Abstract
Background
Patients who suffer severe burns are at higher risk for local and systemic infections. In recent years, emerging resistant pathogens have forced burn care providers world wide to search for alternative forms of treatment. Multidrug-resistant Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter spp., and various fungal strains have been the major contributors to the increase in morbidity and mortality rates. Multi-drug-resistant S. aureus remains the major cause of gram-positive burn wound infections world wide. Treatment strategies include rigorous isolation protocols and new types of antibiotics where necessary.
Methods
We reviewed 398 severely burned patients (burns >40% total body surface area [TBSA]) admitted to our hospital between 2000 and 2006. Patients who did not contract multi-drug-resistant gram-negative organisms during their hospital course and received our standard antibiotic regimen—vancomycin and piperacillin/tazobactam—served as controls (piperacillin/tazobactam; n = 280). The treatment group consisted of patients who, during their acute hospital stay, developed infections with multi-drug-resistant gram-negative pathogens and were treated with vancomycin and colistin for at least three days (colistin; n = 118).
Results
Gram-negative organisms continue to cause the most severe infections in burn patients. Colistin has re-emerged as a highly effective antibiotic against multiresistant Pseudomonas and Acinetobacter infections of burns. Patients who required colistin therapy had a significantly larger average total and full-thickness burn than patients treated with piperacillin/tazobactam and vancomycin, and the mortality rate was significantly higher in the colistin group (p < 0.05). However, there was no significant difference between the colistin and piperacillin/tazobactam groups in the incidence of neurotoxicity, hepatic toxicity, or nephrotoxicity. The main fungal pathogens in burn patients are Candida spp., Aspergillus spp., and Fusarium spp. A definitive diagnosis is more difficult to obtain than in bacterial infections. Amphotericin B and voriconazole remain the two most important anti-fungal substances in our practice.
Conclusions
Innovations in fluid management, ventilatory support, surgical care, and antimicrobial therapy have contributed to a significant reduction in morbidity and mortality rates in burn patients. Vancomycin and clindamycin are the two most important reserve antibiotics for methicillin-resistant Staphylococcus aureus infection. Oxazolidinones and streptogramins have showed high effectiveness against gram-positive infections. Colistin has re-emerged as a highly effective antibiotic against multiresistant Pseudomonas and Acinetobacter infections. Current challenges include Candida, Aspergillus, and molds. The development of new agents, prudent and appropriate use of antibiotics, and better infection control protocols are paramount in the ongoing battle against multi-resistant organisms.
When Doctor G. Tom Shires began his career as a surgeon at the Parkland Memorial Hospital in Dallas, Texas, in 1948, burn care and treatment had only recently emerged from its status as a neglected subspecialty of trauma, becoming under his leadership one of the most prominent fields of clinical and basic research in trauma. On April 16, 1947, two freighters loaded with ammonium nitrate fertilizer exploded at a dock in Texas City, 300 miles south of Dallas, killing 560 people and injuring more than 3,000 in what is still the deadliest industrial accident in American history [1]. Doctor Truman G. Blocker spearheaded the mobilization of the efforts to treat the burn injuries and, subsequently, created the first dedicated burn center in the United States at The University of Texas Medical Branch in Galveston. In the 1960s, when Doctor Shires became Chairman of the Surgery Department at the Southwestern Medical School in Dallas, major improvements were made in burn care; however, shock, sepsis, and multi-organ dysfunction caused a 50% mortality rate in burns exceeding one-half of the total body surface area (TBSA) [2], and the mortality rate attributable to bacterial sepsis in burns >50% TBSA reached 60–80% [1]. Over the next decades, Doctor Shires and his colleagues worked tirelessly to improve the surgical approach to burn wounds, fluid resuscitation, control of infection, support of the hypermetabolic response, nutritional support, treatment of inhalation injury, and rehabilitation [1,3,4]. One of his prominent achievements was the Parkland formula for fluid resuscitation after burn injury [5], today the most widely used schema, which recommends 4 mL of Ringer's lactate/kg/% TBSA in the first 24 h after a burn [5]. Doctor Shires also established burn centers at the University of Washington Harborview Medical Center in Seattle and The New York Hospital-Cornell Medical Center in New York City, where he was Chairman of Surgery from 1976 to 1991 (Fig. 1). Burn survival subsequently improved dramatically, and the size of burn that causes a 50% mortality rate has increased to 98% (Table 1).
FIG. 1.
G. Tom Shires (1926–2007) (with friendly permission from Medical Center Archives of New York-Presbyterian/Weill Cornell.
Table 1.
Percent Total Body Surface Area Burn Associated with an Expected Mortality Rate of 50% in 1952, 1993, and 2006
Bull JP, Fisher AJ. A study of mortality in a burns unit: A revised estimate. Ann Surg 1954;139:269–274.
Unpublished data; Shriners Hospital for Children and The University of Texas Medical Branch, Galveston, Texas.
Pereira CT, Barrow RE, Sterns AM, et al. Age-dependent differences in survival after severe burns: A unicentric review of 1,674 patients and 179 autopsies over 15 years. J Am Coll Surg 2006;202:536–548 and unpublished data. Adapted from Herndon DN, ed. Total Burn Care, 3rd edition. Philadelphia: WB Saunders, 2007.
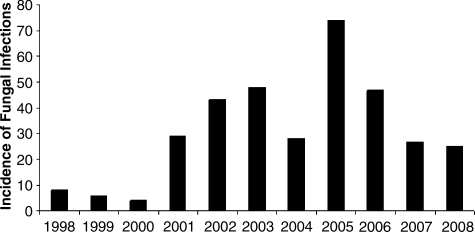
All progress notwithstanding, infection remains the main cause of death among burn patients [3,4]. The loss of the skin barrier and the immune deficiency associated with large burns make these patients especially susceptible to sepsis [6,7]. The Nosocomial Infection Surveillance System from the U.S. Centers for Disease Control and Prevention (CDC) demonstrated that burn intensive care units (ICUs) have the highest rates of primary blood stream infection in patients with central venous catheters among all ICUs [6,8]. Recently, emerging multi-drug-resistant strains of bacteria and fungi have caused an unexpected rise in burn wound infections, sepsis, and associated death worldwide [9–13]. At the Galveston Shriners Hospital, multiresistant Acinetobacter spp. and Fusarium spp. caused an epidemic in the burn unit in the early 2000s (Fig. 2), and multiresistant Pseudomonas spp. emerged more recently.
