Abstract
Altered macrophage kinetics is a pivotal mechanism of visceral obesity-induced inflammation and cardiometabolic risk. Because monocytes can differentiate into either proatherogenic M1 macrophages or anti-inflammatory M2 macrophages, approaches that limit M1 while promoting M2 differentiation represent a unique therapeutic strategy. We hypothesized that adiponectin may prime human monocytes toward the M2 phenotype. Adiponectin promoted the alternative activation of human monocytes into anti-inflammatory M2 macrophages as opposed to the classically activated M1 phenotype. Adiponectin-treated cells displayed increased M2 markers, including the mannose receptor (MR) and alternative macrophage activation-associated CC chemokine-1. Incubation of M1 macrophages with adiponectin-treated M2-derived culture supernatant resulted in a pronounced inhibition of tumor necrosis factor-α and monocyte chemotactic protein-1 secretion. Activation of human monocytes into M2 macrophages by adiponectin was mediated, in addition to AMP-activated protein kinase and peroxisome proliferator-activated receptor (PPAR)-γ, via PPAR-α. Furthermore, macrophages isolated from adiponectin knockout mice demonstrated diminished levels of M2 markers such as MR, which were restored with adiponectin treatment. We report a novel immunoregulatory mechanism through which adiponectin primes human monocyte differentiation into anti-inflammatory M2 macrophages. Conditions associated with low adiponectin levels, such as visceral obesity and insulin resistance, may promote atherosclerosis, in part through aberrant macrophage kinetics.
Keywords: adiponectin, obesity, macrophage activation
atherosclerosis is regarded as a dynamic and progressive disease arising from the combination of abnormal lipid metabolism, endothelial dysfunction, and inflammation (35). A crucial step in this inflammatory process is the infiltration of monocytes into the subendothelial space of large arteries and their differentiation into tissue macrophages, whose activation and function are influenced by the cytokines within the inflammatory milieu of the atherosclerotic lesion.
Proinflammatory cytokines such as interferon (IFN)-γ and interleukin (IL)-1β, and inducers of tumor necrosis factor (TNF)-α such as lipopolysaccharide (LPS), promote macrophage differentiation to a “classical” activation pattern (M1) (11). M1 macrophages are associated with inflammation and tissue destruction, produce proinflammatory cytokines such as TNF-α, IL-6, and IL-12, and increase the production of reactive oxygen species sustaining the process of atherogenesis (12). In contrast, “alternative” activation program (M2) macrophages, which are induced in response to IL-4 and IL-13, dampen the inflammatory process by producing anti-inflammatory factors such as IL-10 and transforming growth factor-β, generating IL-1 receptor antagonist, scavenging apoptotic cell debris, and promoting angiogenesis and tissue repair (11, 31). A reduction in anti-inflammatory M2 macrophages and an increase in genes associated with proinflammatory M1 macrophages occur in obese states (20).
Obesity directly contributes to the pathogenesis of insulin resistance, type 2 diabetes, atherosclerosis, and cardiovascular diseases (9). Obesity-associated inflammation is characterized by an increased abundance of macrophages in the adipose tissue, which are postulated to be major sources of several molecular mediators such as TNF-α, IL-6, and C-reactive protein (CRP), which directly contribute to the proinflammatory milieu mediating vascular injury (14, 18, 19, 23). However, the adipose tissue also secretes adiponectin, an adipokine believed to confer protection against inflammation and obesity-linked insulin resistance (34). Circulating levels of adiponectin are decreased in patients with obesity, type 2 diabetes, or coronary artery disease and are inversely correlated to circulating levels of CRP and IL-6 (7, 8, 13). Similarly, adiponectin levels in human adipose tissue are also inversely affected by TNF-α and IL-6 (3). In cultured endothelial cells, adiponectin treatment inhibits the expression of adhesion molecules, whereas, in macrophages, adiponectin treatment appears to decrease TNF-α production, limits the transformation to foam cells, and stimulates the secretion of the anti-inflammatory cytokine IL-10 (16, 26, 27). Thus adiponectin appears to have anti-inflammatory and anti-atherogenic properties.
