Abstract
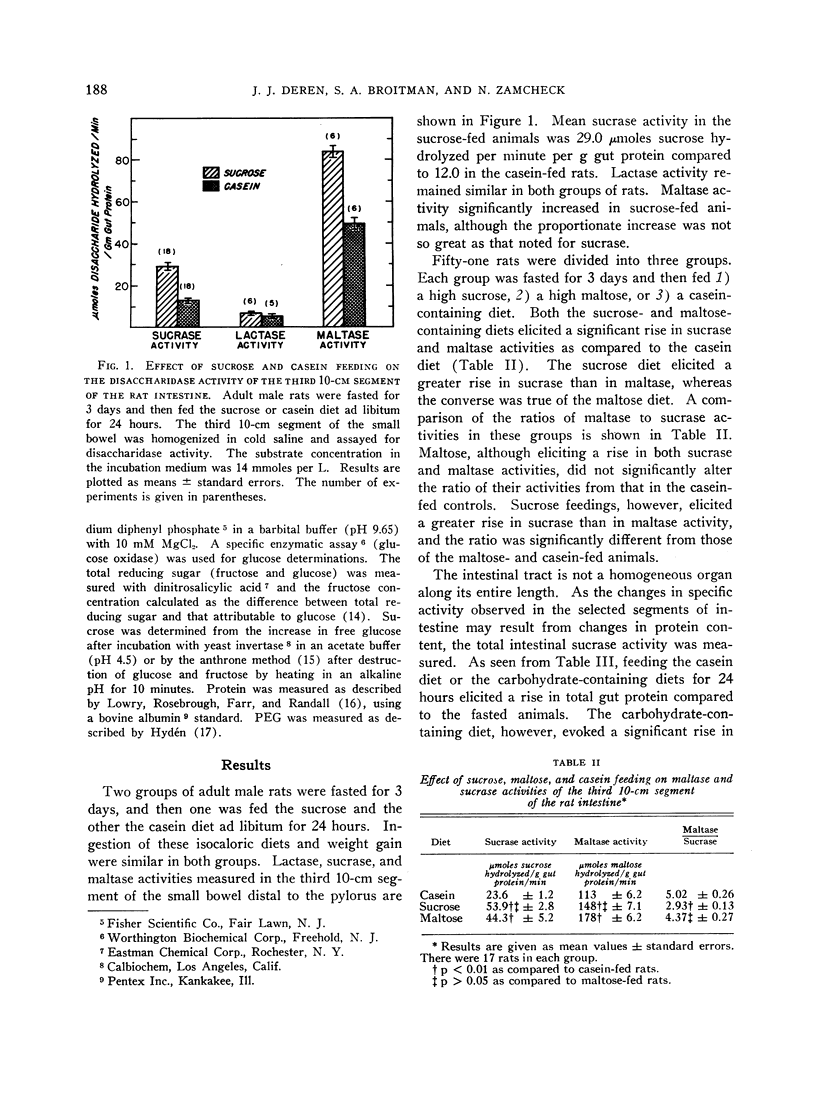
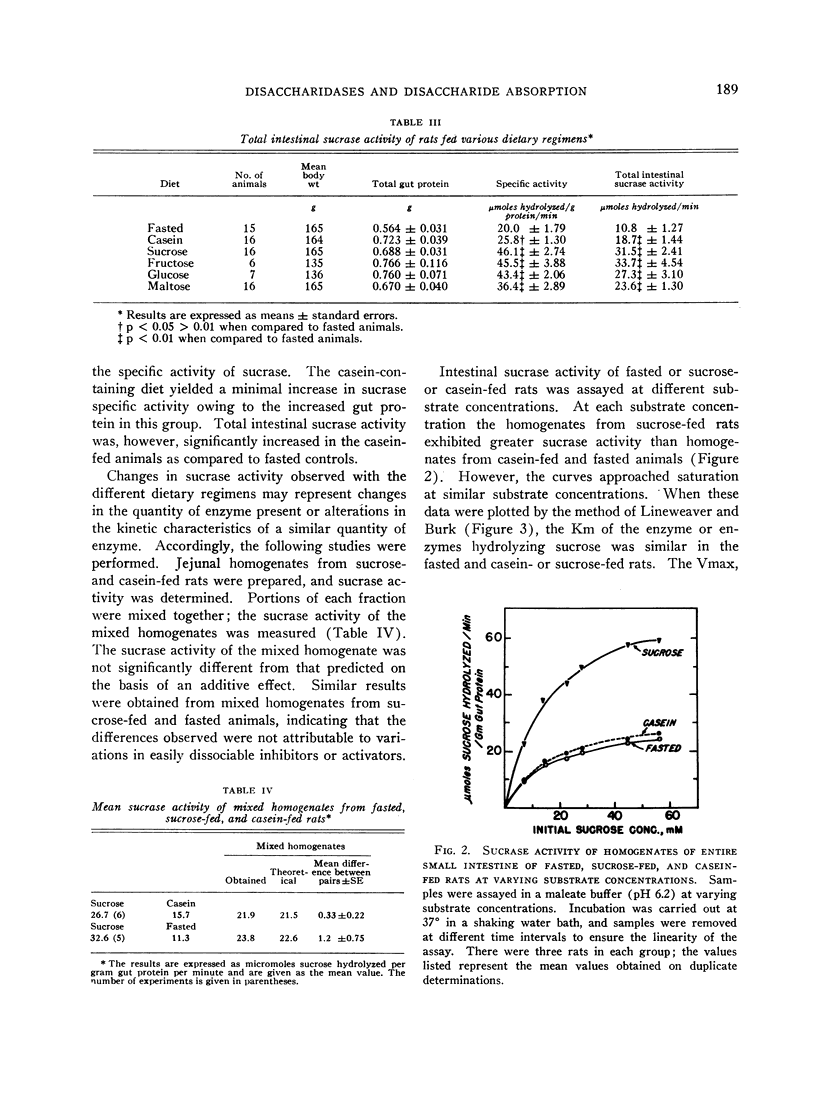
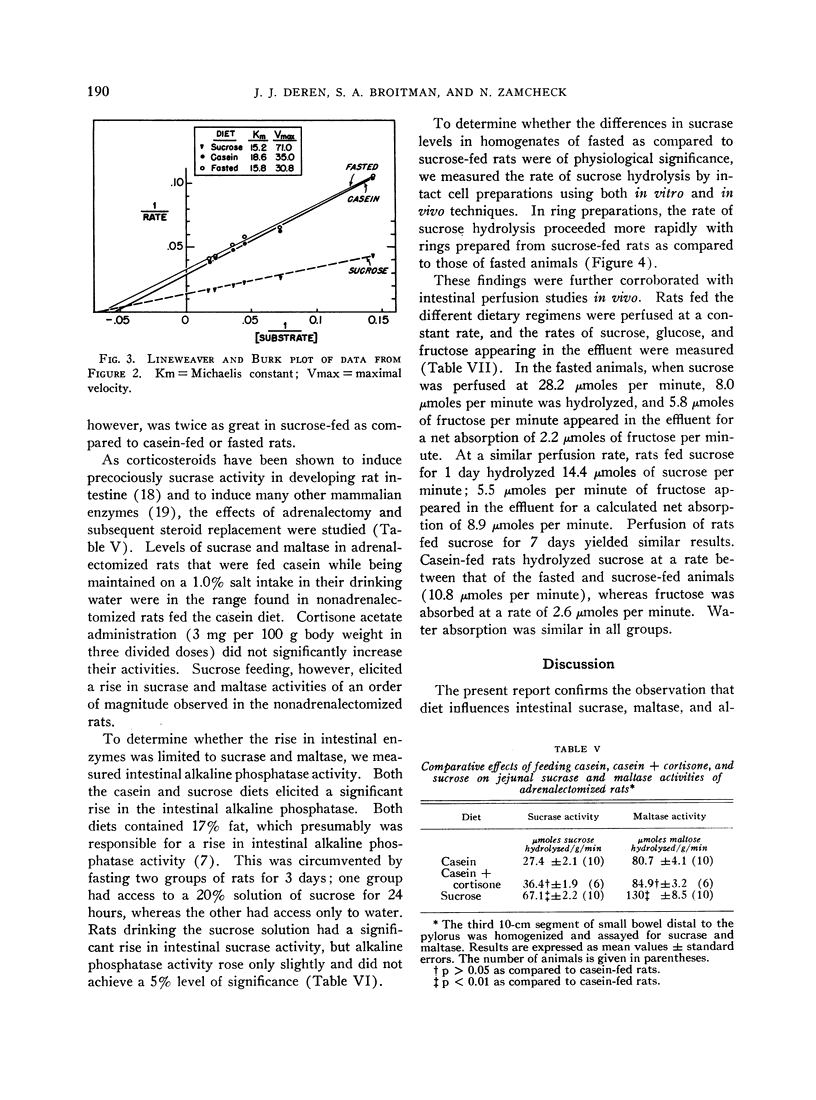
The administration of a carbohydrate-containing diet for 24 hours to rats previously fasted for 3 days led to a twofold increase in total intestinal sucrase and sucrase specific activity. The specific activity of maltase was similarly increased, but lactase activity was unaffected. The sucrose-containing diet led to a greater increase in sucrase than maltase activity, whereas the converse was true of the maltose-containing diet. A carbohydrate-free isocaloric diet led to a slight increase in the total intestinal sucrase, but sucrase specific activity was unchanged. Assay of sucrase activity of mixed homogenates from casein-fed and sucrose-fed rats or fasted and sucrose-fed animals yielded activities that were additive. The Michaelis constant (Km) of the enzyme hydrolyzing sucrose was similar in the fasted, casein-fed, and sucrose-fed rats. The maximal velocity (Vmax) was twice greater in sucrose-fed as compared to casein-fed or fasted rats, suggesting an increased quantity of enzyme subsequent to sucrose feeding.
Adrenalectomized rats maintained on 1.0% salt intake had sucrase and maltase levels comparable to those of controls. Steroid administration did not significantly increase their activities. The response to sucrose feeding was similar in both control and adrenalectomized rats, indicative of the absence of steroidal control on sucrase and maltase activity in the adult animal.
