnormal stem cell cycling in transgenic mice (Kirstetter et al., 2006; Scheller et al., 2006); β/γ catenin−/− (inducible) had no hematopoietic phenotype (Koch et al., 2008); continued Wnt signaling detected in β/γ catenin−/− (Jeannet et al., 2007); stem cell renewal defects in serial transplants of β-catenin−/− (Vav-Cre) (Zhao et al., 2007) |
canonical |
The significance of seven Wnt ligands, sFRP1, Dkk1, and β-catenin is summarized relevant to hematopoiesis. Also shown are hematopoietic sites of expression and known Wnt pathways stimulated by the molecules. G.F., growth factor; F.L., fetal liver; A.B.M., adult bone marrow; H.S.C., hematopoietic stem cell.
Figure 1. An Overview of the Wnt Family of Proteins and Three Signaling Pathways.
The 19 Wnt ligands are recognized by cell surface receptors (Frizzled, LRP5/6, and ROR2). These receptor-ligand interactions induce many intracellular signaling/nuclear transcription events as indicated. Also shown are extracellular Wnt ligand modulators (sFRPs and WIF) and other Wnt receptor interacting ligands (R-spondin, Norrin, and Dkk).
Actions of the Wnt ligands are frequently opposed by other Wnts, soluble receptors, and antagonists (see below). Furthermore, biological responses are dictated by which of the alternate signaling mechanisms are utilized. Two noncanonical pathways, Wnt-Ca2+ and Wnt-JNK, do not (normally) stabilize β-catenin pools. Rather, Wnt-Fzd receptor interactions activate membrane-associated G protein complexes and Disheveled (Dsh) to either increase intracellular Ca2+ levels through inositol-3-phosphate (IP3) or induce the JNK pathway through Rho/Rac GTPases. As a result, noncanonical signals can influence actin-dependent cytoskeletal reorganization. Other studies suggest crosstalk/competition between canonical and noncanonical Wnt pathways. For example, activation of the canonical Wnt pathway is observed in mice deficient in the noncanonical Wnt5a ligand (Topol et al., 2003). Further, activation of noncanonical pathways can sometimes block canonical signaling (Nemeth et al., 2007).
Wnt Ligands Influence Stem and Progenitor Cells
The discovery that Wnt genes are expressed in hematopoietic tissues was soon followed by attempts to determine their contribution to blood cell formation (see Table 1, for a summary, and references). A variety of hematopoietic cell fractions were exposed in culture to recombinant proteins in conditioned medium, commercially obtained materials or purified ligands. Other experimental paradigms placed stem cells and progenitors in culture with Wnt producing stromal cells. Different forms of presentation, doses, and posttranslational modifications are likely to have affected potencies of specific ligands and their corresponding outcomes. Furthermore, it is becoming clear that the nature of the target cells is an important parameter in determining the specific response to a given ligand, either alone or in combination.
An example of this context-dependent response of blood progenitors is found in an early report that showed that Wnt1, Wnt5a, or Wnt10b stimulated the expansion of fetal hematopoietic cells in culture (Austin et al., 1997). Most of the experiments were conducted with Wnt5a, and 2- to 4-fold increments in cell production were observed with addition of the factor or retroviral introduction of a Wnt5a cassette. In another study, human CD34+ progenitors responded to a similar degree when Wnt5a, Wnt2b, or Wnt10b were provided by transduced stromal cells, but it is noteworthy that recombinant human SCF also gave a similar result (Van Den Berg et al., 1998). Much more impressive results were reported by Reya, Weissman, and colleagues, where HSC numbers increased 10- to 50-fold on addition of purified Wnt3a to cultures that contained SCF (Willert et al., 2003). Although dramatic results were obtained in primary experiments conducted with stem cells from Bcl-2 transgenic mice, the authors provided more limited supplemental data generated using marrow from normal animals. While similar trends were observed, expansion of long-term repopulating stem cells from conventional mice was not as robust nor vigorously demonstrated (Reya et al., 2003). More recent studies concluded that Wnt3a was detrimental to stem cell survival and expansion in short-term cultures, even when Bcl-2 transgenic cells were used (Nemeth et al., 2007; Malhotra et al., 2008). Wnt5a was shown to function as an antagonist of canonical Wnt signaling in hematopoietic cells and caused some expansion of transplantable stem cells (Nemeth et al., 2007). Retroviral introduction of Wnt3a prevented reconstitution, but Wnt5a was without influence when expressed using the same approach. The negative result could be due to the use of a potentially toxic DsRed fluorochrome (Tao et al., 2007) as an expression marker. Alternatively, Wnt5a might normally be expressed in sufficient amounts in marrow niches such that elevated exposure to the ligand had no effect. However, injection of purified Wnt5a into NOD/SCID mice increased engraftment of human hematopoietic progenitors (Murdoch et al., 2003).
