Abstract
Basic and clinical research provide evidence that inflammatory mechanisms play a central role in the pathogenesis and progression of atherosclerosis, plaque rupture, thrombosis, and stroke. Inflammatory biomarkers such as high-sensitivity C-reactive protein (hsCRP) have been identified as predictors of first stroke and prognosis after stroke. The value of hsCRP and other markers may depend on the characteristics of the study population; their utility may be less among populations with high vascular risk. A recent randomized clinical trial suggests that the use of rosuvastatin therapy in otherwise healthy patients with hsCRP > 2 mg/dl can reduce the risk of a first stroke by 50%. The prognostic role of hsCRP among patients after a stroke, however, is less clear, and other biomarkers, including lipoprotein-associated phospholipase A2, may provide complementary information about risk of stroke recurrence. Infections, moreover, may contribute to inflammation and stroke risk. While no single infectious organism is likely to be identified as the direct cause of atherosclerosis, summary measures of multiple chronic infectious exposures, or “infectious burden,” have been associated with risk of stroke and atherosclerosis affecting carotid arteries. Acute infections have also been found to serve as stroke triggers in epidemiological studies. Recommendations to vaccinate patients with cardiovascular disease against influenza represent the first specific anti-infective strategy to be employed in vascular prophylaxis. Further studies are needed to determine the role of treatment of inflammation and infection in stroke prevention.
Keywords: atherosclerosis, inflammation, infection, infectious burden, statins, stroke, cerebral thrombosis, risk factors
Introduction
Basic and clinical research provide evidence that inflammatory mechanisms play a central role in the pathogenesis and progression of atherosclerosis, plaque rupture, thrombosis, and stroke. Inflammatory biomarkers such as high-sensitivity C-reactive protein (hsCRP) have been identified as predictors of stroke and prognosis after stroke. Infections, moreover, may contribute to inflammation and stroke risk. While no single infectious organism is likely to be identified as the direct cause of atherosclerosis, summary measures of multiple chronic infectious exposures, or “infectious burden,” have been associated with risk of stroke and atherosclerosis affecting carotid arteries. Acute infections have also been found to serve as stroke triggers. This article, based on a presentation given at the 2010 Princeton conference, focuses on recent epidemiologic and clinical studies evaluating the hypotheses that inflammatory biomarkers and infections are associated with risk of stroke, with an emphasis on the author’s own investigations.
Inflammatory biomarkers in primary prevention
Epidemiological studies of hsCRP and stroke risk
Acute phase proteins have been extensively studied as markers of coronary artery disease (CAD), and to a lesser extent, stroke. HsCRP, in particular, has many features that recommend it as a molecular marker of the risk of stroke associated with inflammation. Disadvantages to the assay are that it very non-specific and so acute increases in levels of hsCRP may occur in the setting of acute infection or other illness.1
HsCRP predicts incident cardiovascular events in several generally healthy populations.2,3,4 In multivariate models, hsCRP may improve the predictive ability of models over those containing lipid values and other risk factors alone (p<0.001).5,6,7
The relationship of hsCRP to incident stroke risk probably depends on the study design and population studied. In the Cardiovascular Health Study, among the elderly, hsCRP predicted incident ischemic stroke,8 though the effect was modest. In a study among elderly European subjects, hsCRP was associated with an increased risk of fatal stroke, but also with a risk of death from all causes.9 A large individual person meta-analysis of 54 prospective cohort studies (n=160,309) was recently performed.10 The risk ratio of ischemic stroke per a one standard deviation (SD) increase in the logeCRP was 1.44 (95% CI 1.32–1.57) when adjusted for age and sex, but was attenuated to 1.27 (95% CI 1.15–1.40) when further adjusted for other risk factors. Of note, however, non-vascular mortality, including cancer, was also increased significantly, and by a greater magnitude, in these analyses (adjusted RR 1.54, 95% CI 1.40–1.68). These results suggest that elevated CRP may be a marker of general illness rather than a specific marker of vascular disease risk.
Other studies do not confirm a consistent independent relationship of hsCRP to stroke risk. In the Framingham study, during >10 years of follow-up, men in the highest quartile of CRP had twice the risk of stroke of those in the lowest and women had three times the risk.11 For men, however, there was no increased risk after adjusting for confounders. Among healthy Japanese-American men in the Honolulu Heart Program, investigators found an almost fourfold increase in stroke risk among those in the highest compared to the lowest quartile of hsCRP.12 The associations were strongest among those ≤55 years, and those without history of hypertension or diabetes.