FIG. 2.
Incidence of burn wound infections at the Shriners Hospital for Children, Galveston Burn Intensive Care Unit, 1998–2008.
The purpose of this paper is to outline the major contributors to infection-related burn deaths—methicillin-resistant Staphylococcus aureus, Pseudomonas and Acinetobacter spp., and fungal infections—and to describe the development of multiresistant strains and the methods of treatment.
Methicillin-Resistant Staphylococcus aureus
Burn wound infections can be caused by bacteria, fungi, or viruses [9]. Historically, group A beta-hemolytic Streptococcus was the most frequent cause of life-threatening burn wound and systemic infections [14]. The use of penicillin altered the spectrum of gram-positive pathogens, leading to the emergence of S. aureus as the most common gram-positive early colonizer of the burn wound [8,15].
In burns, S. aureus has been a major cause of morbidity and death [16]. The disruption of the normal skin barrier and the immunocompromised state makes burns an easy target for colonization. Additionally, prolonged hospitalization and antibiotic therapy are risk factors for the development of methicillin-resistant S. aureus (MRSA) colonization and infection [16,17]. Staphylococcus aureus penetrates the eschar and invades the unburned underlying subcutaneous tissues to form abscesses with thick walls that obstruct host defenses and antibiotic therapy, leading to hematogenous dissemination of the infection [8]. Staphylococci also are responsible for graft loss when the colony count of the graft bed exceeds 105 colony-forming units (cfu)/g of tissue [18].
Burn centers around the world have studied the emerging incidence and prevalence of multiresistant S. aureus infections. De Macedo and Santos described S. aureus as the most prevalent infecting organism in Brazil in the first week after injury [15]. More recently, a 20-year review of the changes in bacterial isolates from burn wounds and their antibiograms in a single center in Europe showed that S. aureus remains the most frequent isolate [19]. These findings have been consistent worldwide [8,12,20]. Staphylococcus aureus also has been reported as the most common organism isolated from blood culture in burned patients with sepsis [21]. A study of the bacteriological profile and antibiotic resistance in a burn unit in France established S. aureus as the most frequent species, with a methicillin-resistance rate of 68.1% [22]. In a burn center in Oman, Prasanna and Thomas reported that more than 50% of the patients developed an MRSA infection during their ICU stays [23].
Pharmacologic treatment
Therapy for S. aureus has been challenged by the development of drug resistance. The first resistant isolate was recovered only two years after the introduction of penicillin in 1944 [24]; a similar two-year period elapsed between the introduction of the semisynthetic penicillin, methicillin and the emergence of the first MRSA [25]. Guggenheim et al. calculated the decline of susceptibility of S. aureus to broad-spectrum first-line drugs, such as ciprofloxacin or penicillinase-stable penicillins (oxacillin or methicillin) over the past 20 years [19]. Vancomycin alone or in conjunction with other antibiotics generally has been considered the treatment of choice for infections caused by MRSA. Even though vancomycin is used only as a last resort in treating MRSA infections, resistance recently has developed to this agent also. Vancomycin-intermediate susceptible S. aureus (VISA) was isolated in 1997 and has since appeared as analogous strains in numerous countries [26].
At this time, no antibiotic class is uniformly effective against S. aureus [26]. The continuously emerging resistance against existing drugs underscores the importance of the development of new antibiotics. New classes of agents have been developed, including inhibitors of protein synthesis such as oxazolidinones (linezolid) and streptogramins (quinupristin/dalfopristin); tigecycline; a bactericidal drug that acts on the cell membrane (daptomycin); and an inhibitor of peptidoglycan synthesis in cell walls (dalbavancin) [27].
In an internal study performed in 2005 at the Galveston Shriners Hospital, only 31% of S. aureus burn wound isolates and none of the S. epidermidis and S. haemolyticus isolates were sensitive to oxacillin [9]. Other antibiotics, such as cotrimoxazole, netilmicin, and rifampin, have, however, retained surprisingly high levels of efficiency. Clindamycin has remained effective, with susceptibility rates exceeding 90% [9]. This susceptibility pattern is consistent with community-acquired MRSA (CA-MRSA).
Prevention
The transmission of MRSA and other infectious agents can be reduced by applying standard infection control measures of hand washing and barrier nursing, efficient cleaning and decontamination of hospital equipment, and actions such as mechanical scrubbing and periodic use of strong disinfectants [9]. In an MRSA-colonized or -infected patient, current recommendations call for patient-dedicated equipment, cohort nursing, regular changes of intravascular catheters, and hydrotherapy [16]. Finally, treatment of infection rather than mere colonization is a major prevention strategy against emergence of more resistant S. aureus [23].
Recent developments
Appropriate infection control practices remain the most important components of the fight against MRSA. A recent study from Rhode Island Hospital showed that it is necessary to screen both patients and health care workers for MRSA, as unrecognized infection in health care workers may function as a reservoir that could impair other control measures [28]. Dansby et al. recently reported that an epidemic of MRSA continued at the Parkland Hospital Burn Unit despite prompt rigorous isolation of identified patients and largely negative personnel cultures [29]. It was only after a renovation that allowed door closure during dressing changes that a sustained decrease in MRSA cases occurred. The authors concluded that rigorous infection control measures, paired with logistic burn unit considerations, are paramount to battle the increasing occurrence of MRSA [29].
Wibbenmeyer et al. examined an outbreak of infection with USA300, a CA-MRSA strain, at the University of Iowa Burn Center [13]. This strain is found in a variety of skin and soft tissue infections, particularly in younger patients. The outbreak caused numerous simultaneous MRSA abscesses. A comparison with a control group, not affected by the aforementioned strain, revealed that infected patients were more likely to have been hospitalized or to have had an operation in the six months before they were hospitalized. The authors concluded that burn patients may be at particular risk for numerous abscesses with USA300. They again stressed that contact precautions and appropriate training of all burn care providers are key to lowering infection rates [13].