Wnt3a Impact on HSCs and Progenitors
Given the importance of Wnt3a for embryonic development, it has been challenging to assess its contribution to hematopoiesis in genetically manipulated animal models. However, Staal and colleagues recently found that it is possible to study viable embryos at 12.5 days of gestation (Luis et al., 2008). In these mice, the percentages of cells in the HSC-containing “LSK” fraction of fetal liver were reduced, as was the frequency of cells capable of short-term repopulation when transplanted into sublethally irradiated recipients. The number of early myeloid progenitors was also below normal, whereas mature myeloid cells were present in the embryo, and no intrinsic defects were found in erythroid or B lineage precursors. While primary transplantation to lethally irradiated recipients did not reveal stem cell defects, repopulation was severely impaired following secondary transplantation. The authors conclude that Wnt3a−/− HSCs recovered from E12.5 fetal livers can generate all types of blood cells but are permanently abnormal with respect to self-renewal. The study also demonstrated an important requirement for thymic stroma-derived Wnt3a for T lymphopoiesis. While Notch1 may be associated with canonical Wnt signaling in some contexts (Duncan et al., 2005), the same was not the case for stem cell defects observed in Wnt3a−/− embryos. While the Luis et al. study indicates that Wnt3a is required for normal fetal hematopoiesis and long term HSC function, results from the Wnt3a-deficient mouse do not reveal which cells or fetal tissues require Wnt3a expression. That is, Wnt3a may be required for the normal function of niches that support HSC; this model is supported by the observation that stromal cells are responsive to Wnt3a in culture (Yamane et al., 2001). In addition, canonical Wnt signaling dramatically influences adhesion molecule expression in several cell types (Malhotra and Kincade, 2008). Alternatively, or in addition, fetal HSC could be direct Wnt3a targets. Many differences in fetal and adult hematopoiesis have been observed, one notable example being that fetal HSCs are actively cycling (Bowie et al., 2007) versus the predominant quiescent phenotype of adult HSCs isolated at steady states. However, it is interesting to compare the Luis et al. results using Wnt3a-targeted fetal liver cells to experiments in which adult stem cell niches were made Wnt deficient (Fleming et al. [2008] and see below). In both circumstances, stem cell abnormalities were only revealed after repeated rounds of expansion via serial transplantation.
Wnt3a and Wnt5a Impact on Lymphoid Progenitors
The role of Wnt family proteins in regulating additional aspects of hematopoiesis has also been investigated. B lymphopoiesis was abnormal in mice whose Wnt5a gene was targeted, and the same ligand appeared to interfere with pro-B responses to IL-7 (Liang et al., 2003). The authors concluded from transplant experiments that lymphopoietic abnormalities were intrinsic to hematopoietic cells in Wnt5a−/− mice. We recently used stromal cells to present Wnt3a or Wnt5a to hematopoietic cells (Malhotra et al., 2008). While Wnt3a inhibited HSC expansion and progression in the B lymphocyte lineage, opposite results were obtained when Wnt5a was provided. Of particular importance, responsiveness to these ligands declined as lymphoid differentiation proceeded. This stage-specific sensitivity is consistent with a recent report that pre-B cells were unaffected by CD19-Cre-driven conditional deletion of β-catenin (Yu et al., 2008). As noted above, several studies report that mild to severe elevations in canonical signaling at the stem cell level cause corresponding reductions in the differentiation capacity of the HSCs (Reya et al., 2003; Baba et al., 2006; Nemeth et al., 2006; Kirstetter et al., 2006; Qian et al., 2008).
Additional Wnt Ligands
While Wnt3a and Wnt5a are arguably the most studied Wnt ligands in the hematopoietic context, other Wnts have also been examined. For example, perturbation of Wnt4 influenced B and T lymphopoiesis without canonical pathway signaling (Louis et al., 2008). In this study, Wnt4−/− and Wnt4+/− neonates had small thymuses, and the LSK fraction of bone marrow, which contains HSCs, was underrepresented in these animals. It was not clear from these findings whether the absence of Wnt4 influenced overall body size. However, enforced expression of Wnt4 in transduced fetal liver cells was sufficient to increase thymus size and also elevated the frequency of marrow LSK cells in transplant recipients. These changes must have resulted from secreted Wnt4, rather than due to a cell-intrinsic effect of the transduced gene, since very few GFP reporter-positive cells were detected in transplanted recipients (approximately 10% of cells in marrow and thymus) and this frequency varied widely. The specific impact of the stimulation on cells that responded to Wnt4 was not investigated.
Hox, homeodomain proteins, are implicated in hematopoiesis and have been found in primitive hematopoietic cells. Hox9 and Hox10 can regulate expression of Wnt10b in human CD34+ progenitors (Ferrell et al., 2005). Wnt10b is upregulated in stem and stromal cells of bone marrow following hematopoietic injury where it could theoretically aid in hematopoietic regeneration. In addition, this ligand can signal via the canonical pathway in at least some circumstances (Bennett et al., 2005). However, retroviral introduction of Wnt10b had a very modest influence on HSC function (Congdon et al., 2008).
Collectively, these reports still only scratch the surface of Wnt ligands that stem and progenitor cells may encounter within the bone marrow and also do not account for all of the receptors potentially available to the cells for ligand recognition (Table 1). No less complex are the various signaling pathways that can be utilized, and the range of modifications that can be applied to affect the overall outcome of each cascade.