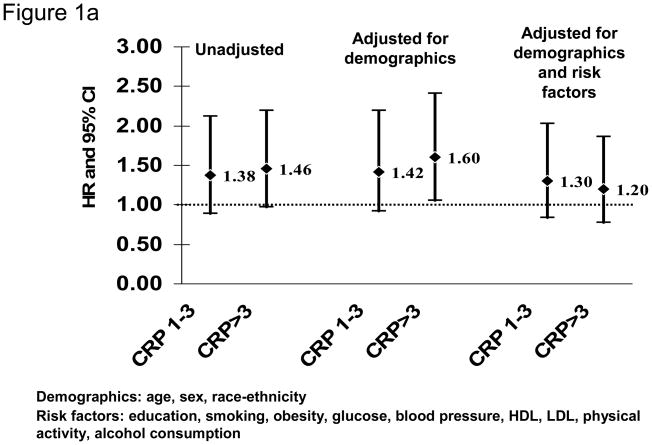
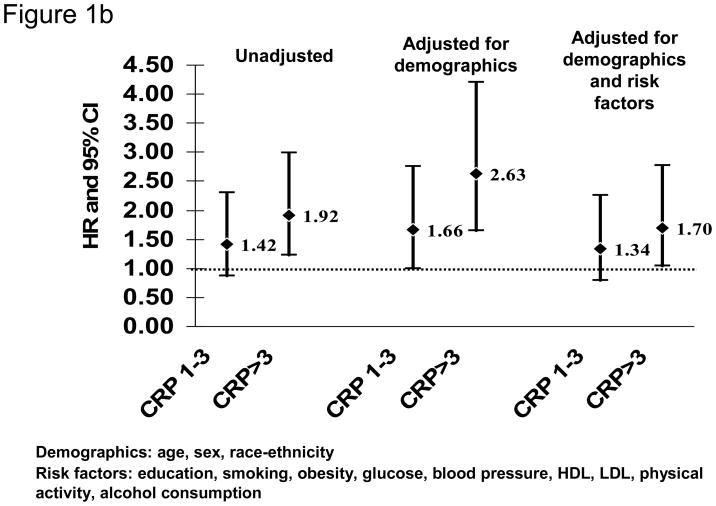
Our recent analysis of data from the Northern Manhattan Study (NOMAS) similarly did not confirm the ability of hsCRP to predict first stroke (Figure 1).13 NOMAS represents a stroke-free, multi-ethnic, community-based cohort study in participants aged ≥40 years. HsCRP measurements were available in 2240 participants (mean age 68.9±10.1 years; 64.2% women; 18.8% white, 23.5% black, and 55.1% Hispanic). After a median follow-up of 7.9 years, compared to those with hsCRP<1 mg/L, those with hsCRP>3 mg/L were at increased risk of ischemic stroke in a model adjusted for demographics (HR 1.60, 95% CI 1.06 to 2.41), but the effect was attenuated after adjusting for other risk factors (adjusted HR 1.20, 95% CI 0.78 to 1.86; Figure 1A). Of note, hsCRP>3 mg/L was associated with risk of MI (adjusted HR 1.70, 95% CI 1.04 to 2.77; Figure 1B) and death (adjusted HR 1.55, 95% CI 1.23 to 1.96) in the cohort. Other studies have similarly failed to confirm an association of hsCRP levels with stroke risk in the elderly.14
Figure 1.
HsCRP levels and risk of ischemic stroke (A) and myocardial infarction (B) in the Northern Manhattan Study (N=2240; reference group hsCRP<1 mg/L)
The association between hsCRP and ischemic stroke is thus probably attenuated in certain older populations and those with more risk factors.15 The findings of an association between hsCRP and stroke risk may depend on both the degree to which other risk factors are included in analyses, as well as on the age and absolute risk of stroke in the population. Those studies in which predictive associations are found, for example, tend to include relatively young, healthy cohorts.
The relationship between hsCRP and measures of subclinical cerebrovascular disease, as assessed by brain MRI, is also uncertain. In NOMAS, an association between hsCRP and white matter hyperintensity volume was not found, though other inflammatory biomarkers were associated with white matter disease.16
Role of statins in preventing first stroke among patients with evidence of inflammation
The hypothesis that statin treatment among apparently healthy men and women with elevated hsCRP may be associated with reduction in risk of vascular events was recently tested in a large randomized clinical trial (JUPITER). Patients were eligible for enrollment if they had no evidence of cardiovascular disease, diabetes, or hyperlipidemia, but had hsCRP ≥2.0 mg/L. They were randomized to rosuvastatin or placebo. The study was stopped early because of evidence of benefit from rosuvastatin therapy, with a significant reduction in the incidence of major cardiovascular events, including stroke.17 There was a 48% relative reduction in risk of stroke (hazard ratio 0.52, 95% CI 0.34–0.79) among those on rosuvastatin.