Pseudomonas aeruginosa and Other Gram-Negative Pathogens
Gram-negative pathogens continue to cause the most severe infections in burn patients. Among these organisms, P. aeruginosa is the most commonly encountered source of chronic or acute burn wound infection in the United States [8,9,30]. In a recent survey of 104 U.S. burn units, 44% of the respondents identified P. aeruginosa as the most prevalent gram-negative pathogen, followed by Acinetobacter baumannii and Enterococcus spp. [31]. The picture is slightly different in Asian countries such as China, where A. baumannii and Proteus mirabilis are the most common causes of burn infection, with P. aeruginosa in third place [32]. In Europe, P. aeruginosa and Escherichia coli are the two most common pathogens, with a frequency for each at 13% of all gram-negative infections [19]. Pseudomonas aeruginosa has a predilection for moist and warm wound environments, thus posing a major challenge for burn patients [9]. This organism is the most common cause of nosocomial pneumonia in patients on a ventilator and is associated with a high mortality rate, especially in pediatric patients [33,34]. Wound infections caused by P. aeruginosa are particularly troublesome. These infections usually start as a localized, superficial lesion with characteristically greenish pus and a sweet smell, and can spread into deeper tissues rapidly and cause sepsis, resulting in substantial mortality rates [34]. The exact reason for the rapid spread of Pseudomonas is unknown; however, some work indicates that sepsis-induced neutropenia may be the underlying cause of rapid progression [35]. A serious complication of invasive Pseudomonas infection in burns is ecthyma gangrenosum, characterized by purple-bluish black spots in previously healthy tissue. Histologically, this complication demonstrates thrombosis of vessels with perivascular hemorrhage [9].
Other common gram-negative microbial isolates in the burn population at the Shriners Hospital in Galveston are E. coli, Klebsiella pneumoniae, and Enterobacter cloacae. The other major multi-resistant strain is A. baumannii/haemolyticus [9].
Pharmacologic treatment
Aminoglycosides, in particular gentamicin, were historically the antibiotics of choice for the treatment of gram-negative infections. The synergistic activity with penicillinase-resistant penicillins and vancomycin in the treatment of staphylococcal infections standardized their premier status before the advent of newer extended-spectrum penicillins, fourth-generation cephalosporins, monobactams, carbapenems, and quinolones [9]. In a study conducted at the Galveston Shriners Hospital, aztreonam was more effective than amikacin and piperacillin (58.4% vs. 45.8%, respectively) against Enterobacteriaceae. Pseudomonas aeruginosa remained susceptible to aztreonam and piperacillin in 90% of cases, whereas it was resistant to aminoglycosides in 79% of cases [36]. When choosing an antimicrobial drug for a suspected P. aeruginosa burn infection, therefore, aztreonam and piperacillin are now considered the first-line agents [36] at our facility.
Treatment of multi-resistant pathogens
Some strains of P. aeruginosa and Acinetobacter now encountered in burn units are resistant to all the aforementioned antibiotic classes. Over the last 20 years, an increase in resistance of P. aeruginosa to reserve antibiotics such as ceftazidime, and a dramatic decrease in susceptibility of Acinetobacter spp. to ceftazidime and ciprofloxacin, has developed [19]. Meropenem has been identified as an important reserve antibiotic to which most P. aeruginosa and Acinetobacter are susceptible [19], but resistance to the carbapenems has developed [37–39]. A study at eight centers in the United States found that 15% of P. aeruginosa isolates recovered from burn ICUs were resistant to ceftazidime and 20% were resistant to imipenem [40]. Pseudomonas isolates with resistance to ciprofloxacin also have been reported [41]. Outbreaks of P. aeruginosa resistant to most available beta-lactams, aminoglycosides, and fluoroquinolones have been reported in burn units, neurosurgical ICUs, and cancer centers and among patients with cystic fibrosis [42].
For the treatment of emerging multiresistant Pseudomonas and Acinetobacter at the Galveston Shriners Hospital, we were forced to re-introduce an old drug class, the polymyxins. Polymyxins are amphipathic molecules that interact with the lipopolysaccharide in the bacterial outer membrane; insertion of the antibiotic into the membrane disrupts it and releases lipopolysaccharide into the surrounding milieu. They also have potent antiendotoxic properties and antibacterial activity against P. aeruginosa and many of the Enterobacteriaceae [9]. Colistin, or polymyxin E, is a multicomponent polypeptide antibiotic comprised mainly of colistins A and B. It became available for clinical use in the 1960s. There are two forms of colistin available: Colistin sulfate for oral and topical use and colistimethate sodium for parenteral use [43].
According to Storm et al., the polymyxins are bacteriostatic at low concentrations and bactericidal at high concentrations [44]. In early studies, Evans et al. and Nord and Hoeprich reported that at a concentration of 0.01 mcM/mL, polymyxin B sulfate was bactericidal for 88% of the P. aeruginosa strains [42,45]. Full bactericidal activity against P. aeruginosa is not seen until the colistin concentration reaches 0.1 mcM/mL [42]. In susceptibility testing performed at the Galveston Shriners Hospital from 2005 to 2008, A. baumannii/haemlyticus, E. cloacae, E. coli, and K. pneumoniae all showed 100% susceptibility to colistin and polymyxin B, whereas P. aeruginosa showed 96% and 99% susceptibility to colistin and polymyxin B, respectively.
