Summary
Dendritic cells present exogenous proteins to MHC class I restricted CD8+ T cells. This function does not require endogenous antigen synthesis within DC, providing the potential to elicit CD8+ T cell responses to immune complexes, inactivated microbes, dying cells and proteins like ovalbumin. In mice, the CD8+ or DEC-205+ DC are specialized for cross-presentation, and this subset can be increased 10 fold in numbers following Flt3L treatment in vivo. Therefore we studied cross-presentation by abundant Flt3L DC using HIV gag protein. When enriched by positive selection with anti-CD11c beads, cells from Flt3L mice are not only more abundant but are more highly enriched in CD11c high DC, particularly the DEC-205+ subset. DC cross-present HIV gag to primed CD8+ T cells, but when the antigen is delivered within an antibody to DEC-205 receptor, cross-presentation becomes 100 fold more efficient than non-targeted antigen. This finding requires gag to be engineered into anti-DEC antibody, not just mixed with antibody. Flt3L DC are a valuable tool to study cross-presentation, since their use overcomes the obstacle posed by the low number of cross-presenting DC in the steady state. These findings support future experiments to use Flt3L to enhance presentation of DC-targeted vaccines.
Keywords: DC, Flt3L, poly IC
Introduction
Induction of strong CD8+ T cell responses is a major goal in the development of preventive and therapeutic vaccines against persistent viruses and tumors. Dendritic cells (DC) can initiate CD8+ T cell responses through either direct priming or cross-priming. Direct priming refers to the generation of peptide-MHC class I complexes from endogenously synthesized proteins, while cross-priming involves processing of exogenous proteins acquired from the extracellular environment. Cross-priming has been shown to be important in initiating MHC class I- restricted responses to tumors, peripheral self, viral and bacterial antigens [1].
The efficiency of cross-presentation can be enhanced when dendritic cells (DC) take up antigens through different receptor-mediated pathways particularly antigen-antibody complexes [2-4], dying cells [5, 6], and proteins targeted within antibodies to specific receptors like DEC-205 [7], DC-SIGN [8], MMR [9]; Langerin [10], LOX1 [11], and CLEC9A [12]. In our research, we have been studying cross-presentation by genetically engineering the sequences for protein antigens into the heavy chain of monoclonal antibodies (mAb) to DC receptors, particularly DEC-205 [7].
Much of the research in this field has used ovalbumin (OVA) as a tool and the CD8+ OT-I TCR transgenic line specific for OVA presented on H-2Kb molecules. This is because the SIINFEKL peptide from OVA is presented efficiently, with only picomolar levels of peptide being active when DC are the antigen presenting cells [13]. Nevertheless, DC can cross-present other proteins like malaria circumsporozoite protein [13-16], HIV gag [17, 18], HSV and influenza proteins [19-21], and tumor antigens [12, 22-24], although this cross-presentation is often studied with TCR transgenic T cells. Vaccine science requires that the cross-presentation pathway be extended to candidate vaccine antigens and non-transgenic T cells.
In mouse lymphoid tissues, a subset of DC, marked by expression of CD8α homodimer and the DEC-205/CD205 endocytic receptor, is more active in cross-presentation [4, 6, 25]. Here we have studied these cross-presenting DC using mice exposed to Flt3L, which is known to expand several different subsets of DC including a relatively high proportion of CD8+ DC, increasing total numbers of this subset ∼ 30 fold [26, 27].
With the most commonly studied protein, OVA, concentrations higher than 50 μg/ml of protein are required to detect cross-presentation [9, 13, 25, 28-34]. Here we test whether cross-presentation of an exogenous HIV protein antigen, the gag p24 protein, to primed CD8+ T cells can be enhanced by receptor mediated uptake. We will show that Flt3L DC efficiently cross-present HIV gag via the DEC-205 receptor, about 100 times more effective than non-targeted gag. 0.1 μg/ml of anti-DEC gag is as effective as 25 μg/ml of gag, suggesting that this strategy for increasing DC numbers be used to identify cross-presentation mechanisms and improve protein vaccines.
Results
Expansion and ready enrichment of CD11c+ DC from mice harboring a Flt3L expressing B16 melanoma
We used anti-CD11c coated magnetic beads to enrich splenic CD11c+ DC from CxB6F1 mice injected with B16 Flt3L secreting melanoma cells. As previously reported, Flt3L greatly expanded total DC numbers ∼10 fold [26, 35, 36]. In our experiments 4-5 ×106 cells were isolated with anti-CD11c beads from one spleen of an untreated mouse, whereas ∼ 60-70 ×106 cells could be enriched from the spleen of a mouse harboring a growing B16-Flt3L melanoma. When the DC from untreated and Flt3L mice were compared by flow cytometry in surface antigen expression (Suppl. Fig. 1 for gating conditions), the populations from Flt3L mice were much more enriched in CD11c high cells, ∼95% vs. ∼25% in untreated mice (Fig. 1). The CD11c-selected population from Flt3L treated mice was PDCA-1low, B220low but CD11chigh, indicating a paucity of plasmacytoid DC (Fig. 1). The majority of the cells expressed MHC class II molecules, while the representation of the CD8α and DEC-205 subset was increased relative to untreated mice. However in three different experiments we observed that this increase was biased toward DEC-205+ DC over CD8+ cells, possibly because of the presence of a recently described CD8α- CD24hi population of precursors to CD8α+ DC [37].
Figure 1. Cell surface markers of splenic CD11c+ DC by flow cytometry.
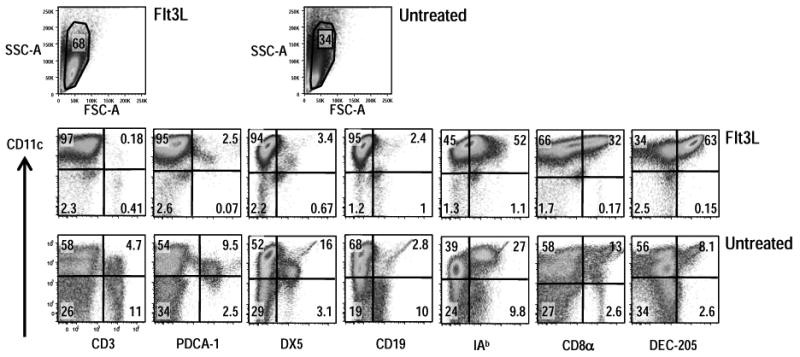
Splenic CD11c+ cells were enriched by positive selection on anti-CD11c beads from CxB6 F1 mice that had been injected with B16 Flt3L melanoma cells or from untreated F1 mice. A six-color flow cytometry panel was used to simultaneously analyze surface markers on CD11c-selected cells. Appropriate isotype-matched, non-binding control Ig's were used to set the quadrant lines (not shown). DC were analyzed by gating against low scatter debris, followed by a live/dead exclusion gate. Analysis is representative of three independent experiments that all examined pooled splenic DC from at least two mice.