There are several limitations to the study design, however, which have curbed enthusiasm regarding use of statins as an anti-inflammatory therapy to prevent vascular disease. First, the benefit was modest in absolute terms. Second, it was not tied to baseline levels of hsCRP, though there was some evidence that the greatest benefit was seen in those whose hsCRP was reduced below 2.0 mg/L. Third, it remains uncertain whether the mechanism through which statins appear to work is related to inflammation or to some other effect, with the effect on hsCRP a bystander or epiphenomenon. JUPITER provides only indirect evidence therefore that statins are of benefit among those with elevated hsCRP, as the results may simply reflect a general benefit of statin therapy among all patients, with the greatest magnitude seen among those at higher risk.
Inflammatory biomarkers in secondary stroke prevention
The utility of hsCRP measured after stroke as a predictor of long-term risk of recurrence is unsettled. In a secondary nested case-control analysis from a multicenter secondary stroke prevention trial, those in the highest tertile of hsCRP had a modest increase in risk of recurrent ischemic stroke (OR 1.39, 95% CI 1.05–1.85).18 Results were not, however, fully adjusted for all risk factors. In data from NOMAS, elevated levels of hsCRP (top quartile) were associated with a doubling of the risk of death over several years after first ischemic stroke, but were not associated with an increase in risk of recurrent stroke.19 Lipoprotein-associated phospholipase A2 (LpPLA2) was associated with risk of recurrent stroke and other vascular events. Because hsCRP was associated with stroke severity, however, while Lp-PLA2 was not, it seems likely that hsCRP serves as a measure of general illness and stroke severity, while Lp-PLA2 may be more specific to vascular inflammation. Both markers may therefore provide complementary information.
Timing of measurement of hsCRP and LpPLA2 may have important implications for the results of these studies. HsCRP levels increase acutely after stroke and remain elevated for 28 days or more, while levels of LpPLA2 decrease.20,21 These changes imply that measurements made soon after stroke and MI are not reflective of pre-stroke levels and may be less reliable for long-term risk stratification.
The decision to measure hsCRP or other markers in stroke patients may be based on CDC/AHA guidelines1 until further data are available. No data yet demonstrate the validity of such an approach, however, and no current guidelines recommend measurement of inflammatory markers in patients with stroke or even provide appropriate levels to determine absolute risk. Because of limitations in available data, members of the CRP Pooling Project concluded that there was not yet enough data to routinely recommend hsCRP testing for prognostication in stroke patients. 22 Ongoing studies are designed to test the prognostic utility of inflammatory measures after stroke (e.g., the Levels of Inflammatory Markers in the Treatment of Stroke study, or LIMITS).23
Chronic infection as a risk factor for atherosclerosis and stroke
Among potential pro-inflammatory causes of atherosclerosis and stroke, infection remains one of the most controversial. Infection could contribute to risk in at least two ways. First, infections could serve as a chronic risk factor, through effects on the vascular wall accumulating over many years, much like conventional risk factors such as hypertension. Alternatively, acute infections could contribute to short-term stroke risk (i.e., stroke trigger), a possibility considered in the next section.24
With regard to infection as chronic risk factor, individual organisms have been associated with atherosclerosis and stroke risk. Electron microscopy, immunocytochemistry, and polymerase chain reaction have demonstrated Chlamydia pneumoniae in diseased blood vessels, including cerebral and carotid arteries.25,26 Viable organisms have been cultured from carotid plaques.27,28 C. pneumoniae is found more commonly in atherosclerotic tissue (52%) than in non-atherosclerotic tissue (5%).29 Data from seroepidemiologic studies provide conflicting evidence of an association between C. pneumoniae and CAD.30 More recently, both case-control31,32,33,34 and prospective studies35 have found evidence for an association between serological evidence of C. pneumoniae and stroke risk, though other studies have not confirmed these findings.36,37,38
Viruses have also been associated with atherosclerosis.39 Herpes simplex virus (HSV) has been found in human early aortic atherosclerotic lesions.40 Cytomegalovirus (CMV) is a contributor to vasculopathy in heart transplant recipients,41 and CMV infection is also more common in CAD.42 Elevated CMV titers are associated with early carotid atherosclerotic changes (increased intima-media thickness), and later carotid stenosis.43 Prospective clinical studies, however, have not confirmed that CMV titers predict increased risk of clinical atherosclerotic events.44
Infectious burden
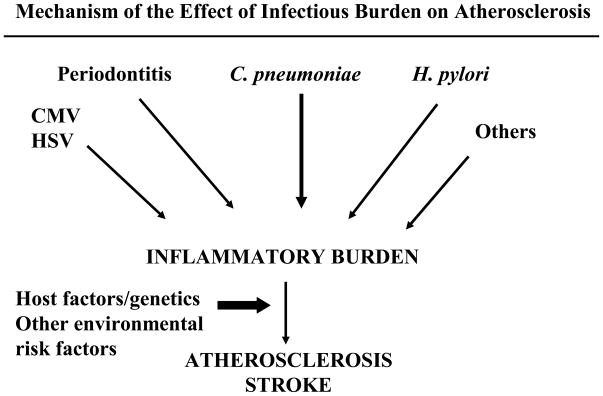
The variable results from these studies indicate that it is unlikely that a single “stroke germ” will be found. Instead, if infection plays a role at all, it is likely to be in a cumulative fashion. The concept of “infectious burden” (IB) has been used to explain the role that infections in aggregate may play in atherosclerosis. According to this hypothesis, infections contribute to the overall inflammatory milieu of atherosclerotic plaque, together with other risk factors, and individuals with the greatest exposure to different infections throughout life are most likely to develop atherosclerosis and stroke (Figure 2). It is likely also the case that those individuals with a more robust inflammatory response to these organisms, perhaps due to polymorphisms in infection-response genes, are more likely to show vascular changes related to infection.
