Abstract
Cisplatin is one of the most effective anticancer agents widely used in the treatment of solid tumors. It is generally considered as a cytotoxic drug which kills cancer cells by damaging DNA and inhibiting DNA synthesis. How cells respond to cisplatin-induced DNA damage plays a critical role in deciding cisplatin sensitivity. Cisplatin-induced DNA damage activates various signaling pathways to prevent or promote cell death. This paper summarizes our current understandings regarding the mechanisms by which cisplatin induces cell death and the bases of cisplatin resistance. We have discussed various steps, including the entry of cisplatin inside cells, DNA repair, drug detoxification, DNA damage response, and regulation of cisplatin-induced apoptosis by protein kinases. An understanding of how various signaling pathways regulate cisplatin-induced cell death should aid in the development of more effective therapeutic strategies for the treatment of cancer.
1. Introduction
Cisplatin was discovered fortuitously by Dr. Rosenberg in 1965 while he was examining the effect of electromagnetic field on bacterial cell growth [1, 2]. Since the active principle that inhibited bacterial cell division was identified to be cisplatin, he anticipated that it would also inhibit the proliferation of rapidly dividing cancer cells. Cisplatin was indeed demonstrated to possess antitumor activity in a mouse model [3] and was first used in the clinical trial almost 30 years ago. Since its approval by the Food and Drug administration in 1978, cisplatin continues to be one of the most effective anticancer drugs used in the treatment of solid tumors.
Cisplatin has been used as a first-line therapy for several cancers, including testicular, ovarian, cervical, head, and neck and small-cell lung cancers either alone or in combination with other anticancer agents. It is also used as an adjuvant therapy following surgery or radiation. In addition to cisplatin, its analogs, such as carboplatin and oxaliplatin, are also currently being used in the clinic. However, patients who initially respond to cisplatin therapy often develop resistance to the drug during the course of the treatment.
The success of cisplatin therapy is compromised due to dose-limiting toxicity, especially nephrotoxicity as well as resistance by tumor cells to cisplatin. Cellular resistance to cisplatin could be either intrinsic or acquired. The clinically acquired resistance can be caused by decreased drug accumulation which includes reduced uptake or increased efflux of cisplatin, increased drug detoxification by cellular thiols, increased DNA repair or tolerance of cisplatin-damaged DNA and the ability of the cancer cells to evade cisplatin-induced cell death. Numerous studies have focused on the drug-target interactions, cellular pharmacology, and pharmacokinetics of cisplatin. Another active area of research has been to develop analogs of cisplatin to minimize toxicity and circumvent cisplatin resistance.
The antitumor activity of cisplatin is believed to be due to its interaction with chromosomal DNA [4]. Only a small fraction of cisplatin, however, actually interacts with DNA and the inhibition of DNA replication cannot solely account for its biological activity [5]. In addition, the efficacy of chemotherapeutic drugs depends not only on their ability to induce DNA damage but also on the cell's ability to detect and respond to DNA damage [6]. Following DNA damage, cells may either repair the damage and start progressing through the cell cycle or if they cannot repair the damage, cells proceed to die [5]. Cisplatin, like many other chemotherapeutic drugs, can induce apoptosis. Thus, the signaling pathways that regulate apoptosis have significant impact on deciding cellular responsiveness to cisplatin. There are many excellent reviews on cisplatin and its analogues [7–15]. In this paper, we primarily focused on recent studies on cellular responses to cisplatin-induced DNA damage although we briefly discussed steps leading to cisplatin-induced DNA damage. This comprehensive paper should not only benefit researchers in the field of cisplatin but also benefit those interested in mechanisms of chemoresistance and targeted therapy.
2. Biotransformation of Cisplatin
Cisplatin or cis-diamminedichloroplatinum(II) is a neutral, square-planar, coordination complex of divalent Pt [8]. The cis configuration is required for its antitumor activity [16]. It has two labile chloride groups and two relatively inert amine ligands. Cisplatin undergoes hydrolysis in water. The chloride concentration is an important factor in determining the hydrolysis or aquation of cisplatin. The high chloride concentration (~103 mM) of blood plasma prevents the hydrolysis of cisplatin. Upon entering the cell, the chloride concentration drops down to 4 mM which facilitates the aquation process [17]. The aquated form of cisplatin is a potent electrophile and reacts with a variety of nucleophiles, including nucleic acids and sulfhydryl groups of proteins.