These results extend prior work on Flt3L [27] showing that the expansion induced by this hematopoietin allows for an increase in the purity of CD11c selected cells that are also enriched in DEC-205+ DC.
Spleen CD11c+ DC from Flt3L treated mice are functional antigen presenting cells
Since the DC generated from Flt3L treated mice expressed intermediate levels of I-Ab molecules (Fig. 1), we expected that the cells would be functionally immature. A distinguishing feature of mature DC is their capacity to stimulate naïve T cells in an allogenic mixed leukocyte reaction (MLR). Thus we tested whether DC isolated from Flt3L-treated mice exhibited allo-stimulatory activity, comparing it to untreated spleen DC (Fig. 2A). We evaluated the DC with or without direct in vivo activation with poly IC, which is known to mature DC [38]. Accordingly, Flt3L or untreated CxB6 F1 mice were injected with 50 μg of poly IC, and 15 h later, DC were enriched with anti-CD11c beads, fixed with para-formaldehyde to prevent further maturation in culture, washed, and added in graded doses to allogeneic T cells isolated from C57BL/6 mice, which had been labeled with CFSE. In Fig. 2A, we show that both DC populations, upon in vivo maturation, stimulated CD4+ and CD8+ T cells to proliferate. We also found that the Flt3L-mobilized DC had a stronger stimulating capacity being about 5 times more active than normal DC. This result can be explained by the enrichment in CD11chigh cells in DC preparations from Flt3L treated mice. Populations from Flt3L mice were almost entirely CD11chigh and MHC IIhigh DC, whereas populations selected with anti-CD11 beads from normal spleen were roughly 30% CD11chigh and MHC IIhigh DC and clearly had contaminating DX5+ and CD19+ NK and B cells (Fig. 1).
Figure 2. Comparision of antigen-presentation by Flt3L and untreated CD11c+ DC.
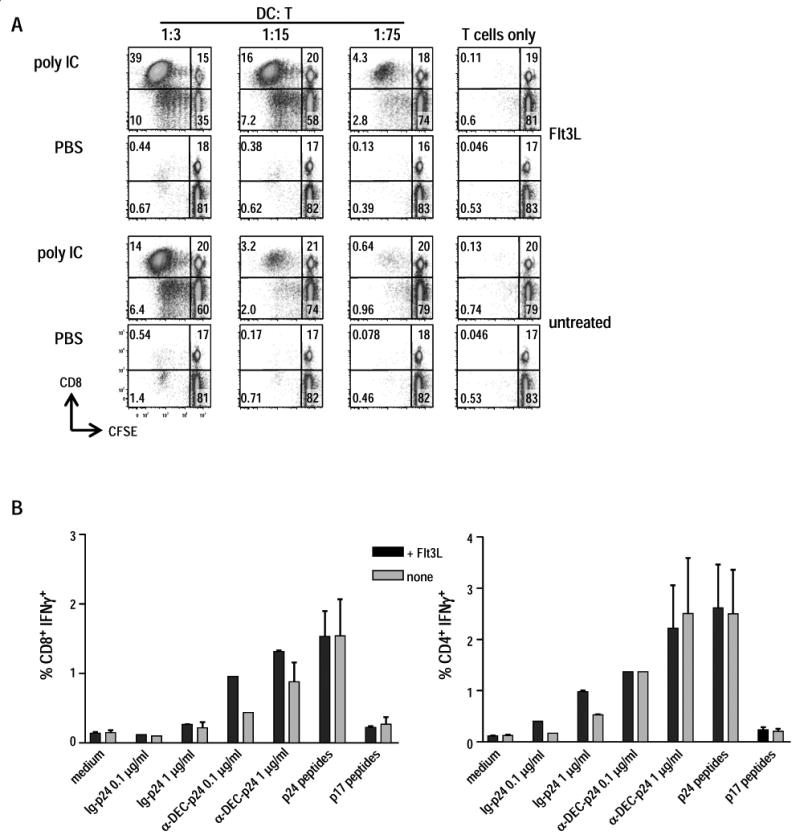
(A) B16 Flt3L-injected or untreated CxB6 F1 mice were stimulated with 50 μg of poly IC for 15 h and allo-stimulatory capacity of splenic CD11c+ DC was compared. Both populations of DC were fixed with 1% para-formaldehyde, washed 3 times, and cultured at various numbers in the presence of a constant number (3×105) of T cells enriched from allogenic C57BL/6 mice. T cell proliferation was detected after 4 days of culture by CFSE dilution of CD8+ and CD4+ T cells. Similar results were obtained using allogeneic T cells from the SJL mouse strain. Data are representative of two independent experiments. (B) Splenic CD11c+ cells purified from Flt3L or untreated F1 mice were pulsed in vitro for 4-5 h with anti-DEC-p24, control Ig-p24 at 0.1 and 1 μg/ml, p24 or p17 15-mer peptides mix (1 μg/ml), followed by 25 μg/ml of poly IC. Fifteen hours later, 1×106 washed DC were added to 3×106 T cells for 6 h in the presence of BFA (10 μg/ml) to detect IFN-γ secretion by intracellular cytokine staining. The T cells were isolated from F1 mice that were primed with Adenovirus gag and boosted with anti-DEC-p24 and poly IC (Methods). Data show mean ± SD of the percentage of CD8+ IFN-γ+ T cells (left) or CD4+ IFN-γ+ T cells (right) representative of two independent experiments.