Figure 2.
Mechanism of the Effect of Infectious Burden on Atherosclerosis and Stroke
Some45,46,47,48,49 but not all50,51 studies have begun to provide evidence of an association between different measures of IB and subclinical measures of atherosclerosis and vascular disease. For stroke specifically, in a case-control analysis,52 cough with phlegm during ≥3 months per year (chronic bronchitis) was associated with stroke or TIA independent of smoking history, other risk factors, and school education (OR 2.63, 95% CI 1.17 – 5.94). Frequent flu-like infections (>2/year) were also associated with stroke/TIA.
Limitations of these studies include post-hoc determinations of appropriate thresholds for IB, and use of simple scoring systems that attribute equal weight to each infection. To address the possibility that different infections are associated with different magnitudes of risk, we created in NOMAS a quantitative index of IB based on the individual association of each of 5 common pathogens with stroke risk.53 Serologies against C. pneumoniae, H. pylori, CMV, HSV1, and HSV2 were measured in 1625 participants followed for a median of 8 years. Each individual infection was positively though not significantly associated with stroke risk after adjusting for other risk factors (Table). Individual unadjusted parameter estimates were then added to generate a weighted IB index. The mean IB index was higher in non-Hispanic blacks and Hispanics compared to non-Hispanic whites (p<0.0001 for both comparisons). This IB index was associated with an increased risk of all strokes (adjusted HR per SD 1.39, 95% CI 1.02 – 1.90) after adjusting for risk factors (Table). Results were similar after adjusting for inflammatory biomarkers. The combined endpoint of all stroke, MI and deaths (adjusted HR per SD 1.15, 95% CI 1.03–1.29) was also associated with this IB index. This same IB index was also associated with carotid plaque thickness in NOMAS, with an increase of 0.09 mm (95% CI 0.03–0.15 mm) per SD increase of IB index, after adjusting for risk factors.54
Table.
Risk of Stroke Associated with Positive Serologies for C. pneumoniae, H. Pylori, CMV, HSV 1, and HSV 2
| Infectious exposure | Adjusted HR (95 % CI)* |
|---|---|
| Individual infections | |
| Chlamydia pneumonia IgA | 1.30 (0.75 – 2.25) |
| Helicobacter pylori IgG | 1.13 (0.68 – 1.89) |
| Cytomegalovirus IgG | 2.19 (0.84 – 5.70) |
| Herpes simplex virus 1 IgG | 1.35 (0.59 – 3.07) |
| Herpes simplex virus 2 IgG | 1.59 (0.91 – 2.76) |
| Infectious burden index (per standard deviation) | 1.39 (1.02 – 1.90) |
Adjusted for age, sex, race-ethnicity, high school education, systolic blood pressure, high density lipoprotein, low density lipoprotein, blood sugar, moderate alcohol use, cigarette smoking status, waist circumference, physical activity, and coronary artery disease.
These analyses provide evidence that more sophisticated measures of infectious burden may have a role in assessing the risk of vascular disease. They further support the notion that past exposure to common infections contributes to atherosclerosis, perhaps by exacerbating inflammation. Future studies are needed to validate these novel approaches to measuring infectious burden, define optimal measures of infectious burden as a vascular risk factor, and elucidate host factors, including genetics, that modify the infection-associated risk of vascular disease.