3. Accumulation of Cisplatin Inside Cells
Cisplatin and its analogues were initially thought to enter cells by passive diffusion because cisplatin uptake was linear, nonsaturable and could not be competed with platinum analogs [4–6, 17]. Although decreased accumulation of cisplatin is often associated with acquired resistance to cisplatin, few or no changes were observed in the plasma membrane function in the cisplatin-resistant cell lines as compared to the parental cells [18–20]. In 1981, it was first proposed that cisplatin could be transported actively via the carrier-mediated transport [21]. Several transporters, including the Na+, K+-ATPase [22] and members of solute carrier (SLC) transporters [11] have been implicated in facilitating the entry of cisplatin into the cells. The plasma membrane copper transporter-1 (CTR1), a member of the SLC family, gained particular attention since a defect in Ctr1 gene decreased cisplatin accumulation in yeast [23, 24]. In addition, cisplatin and carboplatin accumulation was attenuated in mouse embryonic fibroblasts from ctr1 −/− animals compared to wild-type animals [18]. Interestingly, both copper and cisplatin were shown to cause rapid downregulation of CTR1 in ovarian cancer cells by the proteasome-mediated pathway [19]. While CTR1 appears to transport cisplatin and its analogs, there is little decrease in CTR1 when cells acquire resistance to cisplatin. A recent study demonstrated that copper transporter-2 or CTR2 limits accumulation of cisplatin and the level of CTR2 correlates with the sensitivity of ovarian carcinoma cells to cisplatin [25]. The organic cationic transporters SLC22 family of proteins have also been shown to participate in cisplatin influx [11]. Thus, cisplatin can enter cells by passive or facilitated diffusion and by active transport. Depending on the cellular context, multiple transporters may be involved in cisplatin uptake. Therefore, it is difficult to correlate cisplatin sensitivity/resistance with a particular transporter.
Many cell lines with acquired resistance to cisplatin often exhibit reduced drug accumulation. Unlike multidrug resistance (MDR), drug efflux does not appear to be the major cause of cisplatin resistance. Kawai et al. first reported that a 200-kDa plasma membrane glycoprotein is overexpressed in murine thymic lymphoma cells selected for resistance to cisplatin, which correlated with reduced accumulation of cisplatin in the cells [26]. The increased expression of this protein correlated with the degree of cisplatin resistance [26]. There was, however, no follow-up study to establish the importance of this protein in conferring cisplatin resistance. The ATP-dependent glutathione-conjugated efflux pump and copper transporters ATP7A and ATP7B have been implicated in cisplatin export [20]. It is generally believed that reduced cisplatin accumulation in cisplatin resistant cells is due to decrease in uptake of cisplatin rather than an increase in drug efflux [7, 27].
4. Formation and Repair of Cisplatin DNA Adduct
DNA is thought to be the primary biological target of cisplatin [17, 28, 29]. The platinum atom of cisplatin forms covalent bonds with the N7 position of purine bases to form 1,2- or 1,3-intrastrand crosslinks and a lower percentage of interstrand crosslinks. Cisplatin resembles bifunctional alkylating agents. The intrastrand crosslink between two adjacent G residues is believed to be the critical lesion responsible for cisplatin cytotoxicity. Formation of cisplatin-DNA adducts interferes with DNA replication and transcription. The interstrand and intrastrand crosslinks disrupt the structure of the DNA. This alteration in the structure is recognized by the cellular proteins to repair cisplatin-induced DNA damage. Increased repair of cisplatin-induced DNA damage has been associated with cisplatin resistance.
4.1. Cisplatin and Nucleotide Excision Repair Pathway
Since the intrastrand cross-link is the major lesion caused by cisplatin-induced DNA damage, it is primarily repaired via the nucleotide excision repair (NER) system. Xeroderma Pigmentosum (XP) is a disorder caused by deficiency of genes involved in NER. Cells derived from XP patients are exquisitely sensitive to cisplatin [30]. In addition, the favorable response of testicular cancer to cisplatin was associated with low levels of XP complementation group A (XPA) and excision repair cross-complementation group I (ERCCI), which participate in NER [31, 32]. A number of studies correlated the overexpression of ERCC1 or XPA proteins with cisplatin resistance [31–34]. The existence of ERCC1 exon VIII alternative splicing was observed in ovarian cancer cells [35]. Although this splicing did not affect the level of ERCC1, it decreased its excision repair function. In addition, epigenetic changes, such as hypermethylation of ERCC1, which inversely correlated with ERCC1 mRNA levels, have been suggested as a mechanism for enhanced cisplatin sensitivity [36]. A recent study demonstrated that XPA binding domain of ERCC1 was required for the repair of cisplatin-damaged DNA [37]. A double knockdown of XPF/ERCC1 complex was shown to be very effective in enhancing cisplatin sensitivity in non-small cell lung cancer cells [38]. An antisense DNA against XPA sensitized lung adenocarcinoma cells to cisplatin [34]. Recently, it has been reported that treatment of rat spiral ganglion neurons with cisplatin induced the mRNA levels of XPA and XPC along with nuclear translocation of these enzymes, thus decreasing the rate limiting step in the NER pathway [39]. This can provide a plausible mechanism by which cisplatin induces NER. Kang et al. made an interesting observation that XPA was regulated in a circadian fashion in the mouse liver, but not in the testis [40]. Removal of cisplatin-DNA adducts also followed a circadian pattern in the extracts derived from the liver. The authors proposed that chronochemotherapy could be more effective in the treatment of cancers in which XPA removes cisplatin-DNA adducts in a circadian fashion. Thus, the cisplatin-induced DNA repair employing the NER process is multilayered including epigenetic, transcriptional, and posttranslational regulation.