Dosing of polymyxins must be altered in patients with renal impairment, as the kidney is their principal route of elimination [43]. Distribution into pleural fluid, joints, and cerebrospinal fluid is poor [42]. Nephrotoxicity and neurotoxicity are the most common adverse effects of colistin. Close monitoring of the dose-dependent nephrotoxicity and central nervous system toxicity associated with its systemic use therefore is necessary. When colistin is given to animals or humans, it binds, via free amino acid groups, to negatively charged phospholipids in tissues. Kunin and Bugg showed that binding is greatest to kidney and brain tissues, followed by liver, muscle, and lung [46,47]. After repeated doses, the drug accumulates in tissues to concentrations four to five times higher than the peak serum concentrations and persists for at least five to seven days [46,47]. Drug removal by dialysis can be difficult because of the extensive tissue binding.
Colistin in severely burned pediatric patients: A six-year review
To investigate whether the use of colistin can moderate multi-resistant infections, and to elucidate whether it is associated with a greater number of adverse effects or a higher mortality rate in burn patients, we reviewed patients treated at the Galveston Shriners Hospital (previously unreported data). Three hundred ninety-eight patients with burns exceeding 40% TBSA admitted to our hospital between January 2000 and April 2006 were included. Patients who did not contract multi-drug-resistant gram-negative organisms during their hospital courses and received our standard antibiotic regimen—vancomycin and piperacillin/tazobactam—served as controls (piperacillin/tazobactam group; n = 280)(Table 1). The treatment group consisted of burn patients who, during their acute hospital stays, developed infections with multidrug-resistant P. aeruginosa/fluorescence/putida, A. baumannii, E. coli, or K. pneumoniae and who were treated with vancomycin and colistin for at least three days (colistin group; n = 118). Colistin was given at a mean dose of 4.4 ± 0.9 mg/kg divided into three (or, in rare cases, two) doses over 24 h. The primary outcome measures were death; neuronal complications (defined by neuropathies, dizziness, vertigo, apnea, altered mental status, or neuromuscular blockade); hepatic complications (defined by elevated liver enzyme concentrations); and renal dysfunction (defined as an increase in the serum creatinine concentration to more than 50% above the baseline value or a decline in function necessitating renal replacement therapy).
Patients who had multi-resistant gram-negative infections and required colistin therapy had a significantly larger average total and full-thickness burn than patients treated with piperacillin/tazobactam and vancomycin (p < 0.05; Table 2). There was no significant difference between the colistin and piperacillin/tazobactam groups in the incidence of neurotoxicity, hepatic toxicity, or nephrotoxicity (Table 3). However, the mortality rate was significantly higher in the colistin group (p < 0.05; Table 3).
Table 2.
Colistin Study Demographics
| Control (N = 280) | Colistin (N = 118) | P value | |
|---|---|---|---|
| Mean age ± SD (years) | 8 ± 5 | 9 ± 6 | NS |
| Female/male | 87/193 | 43/75 | NS |
| Mean burn size ± SD (% TBSA) | 58 ± 15 | 64 ± 19 | <0.001 |
| Mean full-thickness burn size ± SD (% TBSA) | 44 ± 23 | 52 ± 18 | <0.001 |
| Burn type (%) | NS | ||
| Flame | 63 | 67 | |
| Scald | 22 | 20 | |
| Other | 15 | 13 | |
| Infecting organism (%)a | |||
| Pseudomonas spp. | – | 57.6 | |
| Acinetobacter spp. | – | 33.7 | |
| Other gram-negative spp. | – | 8.7 | |
| Percent with multiple infections | – | 19.5 | |
| Median duration of colistin treatment (days) (25%–75% range) | – | 12 (7–27) | |
| Mean colistin dosage (mg/kg/day ± SD) | – | 4.4 ± 0.9 | |
Patients infected with these organisms received colistin.
NS = not significant; SD = standard deviation; TBSA = total body surface area.
Table 3.
Toxicity and Mortality Associated with the Use of Colistin Compared to Controls
| Control (n = 280) | Colistin (n = 118) | P value | |
|---|---|---|---|
| Neurotoxicity | 1.4 | 0.8 | 0.9 |
| Nephrotoxicity | 15 | 19 | 0.4 |
| Hepatotoxicity | 1.8 | 2.5 | 0.9 |
| Death | 5 | 19 | <0.001 |
Our data indicate that contracting multi-resistant gram-negative infection causes a higher mortality rate in burn patients than does infection caused by susceptible organisms. Colistin is a safe and efficacious antimicrobial therapy without a marked incidence of in toxic side effects. In a recent review article on the re-emergence of colistin as the antibiotic of choice for multidrug-resistant bacterial infections, Li et al. suggested that the toxicity observed in studies from the 1960s and 1970s may have been caused by a lack of understanding of the drug's pharmacokinetics and toxicodynamics [43]. Most of the studies were performed in pediatric patients who suffered from cystic fibrosis-associated pneumonia caused by multi-drug-resistant organisms. However, recent findings show that close monitoring of renal function and, in patients with kidney failure, measurement of drug concentrations enables avoidance of toxicity [43].
The higher mortality rate in the colistin group at the Galveston Shriners Hospital indicates that multi-resistant organisms are aggressive and a major contributor to burn-related death. The significantly larger burns in the colistin group certainly are the main reason for this finding. This study indicates that treatment of the pediatric burn population with colistin can be safe, as it did not increase the overall incidence of adverse effects. However, colistin should be used only under close monitoring of renal function.
Fungal Infections
The incidence of fungal infections has increased over recent years and represents a major issue in surgical and burn ICUs. Between 1979 and 2000, the rate of sepsis attributable to fungal organisms in ICUs tripled [48], in line with an increase in the incidence of sepsis and the total number of sepsis-related deaths. Candida spp. are the most common cause of fungal sepsis [49] and the fourth most common organism causing infection overall. The CDC has reported an increase in fungal infections from 2.5/1,000 to 5.6/1,000 ICU discharges [50]. The morbidity and mortality rate associated with these infections is striking, with the median ICU stay being increased by as much as 30 days and mortality rates of 30–80% [51–53]. The latest National Healthcare Safety Network Report from the CDC, which summarized surveillance data for 2006 from 14 burn units [54], found that burn ICUs had the highest rate of central-line-associated blood stream infections. At the Galveston Burn ICU, the incidence of fungal infections spiked in 2005, with a subsequent decline until 2008 (Fig. 2).