Canonical Wnt Signaling and Hematopoiesis
As discussed above, there is a multitude of Wnt signaling cascades, but the most well-known is the canonical β-catenin pathway, which has been implicated to play a regulatory role in hematopoiesis. In one dramatic example, HSC of BCL-2 transgenic mice were transduced with a constitutively active-β-catenin cassette, promoting their expansion almost 100-fold (Reya et al., 2003). In fact, even after a 1 week culture period, the transduced cells retained a robust ability to engraft the marrow of irradiated recipients and differentiated in all blood lineages. The culture conditions that were sufficient to achieve this result were remarkably simple, but it remains unclear whether the β-catenin gene provided was itself responsible for the observed growth promotion of long-term repopulating HSC. While this hypothesis is certainly an option, it is also conceivable that appropriate levels of canonical Wnt signaling may have simply preserved stem and/or multipotent progenitor integrity and maintained the cultured cells in an undifferentiated state (see additional comments below). We and others have since found that some, but not all, stem cell characteristics can be retained for at least 5 months when primitive hematopoietic cells were transduced with constitutively active β-catenin (Baba et al., 2006; Templin et al., 2008). That is, the cell lines were multipotent and self-renewing but unable to efficiently reconstitute the thymus or rescue lethally irradiated mice. It is noteworthy that both introduced and endogenous β-catenin levels declined whenever our multipotent cell lines acquired lineage-associated markers (Baba et al., 2006). Wnts form activity gradients, and signaling levels might have to be within a precise range to dictate stem cell renewal versus differentiation. Consistent with that interpretation, two lines of transgenic mice that overexpressed large amounts of β-catenin in hematopoietic cells experienced marrow failure (Kirstetter et al., 2006; Scheller et al., 2006). Canonical signaling was also increased in Hmgb3 knockout mice, where progression in hematopoietic lineages was again diminished (Nemeth et al., 2006).
Adenomatous polyposis coli (APC) is another essential component of the canonical Wnt pathway signaling, functioning in the APC-Axin-GSK3-β complex that causes β-catenin degradation. A recent study used interferon-triggered deletion to assess the contribution of APC to hematopoiesis (Qian et al., 2008). The targeted knockout resulted in marrow failure within 2 to 3 weeks, marked by greatly reduced numbers of the stem cell-containing LSK subset as well as more mature progenitors in all lineages. Moreover, the APC-deficient HSCs were intrinsically defective and unable to sustain any type of blood cell formation when transplanted. The analyses revealed abnormally increased proliferation of stem and progenitor rich subsets as well as a tendency to undergo apoptosis. As would be expected, levels of β-catenin were very high in APC-deficient cells, and most of the abnormalities were consistent with extraordinary high levels of canonical Wnt signaling (Kirstetter et al., 2006; Scheller et al., 2006). It is noteworthy that APC is also involved in Wnt-independent functions, and the authors speculated that β-catenin driven phenomena might not alone account for the rapid apoptosis and stem/progenitor depletion they observed. This hypothesis is similar to the Wnt pathway-independent proapoptotic function proposed for axin (Hsu et al., 2001). In any case, this report again demonstrates that stem cell function requires canonical Wnt signaling to be kept within a critical physiological range.
Glycogen synthase kinase 3 (GSK3) is another key component of the canonical signaling pathway. GSK3 is a serine-threonine kinase that phosphorylates cytosolic β-catenin and prevents it from translocating to the nucleus (Figure 1). Inhibition of GSK3 by treatment with a low MW compound increased the repopulation ability of murine as well as human HSC in immunodeficient NOD/SCID mice (Trowbridge et al., 2006). The treatment also expanded stem cells in vitro. In contrast to this result, another group recently found that GSK3 inhibition delayed ex vivo expansion of human CD34+ cord blood cells, while preserving a stem-like phenotype (Holmes et al., 2008). It is important to note that, in addition to Wnt, GSK3 is involved in other signaling pathways such as Insulin, Hedgehog and Notch, some of which have consequences for hematopoiesis (Kockeritz et al., 2006) (Doble and Woodgett, 2003).