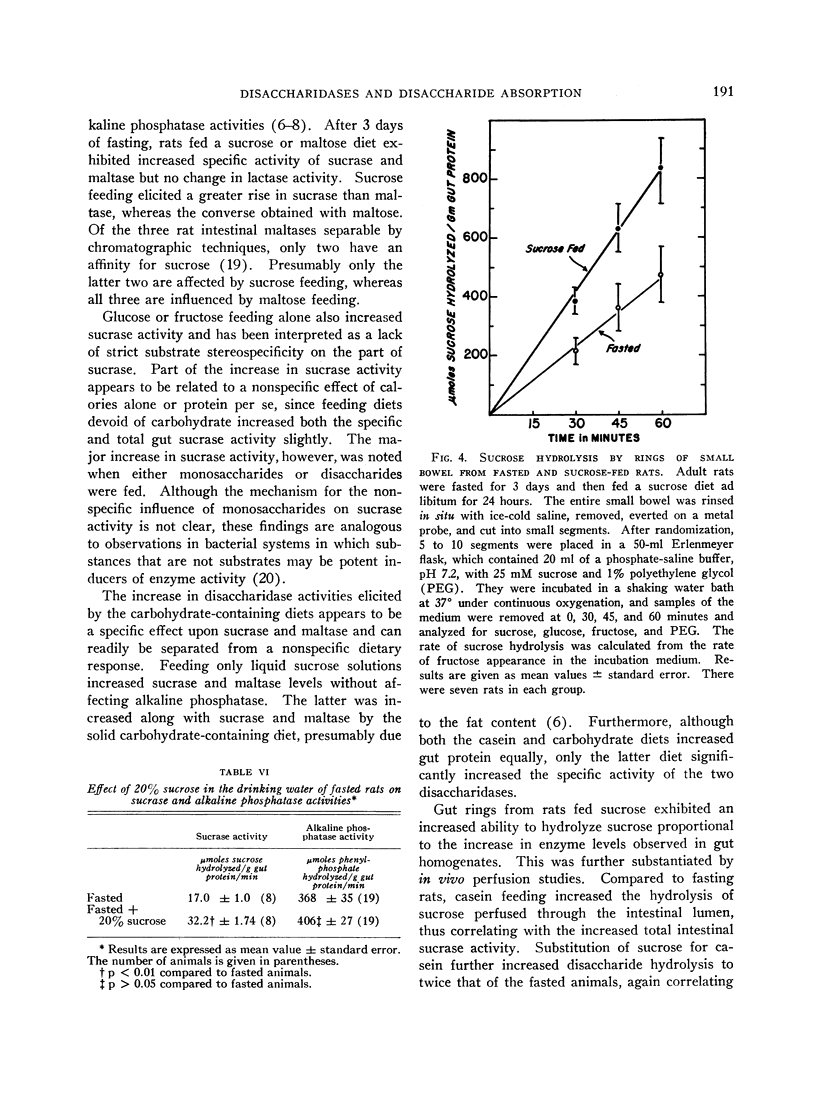
Studies using intestinal ring preparations indicated that sucrose hydrolysis by the intact cells proceeded more rapidly when animals were fed sucrose. Additional corroboration of the physiologic significance of the increased enzyme levels in homogenates was afforded by intestinal perfusion studies. Sucrose hydrolysis increased twofold and fructose absorption fourfold in animals fed sucrose when compared to either fasted or casein-fed rats.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGAR W. T., HIRD F. J., SIDHU G. S. The uptake of amino acids by the intestine. Biochim Biophys Acta. 1954 May;14(1):80–84. doi: 10.1016/0006-3002(54)90134-1. [DOI] [PubMed] [Google Scholar]
- BLAIR D. G., YAKIMETS W., TUBA J. Rat intestinal sucrase. II. The effects of rat age and sex and of diet on sucrase activity. Can J Biochem Physiol. 1963 Apr;41:917–929. [PubMed] [Google Scholar]
- CUATRECASAS P., LOCKWOOD D. H., CALDWELL J. R. LACTASE DEFICIENCY IN THE ADULT. A COMMON OCCURRENCE. Lancet. 1965 Jan 2;1(7375):14–18. doi: 10.1016/s0140-6736(65)90922-0. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. Rat-intestinal dextranase. Localization and relation to the other carbohydrases of the digestive tract. Biochem J. 1963 Jan;86:72–76. doi: 10.1042/bj0860072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. Specificity of the human intestinal disaccharidases and implications for hereditary disaccharide intolerance. J Clin Invest. 