A recently published multi-center retrospective study reviewed all patients admitted to 15 burn centers over a two-year period who had positive fungal cultures [55]. Patients were divided into three groups on the basis of the treatment: Prophylactic only (topical or oral nystatin), non-systemic treatment including burn excision plus topical agents, and systemic treatment. Fungal infection rates differed by burn unit, ranging from 0.7% to 24%. Patients requiring systemic treatment showed the highest mortality rate (21.2%), tended to be older and have a larger TBSA, and had a higher incidence of inhalation injury. The mortality rate was highest for patients with mold or Aspergillus spp. on culture. Candida was the most frequently cultured fungal organism, although associated with the lowest mortality rate.
Patients may be at risk of acquiring and transmitting fungal organisms from their immediate surroundings on a burn unit, as well as at the time of injury or prior to arrival. The same organisms cultured from burn patients, including Aspergillus, Penicillium, and Zygomycetes spp., are more frequently in the burn unit than in other areas of a hospital [56], emphasizing the importance of strict infection control procedures.
In combination with early burn excision and skin grafting, the development of effective topical antimicrobials in the 1960s had a major impact in reducing bacterial colonization, invasive burn wound sepsis, and death [57–60]. These agents now are standard care and include silver sulfadiazine cream (Silvadene®, King Pharmaceuticals, Inc., Bristol, TN), effective against organisms including Pseudomonas with minimal side effects, and mafenide acetate (Sulfamylon®, UDL Laboratories, Rockford, IL), which has broad-spectrum activity and penetrates full-thickness eschar. It has been postulated that the use of topical antimicrobial agents has contributed to a shift in the spectrum of organisms toward the greater importance of fungi as pathological organisms in burn units [61,62].
Opportunistic mycoses occur because of altered and compromising immunologic situations in the host. Burn patients exhibit multiple risk factors for fungal infection. Patients with an Acute Physiology and Chronic Health Evaluation (APACHE) II scores exceeding 10 or ventilator use for longer than 48 h are more susceptible to fungal infections [63]. Also, the use of broad-spectrum antibiotics alters the intestinal and skin flora and promotes overgrowth of yeasts such as Candida. Central access catheters are a notorious entry pathway for fungi. Patients in the ICU who are receiving total parenteral nutrition are at risk for fungal invasion. Finally, burn-induced immunosuppression is a major risk factor for fungal infections in burns, as T lymphocytes, natural killer cells, and phagocytic immunity are required to combat colonization and deeper infection [63]. In burn patients, the most common site for fungal infection is the burn itself, although patients are also at risk for infection of the respiratory, urinary, and gastrointestinal tracts [9].
Fungal pathogens
Candida spp. are the fungal pathogens most commonly encountered in the burn patient. Features identified as independent risk factors for disseminated candidiasis in ICU patients are multiple or prolonged courses of antimicrobial treatment, central intravascular catheters, renal dysfunction and hemodialysis, and Candida spp. found in a culture other than blood [64].
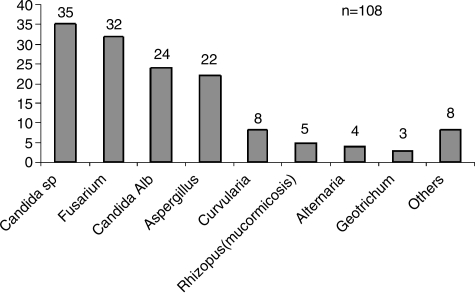
Non-albicans Candida spp, such as C. tropicalis, C. parapsilosis, C. krusei, and C. glabrata are the other most commonly isolated species responsible for surgical infections [63]. Particularly virulent fungal organisms include Aspergillus, Fusarium, and Mucormycosis, as summarized in Table 4. At the Galveston Shriners Hospital, Candida albicans, other Candida spp., and Fusarium are the fungal pathogens isolated most frequently (Fig. 3).
Table 4.
Features and Treatment of Fungal Infections Commonly Encountered in Burn Patients
| Fungus | Features | Suggested therapy | Notes |
|---|---|---|---|
| Candida albicans | Commensal; exists as yeast or mycelial form. Yeast forms blastosphere that buds to form pseudohyphae with branching | Caspofungin Amphotericin Ba |
Most commonly isolated Treatment generally not indicated for isolated urinary tract infection |
| Non-albicans Candida (including C. tropicalis, glabrata, parapsilosis, krusei, and guilliermondii) | Surgery, renal failure, vascular catheter, and fluconazole prophylaxis C. tropicalis associated with characteristic embolic lesions C. glabrata produces only yeast cells (no hyphae) |
Amphotericin B for C. krusei and C. glabrata (fluconazole resistant) Voriconazole for C. parapsilosis and C. guilliermondii (echinocandin and polyene resistance reported) |
Antifungal susceptibility differs significantly, in contrast to C. albicans, and treatment should be guided by species and in vitro susceptibility findings |
| Aspergillus spp. | High mortality rate for invasive aspergillosis Septate hyphae, suppurative inflammation with ischemic necrosis Blood vessel invasion |
Voriconazole Amphotericin B (lipid formulation or deoxycholate) Caspofungin |
Combination treatment could be considered in severe or refractory cases Polyene resistance reported for Aspergillus terreus |
| Fusarium spp. | Hyphae Virulent organism creates wave-front of necrosis Reported in hospital water systems |
Amphotericin B (lipid formulation or deoxycholate) Voriconazole Consider amphotericin B plus caspofungin (intrinsically resistant to caspofungin but synergy reported) |
One of the most drug-resistant fungi, with resistance to polyene, azole, and echinocandin drug classes |
| Zygomycetes (including Rhizopus and Mucor spp.) | Sparsely septate hyphae, suppurative inflammation Arterial invasion with embolization, thrombosis, and infarction (angio-invasive) Sterile bread can be used for isolation Rhinocerebral infection associated with diabetes |
Amphotericin B (lipid formulation or deoxycholate) Itraconazole Ketoconazole Voriconazole |
Azole and echinocandin resistance |
FIG. 3.