All of these experimental approaches caused canonical Wnt signaling to be abnormally elevated, and reciprocal results have not always been obtained in knockout studies. Targeted deletion of either β- or γ- catenin genes results in embryonic lethality, so several studies made use of inducible gene deletions. Hematopoietic cells deficient in one (Cobas et al., 2004) or both (Koch et al., 2008; Jeannet et al., 2007) catenin genes had no obvious defects. These impressive studies were hailed by many as definitive demonstrations that the canonical pathway is not essential for hematopoiesis, but Held and colleagues subsequently showed that TCF/LEF-dependent transcription continued in the combined absence of β- and γ-catenin (Jeannet et al., 2007). It has been speculated that partially functional β-catenin protein might persist in these animals (Staal and Sen, 2008). As another possibility, the reporters used to assess Wnt-dependent signaling in the Jeannet study could have been triggered by non-Wnt ligands such as TGF-β (Barolo, 2006). Furthermore, each of the conditional deletion experiments discussed above utilized an interferon-inducible Cre model to induce gene deletion in adults. In contrast, Zhao et al. subsequently used Vav-cre for β-catenin gene deletion from fetal life and observed significant hematopoietic abnormalities (Zhao et al., 2007). Therefore, the combined set of experiments beg the question of whether the unique proliferative activity and expansion demands placed on fetal stem cells reveal a requirement for Wnt signaling that is otherwise obscured in adults and during homeostasis. As with other models of Wnt blockage or deficiency (Fleming et al., 2008; Luis et al., 2008), stem cell renewal defects were only obvious in secondary transplantation assays. Thus, Wnt-dependent influences on hematopoietic cells can be subtle and only appreciated when very rigorous tests are performed.
Competition among Wnt Family Molecules
Responses to Wnts are buffered by the complexity of interacting ligands and antagonists. Also, there are many examples of crosstalk and competition between Wnt signaling pathways (Mikels and Nusse, 2006; Nemeth et al., 2007; Baksh et al., 2007). As described above, HSCs respond to Wnt5a, and the same ligand also promotes lineage progression of primitive lymphoid progenitors. Wnt5a is usually considered to be a noncanonical Wnt ligand and can antagonize Wnt3a signals via GSK-3-independent β-catenin degradation. For that reason, results obtained with Wnt5a−/− mice could reflect the absence of Wnt5a, an increase in canonical Wnt signaling, or both. As an example of that complexity, transition of HSC into early lymphoid cells is inhibited by the canonical Wnt3a ligand and promoted by Wnt5a (Malhotra et al., 2008). Environmental context and the nature of target cells are important because Wnt5a can be an inhibitor of later stages of lymphoid differentiation (Liang et al., 2003).
A number of secreted molecules modulate Wnt functions in positive and negative ways (Kawano and Kypta, 2003). The Dickkopf (Dkk) proteins may inhibit proximal events in Wnt responses by binding to LRP coreceptors, and this property was recently exploited to implicate Wnt signaling in stem cell maintenance (Fleming et al., 2008). Transgenic expression of Dkk1 with a collagen promoter directed it to subendosteal stem cell niches, reducing canonical Wnt pathway signaling in hematopoietic cells at least 100-fold. While numbers of hematopoietic cells were marginally affected, there were dramatic and durable defects in transplantable stem cells. That is, fewer HSCs were quiescent and bone marrow function was compromised with respect to reconstitution. Remarkably, even temporary residence in Wnt-deficient marrow caused HSC abnormalities.
Wnt equilibrium in normal hematopoietic tissues could theoretically involve one or more of the Dkk proteins. Dkk1 is essential to normal bone development and bone pathology results when it is elevated (Mukhopadhyay et al., 2001; Pinzone et al., 2008). Experimental manipulation of inhibitor levels in the stem cell niche could indicate how Wnts in that site are normally countered by Dkks (Fleming et al., 2008).The Sox17 transcription factor is expressed only during fetal and neonatal life, and this disparity may account for some fetal/adult stem cell differences observed in the hematopoietic system (Kim et al., 2007). It is interesting that Dkk1 was dramatically upregulated in embryos whose Sox17 gene was knocked out, given that HSC also failed to develop in these animals. If Dkk1 normally contributes to Wnt-dependent homeostasis in adult bone marrow, there must be Sox17-independent mechanisms for regulating its expression. Again, it must be considered that requirements for particular Wnt family molecules in fetal and adult life may be different.
In addition to Dkk proteins, several other soluble molecules are important modulators of Wnt functions. For example, secreted Frizzled-related proteins (sFRPs) can bind Wnt ligands, prevent their interaction with transmembrane receptors, and thus, function as antagonists to Wnt signaling (Uren et al., 2000). However, the impact of this class of inhibitors is highly dose dependent, and small amounts of sFRP1 actually enhance Wnt stimulation. We recently discovered that stromal cells release sFRP1 when exposed to estrogen, and this production accounts in part for hormone-mediated suppression of B lymphopoiesis (Yokota et al., 2008). That is, sFRP1 could function similar to Wnt3a as a canonical ligand, at least at a given concentration. However, Wnt3a and sFRP1 were mutually antagonistic when added together at an appropriate ratio. Wnts are known to be matrix bound, while the stability and availability of sFRP1 is heparin dependent (Uren et al., 2000; Zhong et al., 2007). Therefore, proteoglycan-tethered gradients of these molecules could be important for determining stem and progenitor cell functions. The probability of a given stem cell responding via a particular signaling pathway within bone marrow is, therefore, likely to be determined by multiple parameters (Figure 2).
Figure 2. Wnt Proteins Function in Equilibrium within Hematopoietic Stem Cell Niches.
