Abstract
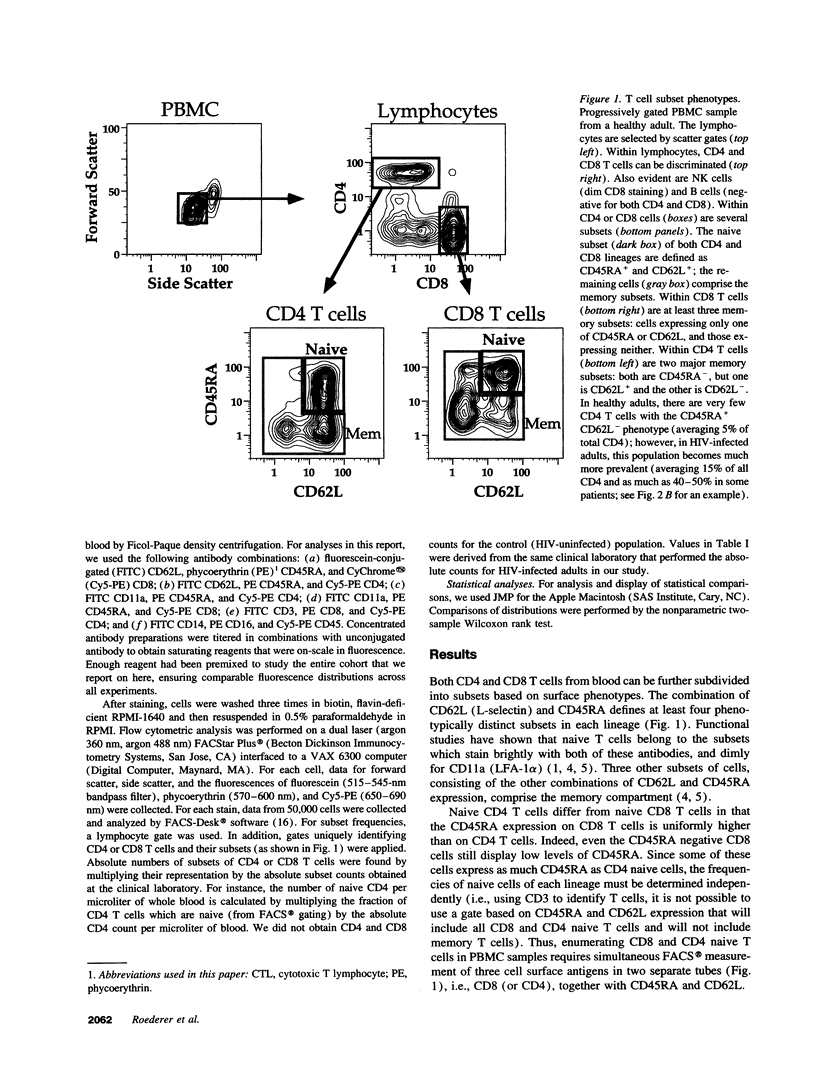
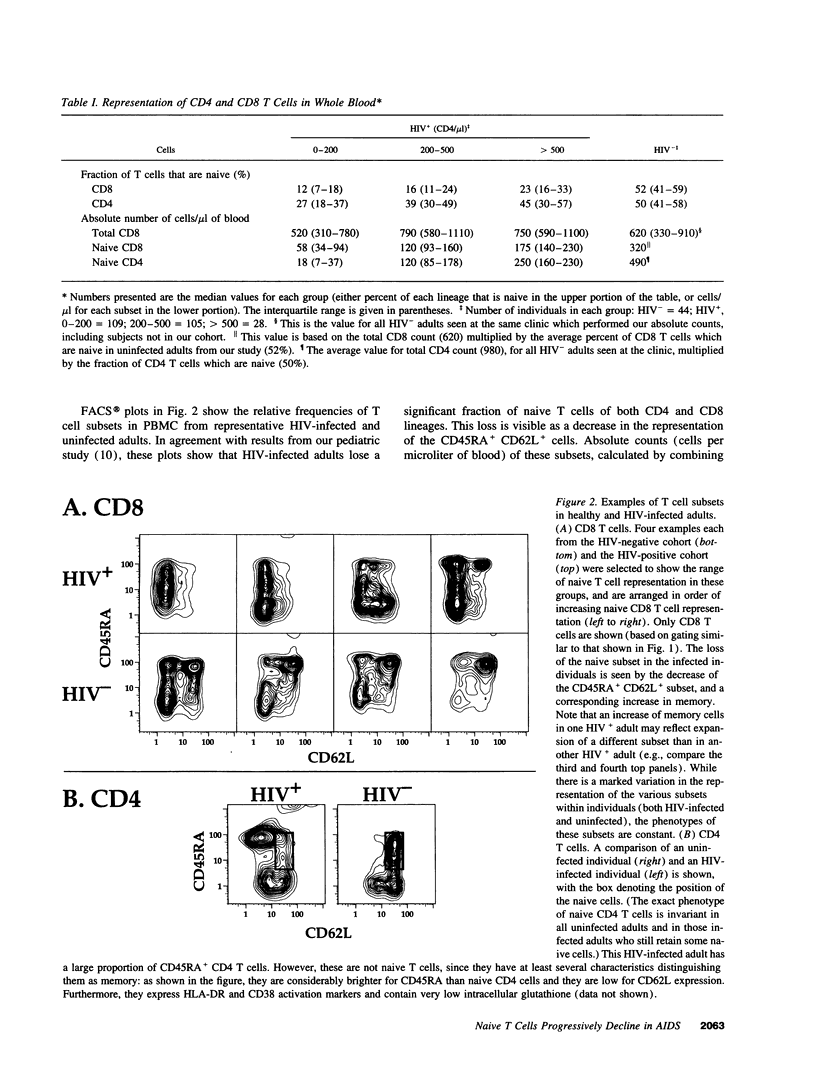
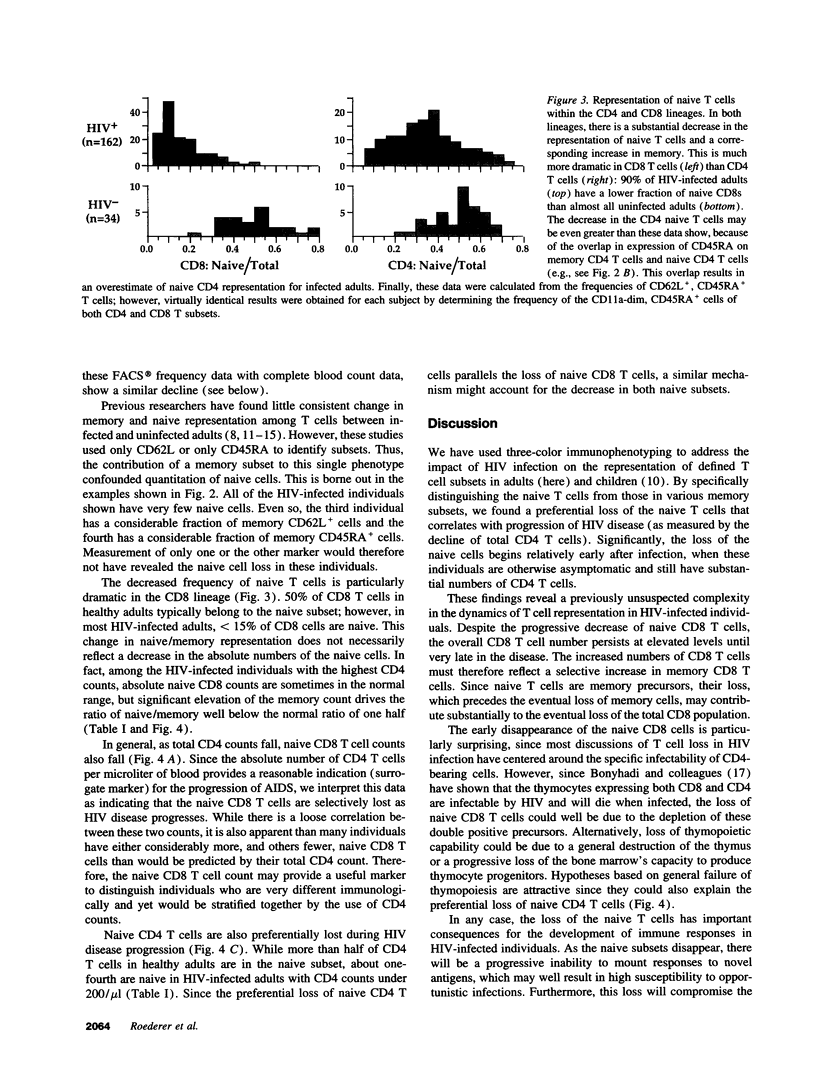
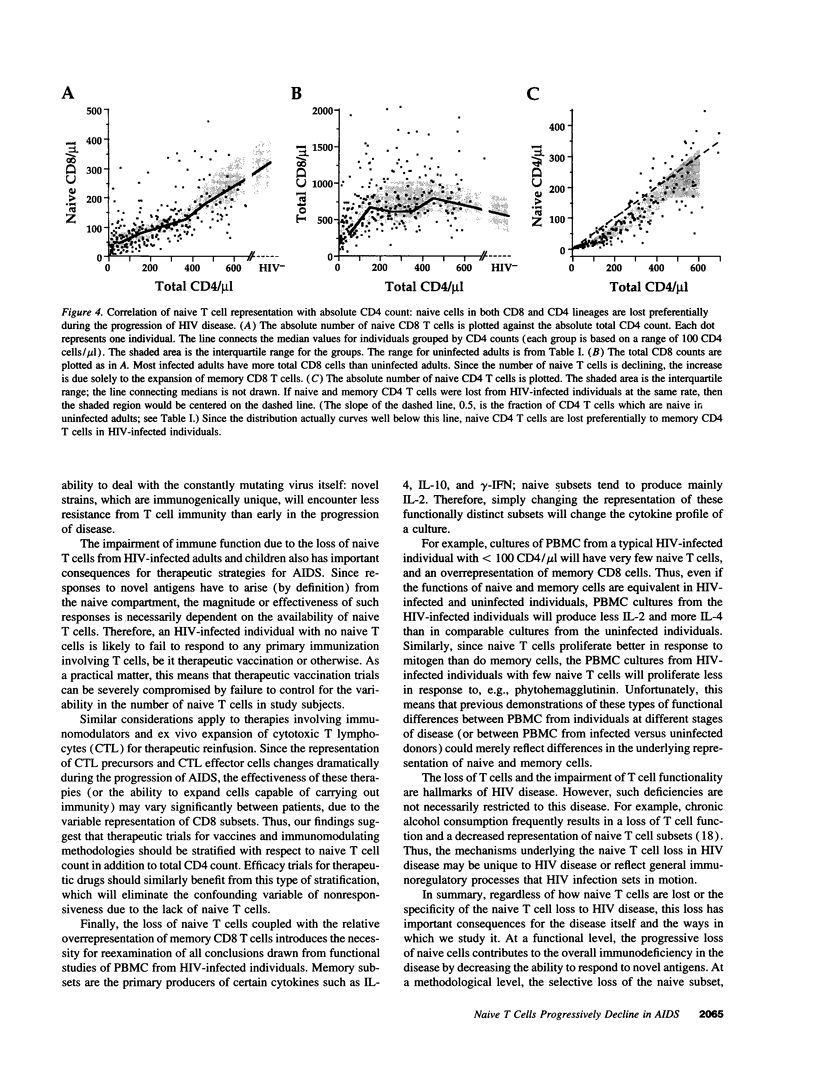
We show here that CD8 naive T cells are depleted during the asymptomatic stage of HIV infection. Although overall CD8 T cell numbers are increased during this stage, the naive CD8 T cells are progressively lost and fall in parallel with overall CD4 T cell counts. In addition, we show that naive CD4 T cells are preferentially lost as total CD4 cell counts fall. These findings, presented here for adults, and in the accompanying study for children, represent the first demonstration that HIV disease involves the loss of both CD4 T cells and CD8 T cells. Furthermore, they provide a new insight into the mechanisms underlying the immunodeficiency of HIV-infected individuals, since naive T cells are required for all new T cell-mediated immune responses. Studies presented here also show that the well-known increase in total CD8 counts in most HIV-infected individuals is primarily due to an expansion of memory cells. Thus, memory CD8 T cells comprise over 80% of the T cells in PBMC from individuals with < 200 CD4/microliter, whereas they comprise roughly 15% in uninfected individuals. Since the naive and memory subsets