In the present study, we hypothesized that adiponectin may exert novel effects to prime human monocytes toward an anti-inflammatory alternative M2 phenotype. To this aim, we evaluated the effects of adiponectin on human monocyte differentiation, in addition to studying macrophage phenotypes in adiponectin-deficient mice.
MATERIALS AND METHODS
Materials.
Recombinant adiponectin, IL-4, and enzyme-linked immunosorbent assay (ELISA) kits were from R & D. Antibodies for flow cytometry (all from BD) included APC mouse anti-human CD206 (clone 19.2), APC mouse IgG1κ isotype control, phycoerythrin (PE) mouse anti-human CD163 (clone GHI/61), and PE mouse IgG1κ isotype control. Antibodies for Western blots were from Abcam [peroxisome proliferator-activated receptor (PPAR) α], Cell Signaling [AMP-activated protein kinase (AMPK) α, phospho (p)-AMPKα, IkBα, pIκBα (Ser32/36), NF-κB p65, pNF-κB p65, and PPARγ], Millipore (actin), and Santa Cruz (CD206). All other reagents were from Sigma.
Isolation of human peripheral blood monocytes.
Approval was received from the Research Ethics Board of St. Michael′s. Participation was voluntary, and all subjects were healthy and provided informed consent. Peripheral blood mononuclear cells were isolated with Ficoll-Paque prefilled Leucosep tubes (Greiner Bio-One). Cells were suspended in RPMI 1640 (supplemented with 10% human serum, 40 μg/ml gentamicin, and 2 mM glutamine), seeded at a density of 5 × 106 cells/well in six-well plates, and incubated for 2 h at 37°C with 5% CO2. Nonadherent cells were discarded, and adherent monocytes maintained in RPMI 1640 for 7 days.
Monocyte differentiation.
Monocyte differentiation into resting macrophages (RM) occurred after 7 days in culture. M2 macrophages were obtained by incubating freshly isolated monocytes with IL-4 (15 ng/ml) for 7 days. In some experiments, monocytes were coincubated for 7 days with IL-4 (15 ng/ml) and adiponectin (10 μg/ml) or its vehicle. RM differentiation into the M1 phenotype was achieved with LPS (100 mg/ml). Foam cell formation, in the presence of adiponectin (10 μg/ml) or its vehicle, was induced with acetylated low density lipoprotein (50 μg/ml).
Real-time PCR.
First-strand cDNA was prepared with the FastLane Cell cDNA Kit (Qiagen). Primers were designed with ProbeFinder (Roche), and the sequences are provided in Supplemental Table 1 (Supplemental material for this article may be found on the American Journal of Physiology: Heart and Circulatory Physiology website.). Amplifications were performed on the Applied Biosystems StepOne Plus Real-Time PCR system. mRNA expression was analyzed using the method of Pfaffl (28), and all values were normalized against the corresponding glyceraldehyde-3-phosphate dehydrogenase levels.
Western blots.
Proteins from whole cell lysates of macrophages were separated on 4–12% Tris-glycine gels (Invitrogen) and transferred to nitrocellulose membranes (Invitrogen). Membranes were probed with primary antibodies and then incubated with the appropriate horseradish peroxidase-associated secondary antibodies before signals were visualized by enhanced chemiluminescence (Amersham Bioscience).
Flow cytometry.
RMs and M2 macrophages were collected by gentle scrapping, washed in PBS, and suspended in 1% BSA-supplemented PBS (BSA-PBS) with the appropriate antibody or isotype control (1:100). After 1 h on ice, cells were washed two times before suspension in BSA-PBS. Antibody-tagged cells were enumerated on a FACSCalibur (BD) system, and the results were evaluated with the FACSDiva and FlowJo software (BD). Nonspecific binding was avoided by incubating macrophages with PBS/10% human serum for 20 min.
Inflammatory cytokine and chemokine measurements.
M2 macrophages were cultured in the presence or absence of adiponectin (10 μg/ml) for 7 days before being maintained in fresh medium for the following 24 h. The medium (20%) was subsequently introduced to 7-day-old RMs for 24 h before the cells were activated with LPS (100 ng/ml) for 4 h. The resultant M1 macrophages were cultured for a further 24 h in fresh medium before TNF-α, monocyte chemotactic protein-1 (MCP-1), and CCL-3 levels in the supernatants were measured by ELISA.
Animal studies.