Spectrum of burn wound pathogens at the Shriners Hospital for Children, Galveston, 2006–2008.
Various specialized culture media are used to grow and identify fungal organisms. Several weeks often are required to receive laboratory confirmation and drug sensitivity reports for specific isolates [9]. Microscopic analysis of tissue biopsies provides an alternative diagnostic method. However, correlation between histopathology samples and burn culture identification appears inconsistent in determining fungal infection [62]. This report highlighted the fact that histopathology study alone often was inadequate in distinguishing fungal colonization from infection and emphasized the importance of sending concurrent samples for culture as well as histopathology study in order to identify species and guide therapy [62].
Pharmacologic treatment
The features and therapies for some of the most common fungal organisms encountered in burn patients are summarized in Table 4. Of note, azoles and echinocandins are efficacious and less toxic than amphotericin B for the treatment of patients with candidemia [65]. Although they are better tolerated, there is no evidence that lipid formulations of amphotericin B are superior to amphotericin B deoxycholate for the treatment of candidemia [65]. Voriconazole improved survival and resulted in fewer severe side effects than amphotericin B deoxycholate in the treatment of invasive aspergillosis. However, transient visual disturbances are common [66].
In patients with severe sepsis or septic shock, empirical antifungal therapy should not be given on a routine basis, although it may be justified in patients at high risk for invasive candidiasis, given the fact that fungal infections account for only 5% of these infections [65].
The enhanced efficacy and reduced toxicity of newer drugs offers an improvement in the treatment of serious fungal infections. However, further studies are required to develop and enhance management guidelines for fungal infections in the burn care setting.
Summary
Innovations and developments in fluid management, ventilatory support, surgical care, and antimicrobial therapy have contributed to major progress in burn care and a significant reduction in associated mortality and morbidity rates. Methicillin-resistant S. aureus-associated infections remain a challenge because of the emergence of more resistant strains and the changing spectrum of available drugs. Vancomycin and clindamycin are the two most important reserve antibiotics for MRSA infection, with susceptibility rates above 90%. Oxazolidinones and streptogramins are two new drug classes that have showed efficacy against gram-positive infections. Colistin has re-emerged as a highly effective antibiotic for multiresistant Pseudomonas and Acinetobacter infections in burns. Its use is not associated with an increase in neurologic, hepatic, or nephrologic complications. Fungal infections in burn patients are on the rise and can be particularly fast-spreading; current challenges include Candida, Aspergillus. A definitive diagnosis is more difficult than in bacterial infections. Amphotericin B and voriconazole remain the two most important antifungal substances. The development of new agents, prudent and appropriate use of antibiotics, and better infection control protocols are paramount in the continuing battle against multiresistant organisms.
Footnotes
Presented at the Memorial Celebration and Festschrift for Doctor G. Tom Shires, New York, New York, October 25, 2008.
Acknowledgments
We would like to express our gratitude to the Postdoctoral Training in Trauma and Burns program (No. T32-GM08256), Clayton Foundation for Research, American Surgical Association Foundation, Anderson Foundation, and Shriners Hospital for Children (Grants 8660 and 8460) for their generous support. LKB is supported by a Shriners Hospitals for Children Research Fellowship (No. 8505). The authors acknowledge Doctors Arthur P. Sanford, Jong A. Lee, and Natalie Williams-Bouyer and the research nursing staff for their invaluable support in the collection and analysis of the data.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Herndon DN. Barrow RE. History of treatments of burns. In: Herndon DN, editor. Total Burn Care. 3rd. Philadelphia: WB Saunders; 2007. pp. 1–8. [Google Scholar]
- 2.Bull JP. Fisher AJ. A study of mortality in a burns unit: A revised estimate. Ann Surg. 1954;139:269–274. doi: 10.1097/00000658-195403000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller MJ. Herndon DN. The challenge of burns. Lancet. 1994;343:216–220. doi: 10.1016/s0140-6736(94)90995-4. [DOI] [PubMed] [Google Scholar]
- 4.Saffle JR. Davis B. Williams P. Recent outcomes in the treatment of burn injury in the United States: A report from the American Burn Association Patient Registry. J Burn Care Rehabil. 1995;16:219–232. doi: 10.1097/00004630-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Baxter CR. Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann NY Acad Sci. 1968;150:874–894. doi: 10.1111/j.1749-6632.1968.tb14738.x. [DOI] [PubMed] [Google Scholar]
- 6.Richards C. Emori TG. Edwards J, et al. Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance System 1999. Am J Infect Control. 2001;29:400–403. doi: 10.1067/mic.2001.118408. [DOI] [PubMed] [Google Scholar]
- 7.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 8.Pruitt BA., Jr McManus AT. Kim SH, et al. Burn wound infections: Current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher JJ. Williams-Bouyer N. Villarreal C, et al. Treatment of infection in burns. In: Herndon DN, editor. Total Burn Care. 3rd. Philadelphia: WB Saunders; 2007. pp. 136–176. [Google Scholar]