To deliver HIV antigens to DC, Trumpfheller et al. cloned HIV gag p24 protein within the heavy chain of a mAb specific for DEC-205 endocytic receptor [17]. We tested the ability of the Flt3L DC to mediate presentation to HIV specific CD8+ and CD4+ T cells after in vitro pulsing of DC with anti-DEC-205 HIV gag p24 fusion mAb (anti-DEC-p24) or a control Ig-p24 fusion antibody. Specifically, CD11c+ populations were isolated from Flt3L treated and non treated mice. Then the DC were cultured overnight with poly IC in the presence of the indicated sources of gag antigen, washed to remove excess antigen and added to HIV gag specific T cells for 6 h. We found (Fig. 2B) that both DC populations stimulated IFN-γ production from HIV-gag primed CD8+ and CD4+ T cells isolated from mice primed with Adenovirus-gag p24 and boosted with anti-DEC-p24 and poly IC. Adenovirus-p24 primarily primes CD8+ T cells, while anti-DEC-p24 along with poly IC induces CD4+ T cells as described by Trumpfheller et al. [17]. The response to DC pulsed with anti-DEC-p24 was similar to a pool of pre-processed HIV gag 15 mer peptides, and anti-DEC-p24 antibody resulted in stronger responses than control Ig-p24 (Fig. 2B). There was a small difference between the DC from Flt3L treated mice and those from normal mice in their ability to stimulate antigen-primed T cells. Thus, Flt3L treatment induced high numbers of CD11c+ DC that were functionally comparable to normal DC in their capacity to process and present in vitro anti-DEC-p24 mAb.
Altogether these results demonstrate that CD11c selected DC isolated from Flt3L-mobilized mice were functional in inducing MLR and in exogenous MHC class I and class II antigen-presentation pathways.
Cross-presentation of anti-DEC-p24 is promoted by in vitro maturation of Flt3L CD11c+ DC and restricted to DEC-205+ DC
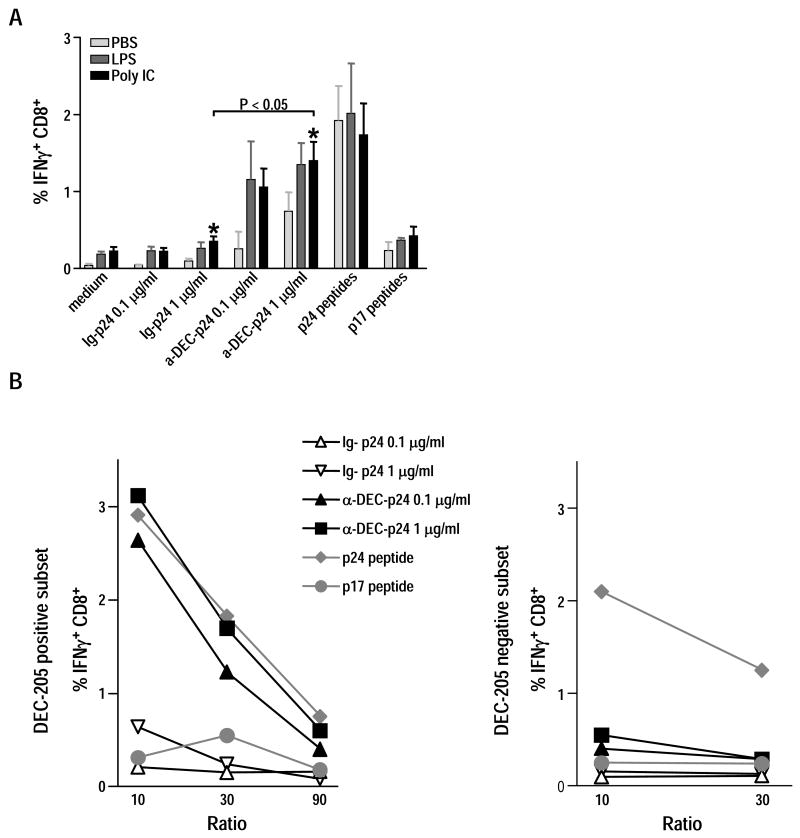
Effective T cell stimulation only occurs if in parallel to antigen uptake, DC undergo maturation, a process that can be triggered by different pathogens or mimics of microbial agonists, such as poly IC for double stranded RNA or lipopolysaccaride respectively. In vitro studies have indicated that DC maturation leads to enhanced cross-presentation as well as expression of the costimulatory molecules required for activation of CD8+ T cells [30, 39-42]. To confirm the capacity of maturation to promote cross-presentation, we enriched splenic CD11c+ DC from mice treated with B16 Flt3L melanoma cells, added different sources of HIV gag protein or peptides for 5 h, and then cultured the cells overnight without or with 0.1 μg/ml of LPS or 25 μg/ml of poly IC as maturation stimuli. After extensive washing, the cells were added to HIV gag specific T cells in the presence of BFA, and cross-presentation was assessed 6 h later by intracellular cytokine staining for IFN-γ secretion by CD8+ T cells.
In three different experiments (Fig. 3A), LPS or poly IC treatment improved cross-presentation relative to PBS treated DC, although the latter could undergo spontaneous maturation in culture as previously reported [43-46]. Although these results cannot be used to assess if immature Flt3L-mobilized DC have any cross-presenting function for stimulation of IFN-γ production from T cells, the results indicate that anti-DEC-p24 mAb is efficiently cross-presented by more mature Flt3L DC, and all our subsequent studies used poly IC to stimulate the antigen-pulsed Flt3L DC.
Figure 3. Cross-presentation of anti-DEC-gag p24 is enhanced by poly IC and is restricted to DEC-205+ DC.
(A) Splenic Flt3L-mobilized CD11c+ DC were pulsed in vitro with 0.1 and 1 μg/ml of anti-DEC-p24, control Ig-p24, or p24 or p17 15-mer peptides (1 μg/ml). Five hours later, medium or LPS (0.1 μg/ml) or poly IC (25 μg/ml) were added. After overnight culture, the yields of cells were comparable. The cells were washed and added for 6 h to HIV gag primed T cells at a ratio of 1:3 with 10 μg/ml BFA. Data show mean ± SD percentage of CD8+ IFN-γ+ T cells pooled from three independent experiments. *p<0.05, paired Student's t-test, 1 μg/ml of anti-DEC-p24 vs. 1 μg/ml of control-Ig-p24 after maturation with poly IC. (B) CD11c+ cells from mice injected with B16 Flt3L melanoma cells were enriched by MACS positive selection and then separated into DEC-205+ and DEC-205- CD11chigh DC subsets by FACS. Sorted cells were washed and pulsed with either medium, anti-DEC-p24 vs. control Ig-p24 at 0.1 or 1 μg/ml, or p24 vs p17 peptides at 1 μg/ml. Five hours later, without washing off the antigens, poly IC at 25 μg/ml was added. After 15 h, graded doses of DEC-205+ and DEC205- cells (x-axis for DC:T cell ratio) were added to 3×106 HIV gag primed T cells, and IFN-γ secretion was assessed by intracellular cytokine staining 6 h later. Frequencies of CD8+ IFN-γ+ T cells from two independent experiments are shown after stimulation by DEC-205+ (left panel) and DEC-205- cells (right panel).