All procedures were performed in accordance with the guidelines of the Canadian Council on Animal Care and approved by the St. Michael's Hospital Animal Care Committee. Macrophages were isolated from the peritoneal cavity of male adiponectin knockout mice (Adipoq−/−) and their wild-type littermates (Adipoq+/+) as previously described (10, 21). Briefly, peritoneal cavities were lavaged with cold PBS, and the cells were washed two times before suspension in RPMI 1640. Macrophages, pooled from five mice, were seeded at a density of 2 × 105 cells/ml and treated with or without recombinant rat IL-4 for 3 days in the presence of adiponectin or its vehicle.
Statistical analysis.
Results are presented as means ± SE. Differences between two groups were compared with the Student's t-test. Intergroup comparison of means was performed by ANOVA followed by the Student's t-test. Significance was set at P < 0.05.
RESULTS
Adiponectin promotes the alternative activation of human monocytes into M2 macrophages.
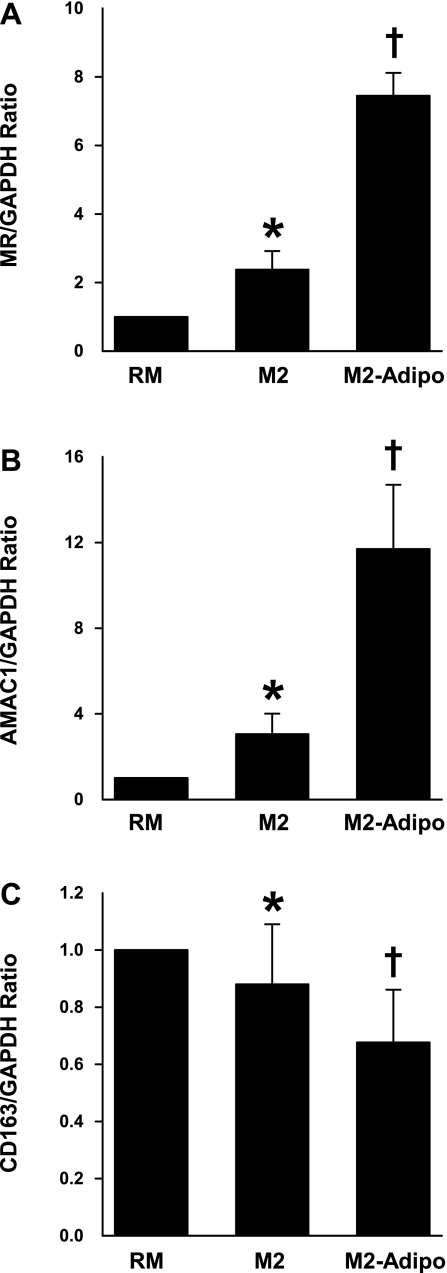
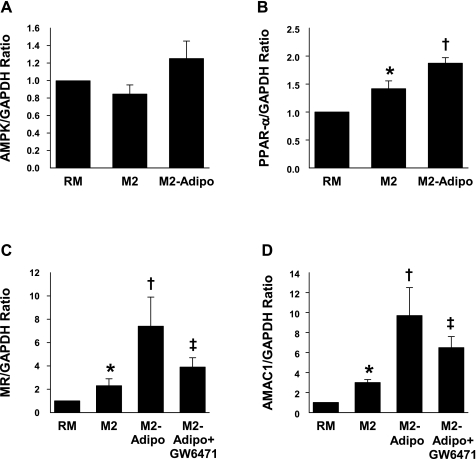
To evaluate the effect of adiponectin on M2 differentiation, primary human monocytes were differentiated into alternative macrophages with IL-4 alone (M2) or in the presence of adiponectin (M2-Adipo). Signaling through the IL-4 receptor, as well as the IL-13 receptor, leads to activation of the mannose receptor (MR, CD206) and alternative macrophage activation-associated CC chemokine-1 (AMAC-1) and downregulation of CD163 expression (10, 15, 33). To characterize the macrophage activation phenotype, we evaluated the mRNA expressions of these markers in M2 and M2-Adipo cells. Both MR and AMAC-1 expressions were strongly induced by IL-4 in M2 macrophages, and their levels were further amplified by adiponectin cotreatment (Fig. 1, A and B). In contrast, expression of the macrophage scavenger receptor CD163 was significantly reduced after IL-4 stimulation and was further suppressed with concomitant adiponectin treatment (Fig. 1C). Macrophage CD163 expression appears to be elevated in type 2 diabetes and obesity, and our findings are consistent with a previous report that suggested adiponectin reduces CD163 expression in monocytes (32).