1962 Mar;41:463–470. doi: 10.1172/JCI104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A., THOMSON D. L. THE HYDROLYSIS OF SUCROSE BY INTACT AND HOMOGENIZED CELLS OF RAT SMALL INTESTINE. INFLUENCE OF PH AND SUBSTRATE CONCENTRATION. Biochim Biophys Acta. 1964 Oct 23;92:99–104. doi: 10.1016/0926-6569(64)90273-1. [DOI] [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. INTESTINAL INVERTASE: PRECOCIOUS DEVELOPMENT OF ACTIVITY AFTER INJECTION OF HYDROCORTISONE. Science. 1964 Jan 3;143(3601):42–44. doi: 10.1126/science.143.3601.42. [DOI] [PubMed] [Google Scholar]
- DURAND P. Lattosuria idiopatica in una paziente con diarrea cronica ed acidosi. Minerva Pediatr. 1958 Jul 14;10(27-28):706–711. [PubMed] [Google Scholar]
- FISCHER J. E. Effects of feeding a diet containing lactose upon beta-D-galactosidase activity and organ development in the rat digestive tract. Am J Physiol. 1957 Jan;188(1):49–53. doi: 10.1152/ajplegacy.1956.188.1.49. [DOI] [PubMed] [Google Scholar]
- GIRARDET P., RICHTERICH R., ANTENER I. ADAPTATION DE LA LACTASE INTESTINALE 'A L'ADMINISTRATION DE LACTOSE CHEZ LE RAT ADULTE. Helv Physiol Pharmacol Acta. 1964;22:7–14. [PubMed] [Google Scholar]
- GREENGARD O., FEIGELSON P. The activation and induction of rat liver tryptophan pyrrolase in vivo by its substrate. J Biol Chem. 1961 Jan;236:158–161. [PubMed] [Google Scholar]
- GREENGARD O., SMITH M. A., ACS G. Relation of cortisone and synthesis of ribonucleic acid to induced and developmental enzyme formation. J Biol Chem. 1963 Apr;238:1548–1551. [PubMed] [Google Scholar]