Splenic CD8α+ DEC-205+ DC are specialized to cross-present cell-associated and protein antigens [4, 19, 21, 25, 47]. In Fig. 1 we have shown that CD8α+ DEC-205+ cell numbers expanded considerably after Flt3L treatment with a profound increase skewed toward the DEC-205+ subset (Fig. 1). To verify that DEC-205+ DC were responsible for DEC-205 mediated cross-presentation of HIV gag, CD11c+ DEC-205+ and CD11c+ DEC-205- cells were purified and sorted by FACS from the spleens of mice inoculated with B16 Flt3L melanoma cells (Methods and Suppl. Fig. 1). Sorted cells were pulsed with anti-DEC-p24, then poly IC was added. After 15 h, graded doses of antigen-pulsed and matured DEC-205+ and DEC-205- cells were added to HIV gag primed T cells, and IFN-γ secretion was assessed 6 h later.
Both DC subsets were able to stimulate CD8+ T cells to secrete IFN-γ after in vitro incubation with a pool of HIV gag p24 15 mer peptides (Fig. 3B). However, when we analyzed cross-presentation of gag protein within anti-DEC-p24 mAb, only the DEC-205+ DC efficiently cross-presented HIV gag to primed CD8+ T cells over a wide range of DC to T cell doses, 1:10-1:90 (Fig. 3B). In contrast, there was no presentation of control Ig-p24, indicating that the DEC+ DC subset selectively cross-presents DEC-targeted protein.
With these results, we proceeded to carry out quantitative assays to assess the efficiency of HIV gag p24 cross-presentation by Flt3L-mobilized DC.
DEC-205 greatly increases the efficiency of HIV gag cross-presentation
To better evaluate the efficiency of cross-presentation by Flt3L-mobilized DC, we carried out more detailed studies of antigen and DC dose. When we pulsed Flt3L CD11c+ DC with different doses of anti-DEC-p24 vs control Ig-p24 overnight, along with maturation by poly IC, escalating doses of anti-DEC-p24 resulted in an increased percentage of IFN-γ+ CD8+ T cells, reaching a plateau at only 1 μg/ml of the fusion antibody (Fig. 4A). When used to stimulate HIV gag primed T cells at a DC:T cell ratio of 1:3, the Flt3L DC pulsed with 0.1 μg/ml of anti-DEC-p24 induced higher levels of IFN-γ secretion by CD8+ T cells than DC pulsed with 1 μg/ml control Ig-p24 (Fig. 4A). In Fig. 4B, we displayed the same results but from 4 different experiments.
Figure 4. Efficient gag-presentation to CD8+ and CD4+ T cells following in vitro targeting of Flt3L DC with anti-DEC-gag p24 mAb.
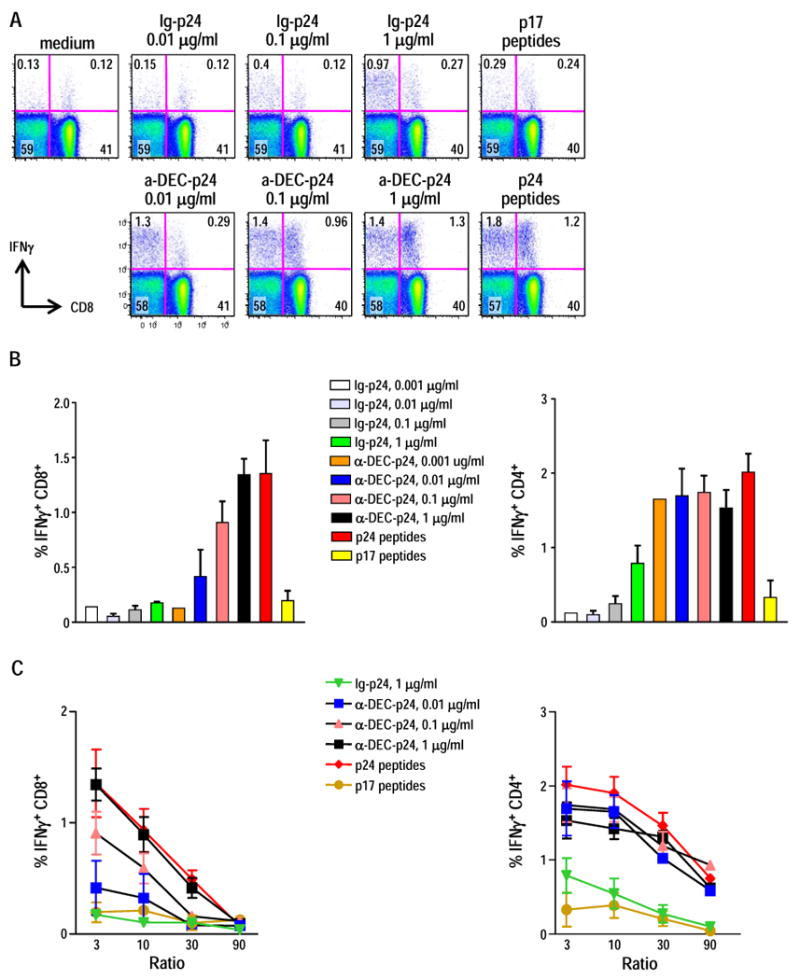
(A) Splenic Flt3L CD11c+ DC pulsed with anti-DEC-p24, control Ig-p24, p24 or p17 peptides at the concentration indicated, matured overnight with poly IC 25 μg/ml were used to stimulate IFN-γ secretion by HIV gag primed T cell in a 6 h assay at 1:3 DC: T cell ratio. Dot plots are representative of three independent experiments. (B) As in A, but Flt3L DC were pulsed with limiting dilutions of anti-DEC-p24 and control Ig-p24, matured with poly IC and cocultured for 6 h with primed HIV gag T cells. IFN-γ secretion from CD8+ T cells and CD4+ T cells was assessed by intracellular cytokine staining. Data show mean ± SD for the percentage of CD8+ IFN-γ+ T cells (left) or CD4+ IFN-γ+ T cells (right) from four independent experiments. (C) Graded doses of Flt3L DC, pulsed and matured as described in B, were cocultured for 6 h with primed HIV gag T cells (x-axis for DC:T cell ratio). IFN-γ secretion from CD8+ T cells (left) and CD4+ T cells (right) was assessed by intracellular cytokines. Mean ± SD of the frequencies of IFN-γ producing CD8+ and CD4+ T cells in three to four independent experiments are indicated.