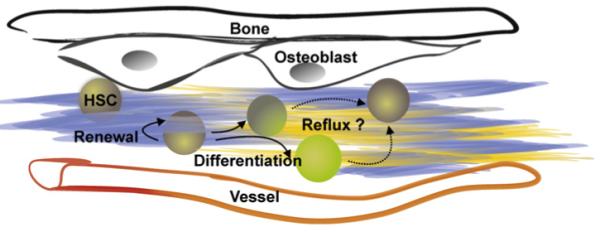
The net biological activity within a discrete marrow location is determined by factors related to synthesis, secretion, and presentation of multiple Wnt proteins as well as patterns of receptor expression on the hematopoietic cells. Functional gradients of these molecules are also determined by competition between them. Matrix-associated agonists and antagonists in the Wnt family are indicated in this model as blue and yellow, respectively. All of these parameters may need to be within carefully regulated ranges, and stem cells (HSC, depicted by gray shaded circles) can physically move into, within, and from niches. Dormant stem cells may preferentially associate with niches such as those provided by subendosteal osteoblasts. We speculate that a degree of reflux occurs as stem cells “test the waters” before either progressing in a blood cell lineage (shown by green shaded circles) or returning to a self-renewing or dormant state. That testing process might correspond to movement within a Wnt gradient followed by resettling in a niche. The only evidence for Wnt driven dedifferentiation comes from culture experiments as discussed in the text.
Retention and Migration of HSC in Response to Wnts?
Wnts were discovered within the context of cellular patterning/movement, and β-catenin has well-known roles in cell adhesion junctions (Daugherty and Gottardi, 2007). Thus, it is interesting that canonical Wnt signaling elicited by Wnt3a or introduction of a constitutively active β-catenin gene dramatically reduced expression of cell adhesion molecules (Malhotra and Kincade, 2008). VCAM-1 was diminished in stromal, endothelial, and hematopoietic cells. This adhesion molecule is important for the homing of HSC to bone marrow and is thought to help retain progenitor cells in appropriate niches (Ulyanova et al., 2007; Leuker et al., 2001; Koni et al., 2001). Consistent with the requirement on stromal rather than hematopoietic cells, HSC that had been exposed to Wnt3a and as a result no longer expressed VCAM-1 were still able to engraft transplant recipients. ICAM-1 levels were also reduced on Wnt-stimulated stromal cells (Malhotra and Kincade, 2008). Proximity of hematopoietic cells to appropriate Wnt concentrations is likely to be a critical parameter, and Wnts can control this access by modulating cell adhesion and migration-related molecules. For example, one can imagine a stem cell approaching a given niche and then being encouraged to stay or exit because of the particular Wnt gradient present in that location (Figure 2).
Wnt Control of Stem Cell Proliferation?
Wnts initially appeared to regulate hematopoiesis in a similar fashion as other established growth and differentiation factors. For example, exposure to Wnt proteins potentiated colony formation in cultures that had also been supplemented with colony-stimulating factors (Austin et al., 1997). One might reach the same conclusion based on reports that (1) HSC could be extensively expanded under the influence of recombinant Wnt3a and (2) that the stem/progenitor compartment was abnormally cycling in transgenic mice overexpressing β-catenin (Willert et al., 2003; Scheller et al., 2006; Kirstetter et al., 2006; Qian et al., 2008). However, fetal hematopoietic cells that were already rapidly dividing were used in some of the situations where Wnt family molecules were said to stimulate proliferation (see Table 1). Also, colony numbers in progenitor assays were usually not higher than what could be obtained with combinations of other factors (Van Den Berg et al., 1998). In our hands, exposure of HSC to Wnt3a-producing stromal cells limited their expansion (Malhotra et al., 2008). Furthermore, HSC mitotic activity was high in mice with a Wnt deficiency caused by a Dkk-1 transgene (Fleming et al., 2008). While it remains possible to attribute these discrepancies to varying dose and context of Wnt presentation, we consider it more likely that the primary influence of Wnts is on the differentiation of hematopoietic cells rather than replication. Of particular interest is the possibility that Wnts control the direction of differentiation.
Maintaining and Regaining an Undifferentiated State
Differentiation from long-term repopulating HSC to short-term multipotent and restricted progenitor cells is thought to be unidirectional under normal circumstances. In fact, the best examples of dedifferentiation of blood cells involve malignancies and genetic engineering (Cobaleda and Busslinger, 2008). Similarly, introduction of constitutively active β-catenin had a dramatic effect on partially differentiated hematopoietic cells (Baba et al., 2005). That is, lymphoid committed progenitors could give rise to myeloid cells, and myeloid progenitors could differentiate into lymphoid cells under the influence of canonical, β-catenin-dependent signaling. We speculated that excessive β-catenin caused epigenetic changes that, in turn, diminished lineage-restricting mechanisms. Cobaleda and Busslinger recently noted that “lineage infidelity” has been well documented in follicular lymphoma and suggest that changes in levels of stabilizing transcription factors could be involved (Cobaleda and Busslinger, 2008). Similarly, Lef-1 is a key transcriptional mediator in Wnt signaling, and its deregulation produces tumor stem cells with potential for lymphoid and myeloid differentiation (Petropoulos et al., 2008). An imbalance between Wnt family molecules occurs in some human leukemias (Nemeth and Bodine, 2007).