- 10.Greenhalgh DG. Saffle JR. Holmes JHT, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 11.D'Avignon LC. Saffle JR. Chung KK, et al. Prevention and management of infections associated with burns in the combat casualty. J Trauma. 2008;64(3 Suppl):S277–S286. doi: 10.1097/TA.0b013e318163c3e4. [DOI] [PubMed] [Google Scholar]
- 12.Miranda BH. Ali SN. Jeffery SL, et al. Two stage study of wound microorganisms affecting burns and plastic surgery inpatients. J Burn Care Res. 2008;29:927–932. doi: 10.1097/BCR.0b013e31818ba15f. [DOI] [PubMed] [Google Scholar]
- 13.Wibbenmeyer LA. Kealey GP. Latenser BA, et al. Emergence of the USA300 strain of methicillin-resistant Staphylococcus aureus in a burn-trauma unit. J Burn Care Res. 2008;29:790–797. doi: 10.1097/BCR.0b013e3181848b8f. [DOI] [PubMed] [Google Scholar]
- 14.Durtschi MB. Orgain C. Counts GW, et al. A prospective study of prophylactic penicillin in acutely burned hospitalized patients. J Trauma. 1982;22:11–14. doi: 10.1097/00005373-198201000-00003. [DOI] [PubMed] [Google Scholar]
- 15.de Macedo JL. Santos JB. Bacterial and fungal colonization of burn wounds. Mem Inst Oswaldo Cruz. 2005;100:535–539. doi: 10.1590/s0074-02762005000500014. [DOI] [PubMed] [Google Scholar]
- 16.Cook N. Methicillin-resistant Staphylococcus aureus versus the burn patient. Burns. 1998;24:91–98. doi: 10.1016/s0305-4179(97)00114-9. [DOI] [PubMed] [Google Scholar]
- 17.Phillips LG. Heggers JP. Robson MC. Burn and trauma units as sources of methicillin-resistant Staphylococcus aureus. J Burn Care Rehabil. 1992;13:293–297. doi: 10.1097/00004630-199203000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Robson MC. Krizek TJ. Heggers JP. Biology of surgical infection. In: Ravitch MM, editor. Current Problems in Surgery. Chicago: Year Book Medical Publishers; 1973. pp. 1–62. [DOI] [PubMed] [Google Scholar]
- 19.Guggenheim M. Zbinden R. Handschin AE, et al. Changes in bacterial isolates from burn wounds and their antibiograms: A 20-year study (1986–2005) Burns. 2009;35:553–560. doi: 10.1016/j.burns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Schuster KM. Wilson D. Schulman CI, et al. Continuous-infusion oxacillin for the treatment of burn wound cellulitis. Surg Infect. 2009;10:41–45. doi: 10.1089/sur.2007.081. [DOI] [PubMed] [Google Scholar]
- 21.de Macedo JL. Rosa SC. Castro C. Sepsis in burned patients. Rev Soc Bras Med Trop. 2003;36:647–652. doi: 10.1590/s0037-86822003000600001. [DOI] [PubMed] [Google Scholar]
- 22.Thabet L. Turki A. Ben Redjeb S, et al. [Bacteriological profile and antibiotic resistance of bacteria isolates in a burn department](Fre) Tunis Med. 2008;86:1051–1054. [PubMed] [Google Scholar]
- 23.Prasanna M. Thomas C. A profile of methicillin-resistant Staphylococcus aureus infection in the burn center of the Sultanate of Oman. Burns. 1998;24:631–636. doi: 10.1016/s0305-4179(98)00108-9. [DOI] [PubMed] [Google Scholar]
- 24.Barber M. Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;2:641–644. doi: 10.1016/s0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- 25.Jevons MP. Coe AW. Parker MT. Methicillin resistance in staphylococci. Lancet. 1963;1:904–907. doi: 10.1016/s0140-6736(63)91687-8. [DOI] [PubMed] [Google Scholar]
- 26.Enright MC. The evolution of a resistant pathogen—the case of MRSA. Curr Opin Pharmacol. 2003;3:474–479. doi: 10.1016/s1471-4892(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 27.Metzger R. Bonatti H. Sawyer R. Future trends in the treatment of serious gram-positive infections. Drugs Today. 2009;45:33–45. doi: 10.1358/dot.2009.45.1.1315922. [DOI] [PubMed] [Google Scholar]
- 28.Ben-David D. Mermel LA. Parenteau S. Methicillin-resistant Staphylococcus aureus transmission: The possible importance of unrecognized health care worker carriage. Am J Infect Control. 2008;36:93–97. doi: 10.1016/j.ajic.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Dansby W. Purdue G. Hunt J, et al. Aerosolization of methicillin-resistant Staphylococcus aureus during an epidemic in a burn intensive care unit. J Burn Care Res. 2008;29:331–337. doi: 10.1097/BCR.0b013e3181667583. [DOI] [PubMed] [Google Scholar]
- 30.Mayhall CG. The epidemiology of burn wound infections: Then and now. Clin Infect Dis. 2003;37:543–550. doi: 10.1086/376993. [DOI] [PubMed] [Google Scholar]
- 31.Hodle AE. Richter KP. Thompson RM. Infection control practices in U.S. burn units. J Burn Care Res. 2006;27:142–151. doi: 10.1097/01.BCR.0000203493.31642.79. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z. Rong XZ. Zhang T, et al. [Distribution and drug resistance analysis of bacteria in different wound infections](Chi) Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:82–83. [PubMed] [Google Scholar]
- 33.Zar HJ. Cotton MF. Nosocomial pneumonia in pediatric patients: Practical problems and rational solutions. Paediatr Drugs. 2002;4:73–83. doi: 10.2165/00128072-200204020-00001. [DOI] [PubMed] [Google Scholar]
- 34.Murray PR. Baron EJ. Jorgensen JH, et al. Manual of Clinical Microbiology. 8th. Washington, DC: ASM Press; 2003. [Google Scholar]
- 35.Shoup M. Weisenberger JM. Wang JL, et al. Mechanisms of neutropenia involving myeloid maturation arrest in burn sepsis. Ann Surg. 1998;228:112–122. doi: 10.1097/00000658-199807000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walton MA. Villarreal C. Herndon DN, et al. The use of aztreonam as an alternate therapy for multiresistant Pseudomonas aeruginosa. Burns. 1997;23:225–227. doi: 10.1016/s0305-4179(96)00126-x. [DOI] [PubMed] [Google Scholar]