Next, we assessed the role of DC dose. Flt3L DC were pulsed with a fixed dose of control Ig-p24 vs increasing concentrations of anti-DEC-p24 or gag peptides. After maturation with poly IC, DC were added in graded numbers to HIV gag primed/boosted T cells. In vitro cross-presentation of anti-DEC-p24 by Flt3L DC occurred in a DC-dose dependent manner. Flt3L DC pulsed with anti-DEC-p24 stimulated IFN-γ secretion of CD8+ T cells over a range of DC to T cell doses from 1:3 up to 1:90 (Fig. 4C, left panel). A similar DC dose dependence was obtained when we analyzed stimulation of CD4+ T cells after anti-DEC-p24 in vitro pulsing (Fig. 4C, right panel).
Together the results indicate that a low concentration of anti-DEC-p24 antibody mediates presentation of gag protein to both HIV specific CD8+ and CD4+ T cells in a DC-dose dependent fashion.
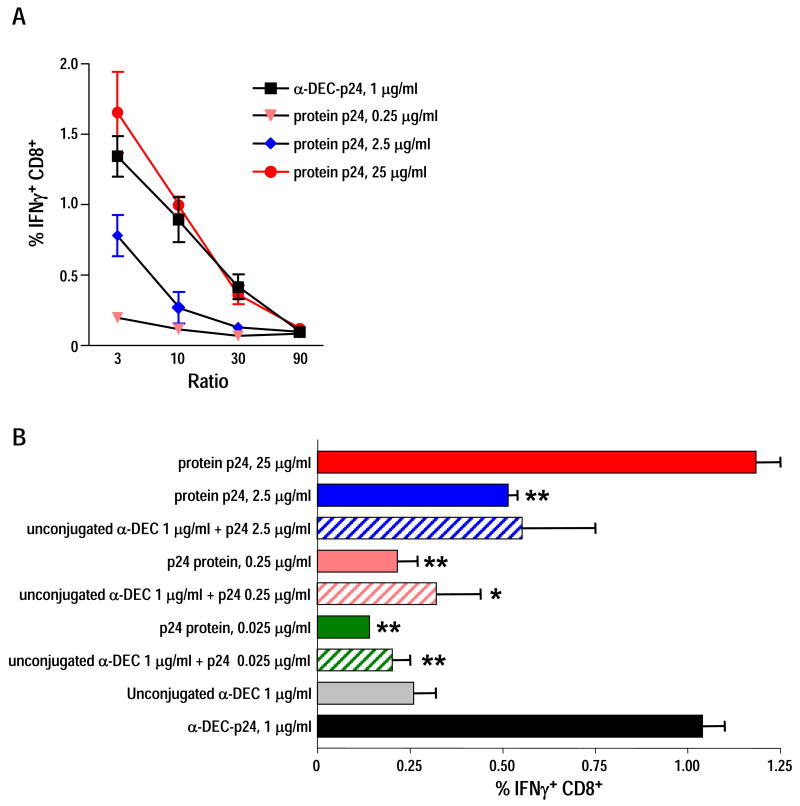
DEC-205 targeting greatly enhanced cross-presentation of soluble HIV gag p24 protein
Previous studies showed that only relatively high doses of antigens e.g., 50-100 μg/ml OVA, are able to bring about cross-presentation through the exogenous MHC-I pathway. We therefore wondered if DEC targeting could increase presentation of protein that was not coupled to the anti-DEC antibody. We first compared cross-presentation of anti-DEC-p24 mAb with soluble HIV gag p24 protein. In Fig. 5A we found that cross-presentation of soluble HIV gag protein to specific CD8+ T cells by Flt3L DC took place in a dose dependent manner, and that 25 μg/ml of gag was comparable to 1 μg/ml of anti-DEC-gag (or 0.25 μg/ml gag protein within the antibody). These results demonstrate that cross-presentation of anti-DEC-p24 by Flt3L DC improves efficiency almost 100 times relative to non-targeted gag protein.
Figure 5. Conjugation of DEC-205 to HIV gag, but not DEC-205 itself, greatly enhances cross-presentation of soluble HIV gag p24 protein.
(A) Splenic Flt3L CD11c+ DC were pulsed with 1 μg/ml of anti-DEC-p24 or increasing doses of HIV gag p24 soluble protein. The DC were matured with 25 μg/ml poly IC and added at various doses to stimulate primed HIV gag T cells in a 6 h coculture (x-axis for DC:T cell ratio). IFN-γ secretion from CD8+ T cells was assessed by intracellular cytokine staining. Data show mean ± SD of three independent experiments. (B) Splenic Flt3L CD11c+ DC were pulsed with 1 μg/ml of anti-DEC-p24, or 1 μg/ml of unconjugated anti-DEC, or different combinations of HIV gag p24 protein and unconjugated anti-DEC, or limiting doses of HIV gag p24 protein. As above, the DC were matured with poly IC and added at various doses to stimulate primed HIV gag T cells in a 6 h coculture (x-axis for DC:T cell ratio). IFN-γ secretion from CD8+ T cells was assessed by intracellular cytokine staining. Data show mean ± SD of two independent experiments. *p<0.05, **p<0.01, paired Student's t-test; 1 ug/ml of anti-DEC-p24 vs. other samples.