NER is also linked to the cellular signaling pathways. It has been reported that the NER process may prevent cisplatin-induced apoptosis by activating the ataxia telangiectasia mutated (ATM) pathway which is recruited to the damaged DNA through XPC [41]. Lack of functional p53 has been associated with persistence of cisplatin-induced intrastrand cross-links, suggesting the importance of p53 in regulating NER of cisplatin-damaged DNA [42]. Functional NER was also required for cisplatin-induced transcription of Bcl-xL via nuclear factor-kappa B (NF-κB) [43].
In addition to NER, cisplatin can also induce transcription-coupled repair (TCR). The intrastrand crosslink stalls RNA polymerase II to trigger TCR [44]. It has been reported that p53 protects against cisplatin-induced apoptosis in a TCR-dependent manner [30]. In addition, the homology-directed DNA repair (HR) that allows error-free repair of the double-strand breaks caused by the excision of cisplatin-DNA adducts has been implicated in the repair of cisplatin-induced DNA damage [45]. It has been reported that mouse mammary tumors containing irreparable null alleles of Brca1 gene, which is involved in DNA double strand break repair, do not become resistant to cisplatin. Bypass of cisplatin-DNA adduct has also been associated with cisplatin resistance. DNA polymerase-eta could replicate across intrastrand cross-link between cisplatin and two adjacent G residues [46].
4.2. Cisplatin and Mismatch Repair Pathway
Mismatch repair (MMR) system recognizes cisplatin-induced DNA damage, but instead of increasing cell viability, MMR system was shown to be important for cisplatin-mediated cytotoxicity [47]. DNA mismatch repair protein, MutSα recognized DNA lesions formed by cisplatin [48–50], and mutations in MSH1 or MLH1 genes of the MMR system were observed in cisplatin-resistant cells [51–53]. Recently, the proapoptotic function of cisplatin was shown to be mediated in an MSH2/MSH6-dependent manner [54]. MLH1-proficient cells were more sensitive to cisplatin compared to MLH1-deficient cells. Cell death by cisplatin was associated with significant proteolysis of MLH1, caused by destabilization of X-linked inhibitor of apoptosis protein (XIAP), resulting in caspase activation [55]. The repair function of MMR proteins has been reported to be uncoupled from their function in mediating cisplatin-induced cell death [56–58]. Since the primary mechanism of cisplatin involves DNA damage and p53 is also involved in DNA damage signaling, there are many studies that correlate cisplatin and DNA damage and repair with p53 activity [59–62]. It has been reported that cisplatin enhances the interaction between mismatch repair protein MLH1/postmeiotic segregation increased 2 (PMS2) and p73 triggering apoptosis in mismatch repair-proficient cells [60].
5. Interaction of Cisplatin with Cellular Thiols
Although the major target of cisplatin is the nuclear DNA, it exhibits a high affinity towards sulfur donors such as cysteines and methionines forming stable Pt-S bonds. This competes with the affinity towards the nitrogen atom in the DNA thus contributing towards resistance against the cytotoxic action of cisplatin [63]. The abundant intracellular thiols involved in the drug resistance are glutathione and metallothionein.
5.1. Interaction of Cisplatin with Glutathione
When cancer cells are exposed to cisplatin, the platinum atom in cisplatin is chelated by glutathione (GSH) and the glutathione-Pt complex is effluxed from the cell in an ATP-dependent manner by the glutathione transporter family, termed the GS-X pumps [64]. It was initially noted that cells that are resistant to cisplatin have elevated levels of glutathione [65]. However, recent studies with cisplatin-resistant cancer cell lines seem to suggest otherwise [66, 67]. Based on NMR studies, Kasherman et al. reported that the higher levels of GSH do not correlate with decreased sensitivity to cisplatin [68]. In agreement with this study, Chen et al. suggested that increased levels of GSH might sensitize cells to cisplatin by upregulation of the copper transporter hCtr1 [69]. Therefore, whether overexpression of glutathione contributes to or combats cisplatin resistance is still under debate.