Fig. 1.
mRNA levels of mannose receptor (MR, A), alternative macrophage activation-associated CC chemokine-1 (AMAC-1, B), and CD163 (C) in resting macrophages (RM), M2 macrophages, and macrophages in the presence of adiponectin (M2-Adipo) as measured by real-time PCR with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) acting as the housekeeping gene; n = 7 experiments. *P < 0.05 vs. RM. †P < 0.05 vs. M2.
Adiponectin-activated M2 macrophages demonstrate increased MR protein expression.
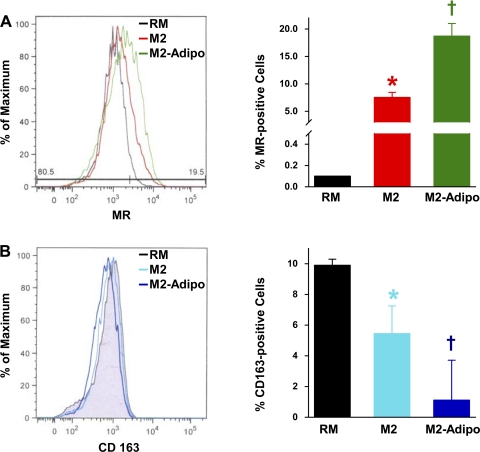
Flow cytometry revealed higher MR protein levels in M2 macrophages compared with RMs. Moreover, MR expression was enhanced in M2-Adipo macrophages. Representative results and the summarized data are shown in Fig. 2A. In contrast, significantly decreased CD163 expression was observed in M2 macrophages with CD163 levels further diminished in M2-Adipo macrophages (Fig. 2B). The observed changes in mRNA expressions and protein levels of M2 markers in M2-Adipo macrophages suggest that adiponectin promotes alternative M2 differentiation.
Fig. 2.
Representative histograms and quantification of allophycocyanin-MR-positive (A) and phycoerythrin-CD163-positive (B) RM, M2, and M2-Adipo macrophages as detected by flow cytometry; n = 3 experiments. *P < 0.05 vs. RM. †P < 0.05 vs. M2.
Adiponectin-stimulated M2 macrophages exert paracrine anti-inflammatory effects on M1 macrophages.
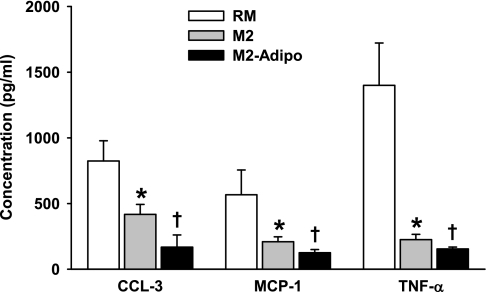
Adiponectin has a crucial role in suppressing macrophage activity, and it has been shown to cause decreased production of proinflammatory cytokines in macrophages, both in vivo and in vitro (37). To determine whether M2-Adipo macrophages can influence the inflammatory status of activated M1 macrophages, indirect coculture experiments were performed, and cytokine and chemokine release by M1 macrophages was subsequently quantified by ELISA. Incubation of M1 macrophages with M2-derived culture supernatant resulted in pronouncedly decreased secretion of the proinflammatory molecules TNF-α, MCP-1, and CCL-3 (Fig. 3). This inhibitory effect was more evident when M1 macrophages were incubated with the supernatant derived from M2 macrophages primed in the presence of adiponectin. These findings indicate that M2 macrophages can suppress the inflammatory status of surrounding M1 macrophages and that M2 macrophage priming with adiponectin enhances the anti-inflammatory properties of M2 macrophages.
Fig. 3.
Cytokine and chemokine secretions from lipopolysaccharide (LPS)-activated M1 macrophages that had previously been exposed to medium from RM, M2, or M2-Adipo macrophage cultures; n = 4 experiments. *P < 0.05 vs. RM. †P < 0.05 vs. M2.