- HOLZEL A., SCHWARZ V., SUTCLIFFE K. W. Defective lactose absorption causing malnutrition in infancy. Lancet. 1959 May 30;1(7083):1126–1128. doi: 10.1016/s0140-6736(59)90710-x. [DOI] [PubMed] [Google Scholar]
- HOOPER C. S., BLAIR M. The effect of starvation on epithelial renewal in the rat duodenum. Exp Cell Res. 1958 Feb;14(1):175–181. doi: 10.1016/0014-4827(58)90224-6. [DOI] [PubMed] [Google Scholar]
- JACOBSON E. D., BONDY D. C., BROITMAN S. A., FORDTRAN J. S. Validity of polyethylene glycol in estimating intestinal water volume. Gastroenterology. 1963 Jun;44:761–767. [PubMed] [Google Scholar]
- Jirsová V., Heringová A. Effect of aldosterone and corticosterone on beta-galactosidase and invertase activity in the small intestine of rats. Nature. 1965 Apr 17;206(981):300–301. doi: 10.1038/206300a0. [DOI] [PubMed] [Google Scholar]
- KENNEY F. T. Induction of tyrosine-alpha-ketoglutarate transaminase in rat liver. III. Immunochemical analysis. J Biol Chem. 1962 May;237:1610–1614. [PubMed] [Google Scholar]
- LEVIN R. J., NEWEY H., SMYTH D. H. THE EFFECTS OF ADRENALECTOMY AND FASTING ON INTESTINAL FUNCTION IN THE RAT. J Physiol. 1965 Mar;177:58–73. doi: 10.1113/jphysiol.1965.sp007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. The digestive function of the epithelium of the small intestine. I. An intracellular locus of disaccharide and sugar phosphate ester hydrolysis. Biochim Biophys Acta. 1961 Sep 16;52:281–293. doi: 