Progenitor-like cystocytes in the ovary and spermatogonial cells in the testis can dedifferentiate to become germline stem cells in Drosophila (Brawley and Matunis, 2004; Kai and Spradling, 2004). While described in this model as an emergency stem cell source, it was long ago proposed that progenitor-to-stem cell retrodifferentiation might be possible for hematopoietic cells (Schofield, 1978). The findings described above indicate that Wnt signaling can be engineered to achieve a degree of reprogramming (Baba et al., 2005). However, it was not clear if dedifferentiation and progression within an alternate lineage would ever occur naturally, and if so, whether it could be observed in noninvasive experimental circumstances. We recently found that exposure to Wnt3a caused committed lymphoid cells to reacquire some stem cell-like characteristics (Malhotra et al., 2008). In this example, early lymphoid progenitors (ELP) were isolated from marrow of RAG-1/GFP knockin mice and placed in culture with Wnt-producing stromal cells. RAG-1 transcripts were extinguished within 18 hr, while Thy1.1 was reacquired and changes in differentiation potential occurred within the next few days. That is, the previously lineage-biased lymphoid cells retained or regained some myeloid colony-forming capacity during the period of culture. Downregulation of erythroid differentiation potential is thought to be one of the earliest events in the stem to progenitor transition (Adolfsson et al., 2005). Thus, it is significant that ELP became responsive to erythroid differentiation signals during exposure to Wnt3a. Pro-lymphocytes appear further along in the lymphoid lineage differentiation pathway, and yet, some of these relatively mature cells even acquired several phenotypic characteristics (Lin−Sca-1+c-KitHiThy1.1LoRag-1−) of stem cells following exposure to Wnt3a. In functional assays, responsiveness to Wnt3a progressively declined with stages of lymphoid differentiation. These remarkable findings indicate that at least some early events in blood cell formation are reversible. It will be interesting to determine whether functional HSCs can be regenerated by manipulation of Wnt signals.
As recently reviewed by Graf and Stadtfeld (2008), HSC are heterogeneous and may oscillate through multiple states. For example, clonal analyses have revealed that individual stem cells exhibit lymphoid/myeloid biases that are heritable through multiple rounds of transplantation (Sieburg et al., 2006; Dykstra et al., 2007). As an example of a different type of heterogeneity, a continuum of HSC subsets was recently proposed to move from “reserved” to “primed” states (Haug et al., 2008). Changes in both directions were noted in this study by staining for expression of N-cadherin. Stem cell dormancy may also be distinguished by lack of CD34 and underappreciated because of experimental artifacts (Wilson et al., 2008). This reserved status might explain why subsets of human stem cells oscillate in terms of marker expression and require considerable time to expand following transplantation (McKenzie et al., 2006, 2007). Furthermore, the engraftment potential of murine HSC fluctuates as they move through the cell cycle (Habibian et al., 1998). We speculate that such reversible transitions are regulated in part by Wnt family molecules. In fact, the multipotent EMCL1 cell line was shown to spontaneously undergo fluctuations in gene expression and differentiation competence (Chang et al., 2008). Canonical Wnt pathway stimulation by introduction of active β-catenin blocked the myelopoietic potential of these cells (Baba et al., 2006). Similarly, manipulation of canonical Wnt signaling has been effectively used to maintain pluripotency of embryonic stem cell (ESC) lines and augment the reprogramming of fibroblasts to create induced ESCs (Ying et al., 2008; Marson et al., 2008).
Wnt proteins are likely to be present in both “osteoblastic” and “vascular” niches in bone marrow (Duncan et al., 2005; Zhang and Li, 2008; Wilson et al., 2007; Celso et al., 2008). However, the net biological activity in any location must be an extremely complex function of overlapping gradients of multiple Wnt ligands and secreted antagonists. Doses and combinations of Wnts, proximity to those molecules, receptors expressed, and cross-competition with other environmental cues are all certain to be important determinants of stem cell responses (Figure 2).
Concluding Remarks
The evidence is compelling that Wnts are required to maintain the integrity of HSC. However, induction of hematopoietic demand by serial transplantation or myeloablation has been required to clearly see abnormalities in HSC exposed to Wnt deficient environments. Thus, Wnts may operate in cooperation with many other components of stem cell niches and contribute most notably under conditions of disease, stress, or aging. On the other hand, at least some ligands for canonical Wnt signaling repress and even reverse differentiation of HSC and ESCs. As suggested above, refluxing between stem and progenitor cell states could be partially regulated by Wnts. Continued assault on this complex group of ligands, receptors, and coreceptors is certain to be useful as well as informative. For example, it may eventually be possible to expand and reprogram normal stem and progenitor cells by exploiting the Wnt family of proteins. Such an approach could improve the ability of HSCs to rebound following myeloablation or engraft following transplantation, and converse treatments may be found to deprive leukemia cells of essential Wnt stimulation.