Apart from GSH, the glutathione S-transferase P1-1 (GSTP1-1) enzyme has also been associated with resistance to cisplatin-based chemotherapy [70, 71]. Pasello et al. demonstrated that increased levels of GSTP1 were associated with cisplatin resistance in osteosarcoma cell lines and a higher relapse rate and poor prognosis in high-grade osteosarcoma patients [72]. In contrast, a recent study by Peklak-Scott et al. suggested that a high level of cisplatin resistance may not be due to conjugation of cisplatin to glutathione by GSTP1 [73]. Other enzymes in the glutathione transferase family, such as the GSTμ or the GSTO1-1, have also been implicated in contributing towards cisplatin resistance [74, 75]. Thus, although a great deal of work has been focused on the correlation of glutathione and its conjugating enzymes towards cisplatin resistance, further studies are needed to explore this in detail.
5.2. Interaction of Cisplatin with Metallothionein
Metallothioneins (MT) are cysteine-rich proteins, which consist of 61-68 amino acids of which 20 are cysteins. The four isoforms of MTs (MT1-MT4) are ubiquitously expressed in humans and are inducible by a variety of drugs, including cisplatin [76]. They are involved in zinc and copper homeostasis, heavy metal detoxification, and protection from apoptosis. Initial reports observed an increased expression of metallothionein correlating with cisplatin resistance in ovarian carcinoma cell lines [77]. We have also seen that a wide variety of human cancer cell lines with acquired resistance to cisplatin overexpressed metallothionein and ectopic expression of metallothionein conferred cisplatin resistance [78]. Recent reports also suggest that the increased expression of metallothionein correlates with cellular resistance against cisplatin [79–81].
The ubiquitously occurring metallothionein isoforms, MT-1 and MT-2, have been shown to react faster with cisplatin [82], compared to glutathione [83, 84]. The basal levels of MT-1 and MT-2 are often significantly increased in cancer cells [78, 85], resulting in even stronger scavenging of divalent platinum, and contributing to acquired resistance against cisplatin [78, 86, 87]. MT-3 isoform was initially thought to be unresponsive to the platinum drugs [81]. Recent reports, however, suggest that MT-3 is overexpressed in hypoxic conditions, and the reaction between MT-3 and Pt(II) is kinetically preferred [81]. The authors further proposed that the Zn(II) released from this reaction can result in the upregulation of the MT-1 and MT-2 isoforms. Thus, metallothionein isoforms play an important role in contributing towards cisplatin resistance.
6. Cisplatin and DNA Damage Signaling
Various stress signals generate DNA lesions that may lead to mutations and genomic instability. Following DNA damage, cell cycle checkpoints are activated to delay cell-cycle progression to provide time for DNA repair or eliminate genetically unstable cells by inducing cell death. It is now recognized that inhibition of DNA replication is not sufficient to explain cisplatin cytotoxicity. How cells respond to cisplatin-induced DNA damage plays a major role in the ultimate decision whether a cell should live or die following cisplatin treatment.
6.1. p53 and Cisplatin-Induced DNA Damage Response
The tumor suppressor protein p53 is considered as the “guardian of genome”. It plays a critical role in eliciting cellular responses to DNA damage. p53 is a short-lived protein which is primarily degraded via the ubiquitin proteasome-mediated pathway [30, 88]. The E3 ubiquitin ligase Mdm2 is a transcriptional target of p53 and regulates p53 expression via a negative feedback loop [30]. DNA damage results in the activation of ATM and/or ATM- and Rad3-related (ATR), resulting in phosphorylation and stabilization of p53 [88]. p53 can transactivate genes involved in cell cycle progression (e.g., p21), DNA repair (e.g., growth arrest and DNA damage-inducible 45, GADD45), and apoptosis (e.g., Bax) [89].
Fujiwara et al. first demonstrated that adenoviral-mediated delivery of p53 into small-cell lung cancer cells induced massive apoptosis both in monolayer cultures and in tumor xenografts upon treatment with cisplatin [90]. Introduction of wild-type p53 by adenovirus vector also sensitized ovarian cancer cells to cisplatin [91–93]. Several proteins, including cyclin-dependent kinase inhibitor p21, ATR, and checkpoint kinase (CHK2) have been implicated in p53-mediated apoptosis [94–97]. In addition, tumor cells lacking functional p53 were more resistant to cisplatin than cells that contained functional p53 and the resistant cell lines were sensitized to cisplatin upon reconstitution with wild-type p53 [98, 99]. p53 itself has been shown to bind cisplatin-modified DNA [100]. In addition, cisplatin was shown to induce nitrosylation of p53 preventing its mitochondrial translocation [101].