Adiponectin does not switch RM, M1, or foam cells into an M2 phenotype.
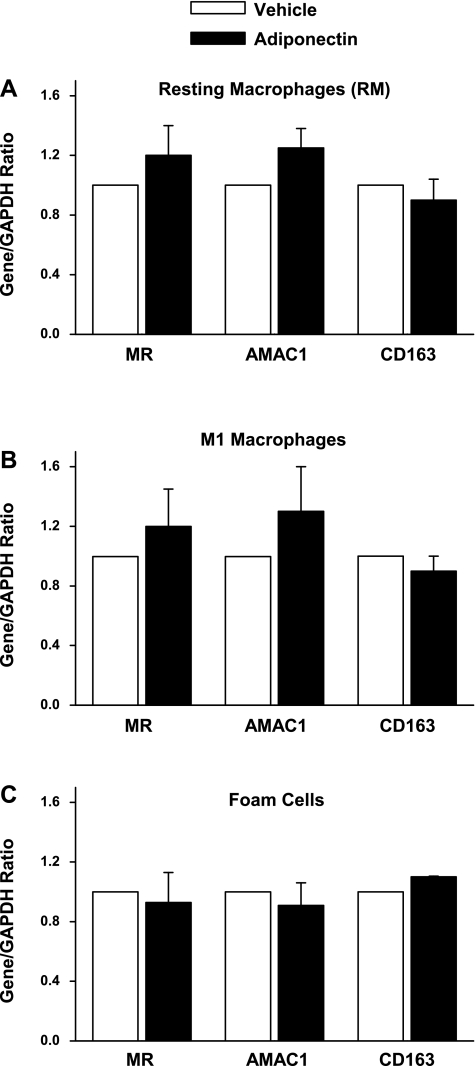
Previous studies have shown that macrophages are plastic cells and can be switched from one activated state to another and vice versa (2, 30). Therefore, we tested whether RM, M1 macrophages, or foam cells can be reverted to the M2 phenotype following adiponectin treatment. M2 phenotype was evaluated by determining the mRNA expressions of MR, AMAC1, and CD163. Adiponectin exposure did not appreciably alter the transcript expressions (Fig. 4) or protein levels (data not shown) of any of these M2 markers, suggesting that it does not influence the expression of M2 markers in RM, M1, or foam cells.
Fig. 4.
mRNA levels of MR, AMAC-1, and CD163 in vehicle- and adiponectin-treated RM (A), LPS-activated M1 macrophages (B), and acetylated low density lipoprotein (AcLDL)-activated foam cells (C) as measured by real-time PCR with GAPDH acting as the housekeeping gene; n = 4 experiments.
Adiponectin activates M2 macrophages via PPAR-α and the NF-κB-IκB signaling module.
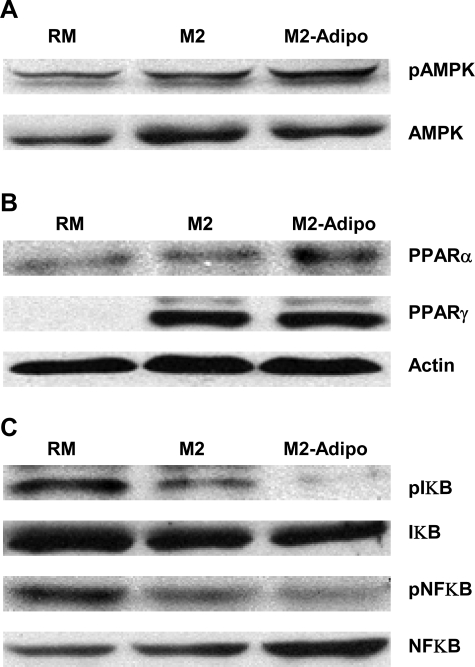
Macrophages constitutively express the adiponectin receptors AdipoR1 and AdipoR2 (6). The downstream targets of these receptors include the PPAR-α, AMPK, and p38 mitogen-activated protein kinase (38). In M2-Adipo macrophages, we found similar AMPK and PPAR-γ transcript expression and protein levels (Figs. 5A, 6A, and 6B) but observed significantly higher levels of PPAR-α transcript and protein (Figs. 5B and 6B) compared with those of both RM and M2 macrophages. We further noted that the PPAR-α antagonist GW-6471 blocked M2-Adipo macrophage differentiation by altering the expression of the M2 macrophage markers MR (Fig. 5C) and AMAC1 (Fig. 5D). As shown in Fig. 6C, we observed suppressed NF-κB and IκB phosphorylation in M2 macrophages, effects that were further accented in M2 macrophages that had been treated with adiponectin. Collectively, our results suggest that adiponectin-activated M2 differentiation appears to be intricately linked with the PPAR-α and PPAR-γ receptors as well as the NF-κB-IκB signaling module.