ACKNOWLEDGMENTS
Work in our laboratory is supported by grants AI020069, AI058162, and AI069024 from the National Institutes of Health. P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J. Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: Expansion of multilineage progenitor cells. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- Baba Y, Garrett KP, Kincade PW. Constitutively active b-catenin confers multilineage differentiation potential on lymphoid and myeloid progenitors. Immunity. 2005;23:599–609. doi: 10.1016/j.immuni.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, Kincade PW. Constitutively active beta-catenin promotes expansion of multipotent hematopoietic progenitors in culture. J. Immunol. 2006;177:2294–2303. doi: 10.4049/jimmunol.177.4.2294. [DOI] [PubMed] [Google Scholar]
- Baksh D, Boland GM, Tuan RS. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J. Cell. Biochem. 2007;101:1109–1124. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat. Immunol. 2002;3:1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25:7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, Hoodless PA, Eaves CJ. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl. Acad. Sci. USA. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Celso CL, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scaddden DE. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2008 doi: 10.1038/nature07434. in press. Published online December 3, 2008. 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobaleda C, Busslinger M. Developmental plasticity of lymphocytes. Curr. Opin. Immunol. 2008;20:139–148. doi: 10.1016/j.coi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon KL, Voermans C, Ferguson EC, DiMascio LN, Uqoezwa M, Zhao C, Reya T. Activation of Wnt signaling in hematopoietic regeneration. Stem Cells. 2008;26:1202–1210. doi: 10.1634/stemcells.2007-0768. [DOI] [PubMed] [Google Scholar]
- Daugherty RL, Gottardi CJ. Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology (Bethesda) 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Døsen G, Tenstad E, Nygren MK, Stubberud H, Funderud S, Rian E. Wnt expression and canonical Wnt signaling in human bone marrow B lymphopoiesis. BMC Immunol. 2006;7:13. doi: 10.1186/1471-2172-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Ferrell CM, Dorsam ST, Ohta H, Humphries RK, Derynck MK, Haqq C, Largman C, Lawrence HJ. Activation of stem-cell specific genes by HOXA9 and HOXA10 homeodomain proteins in CD34+ human cord blood cells. Stem Cells. 2005;23:644–655. doi: 10.1634/stemcells.2004-0198. [DOI] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo CC, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3:480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Habibian HK, Peters SO, Hsieh CC, Wuu J, Vergilis K, Grimaldi CI, Reilly J, Carlson JE, Frimberger AE, Stewart FM, Quesenberry PJ. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J. Exp. Med. 1998;188:393–398. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of b-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Haug JS, He XC, Grindley JC, Wunderlich JP, Gaudenz K, Ross JT, Paulson A, Wagner KP, Xie Y, Zhu R, et al. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell. 2008;2:367–379. doi: 10.1016/j.stem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Holmes T, O’Brien TA, Knight R, Lindeman R, Shen S, Song E, Symonds G, Dolnikov A. Glycogen synthase kinase-3beta inhibition preserves hematopoietic stem cell activity and inhibits leukemic cell growth. Stem Cells. 2008;26:1288–1297. doi: 10.1634/stemcells.2007-0600. [DOI] [PubMed] [Google Scholar]
- Hsu W, Shakya R, Costantini F. Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. J. Cell Biol. 2001;155:1055–1064. doi: 10.1083/jcb.200107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2007;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Koch U, Wilson A, Cobas M, Kemler R, MacDonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3-an overview of an over-achieving protein kinase. Curr. Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lako M, Lindsay S, Lincoln J, Cairns PM, Armstrong L, Hole N. Characterisation of Wnt gene expression during the differentiation of murine embryonic stem cells in vitro: role of Wnt3 in enhancing haematopoietic differentiation. Mech. Dev. 2001;103:49–59. doi: 10.1016/s0925-4773(01)00331-8. [DOI] [PubMed] [Google Scholar]
- Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J. Exp. Med. 2001;193:755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Louis I, Heinonen KM, Chagraoui J, Vainio S, Sauvageau G, Perreault C. The signaling protein Wnt4 enhances thymopoiesis and expands multipotent hematopoietic progenitors through beta-catenin-independent signaling. Immunity. 2008;29:57–67. doi: 10.1016/j.immuni.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Luis TC, Weerkamp F, Naber BA, Baert MRM, de Haas EF, Nikolic T, Heuvelmans S, De Krijger RR, Von Dongen JM, Staal FJT. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2008 doi: 10.1182/blood-2008-06-163774. in press. Published online October 2, 2008. 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, Barth AM, McCrea PD. Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp. Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Macdonald BT, Semenov MV, He X. SnapShot: Wnt/beta-catenin signaling. Cell. 2007;131:1204. doi: 10.1016/j.cell.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp. Hematol. 2008 doi: 10.1016/j.exphem.2008.08.008. in press. Published online October 23, 2008. 10.1016/j.exphem.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Baba Y, Garrett KP, Staal FJ, Gerstein R, Kincade PW. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J. Immunol. 2008;181:3955–3964. doi: 10.4049/jimmunol.181.6.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JL, Gan OI, Doedens M, Wang JC, Dick JE. Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat. Immunol. 2006;7:1225–1233. doi: 10.1038/ni1393. [DOI] [PubMed] [Google Scholar]