p53 can regulate cisplatin-induced cell death by several mechanisms. Degradation of FLIP (FLICE-like inhibitory protein) has been reported to be necessary for p53-induced apoptosis in response to cisplatin [102, 103]. p53 also promotes cisplatin-induced apoptosis by directly binding and counteracting the antiapoptotic function of Bcl-xL [104]. Although the phosphatase and tensin homolog (PTEN) is believed to inhibit phosphoinositide 3-kinase (PI3K)/Akt, overexpression of PTEN was shown to involve p53-mediated apoptotic cascade in cisplatin-resistant ovarian cancer cells independent of PI3K/Akt pathway [105]. The nutrient-sensor AMP-kinase (AMPK) was shown to be activated by cisplatin in AGS and HCT-116 cancer cells and inhibition of AMPK enhanced cisplatin-induced apoptosis by causing hyperinduction of p53 [106].
One of the major side effects of cisplatin therapy is nephrotoxicity and the involvement of p53 in cisplatin-induced nephrotoxicity has been investigated. p53 induced proapoptotic Bcl-2 family member PUMAα in renal tubular cells upon treatment with cisplatin, and dominant-negative p53 suppressed the expression of PUMAα. This study was extended in C57 mice. Acute renal failure upon cisplatin treatment was abrogated in p53-deficient C57 mice and this was associated with little or no induction of PUMAα [107]. CHK2 has also been implicated in apoptosis of renal cells and tissues as a result of cisplatin-induced p53 activation [94]. A study by Yang et al. revealed that caspase-6 and -7 are transcriptional targets of p53 [108]. Thus, induction of p53 by cisplatin resulted in the activation of these caspases contributing to nephrotoxicity [108]. Inhibition of p53 by pharmacological inhibitor or knockout of p53 in mice suppressed caspase-6 and -7 transactivation and protected against nephrotoxicity. Recently, microRNAs have also been shown to play a major role in cisplatin nephrotoxicity. miR-34a is induced by cisplatin via p53 and plays a cytoprotective role in the survival of proximal tubular cells [109].
Although a plethora of literature exists on the role of p53 in contributing towards cisplatin cytotoxicity, p53 has also been associated with cisplatin resistance. MCF-7 breast cancer cells containing wild-type p53 are highly resistant to cisplatin but disruption of p53 by the introduction of human papilloma virus (HPV) in MCF-7 cells sensitized these cells to cisplatin [110]. Ovarian cancer cells selected for cisplatin resistance exhibited higher levels of p53 compared to cisplatin-sensitive counterpart [111]. Although p53 level is low in HeLa cells due to degradation of p53 by HPV, it was elevated in cisplatin-resistant HeLa cells [112]. Studies have also shown that mutations in p53 contributed to cisplatin resistance in different cancer models [113–116].
Although p53 plays an important role in cisplatin-induced DNA damage response, p53-negative cells also respond to cisplatin-induced DNA damage, suggesting alternate pathways of sensing cisplatin-induced DNA damage.
6.2. c-Abl and Cisplatin-Induced DNA Damage Response
The tyrosine kinase c-Abl plays an important role in stress response to DNA damaging agents. It belongs to the nonreceptor tyrosine kinases and contains nuclear localization motifs and nuclear export signals. Thus, it can shuttle between the nucleus and cytoplasm. Nuclear import of c-Abl was shown to be necessary for DNA damage-induced apoptosis [117]. It is activated in response to cisplatin causing activation of c-Jun-N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) [118]. c-Abl-deficient cells fail to activate JNK. Nuclear c-Abl can associate with and phosphorylate MEK kinase 1 (MEKK1) in response to DNA damage resulting in the activation of JNK/SAPK [119]. Nehmé et al. [120] demonstrated that activation of c-Abl and JNK is contingent upon the recognition of cisplatin-induced DNA damage by the MMR system since c-Abl response is absent in MMR-deficient cells. They further demonstrated that the activation by these pathways is specific to cisplatin and not to the cisplatin analogue oxaliplatin, thus highlighting the importance of the MMR system to specifically recognize cisplatin-DNA adducts [120, 121].