Acute infection as a stroke trigger
Case-control studies have also found recent (i.e., within 1 week) infection to be associated with acute stroke.55,56,57,58,59 Case-control studies, however, may be limited by inter-individual confounding. To limit such confounding, we recently undertook a case-crossover analysis among participants in the Cardiovascular Health Study by comparing hospitalization for infection during case periods (90, 30, or 14 days prior to stroke) and control periods (equivalent time periods exactly 1 or 2 years prior to stroke).60 During a median follow-up of 12.2 years, 669 incident ischemic strokes were observed in participants without baseline history of stroke. Hospitalization for infection was more likely during case than control time periods; for 90 days prior to stroke, OR=3.4 (95% CI 1.8–6.5). Risks were higher when examining shorter intervals: for 30 days, OR= 7.3 (95% CI 1.9–40.9), and 14 days, OR=8.0 (95% CI 1.7– 77.3). Survival analyses confirmed these findings.
Similarly, in a prospective analysis among 50,000 patients in the United Kingdom General Practice Research Database, both upper respiratory infections and urinary tract infections were associated with an increased risk of stroke.61 The risk of stroke in the three days after infection was approximately three times as high as during infection-free periods, and gradually diminished during the following three months. There is also evidence from observational studies that vaccination against common infections, particularly influenza, can prevent stroke. Influenza vaccination during the previous season is associated with just over a 50% reduction in risk of stroke.62 This protective effect was not present for vaccinations against other organisms, however. The mechanism of this benefit from flu vaccination is uncertain but may reflect reduced immune activation of atherosclerotic plaque or coagulation.63,64 Alternatively, vaccination could lead to a reduction in illness-associated dehydration and respiratory impairment. Whether other viruses can be similarly implicated in short-term stroke risk remains uncertain. In children, however, varicella infection appears to represent a period of increased stroke risk.65 Further studies, including in stroke patients, are warranted.
Infections as a treatment target
Recent guidelines recommend vaccination against influenza in patients with cardiovascular disease as a means to prevent cardiovascular events. 66,67 This represents the first anti-infective treatment to be championed as a vascular prevention strategy. There is data from a prospective uncontrolled study that suggests, however, that treatment of periodontal infection can lead to a reduction in endothelial dysfunction and intima-media thickness.68 Although pilot clinical trials provided some evidence that antibiotics, particularly macrolide antibiotics directed against chlamydiae, might reduce the risk of recurrent coronary events in patients with atherosclerosis,69 subsequent definitive randomized controlled trials, were unable to confirm these findings.70,71 Currently, therefore, there is no indication for antibiotics in patients with atherosclerotic disease. It should be noted, however, that these studies were largely confined to patients with coronary disease. Similar trials of antibiotics for patients with stroke have not been performed, and it is possible that the effects for stroke would differ.
The identification of a short-term state of elevated stroke risk after acute infection could have direct therapeutic implications, however, independent of use of antibiotics. For example, increased doses of antiplatelet agents or statins may be warranted during times of fever or infection, when benefits may outweigh risks of dose-related side effects. In addition, the period during and soon after hospitalization for infection could constitute a “treatable moment,” during which patients can be evaluated for cardiovascular risk and standard preventive strategies instituted.
References
- 1.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease. Application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Engl J Med. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-Reactive Protein and Low-Density Lipoprotein Cholesterol Levels in the Prediction of First Cardiovascular Events. New Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 8.Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, Polak JF, Sutton-Tyrrell K, Herrington DM, Price TR, Cushman M. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–70. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 9.Gussekloo J, Schaap MC, Frolich M, Blauw GJ, Westendorp RG. C-reactive protein is a strong but nonspecific risk factor of fatal stroke in elderly persons. Arterioscler Thromb Vasc Biol. 2000;20:1047–51. doi: 10.1161/01.atv.20.4.1047. [DOI] [PubMed] [Google Scholar]