Then to rule out a boosting effect of the anti-DEC antibody itself, we analyzed CD8+ T cell responses to Flt3L DC pulsed in vitro with anti-DEC-p24 fusion mAb, unconjugated anti-DEC-205 mAb, the combination of unconjugated anti-DEC-205 with HIV gag protein, or the soluble HIV protein alone. As shown in Fig. 5B, the combination of unconjugated mAb with soluble HIV protein did not enhance gag presentation.
We conclude that introduction of a protein within anti-DEC-205 antibody greatly enhances the capacity of Flt3L-mobilized DC to cross-present a protective microbial antigen, HIV gag.
Discussion
The limited number of DC isolated from mouse spleen has often restricted their use for functional studies, particularly cross-presentation, where the relevant CD8α+ or DEC-205+ DC [4, 19-21, 47] represent a relatively small fraction of CD11c high DC. Flt3L is a regulator of hematopoietic cell development and increases the number of peripheral DC in various tissues of mice [26, 36]. It is now evident that the receptor, Flt-3 or Flt-2 or CD135, is a marker for committed progenitors of DC that form in the bone marrow and then continue to respond to Flt3L after migration via the blood into spleen and lymph nodes [48-53]. Interestingly the expansion in DC numbers was skewed towards the expansion of DEC-205+ cells over CD8+ DC. The CD8- DEC-205+ DC may represent precursors to CD8+ DEC-205+ DC [37]. One emphasis of our current study is that when mice are exposed to Flt3L, it becomes much more feasible to study cross-presentation, since the numbers and purity of cross-presenting DEC-205+ DC are greatly expanded.
The majority of studies of cross-presentation emphasize the clonal expansion of CD8+ TCR transgenic T cells. Polyclonal T cells from HIV gag primed mice also respond to cross-presenting DC, although the efficiency of presentation was increased by targeting the gag protein to the DEC-205 receptor. Previously we found that the targeting of HIV gag protein to the DEC-205 receptor on monocyte-derived human DC enhanced cross-presentation to gag-specific CD8+ T cells from individuals infected with HIV-1 [18]. In that study, we also compared different receptors, DEC-205, MMR and DC-SIGN, and obtained evidence that antigen delivery via DEC-205 was superior to the other endocytic receptors for expanding gag-specific CD8+ T cells [18]. Here our current studies were directed to DEC-205 targeting, where DEC-205 represents one receptor that is clearly expressed in vivo on most T cell area DC of human lymph nodes [54]. Other DC receptors and surface products might be expected to mediate improved presentation with antibody-targeted antigens. Here we provide quantitative information on the capacity of DC to present antigen via DEC-205, which also takes place in DC preparations that are abundant and highly enriched. Flt3L induced expansion of DC should greatly facilitate studies of cross-presentation and set the stage for the use of Flt3L to enhance the efficacy of DEC-205 targeted vaccines in vivo.
Immune responses require DC maturation induced by microbial molecular patterns, in particular by agonists for microbial pattern recognition receptors [55]. These agonists may directly enhance the intracellular mechanism for cross-presentation [29, 40, 42, 56]. The role of maturation is difficult to study with isolated DC because these undergo what is termed “spontaneous” maturation in culture. Nevertheless, we were able to show that maturation of Flt3L DC, either with LPS or with poly IC, improved cross-presentation of anti-DEC-p24.
Traditionally cross-presentation requires high doses of antigens with the most sensitive protein, OVA, typically being used at concentrations of >50 μg/ml or 1 nM to detect a signal even with OT-I TCR transgenic reporter T cells [33, 57]. In our prior experience with mouse DC, cross-presentation of OVA to OT-I T cells under similar culture conditions to the gag experiments in our paper required concentrations of 60-250 μg/ml OVA [13]. Here, by using the DEC-205 receptor to target the HIV gag protein to DC, we find that dose as low as 1 μg/ml of anti-DEC-p24 or 5 pM leads to strong presentation to CD8+ T cells from a polyclonal population. Also, relative to non-targeted gag p24 protein, DEC-205 mediated cross-presentation is 100 fold more efficient. The quantitative aspects of our data highlight the potential value that Flt3L mobilization and receptor mediated targeting potentially play in the use of cross-presentation to present non-replicating antigens to CD8+ T cells.
While the goal of this paper is to highlight the usefulness of Flt3L-mobilized DC in cross-presentation of a microbial protein, Flt3L mobilization may have a role in enhancing presentation of DC targeted vaccines. Others have reported that daily injections of Flt3L over a period of 9 days is able to expand DC numbers several fold in humans [58, 59]. Further experiments are required to assess if there are ways to combine DC mobilization and DC targeting to improve vaccination.
Materials and Methods
Mice
Balb/c × C57Bl/6 (C × B6) F1 mice from Harlan were maintained under specific pathogen-free conditions and used at 6-8 wk of age in accordance with Rockefeller University Animal Care and Use Committee guidelines.
B16 Flt3L melanoma cells
Melanoma cells expressing Fms-like tyrosine kinase 3 ligand (Flt3L), were established via retroviral gene transfer [60] and generously provided by L. Santambrogio (Albert Einstein College of Medicine, New York, NY). B16 Flt3L melanoma cells were cultured with DMEM containing 10% FBS and 5 × 106 were injected s.c into the belly region of mice. After 15-20 days, all major splenic DC subsets had expanded >10 fold as shown previously [36] and reproduced here.
Antibodies and reagents
We purchased from BD Biosciences FITC-antibodies to CD3ε (145-2C11), B220 (RA3-6B2), MHC-II (IAb) and DX5, PE- antibodies to CD8α (53-6.7), PDCA-1 and anti-CD11c (HL3 clone), PerCP-anti-CD4 (RM4-5), PerCP-Cy5.5-anti-CD8α, PE-Cy 7 anti-IFN-γ (XMG1.2), APC-antibodies anti-IFN-γ (XMG1.2) and thy 1.2 (CD 90.2, clone 53-2.1), Alexa Fluor 700 anti-CD3 (500A2), APC-Alexa 647-antibodies to DEC-205 and B220 as well as Cytofix/Cytoperm kit and Stabilizing Fixative. Anti-CD11c beads (N418) were from Miltenyi Biotec. Rat anti MHC class II (TIB120, M5/114.15.2) was from ATCC. Anti-rat IgG Dynalbeads and Live/Dead Fixable Aqua vitality dye were from Invitrogen. Polyinosinic-polycytidylic acid (poly IC) was from Thermo Scientific. Other reagents were LPS from Escherichia coli 0127:B8 and BFA from Sigma-Aldrich.