Interestingly, MMR/c-Abl cooperates with p73, a member of the p53 family, to trigger apoptosis [122]. Cisplatin caused induction of p73 in several cancer cell lines and in mouse embryonic fibroblasts (MEF), which were proficient in mismatch DNA-repair pathway but not in MEF deficient in c-Abl or MMR [122]. Activation of c-Abl in response to cisplatin led to phosphorylation and stabilization of p73 [123, 124]. Phosphorylation of p73 can also increase its proapoptotic function by dissociating itself from p63, another member of the p53 family. p63 can bind to and counteract the proapoptotic function of p73 [125]. In addition, c-Jun was shown to enhance p73 stability and transactivation activity by preventing its degradation via the proteasomal pathway [126]. Binding of the transcription coactivator Yap1 also prevents proteasomal degradation of p73 and results in the recruitment of p300 to trigger transcription of proapoptotic genes. c-Abl can directly phosphorylate Yap1, increasing its stability and affinity for p73 [127]. Phosphorylation of Yap1 can dictate whether p73 will transactivate proapoptotic or growth arrest genes [127]. A recent study suggests that c-Abl can regulate the function of p63. Phosphorylation of p63 at Tyr residue by c-Abl stabilizes it causing an increase in its proapoptotic function [128].
Cisplatin can trigger cleavage of c-Abl which is a substrate for caspase and proteolytic cleavage of c-Abl was shown to be important for cisplatin-induced apoptosis [129]. Activation of p38 MAPK is critical for regulating cisplatin activity. Galan-Moya et al. [130] recently reported that c-Abl activates p38 MAPK independent of its tyrosine-kinase activity but by stabilizing MKK6, the upstream kinase of p38 MAPK. This study provides an explanation why the c-Abl inhibitor imatinib fails to inhibit p38 MAPK [130]. Thus, c-Abl is an important mediator of cisplatin-induced DNA damage response and acts in cooperation with the siblings of p53 and MAPK pathways to trigger cisplatin-induced apoptosis.
7. Regulation of Cisplatin-Induced Cell Death by Protein Kinases
Cisplatin primarily induces cell death by apoptosis and a defect in apoptotic signaling could also confer cisplatin resistance. There are two major pathways of cell death [131, 132]. The extrinsic pathway is initiated when ligands bind to the tumor necrosis factor-α (TNFα) receptor superfamily followed by oligomerization and recruitment of procaspase-8 via adaptor molecules to form the death-inducing signaling complex (DISC). The intrinsic pathway is initiated by cellular stress, such as DNA damage, resulting in release of cytochrome-c from the mitochondria causing activation of procaspase-9 through the interaction with apoptosis promoting activating factor-1 (APAF-1) and formation of an active apoptosome complex. Bcl-2 family proteins regulate DNA damage-induced apoptosis by regulating the release of mitochondrial cytochrome c in response to DNA damage. Cisplatin-induced genotoxic stress activates multiple signal transduction pathways, which can contribute to apoptosis or chemoresistance.
7.1. Cisplatin and Protein Kinase C
Protein kinase C (PKC) is a family of closely related phospholipid-dependent enzymes that play critical roles in signal transduction and cell regulation [133–136]. Based on the structure and biochemical properties they are grouped as conventional (α, βI, βII, and γ), novel (δ, ε, η, and θ) and atypical (ζ and ι) PKCs. Tumor-promoting phorbol esters are potent activators of PKCs but persistent treatment with phorbol esters can induce downregulation or degradation of phorbol ester-sensitive conventional and novel PKCs.
We inadvertently found that the PKC signal transduction pathway can regulate cisplatin sensitivity. In the meantime, Hofmann et al. reported that inhibition of PKC by quercetin or downregulation of PKC by the phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA) could enhance the antiproliferative activity of cisplatin [137]. In contrast, Isonishi et al. [138] and we [139] simultaneously reported that activation of PKC by phorbol esters could enhance sensitivity of human ovarian cancer 2008 and human cervical cancer HeLa cells to cisplatin. There were contrasting reports whether activation or downregulation of PKC was necessary for cisplatin sensitization [138, 140]. Although TPA is a useful tool as a pharmacological agent to study PKC function, it is a tumor promoter and therefore cannot be used in the clinic. We first showed that bryostatin 1, a partial PKC agonist which lacks tumor promoting activity, also sensitized HeLa cells to cisplatin [141]. Based on the preclinical studies, Phase II trial using combination of bryostatin 1 and cisplatin was initiated in advanced recurrent cervical carcinoma but was not very effective [142]. Combination of bryostatin 1 and cisplatin had minimal toxicity in patients with refractory nonhematological malignancies although only four patients achieved an objective response [143]. This may be because bryostatin 1 is a partial agonist and its regulation is complex. One of the caveats with these earlier studies to define the role of PKC in regulating cisplatin sensitivity was that PKC activation and downregulation was monitored based on PKC activity assay which does not discriminate among PKC isozymes. We now know that PKC isozymes may have distinct and even opposite effects on cisplatin-induced cell death [144]. Another shortcoming with these studies was the use of pharmacological agents that lack absolute specificity to PKC.