Fig. 5.
mRNA levels of AMP-activated protein kinase (AMPK) (A), peroxisome proliferator-activated receptor (PPAR)-α (B), MR (C), and AMAC-1 (D) in RM, M2, and M2-Adipo macrophages (incubated in the presence or absence of GW-6471) as measured by real-time PCR with GAPDH acting as the housekeeping gene; n = 4 experiments. *P < 0.05 vs. RM. †P < 0.05 vs. M2. ‡P < 0.05 vs. M2-Adipo.
Fig. 6.
Representative Western blots for phosphorylated (p) and total AMPK (A), PPAR-α and –γ (B), and phosphorylated and total NF-κB and IκB (C) in RM, M2, and M2-Adipo macrophages. Blots are representative of 4 independent experiments.
Adiponectin deficiency alters peritoneal monocyte activation favoring an inflammatory phenotype.
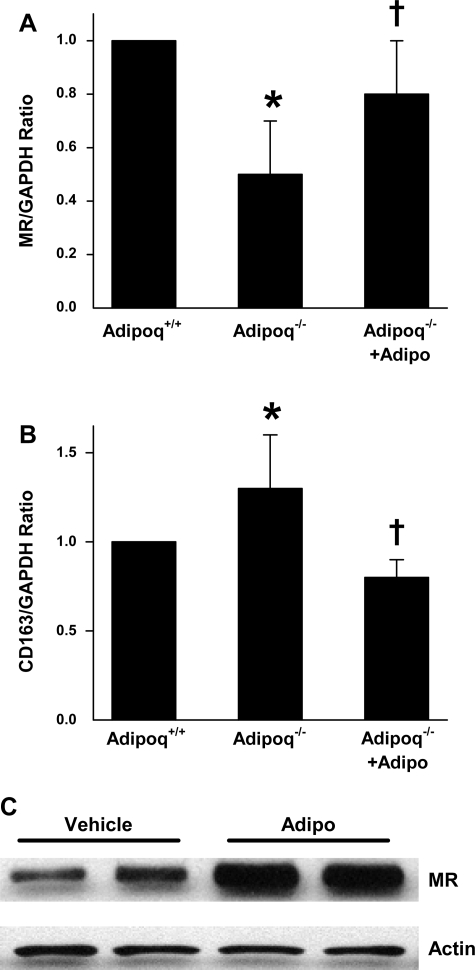
As shown in Fig. 7A, expression of the M2-specific marker MR was significantly lower in macrophages derived from Adipoq−/− mice compared with those from Adipoq+/+ littermates. Adiponectin treatment improved MR transcript and protein expressions in IL-4-treated macrophages from Adipoq−/− mice (Fig. 7, A and C). Macrophage expression of CD163 was greater in Adipoq−/− mice relative to their Adipoq+/+ littermates (Fig. 7B). In contrast, adiponectin-treated Adipoq−/−-derived macrophages demonstrated similar CD163 expression to those from Adipoq+/+ mice. Taken together, these results provide further support for a role for adiponectin in promoting differentiation of monocytes toward an anti-inflammatory M2 phenotype.
Fig. 7.
mRNA levels of MR (A) and CD163 (B) in peritoneal monocytes isolated from wild-type (Adipoq+/+) mice and adiponectin knockout (Adipoq−/−) littermates as measured by real-time PCR with GAPDH acting as the housekeeping gene. C: MR protein levels in peritoneal monocytes from Adipoq−/− mice as detected by Western blotting with actin acting as the housekeeping protein. Monocytes were cultured with IL-4 alone or in the presence of adiponectin or its vehicle for 3 days; n = 5 for A and B, n = 3 for C. *P < 0.05 vs. Adipoq+/+. †P < 0.05 vs. Adipoq−/−.