Fusion HIV gag mAb
The fusion mAb anti-DEC-p24 and the control Ig-p24 were prepared as described [17] and were characterized by SDS-PAGE and Western Blotting (HRP-conjugated anti-mouse IgG or anti-HIV gag). Binding of the fusion mAb to stable DEC-205 transfected CHO cells was tested by FACS analysis as described [17].
Soluble HIV gag p24 protein
The cDNA for the HIV gag p24 (clade B) was fused to the sequence containing a signal peptide and a FLAG epitope tag. This construct named SF-p24 (GenBank accession number GQ304738) was cloned into pCMV expression vector and stably transfected into CHO cells, in order to produce a soluble, FLAG-tagged (SF) protein of gag p24, which was purified without endotoxin contamination from the culture supernatants of CHO/SF-p24 cells, by anti-FLAG® M1 Affinity Gel (Sigma-Aldrich, St. Louis, MO) following manufacturer's instruction.
HIV gag peptide library
Overlapping (staggered by 4 aminoacids) 15 mer peptides spanning the entire HIV gag p17 or p24 sequence were synthesized by H. Zebroski in the Proteomics Resource Center (The Rockefeller University). HIV gag p24 or p17 peptides were resuspended at 1 mg/ml of each peptides in 100% DMSO and added to DC at 1 μg/ml.
Soluble HIV gag p24 protein
The cDNA for the HIV gag p24 (clade B) was fused to the sequence containing a signal peptide and a FLAG epitope tag. This construct named SF-p24 (GenBank accession number GQ304738) was cloned into pCMV expression vector and stably transfected into CHO cells, in order to produce a soluble, FLAG-tagged (SF) protein of gag p24. The SF-p24 protein was purified without endotoxin contamination from the culture supernatants of CHO/SF-p24 cells, by Anti-FLAG® M1 Affinity Gel (Sigma-Aldrich, St. Louis, MO) following the manufacturer's instruction.
Cell preparation
Spleens were removed from Flt3L treated mice, cut in small fragments, and digested into single cell-suspensions with 400 U/ml collagenase D (Roche Applied Science) for 25 min at 37°C. After inhibition of collagenase with 10 mM EDTA, the cells were resuspended in PBS in 2 mM EDTA and 2% FCS. CD11c+ DC were enriched by positive selection using anti-CD11c magnetic beads and MACS columns (Miltenyi Biotec). To purify CD8α+ and CD8α- cells, CD11c+ cells were sorted on a FACSVantage (BD Biosciences) into B220- DX5- CD3- CD11chigh CD8α+ and B220- DX5- CD3- CD11chigh CD8α- fractions. The purity of the DC subsets was >95-99%. Antigen primed T cells, which were from F1 mice primed with Adenovirus gag and boosted 4-6 wks later with anti-DEC-p24 and poly IC, were enriched by excluding MHC class II+ cells using TIB120/M5/114 rat mAb and anti-rat IgG Dynalbeads.
Mixed leukocyte reaction (MLR) assay for DC maturation and function
B16 Flt3L treated or untreated CxB6 F1 mice were injected i.p. with PBS or 50 μg of poly IC. Fifteen hours later spleens were collected and collagenase digested. CxB6 DC were fixed for 20 min on ice and graded numbers were added to 3×105 CFSE labeled (Molecular Probes, Eugene, OR) C57BL/6 T cells. After 4 days of culture, samples were stained with Live/Dead Fixable Violet viability dye (Invitrogen, Carlsbad, CA), Alexa 700 anti-CD3, APC Alexa 780 anti-CD8 and PerCP Cy 5.5 anti-CD4, and acquired on a BD LSR II flow cytometer (BD Biosciences). Data were analyzed with FlowJo Software (Tree Star, Inc.).
Ag-presentation assay to HIV gag specific T cells
2×106 splenic CD11c+ DC, or purified DC subsets, were first pulsed with different antigens (Results) for 5 h in 24-well culture plates in a final volume of 0.5 ml RPMI 1640 containing 5% FCS and the supernatant (3% vol/vol) from J558L cells transduced with murine GM-CSF. Then 25 μg/ml poly IC or 0.1 μg/ml of LPS was added to the cultures for 15-18h. The DC were washed three times with PBS and added to antigen-primed T cells. IFN-γ production during a 6 h DC:T cell coculture in the presence of 10 μg/ml of BFA was monitored by washing the cells, incubating 10 min at 4°C with 2.4G2 mAb to block FcγR, and staining with Live/Dead Fixable Aqua, anti-CD3, anti-CD8 and anti-CD4 mAbs for 20 min at 4°C. Cells were fixed and permeabilized 10 min with Cytofix/Cytoperm and stained with APC-conjugated anti-IFN-γ mAb for 15 min at room temperature and resuspended in stabilizing fixative. 105 live-CD3+ events were acquired using a BD LSR II flow cytometer. Data were analyzed with FlowJo Software.
Statistical analysis
Statistical significance was evaluated using two-tailed Student's t test, 95% confidence interval. Results are expressed as means ± SD. In the figures, p values of 0.05 are labeled with a single asterisk (*), 0.01 (**) or 0.001 (***). Analysis was performed with a Prism 3 program (Graphpad Sofware Inc.).
Supplementary Material
Acknowledgments
We thank Laura Santambrogio (Albert Einstein College of Medicine New York, NY) for Flt3L transduced B16 melanoma cells; Klara Velinzon for expert technical assistance with cell sorting; Henry Zebroski for synthesizing gag peptides; Juliana Idoyaga for anti-DEC-205 APC-Alexa 647; Bei Wang, Cheolho Cheong and Jaehoon Choi for their involvement in the late phase of this work; Olga Mizenina for endotoxin tests; and Judy Adams for help with graphics. This work was supported by grants from NIAID, AI13013 and AI40874 to RMS.
Abbreviations
- DC
dendritic cell
- OVA
ovalbumin
- Flt3L
Fms-like tyrosine kinase 3 ligand
- mAb
monoclonal antibody
- poly IC
polyinosinic:polycytidylic acid
Footnotes
Conflict of interest. RMS is a paid scientific consultant to Celldex Therapeutics which is developing DEC-205-targeted vaccines.