have very different functional activities, this altered naive/memory T cell representation has significant consequences for the interpretation of data from in vitro functional studies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonyhadi M. L., Rabin L., Salimi S., Brown D. A., Kosek J., McCune J. M., Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993 Jun 24;363(6431):728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- Borkowsky W., Moore T., Krasinski K., Ajuang-Simbiri K. O., Holzman R. Evolution of phenotypic memory T cells in HIV-1 infected infants and children. Clin Immunol Immunopathol. 1992 Jun;63(3):280–284. doi: 10.1016/0090-1229(92)90234-f. [DOI] [PubMed] [Google Scholar]
- Cook R. T., Waldschmidt T. J., Ballas Z. K., Cook B. L., Booth B. M., Stewart B. C., Garvey M. J. Fine T-cell subsets in alcoholics as determined by the expression of L-selectin, leukocyte common antigen, and beta-integrin. Alcohol Clin Exp Res. 1994 Feb;18(1):71–80. doi: 10.1111/j.1530-0277.1994.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Ernst D. N., Weigle W. O., Noonan D. J., McQuitty D. N., Hobbs M. V. The age-associated increase in IFN-gamma synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J Immunol. 1993 Jul 15;151(2):575–587. [PubMed] [Google Scholar]
- Froebel K. S., Doherty K. V., Whitelaw J. A., Hague R. A., Mok J. Y., Bird A. G. Increased expression of the CD45RO (memory) antigen on T cells in HIV-infected children. AIDS. 1991 Jan;5(1):97–99. doi: 10.1097/00002030-199101000-00015. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989 Jul;52(1):10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- Okumura M., Fujii Y., Takeuchi Y., Inada K., Nakahara K., Matsuda H. Age-related accumulation of LFA-1high cells in a CD8+CD45RAhigh T cell population. Eur J Immunol. 1993 May;23(5):1057–1063. doi: 10.1002/eji.1830230512. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Treer J. R., Ferguson-Darnell B., Collins P. A., Buck D., Terstappen L. W. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J Immunol. 1993 Feb 1;150(3):1105–1121. [PubMed] [Google Scholar]
- Prince H. E., Kleinman S., Czaplicki C., John J., Williams A. E. Interrelationships between serologic markers of immune activation and T lymphocyte subsets in HIV infection. J Acquir Immune Defic Syndr. 1990;3(5):525–530. [PubMed] [Google Scholar]
- Rabin R. L., Roederer M., Maldonado Y., Petru A., Herzenberg L. A., Herzenberg L. A. Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J Clin Invest. 