- 37.Japoni A. Alborzi A. Kalani M, et al. Susceptibility patterns and cross-resistance of antibiotics against Pseudomonas aeruginosa isolated from burn patients in the South of Iran. Burns. 2006;32:343–347. doi: 10.1016/j.burns.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Ozkurt Z. Ertek M. Erol S, et al. The risk factors for acquisition of imipenem-resistant Pseudomonas aeruginosa in the burn unit. Burns. 2005;31:870–873. doi: 10.1016/j.burns.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Mokaddas EM. Sanyal SC. Resistance patterns of Pseudomonas aeruginosa to carbapenems and piperacillin/tazobactam. J Chemother. 1999;11:93–96. doi: 10.1179/joc.1999.11.2.93. [DOI] [PubMed] [Google Scholar]
- 40.Archibald L. Phillips L. Monnet D, et al. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: Increasing importance of the intensive care unit. Clin Infect Dis. 1997;24:211–215. doi: 10.1093/clinids/24.2.211. [DOI] [PubMed] [Google Scholar]
- 41.Fass RJ. Barnishan J. Ayers LW. Emergence of bacterial resistance to imipenem and ciprofloxacin in a university hospital. J Antimicrob Chemother. 1995;36:343–353. doi: 10.1093/jac/36.2.343. [DOI] [PubMed] [Google Scholar]
- 42.Evans ME. Feola DJ. Rapp RP. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–967. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- 43.Li J. Nation RL. Turnidge JD, et al. Colistin: The re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 44.Storm DR. Rosenthal KS. Swanson PE. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- 45.Nord NM. Hoeprich PD. Polymyxin B and colistin: A critical comparison. N Engl J Med. 1964;270:1030–1035. doi: 10.1056/NEJM196405142702002. [DOI] [PubMed] [Google Scholar]
- 46.Kunin CM. Bugg A. Binding of polymyxin antibiotics to tissues: The major determinant of distribution and persistence in the body. J Infect Dis. 1971;124:394–400. doi: 10.1093/infdis/124.4.394. [DOI] [PubMed] [Google Scholar]
- 47.Kunin CM. Bugg A. Recovery of tissue bound polymyxin B and colistimethate. Proc Soc Exp Biol Med. 1971;137:786–790. doi: 10.3181/00379727-137-35667. [DOI] [PubMed] [Google Scholar]
- 48.Martin GS. Mannino DM. Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 49.Wisplinghoff H. Bischoff T. Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 50.Banerjee SN. Emori TG. Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 51.Burchard KW. Minor LB. Slotman GJ, et al. Fungal sepsis in surgical patients. Arch Surg. 1983;118:217–221. doi: 10.1001/archsurg.1983.01390020065011. [DOI] [PubMed] [Google Scholar]
- 52.Fraser VJ. Jones M. Dunkel J, et al. Candidemia in a tertiary care hospital: Epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992;15:414–421. doi: 10.1093/clind/15.3.414. [DOI] [PubMed] [Google Scholar]
- 53.Wey SB. Mori M. Pfaller MA, et al. Hospital-acquired candidemia: The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 54.Edwards JR. Peterson KD. Andrus ML, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007;35:290–301. doi: 10.1016/j.ajic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Ballard J. Edelman L. Saffle J, et al. Positive fungal cultures in burn patients: A multicenter review. J Burn Care Res. 2008;29:213–221. doi: 10.1097/BCR.0b013e31815f6ecb. [DOI] [PubMed] [Google Scholar]
- 56.Mousa HA. Al-Bader SM. Hassan DA. Correlation between fungi isolated from burn wounds and burn care units. Burns. 1999;25:145–147. doi: 10.1016/s0305-4179(98)00148-x. [DOI] [PubMed] [Google Scholar]
- 57.Al-Mousawi AM. Jeschke MG. Herndon DN. History of metabolic treatments in burn care. Wounds. 2008;20:185–191. [PubMed] [Google Scholar]
- 58.Fox CL., Jr Rappole BW. Stanford W. Control of Pseudomonas infection in burns by silver sulfadiazine. Surg Gynecol Obstet. 1969;128:1021–1026. [PubMed] [Google Scholar]
- 59.Lindberg RB. Moncrief JA. Switzer WE, et al. The successful control of burn wound sepsis. J Trauma. 1965;5:601–616. doi: 10.1097/00005373-196509000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Moyer CA. Brentano L. Gravens DL, et al. Treatment of large human burns with 0.5 per cent silver nitrate solution. Arch Surg. 1965;90:812–867. doi: 10.1001/archsurg.1965.01320120014002. [DOI] [PubMed] [Google Scholar]
- 61.Becker WK. Cioffi WG., Jr McManus AT, et al. Fungal burn wound infection: A 10-year experience. Arch Surg. 1991;126:44–48. doi: 10.1001/archsurg.1991.01410250048008. [DOI] [PubMed] [Google Scholar]
- 62.Schofield CM. Murray CK. Horvath EE, et al. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns. 2007;33:341–346. doi: 10.1016/j.burns.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 63.Dean DA. Burchard KW. Fungal infection in surgical patients. Am J Surg. 1996;171:374–382. doi: 10.1016/S0002-9610(97)89647-X. [DOI] [PubMed] [Google Scholar]
- 64.Munoz P. Burillo A. Bouza E. Criteria used when initiating antifungal therapy against Candida spp. in the intensive care unit. Int J Antimicrob Agents. 2000;15:83–90. doi: 10.1016/s0924-8579(00)00147-3. [DOI] [PubMed] [Google Scholar]
- 65.Bochud PY. Bonten M. Marchetti O, et al. Antimicrobial therapy for patients with severe sepsis and septic shock: An evidence-based review. Crit Care Med. 2004;32(11 Suppl):S495–S512. doi: 10.1097/01.ccm.0000143118.41100.14. [DOI] [PubMed] [Google Scholar]
- 66.Herbrecht R. Denning DW. Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 67.doctorfungus.org. www.doctorfungus.org/thefungi/index.htm. [Mar 12;2009 ]. doctorfungus.orgwww.doctorfungus.org/thefungi/index.htm
- 68.Krcmery V. Barnes AJ. Non-albicans Candida spp. causing fungaemia: Pathogenicity and antifungal resistance. J Hosp Infect. 2002;50:243–260. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- 69.McKinsey DS. Making best use of the newer antifungal agents. Drug Benefit Trends March. 2004:131–147. [Google Scholar]
- 70.Anaissie EJ. Kuchar RT. Rex JH, et al. Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: A new paradigm for the epidemiology of opportunistic mold infections. Clin Infect Dis. 2001;33:1871–1878. doi: 10.1086/324501. [DOI] [PubMed] [Google Scholar]