DISCUSSION
In the present study, we have demonstrated that the adipokine, adiponectin, promotes the differentiation of monocytes into the anti-inflammatory M2 macrophage phenotype. Adiponectin treatment stimulated the expression of markers of M2 activation such as MR and AMAC-1 but did not influence the transition of differentiated macrophages, such as M1 macrophages, RM, or foam cells, toward the anti-inflammatory M2 phenotype. Furthermore, adiponectin enhanced the anti-inflammatory properties of M2 macrophages on M1 macrophages as demonstrated by indirect coculture experiments. PPAR-α activation as well as the NF-κB-IκB signaling module seem to be involved in facilitating monocyte differentiation to the M2 phenotype. Finally, the observation that peritoneal monocytes in adiponectin knockout mice tended to exhibit decreased markers of M2 activation, and that adiponectin supplementation partially abrogated this effect, support our in vitro observations.
Several studies have demonstrated that low concentrations of adiponectin, as observed in obese individuals, are independently associated with the prevalence of coronary artery disease in men and that higher concentrations of adiponectin, independent of glycemic or lipid status, confer a lower risk of myocardial infarction (17, 29). Adiponectin, on a molecular level, appears to exert an antiatherogenic effect upon the vasculature by enhancing endothelial nitric oxide synthesis, attenuating the attachment of monocytes to endothelial cells by inhibiting the proinflammatory TNF-α- and IL-8-induced synthesis of adhesion molecules (e.g., intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin), interfering with NF-κB activation, reducing foam cell formation, and suppressing both the proliferation and migration of human aortic smooth muscle cells (1, 4, 26, 27). However, several features of adiponectin indicate that it may function as a cytokine that modulates immune function. It can regulate immune responses by promoting the clearance of apoptotic cells by macrophages, it decreases macrophage TNF-α production, and it stimulates the secretion of the anti-inflammatory cytokine IL-10 (16, 36). Our findings further support a role for adiponectin in immune function modulation, since it appears to stimulate monocyte differentiation toward an anti-inflammatory phenotype. These observations are consistent with recent observations that adiponectin treatment results in an increase in the levels of M2 markers and a reduction in reactive oxygen species generation (25).
Human atherosclerotic lesions appear to contain both M1 and M2 macrophages (2). M2 macrophages are present at locations distant from the lipid core in more stable zones of the plaque and appear to exert anti-inflammatory properties on M1 macrophages. Our observations support a potential role for adiponectin-activated M2 macrophages to exert a paracrine anti-inflammatory effect on M1 macrophages. Native monocytes, in the presence of an M2 stimulus such as IL-4, can be primed by adiponectin toward an enhanced M2 phenotype. This presents yet another molecular pathway via which adiponectin exerts its anti-inflammatory effects upon the vasculature, inhibiting plaque formation and stabilizing established atherosclerotic plaques. This reduction in lesion inflammation may result in plaque stabilization by increasing collagen content and diminishing fibrous cap thinning. Indeed, in vivo studies conducted in adiponectin knockout mice demonstrate increased neointimal thickening and vascular smooth muscle cell proliferation following vascular injury compared with wild-type controls, with the extent of neointimal proliferation being considerably attenuated after adenovirus-mediated reexpression of adiponectin (24). However, adiponectin does not influence the expression of M2 markers in RM, M1 programmed macrophages, or foam cells that are already differentiated. The underlying reason(s) behind why adiponectin on its own does not propel the activation of RM, M1, and foam cells into a more M2 phenotype remains unknown.
The mechanism through which adiponectin primes monocytes toward the M2 phenotype seems to involve PPAR-α activation and the NF-κB-IκB signaling module. PPAR-α is a nuclear receptor that regulates the expression of genes encoding proteins involved in lipid metabolism, fatty acid oxidation, and glucose homeostasis and appears to exert anti-inflammatory effects in the vascular wall by modulating atherosclerosis-associated inflammatory responses (39). PPAR-α activation has been demonstrated to reduce the production of Th1 cytokines, such as IFN-γ and IL-1β, which induce the classical activation profile M1 (5, 22). NF-κB has been implicated as a promoter of inflammation in multiple experimental models. In our hands, adiponectin treatment was associated with enhanced PPAR-α activation and reduced NF-κB-IKB phosphorylation. Thus adiponectin priming of monocytes via PPAR-α and the NF-κB-IκB signaling module may further enhance the M2 anti-inflammatory phenotype as observed in the current study.