1995 May;95(5):2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. M., Grieco M. H. Quantitative changes in T helper inducer (CD4+ CD45RA-), T suppressor inducer (CD4+ CD45RA+), T suppressor (CD8+ CD11b+), and T cytotoxic (CD8+ CD11b-) subsets in human immunodeficiency virus infection. J Clin Lab Anal. 1991;5(2):96–100. doi: 10.1002/jcla.1860050205. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Sohen S., Rothstein D. M., Tallman T., Gaudette D., Schlossman S. F., Morimoto C. The functional heterogeneity of CD8+ cells defined by anti-CD45RA (2H4) and anti-CD29 (4B4) antibodies. Cell Immunol. 1990 Jun;128(1):314–328. doi: 10.1016/0008-8749(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Swain S. L. Effector functions of helper T cells. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):150–154. doi: 10.1097/00002371-199308000-00011. [DOI] [PubMed] [Google Scholar]
- Teitel J. M., Freedman J. J., Garvey M. B., Kardish M. Two-year evaluation of clinical and laboratory variables of immune function in 117 hemophiliacs seropositive or seronegative for HIV-1. Am J Hematol. 1989 Dec;32(4):262–272. doi: 10.1002/ajh.2830320406. [DOI] [PubMed] [Google Scholar]
- de Jong R., Brouwer M., Miedema F., van Lier R. A. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation. J Immunol. 1991 Apr 1;146(7):2088–2094. [PubMed] [Google Scholar]