10.1016/0006-3002(61)90677-1. [DOI] [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. The digestive function of the epithelium of the small intestine. II. Localization of disaccharide hydrolysis in the isolated brush border portion of intestinal epithelial cells. Biochim Biophys Acta. 1961 Sep 16;52:293–298. doi: 10.1016/0006-3002(61)90678-3. [DOI] [PubMed] [Google Scholar]
- PLOTKIN G. R., ISSELBACHER K. J. SECONDARY DISACCHARIDASE DEFICIENCY IN ADULT CELIAC DISEASE (NONTROPICAL SPRUE) AND OTHER MALABSORPTION STATES. N Engl J Med. 1964 Nov 12;271:1033–1037. doi: 10.1056/NEJM196411122712003. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J., BRODIE B. B., HOGBEN C. A. Absorption of drugs from the rat small intestine. J Pharmacol Exp Ther. 1958 May;123(1):81–88. [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. An analysis of the kinetics of rat liver tryptophan pyrrolase induction: the significance of both enzyme synthesis and degradation. Biochem Biophys Res Commun. 1964 Mar 26;15(3):214–219. doi: 10.1016/0006-291x(64)90148-2. [DOI] [PubMed] [Google Scholar]
- TUBA J., DICKIE N. The role of alkaline phosphatase in intestinal absorption. II. The effects of various carbohydrates on levels of the enzyme in intestinal mucosa. Can J Biochem Physiol. 1954 Nov;32(6):621–624. [PubMed] [Google Scholar]
- TUBA J., ROBINSON M. I. The response of intestinal alkaline phosphatase of fasted rats to forced feeding of fat. J Biol Chem. 1953 Aug;203(2):947–951. [PubMed] [Google Scholar]