In conclusion, our present study revealed that adiponectin primes human monocyte differentiation toward anti-inflammatory M2 macrophages via PPAR-α. Adiponectin-activated M2 macrophages suppress the secretion of proinflammatory molecules by M1 macrophages, an effect that may promote atherosclerotic plaque stability. Our data provide additional insight into how adiponectin deficiency may promote vascular inflammation and atherosclerosis, in part, through aberrant macrophage kinetics and provide an entirely novel biological basis for the atheroprotective effects of this adipokine.
GRANTS
This work was supported by grants from the Heart & Stroke Foundation of Canada to S. Verma (Grant no. NA6665) and the United States National Institutes of Health (Grant no. HL-51586) to L. Chan. P. E. Szmitko is the Merck Frosst Canada Traineeship in Cardiovascular, Metabolic and Renal Biology Fellow. K. K. Singh is the recipient of a Heart & Stroke Foundation of Canada/Pfizer Research Fellowship. H. Teoh is the St. Michael's Hospital-sanofi-aventis Cardiometabolic Risk Initiative Research Fellow. S. Verma is the Canada Research Chair in Atherosclerosis at the University of Toronto.
DISCLOSURES
The authors report no conflict of interest pertinent to the data presented in this manuscript.
Supplementary Material
REFERENCES
- 1.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 105: 2893–2898, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6: 137–143, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 285: E527–E533, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021–45026, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chinetti G, Lestavel S, Fruchart JC, Clavey V, Staels B. Peroxisome proliferator-activated receptor alpha reduces cholesterol esterification in macrophages. Circ Res 92: 212–217, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun 314: 151–158, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Devaraj S, Torok N, Dasu MR, Samols D, Jialal I. Adiponectin decreases C-reactive protein synthesis and secretion from endothelial cells: evidence for an adipose tissue-vascular loop. Arterioscler Thromb Vasc Biol 28: 1368–1374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engeli S, Feldpausch M, Gorzelniak K, Hartwig F, Heintze U, Janke J, Mohlig M, Pfeiffer AF, Luft FC, Sharma AM. Association between adiponectin and mediators of inflammation in obese women. Diabetes 52: 942–947, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Friedman JM. Obesity in the new millennium. Nature 404: 632–634, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JL, Ho YK, Brown MS, Innerarity TL, Mahley RW. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J Biol Chem 255: 1839–1848, 1980 [PubMed] [Google Scholar]
- 11.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gordon S. The macrophage: past, present and future. Eur J Immunol 37, Suppl 1: S9–S17, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Jialal I, Devaraj S. Role of C-reactive protein in the assessment of cardiovascular risk. Am J Cardiol 91: 200–202, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kodelja V, Muller C, Politz O, Hakij N, Orfanos CE, Goerdt S. Alternative macrophage activation-associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1 alpha with a Th2-associated expression pattern. J Immunol 160: 1411–1418, 1998 [PubMed] [Google Scholar]
- 16.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109: 2046–2049, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85–89, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 288: H2031–H2041, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J 74: 213–220, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased beta-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem 277: 34658–34661, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Marx N, Kehrle B, Kohlhammer K, Grub M, Koenig W, Hombach V, Libby P, Plutzky J. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res 90: 703–710, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87: 407–416, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem 277: 37487–37491, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285: 6153–6160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473–2476, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103: 1057–1063, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. J Am Me Assoc 291: 1730–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142: 481–489, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2: 965–975, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Sporrer D, Weber M, Wanninger J, Weigert J, Neumeier M, Stogbauer F, Lieberer E, Bala M, Kopp A, Schaffler A, Buechler C. Adiponectin downregulates CD163 whose cellular and soluble forms are elevated in obesity. Eur J Clin Invest 39: 671–679, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol 292: H1655–H1663, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: part I. Circulation 108: 1917–1923, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 117: 375–386, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta 1771: 972–982, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.