An increase in novel PKCδ or -ε and a decrease in conventional PKCs have been associated with acquired resistance to cisplatin [145]. However, inhibition of PKCα by Gö 6976 and depletion of PKCα by siRNA enhanced sensitivity of both parental and cisplatin-resistant HeLa cells to cisplatin [146]. Antisense oligonucleotides against PKCα enhanced the antitumor activity of cisplatin against human breast cancer MCF-7, prostate cancer PC3, and human small cell carcinoma H69 cells transplanted in nude mice [147]. Additionally, antisense oligonucleotide against PKCα in combination with cisplatin was effective in patients with non-small cell lung cancer [148]. Furthermore, although PKCα was downregulated in cisplatin-resistant A2780 cells, introduction of PKCα in these cells attenuated cisplatin sensitivity [149]. A recent study demonstrated that inhibition of PKCβ by enzastaurin enhanced cisplatin sensitivity via dephosphorylation of p90 ribosomal S6 kinase and Bad [150]. These results suggest that conventional PKCα and -β function as antiapoptotic proteins. It is not clear why a decrease rather than an increase in cPKCs was associated with cisplatin resistance.
The observation that PKCδ is a substrate for caspase-3 [151] established the importance of this PKC isozyme in apoptotic signaling. It has been reported that treatment of cisplatin-resistant human squamous cell carcinoma SCC-25 (SCC25/CP) cells to cisplatin failed to induce caspase-3 activation and cleavage of PKCδ due to an increase in antiapoptotic Bcl-xL [152]. Interestingly, the effect of bryostatin 1 on caspase activation and PKCδ downregulation followed similar biphasic concentration response in both parental and cisplatin-resistant HeLa cells [146, 153]. We have shown that PKCδ not only acts downstream of caspase-3 but it can also regulate cisplatin-induced activation of caspase-3 [154]. These studies were based on the effect of rottlerin, a pharmacological inhibitor of PKCδ on cisplatin-induced apoptosis [155]. Although rottlerin caused downregulation of caspase-2 and inhibition of cisplatin-induced apoptosis, the effect of rottlerin on caspase-2 downregulation was not due to inhibition of PKCδ [156]. The effect of PKCδ on cisplatin-induced apoptosis depends on the cellular context. In gastric cancer MKN28 cells, PKCδ was shown to enhance cisplatin-induced caspase activation and cell death via p53 [157]. Overexpression of PKCδ or caspase cleavage-resistant mutant of PKCδ had little effect on cisplatin-induced cell death in human small-cell lung cancer H69 cells which have mutated p53 [158]. On the other hand, knockdown of PKCδ enhanced cisplatin-induced cell death in thyroid cancer by decreasing fos expression [159].
We have shown that overexpression of PKCε contributes to cisplatin resistance by inhibiting cisplatin-induced apoptosis [160]. Integrative genomic approach has identified PKCι as a potential oncogene for ovarian carcinoma [161]. It has also been associated with chemoresistance of glioblastoma multiforme, an aggressive form of brain cancer [162]. The mechanism of PKCι-mediated chemoresistance involved inhibition of p38 MAPK [162]. A recent study suggests that atypical PKCζ can counteract the ability of cisplatin to decrease matrix metalloproteinase-2 secretion [163]. Thus, the effects of PKC on cellular sensitivity/resistance to cisplatin depend on the pattern of the PKC isozymes as well as on the cellular context.
7.2. Cisplatin and MAPK
Mitogen-activated protein kinases (MAPK) are a family of structurally-related serine/threonine protein kinases that coordinate various extracellular signals to regulate cell growth and survival [164–166]. There are three major subfamilies of MAPK: extracellular signal-regulated kinase (ERK)-1 and -2, stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) and p38 MAPK. All three MAPKs have been implicated in regulating cisplatin-induced cell death.