- McKenzie JL, Gan OI, Doedens M, Dick JE. Reversible cell surface expression of CD38 on CD34-positive human hematopoietic repopulating cells. Exp. Hematol. 2007;35:1429–1436. doi: 10.1016/j.exphem.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur. J. Immunol. 2002;32:967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L, Moon RT, Bhatia M. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth MJ, Bodine DM. Regulation of hematopoiesis and the hematopoietic stem cell niche by Wnt signaling pathways. Cell Res. 2007;17:746–758. doi: 10.1038/cr.2007.69. [DOI] [PubMed] [Google Scholar]
- Nemeth MJ, Kirby MR, Bodine DM. Hmgb3 regulates the balance between hematopoietic stem cell self-renewal and differentiation. Proc. Natl. Acad. Sci. USA. 2006;103:13783–13788. doi: 10.1073/pnas.0604006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc. Natl. Acad. Sci. USA. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Petropoulos K, Arseni N, Schessl C, Stadler CR, Rawat VP, Deshpande AJ, Heilmeier B, Hiddemann W, Quintanilla-Martinez L, Bohlander SK, et al. A novel role for Lef-1, a central transcription mediator of Wnt signaling, in leukemogenesis. J. Exp. Med. 2008;205:515–522. doi: 10.1084/jem.20071875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD., Jr. The role of Dickkopf-1 in bone development, homeostasis and disease. Blood. 2008 doi: 10.1182/blood-2008-03-145169. in press. Published online October 3, 2008. 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Chen L, Fernald AA, Williams BO, Le Beau MM. A critical role for Apc in hematopoietic stem and progenitor cell survival. J. Exp. Med. 2008;205:2163–2175. doi: 10.1084/jem.20080578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke WW, Birchmeier W. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J. Cell Biol. 1996;135:215–225. doi: 10.1083/jcb.135.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat. Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X. SnapShot: noncanonical Wnt signaling pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–2316. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur. J. Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Evans BG, Yao J, Cooper S, Cornetta K, Ballas CB, Hangoc G, Broxmeyer HE. Enhanced green fluorescent protein is a nearly ideal long-term expression tracer for hematopoietic stem cells, whereas DsRed-express fluorescent protein is not. Stem Cells. 2007;25:670–678. doi: 10.1634/stemcells.2006-0553. [DOI] [PubMed] [Google Scholar]
- Templin C, Kotlarz D, Rathinam C, Rudolph C, Schatzlein S, Ramireddy K, Rudolph KL, Schlegelberger B, Klein C, Drexler H. Establishment of immortalized multipotent hematopoietic progenitor cell lines by retroviral-mediated gene transfer of beta-catenin. Exp. Hematol. 2008;36:204–215. doi: 10.1016/j.exphem.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent b-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat. Med. 2006;12:89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulyanova T, Priestley GV, Nakamoto B, Jiang Y, Papayannopoulou T. VCAM-1 ablation in nonhematopoietic cells in MxCre+ VCAM-1f/f mice is variable and dictates their phenotype. Exp. Hematol. 2007;35:565–571. doi: 10.1016/j.exphem.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- Weerkamp F, Baert MR, Naber BA, Koster EE, de Haas EF, Atkuri KR, van Dongen JJ, Herzenberg LA, Staal FJ. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc. Natl. Acad. Sci. USA. 2006;103:3322–3326. doi: 10.1073/pnas.0511299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, III, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C, Essers MA, MacDonald HR, Trumpp A. Dormant and self-renewing hematopoietic stem cells and their niches. Ann. N Y Acad. Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and Repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Yamane T, Kunisada T, Tsukamoto H, Yamazaki H, Niwa H, Takada S, Hayashi SI. Wnt signaling regulates hemopoiesis through stromal cells. J. Immunol. 2001;167:765–772. doi: 10.4049/jimmunol.167.2.765. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Oritani K, Garrett KP, Kouro T, Nishida M, Takahashi I, Ichii M, Satoh Y, Kincade PW, Kanakura Y. Soluble frizzled-related protein 1 is estrogen inducible in bone marrow stromal cells and suppresses the earliest events in lymphopoiesis. J. Immunol. 2008 doi: 10.4049/jimmunol.181.9.6061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Quinn WJ, III, Salay T, Crowley JE, Cancro MP, Sen JM. Role of beta-catenin in B cell development and function. J. Immunol. 2008;181:3777–3783. doi: 10.4049/jimmunol.181.6.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li L. Stem cell niche: microenvironment and beyond. J. Biol. Chem. 2008;283:9499–9503. doi: 10.1074/jbc.R700043200. [DOI] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Desilva T, Lin L, Bodine P, Bhat RA, Presman E, Pocas J, Stahl M, Kriz R. Regulation of secreted Frizzled-related protein-1 by heparin. J. Biol. Chem. 2007;282:20523–20533. doi: 10.1074/jbc.M609096200. [DOI] [PubMed] [Google Scholar]