- 10.The Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, D’Agostino RB, Franzblau C, Wilson PW. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack. Stroke. 2001;32:2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 12.Curb JD, Abbott RD, Rodriguez BL, Sakkinen P, Popper JS, Yano K, Tracy RP. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003;107:2016–20. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]
- 13.Elkind MS, Luna JM, Moon YP, Liu KM, Spitalnik S, Paik MC, Sacco RL. High-sensitivity C-reactive protein predicts mortality but not stroke: The Northern Manhattan Study. Neurology. 2009;73:1300–1307. doi: 10.1212/WNL.0b013e3181bd10bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory Markers and Onset of Cardiovascular Events Results From the Health ABC Study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 15.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-Terminal Pro-Brain Natriuretic Peptide, C-Reactive Protein, and Urinary Albumin Levels as Predictors of Mortality and Cardiovascular Events in Older Adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 16.Wright CB, Moon YP, Paik MC, Brown TR, Rabbani LR, Yoshita M, DeCarli C, Sacco RL, Elkind MSV. Inflammatory Biomarkers of Vascular Risk as Correlates of Leukoariosis. Stroke. 2009;40(11):3466–71. doi: 10.1161/STROKEAHA.109.559567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the Prevention of Stroke Among Men and Women With Elevated Levels of C-Reactive Protein: Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Circulation. 2010;121:143–150. doi: 10.1161/CIRCULATIONAHA.109.874834. [DOI] [PubMed] [Google Scholar]
- 18.Woodward M, Lowe GDO, Campbell DJ, Colman S, Rumley A, Chalmers J, Neal BC, Patel A, Jenkins AJ, Kemp BE, MacMahon SW. Associations of inflammatory and hemostatic variables with the risk of recurrent stroke. Stroke. 2005;36:2143–2147. doi: 10.1161/01.STR.0000181754.38408.4c. [DOI] [PubMed] [Google Scholar]
- 19.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. Lipoprotein-associated phospholipase A2, C-reactive protein, and outcome after ischemic stroke. Arch Int Med. 2006;166:2073–2080. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 20.Elkind MSV, Leon V, Moon YP, Paik MC, Sacco RL. High-sensitivity C-reactive protein and lipoprotein-associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke. 2009;40:3233–3237. doi: 10.1161/STROKEAHA.109.552802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkind MSV, Coates K, Tai W, Paik MC, Boden-Albala B, Sacco RL. Levels of acute phase proteins remain stable after ischemic stroke. BMC Neurology. 2006;6:37. doi: 10.1186/1471-2377-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, Emsley HC, Forconi S, Hopkins SJ, Masotti L, Muir KW, Paciucci A, Papa F, Roncacci S, Sander D, Sander K, Smith CJ, Stefanini A, Weber D. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–29. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 23.Elkind MSV, Luna JM, Coffey CS, McClure LA, Liu KM, Spitalnik S, Paik MC, Roldan A, White C, Hart R, Benavente O. The Levels of Inflammatory Markers in the Treatment of Stroke (LIMITS) Study: Inflammatory Biomarkers as Risk Predictors after Lacunar Stroke. Int J Stroke. 2010;5:117–25. doi: 10.1111/j.1747-4949.2010.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkind MSV. Why Now? Moving from Stroke Risk Factors to Stroke Triggers. Curr Opin Neurol. 2007;20:51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- 25.Virok D, Kis Z, Karai L, Intzedy L, Burian K, Szabo A, Ivanyi B, Gonczol E. Chlamydia pneumoniae in atherosclerotic middle cerebral artery. Stroke. 2001;32:1973–1976. doi: 10.1161/hs0901.094290. [DOI] [PubMed] [Google Scholar]
- 26.Vink A, Poppen M, Schoneveld AH, Roholl PJM, de Kleijn DPV, Borst C, Pasterkamp G. Distribution of Chlamydia pneumoniae in the human arterial system and its relation to the local amount of atherosclerosis within the individual. Circulation. 2001;103:1613–7. doi: 10.1161/01.cir.103.12.1613. [DOI] [PubMed] [Google Scholar]
- 27.Maass M, Bartels C, Engel PM, Mamat U, Sievers HH. Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. JACC. 1998;31:827–32. doi: 10.1016/s0735-1097(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 28.Jackson LA, Campbell LA, Kuo CC, Rodriguez DI, Lee A, Grayston JT. Isolation of Chlamydia pneumoniae from a carotid artery specimen. J Infect Dis. 1997;176(1):292–5. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 29.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–6. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 30.Kalayoglu MV, Libby P, Byrne GI. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA. 2002;288:2724–31. doi: 10.1001/jama.288.21.2724. [DOI] [PubMed] [Google Scholar]