References
- 1.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 2.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu K, Iyoda T, Saternus M, Kimura K, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacken PJ, Joosten B, Reddy A, Wu D, Eek A, Laverman P, Kretz-Rommel A, Adema GJ, Torensma R, Figdor CG. No advantage of cell-penetrating peptides over receptor-specific antibodies in targeting antigen to human dendritic cells for cross-presentation. J Immunol. 2008;180:7687–7696. doi: 10.4049/jimmunol.180.11.7687. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 10.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 11.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 12.Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis ESC. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, Morrot A, Zavala F, Steinman RM, Nussenzweig RS, Nussenzweig MC. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sponaas AM, Cadman ET, Voisine C, Harrison V, Boonstra A, O'Garra A, Langhorne J. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J Exp Med. 2006;203:1427–1433. doi: 10.1084/jem.20052450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 17.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, Granelli-Piperno A, Steinman RM. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, Nussenzweig MC, Piperno AG, Steinman RM. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8α+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 22.Mahnke K, Qian Y, Fondel S, Brueck J, Becker C, Enk AH. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res. 2005;65:7007–7012. doi: 10.1158/0008-5472.CAN-05-0938. [DOI] [PubMed] [Google Scholar]
- 23.Johnson TS, Mahnke K, Storn V, Schonfeld K, Ring S, Nettelbeck DM, Haisma HJ, Le Gall F, Kontermann RE, Enk AH. Inhibition of melanoma growth by targeting of antigen to dendritic cells via an anti-DEC-205 single-chain fragment variable molecule. Clin Cancer Res. 2008;14:8169–8177. doi: 10.1158/1078-0432.CCR-08-1474. [DOI] [PubMed] [Google Scholar]
- 24.van Mierlo GJ, Boonman ZF, Dumortier HM, den Boer AT, Fransen MF, Nouta J, van der Voort EI, Offringa R, Toes RE, Melief CJ. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–6759. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 25.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, Belz GT, Carbone FR, Shortman K, Heath WR, Villadangos JA. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: Multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte- macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 28.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–3039. [PubMed] [Google Scholar]
- 29.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 30.Diener KR, Moldenhauer LM, Lyons AB, Brown MP, Hayball JD. Human Flt-3 ligand-mobilized dendritic cells require additional activation to drive effective immune responses. Exp Hematol. 2008;36:51–60. doi: 10.1016/j.exphem.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Garulli B, Stillitano MG, Barnaba V, Castrucci MR. Primary CD8+ T-cell response to soluble ovalbumin is improved by chloroquine treatment in vivo. Clin Vaccine Immunol. 2008;15:1497–1504. doi: 10.1128/CVI.00166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klotz L, Hucke S, Thimm D, Classen S, Gaarz A, Schultze J, Edenhofer F, Kurts C, Klockgether T, Limmer A, Knolle P, Burgdorf S. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor {gamma} is mediated by up-regulation of B7H1. J Immunol. 2009;183:129–136. doi: 10.4049/jimmunol.0804260. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 34.Merad M, Sugie T, Engleman EG, Fong L. In vivo manipulation of dendritic cells to induce therapeutic immunity. Blood. 2002;99:1676–1682. doi: 10.1182/blood.v99.5.1676. [DOI] [PubMed] [Google Scholar]
- 35.O'Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 36.Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand- treated mice. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 37.Bedoui S, Prato S, Mintern J, Gebhardt T, Zhan Y, Lew AM, Heath WR, Villadangos JA, Segura E. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J Immunol. 2009;182:4200–4207. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- 38.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Gil-Torregrosa BC, Lennon-Dumenil AM, Kessler B, Guermonprez P, Ploegh HL, Fruci D, van Endert P, Amigorena S. Control of cross-presentation during dendritic cell maturation. Eur J Immunol. 2004;34:398–407. doi: 10.1002/eji.200324508. [DOI] [PubMed] [Google Scholar]
- 41.Faure F, M A, Sadaka C, Sedlik C, Jotereau F, Amigorena S. Long-lasting cross-presentation of tumor antigen in human DC. Eur J Immunol. 2009;39:380–390. doi: 10.1002/eji.200838669. [DOI] [PubMed] [Google Scholar]
- 42.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentialy regulated during dendritic cell maturation. J Exp Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson NS, El-Sukkari D, Villadangos JA. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood. 2004;103:2187–2195. doi: 10.1182/blood-2003-08-2729. [DOI] [PubMed] [Google Scholar]
- 44.Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, Muramatsu S, Hodes RJ, Steinman RM. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, Germain RN, Mellman I, Steinman RM. The formation of immunogenic MHC class II- peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–936. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz V, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 48.Curti A, Fogli M, Ratta M, Tura S, Lemoli RM. Stem cell factor and FLT3-Ligand are strictly required to sustain the long-term expansion of primitive CD34(+)DR(-) dendritic cell precursors. J Immunol. 2001;166:848–854. doi: 10.4049/jimmunol.166.2.848. [DOI] [PubMed] [Google Scholar]
- 49.D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from flt3+ lymphoid and myeloid-committed progenitors to flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Randolph GJ, Rudensky AY, Nussenzweig MC. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onai N, Obata-Onai A, Tussiwand R, Lanzavecchia A, Manz MG. Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J Exp Med. 2006;203:227–238. doi: 10.1084/jem.20051645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Granelli-Piperno A, Pritsker A, Pack M, Shimeliovich I, Arrighi JF, Park CG, Trumpfheller C, Piguet V, Moran TM, Steinman RM. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005;175:4265–4273. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74:479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 56.Maurer T, Heit A, Hochrein H, Ampenberger F, O'Keeffe M, Bauer S, Lipford G, Vabulas R, Wagner H. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur J Immunol. 2002;32:2356–2364. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 57.Kurts C, Miller JFAP, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jefford M, Schnurr M, Toy T, Masterman KA, Shin A, Beecroft T, Tai TY, Shortman K, Shackleton M, Davis ID, Parente P, Luft T, Chen W, Cebon J, Maraskovsky E. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102:1753–1763. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 59.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, Caron D, Maliszewski C, Davoust J, Fay J, Palucka K. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 60.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.