- 31.Elkind MS, Lin I-F, Grayston TJ, Sacco RL. Chlamydia pneumoniae and the risk of first ischemic stroke: The Northern Manhattan Stroke Study. Stroke. 2000;31:1521–5. doi: 10.1161/01.str.31.7.1521. [DOI] [PubMed] [Google Scholar]
- 32.Elkind MS, Sciacca R, Tondella MLC, Feikin D, Fields B, DiTullio MR. Antibodies to Chlamydia pneumoniae are associated with risk of ischemic stroke. Neurology. 2003;60(Suppl 1):A256–7. [Google Scholar]
- 33.Madre JG, Garcia JL, Gonzalez RC, Montero JM, Paniagua EB, Escribano JR, Martinez JD, Cenjor RF. Association between seropositivity to Chlamydia pneumoniae and acute ischaemic stroke. Eur J Neurol. 2002;9:303–6. doi: 10.1046/j.1468-1331.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 34.Cook PJ, Honeybourne D, Lip GYH, Beevers DG, Wise R, Davies P. Chlamydia pneumoniae antibody titers are significantly associated with acute stroke and transient cerebral ischemia: The West Birmingham Stroke Project. Stroke. 1998;29:404–10. doi: 10.1161/01.str.29.2.404. [DOI] [PubMed] [Google Scholar]
- 35.Fagerberg B, Gnarpe J, Gnarpe H, Agewall S, Wikstrand J. Chlamydia pneumoniae but not cytomegalovirus antibodies are associated with future risk of stroke and cardiovascular disease. Stroke. 1999;30:299–305. doi: 10.1161/01.str.30.2.299. [DOI] [PubMed] [Google Scholar]
- 36.Glader CA, Stegmayr B, Boman J, Stenlund H, Weinehall L, Hallmans G, Dahlén GH. Chlamydia pneumoniae antibodies and high lipoprotein(a) levels do not predict ischemic cerebral infarctions. Stroke. 1999;30:2013–8. doi: 10.1161/01.str.30.10.2013. [DOI] [PubMed] [Google Scholar]
- 37.Tanne D, Haim M, Boyko V, Goldbourt U, Reshef T, Adler Y, Brunner D, Mekori YA, Behar S. Prospective study of Chlamydia pneumoniae IgG and IgA seropositivity and risk of incident ischemic stroke. Cerebrovasc Dis. 2003;16:166–70. doi: 10.1159/000070597. [DOI] [PubMed] [Google Scholar]
- 38.Heuschmann PU, Neureiter D, Gesslein M, Craiovan B, Maass M, Faller G, Beck G, Neundoerfer B, Kolominsky-Rabas PL. Association between infection with Helicobacter pylori and Chlamydia pneumoniae and risk of ischemic stroke subtypes: Results from a population-based case-control study. Stroke. 2001;32:2253–2258. doi: 10.1161/hs1001.097096. [DOI] [PubMed] [Google Scholar]
- 39.Fabricant CG, Fabricant J, Minick CR, Litrenta MM. Herpesvirus-induced atherosclerosis in chickens. Federation Proc. 1983;42:2476–9. [PubMed] [Google Scholar]
- 40.Benditt EP, Barrett T, McDougall JK. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci USA. 1983;80:6386–9. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ventura HO, Mehra MR, Smart FW, Stapleton DD. Cardiac allograft vasculopathy: current concepts. Am Heart J. 1995;129:791–8. doi: 10.1016/0002-8703(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 42.Melnick JL, Adam E, Debakey ME. Possible role of cytomegalovirus in atherogenesis. JAMA. 1990;263:2204–7. [PubMed] [Google Scholar]
- 43.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Rippin G, Hafner G, Pfeifer U, Meyer J. Are morphological or functional changes in the carotid artery wall associated with Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, or herpes simplex virus infection? Stroke. 2000;31:2127–2133. doi: 10.1161/01.str.31.9.2127. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Hennekens CH, Stampfer MJ, Wang F. Prospective study of herpes simplex virus, cytomegalovirus, and the risk of future myocardial infarction and stroke. Circulation. 1998;98:2796–9. doi: 10.1161/01.cir.98.25.2796. [DOI] [PubMed] [Google Scholar]
- 45.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Rippin G, Victor A, Hafner G, Schlumberger W, Meyer J. Impact of infectious burden on extent and long-term prognosis of atherosclerosis. Circulation. 2002;105:15–21. doi: 10.1161/hc0102.101362. [DOI] [PubMed] [Google Scholar]
- 46.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Victor A, Hafner G, Prellwitz W, Schlumberger W, Meyer J. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33:2581–6. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. doi: 10.1161/01.cir.103.1.45. [DOI] [PubMed] [Google Scholar]
- 49.Smieja M, Gnarpe J, Lonn E, Gnarpe H, Olsson G, Yi Q, Dzavik V, McQueen M, Yusuf S Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:251–7. doi: 10.1161/01.cir.0000044940.65226.1f. [DOI] [PubMed] [Google Scholar]
- 50.Dai DF, Lin JW, Kao JH, Hsu CN, Chiang FT, Lin JL, Chou YH, Hsu KL, Tseng CD, Tseng YZ, Hwang JJ. The effects of metabolic syndrome versus infectious burden on inflammation, severity of coronary atherosclerosis, and major adverse cardiovascular events. J Clin Endocrinol Metab. 2007;92:2532–7. doi: 10.1210/jc.2006-2428. [DOI] [PubMed] [Google Scholar]
- 51.Szklo M, Ding J, Tsai MY, Cushman M, Polak JF, Lima J, Barr RG, Sharrett AR. Individual pathogens, pathogen burden and markers of subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Med (Hagerstown) 2009;10:747–51. doi: 10.2459/JCM.0b013e32832cacab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grau AJ, Preusch MR, Palm F, Lichy C, Becher H, Buggle F. Association of symptoms of chronic bronchitis and frequent flu-like illnesses with stroke. Stroke. 2009;40:3206–3210. doi: 10.1161/STROKEAHA.109.561019. [DOI] [PubMed] [Google Scholar]
- 53.Elkind MSV, Ramakrishnan P, Moon YP, Boden-Albala B, Liu KM, Spitalnik SL, Rundek T, Sacco RL, Paik MC. Infectious Burden and Risk of Stroke: The Northern Manhattan Study. Arch Neurol. 2010;67:33–38. doi: 10.1001/archneurol.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elkind MSV, Luna JM, Moon YP, Boden-Albala B, Liu KM, Spitalnik S, Rundek T, Sacco RL, Paik MC. Infectious Burden and Carotid Plaque Thickness: The Northern Manhattan Study. Stroke. 2010;41:e117–e122. doi: 10.1161/STROKEAHA.109.571299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Syrjanen J, Valtonen VV, Iivanainen M, Kaste M, Huttunen JK. Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients. Br Med J. 1988;296:1156–60. doi: 10.1136/bmj.296.6630.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grau AJ, Buggle F, Heindl S, Steichen-Wiehn C, Banerjee T, Maiwald M, Rohlfs M, Suhr H, Fiehn W, Becher H. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. 1995;26:373–9. doi: 10.1161/01.str.26.3.373. [DOI] [PubMed] [Google Scholar]
- 57.Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke. 1996;27(12):2204–6. doi: 10.1161/01.str.27.12.2204. [DOI] [PubMed] [Google Scholar]
- 58.Grau AJ, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T, Maiwald M, Werle E, Zorn M, Hengel H, Hacke W. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia. Neurology. 1998;50:196–203. doi: 10.1212/wnl.50.1.196. [DOI] [PubMed] [Google Scholar]
- 59.Macko RF, Ameriso SF, Gruber A, Griffin JH, Fernandez JA, Barndt R, Quismorio FP, Jr, Weiner JM, Fisher M. Impairment of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 60.Elkind MS, Carty CL, OMeara ES, Lumley T, Lefkowitz D, Kronmal RA, Longstreth WT. Hospitalizations for Infections Trigger Acute Ischemic Stroke: The Cardiovascular Health Study. Stroke. 2010;41(4):e25. doi: 10.1161/STROKEAHA.110.608588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 62.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36:1501–6. doi: 10.1161/01.STR.0000170674.45136.80. [DOI] [PubMed] [Google Scholar]
- 63.Madjid M, Naghavi M, Litovsky S, Casscells SW. Influenza and cardiovascular disease: a new opportunity for prevention and the need for further studies. Circulation. 2003;108:2730–6. doi: 10.1161/01.CIR.0000102380.47012.92. [DOI] [PubMed] [Google Scholar]
- 64.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation. 2003;107:762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 65.Losurdo G, Giacchino R, Castagnola E, Gattorno M, Costabel S, Rossi A, Amato S, Di Pietro P, Molinari AC. Cerebrovascular disease and varicella in children. Brain Dev. 2006;28:366–370. doi: 10.1016/j.braindev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Advisory Committee on Immunization Practices. Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-10):1–42. [PubMed] [Google Scholar]
- 67.Davis MM, Taubert K, Benin AL, Brown DW, Mensah GA, Baddour LM, Dunbar S, Krumholz HM American Heart Association; American College of Cardiology; American Association of Cardiovascular and Pulmonary Rehabilitation; American Association of Critical Care Nurses; American Association of Heart Failure Nurses; American Diabetes Association; Association of Black Cardiologists, Inc; Heart Failure Society of America; Preventive Cardiovascular Nurses Association; American Academy of Nurse Practitioners; Centers for Disease Control and Prevention and the Advisory Committee on Immunization. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. Circulation. 2006;114:1549–53. doi: 10.1161/CIRCULATIONAHA.106.178242. [DOI] [PubMed] [Google Scholar]
- 68.Piconi S, Trabattoni D, Luraghi C, Perilli E, Borelli M, Pacei M, Rizzardini G, Lattuada A, Bray DH, Catalano M, Sparaco A, Clerici M. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. 2009;23:1196–204. doi: 10.1096/fj.08-119578. [DOI] [PubMed] [Google Scholar]
- 69.Grayston JT. Antibiotic treatment of atherosclerotic cardiovascular disease. Circulation. 2003;107:1228–1230. doi: 10.1161/01.cir.0000056032.56396.89. [DOI] [PubMed] [Google Scholar]
- 70.Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–54. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 71.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, Knirsch C ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–45. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]