ERK is activated in response to growth factors and mitogens. Cisplatin has been shown to cause activation of ERK in several cell types although there are controversies whether activation of ERK prevents or contributes to cisplatin-induced cell death [167–173]. ERK has been shown to function as a prosurvival protein in ovarian cancer [102, 174], melanoma [175], cervical cancer SiHA [176], human myeloid leukemic [177], and gastric cancer [178] cells. High basal nuclear phospho-ERK2 was associated with cisplatin resistance of ovarian cancer OVCAR-3 cells [179]. Furthermore, nanoparticle-mediated delivery of MEK inhibitor PD98059 enhanced antitumor activity of cisplatin in melanoma-bearing mice [180]. Cisplatin-induced ERK activation precedes p53-mediated DNA damage response since ERK directly phosphorylates p53 causing upregulation of p21, GADD45, and Mdm2 [181]. Thus, activation of ERK may cause cell cycle arrest allowing time for the repair of cisplatin-induced DNA damage via p53. ERK also induced phosphorylation of BAD at Ser112 site in response to cisplatin in ovarian cancer cells, and inhibition of ERK by PD98059 or mutation of Ser112 to Ala sensitized cells to cisplatin [174]. In SiHA cells, phosphorylation and activation of NF-κB were associated with the prosurvival function of ERK [176]. Glutathione-mediated cisplatin transport and GSTP1 expression also contributed to the antiapoptotic function of ERK in human myeloid leukemic cells [177] and gastric cancer cells [178], respectively. Recently, it has been reported that ovarian cancer cells grown in three-dimensional cultures acquired resistance to anoikis and apoptosis when exposed to clinically relevant concentrations of cisplatin [167]. This resistance was mediated by the ERK1/2 signaling and the PI3K/Akt pathway. ERK signaling was also shown to be activated when stimulated by inducers such as the cigarette smoke-carcinogen NNK [4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone], causing cisplatin resistance [182].
ERK activation was also shown to be required for cisplatin-induced apoptosis in cervical cancer HeLa cells [168, 169, 173], osteosarcoma and neuroblastoma cells [183], testicular germ cell tumors [184], glioma cells [185], renal epithelial cells [186], nasopharyngeal carcinoma cells [187], and human small cell lung cancer cells [188]. Decrease in ERK level/phosphorylation was associated with cisplatin resistance in HeLa cells [168, 173]. Cisplatin-induced activation of p53 was associated with proapoptotic effect of ERK1/2 in B104 cells [189], whereas cisplatin-induced acute renal failure (ARF) in mice was attributed to increase in TNFα gene expression by ERK and activation of caspase-3 [190]. We have found that knockdown of PKCδ attenuates cisplatin-induced ERK activation and apoptosis [173], suggesting that PKCδ acts upstream of ERK1/2 to trigger cisplatin-induced apoptosis.
7.3. Cisplatin and JNK
c-Jun N-terminal kinase or stress-activated protein kinase is activated by various stress stimuli, including DNA damage. The involvement of the JNK pathway in cisplatin-induced apoptosis began when it was seen that cells defective in JNK pathway were resistant to cisplatin [191]. Although both cis and transplatin activated the JNK pathway, the kinetics of JNK activation was distinct [192]. Slow and persistent activation of JNK by cisplatin as opposed to rapid and transient activation of JNK by transplatin may explain the ability of cisplatin to induce cell death. The observation that p73, a proapoptotic member of the p53 family, forms a complex with JNK leading to cisplatin-induced apoptosis, provides a mechanistic basis of how JNK activation leads to cisplatin-induced apoptosis [193]. A mutation in the binding sites of JNK reduced p73-mediated apoptosis. In addition, JNK has been involved in cisplatin-induced cytotoxicity mediated by the latent membrane protein-1 (LMP-1) of the Epstein-Barr virus [190, 194] and phospholipase A2-activating protein (PLAA) [195]. Furthermore, inhibition of TWIST [196], Snail [197], cytokeratin-8 [198], and the RNA-dependent protein kinase (PKR) [199] has been reported to induce JNK activation leading to cisplatin-mediated cytotoxicity. Studies have also implicated activation of JNK pathway following recognition of cisplatin-induced DNA damage by the MMR [121, 200]. JNK and c-Abl were proposed to be signal transducers involved in MMR system that recognizes the cisplatin-DNA adducts and induce cell death [121]. In addition to its role in regulating anticancer activity of cisplatin, JNK pathway has also been implicated in the nephrotoxicity induced by cisplatin. Inhibition of the JNK pathway was cytoprotective restricting renal cell death and inflammation [201]. Recently, the JNK pathway was also shown to mediate cisplatin-induced nephrotoxicity driven by the Toll-like receptor, TLR4 [202].
7.4. Cisplatin and p38 MAPK
The p38 MAPK family is activated by environmental stress and inflammatory cytokines and is an important mediator of cisplatin-induced apoptosis. The activation of this pathway by cisplatin has been seen in different experimental model systems, resulting in a cisplatin-sensitive phenotype [203]. Inhibition of p38 MAPK rendered cells resistant to cisplatin and restimulation of the p38 MAPK along with JNK sensitized cisplatin resistant ovarian cancer 2008/C13* cells by increasing the expression of FasL [204]. Akt2 has been shown to negatively regulate the p38 MAPK pathway by binding to and phosphorylating one of the p38 family members ASK1, resulting in the inhibition of this pathway and rendering the cells resistant to cisplatin [205]. The p38 MAPK pathway was shown to be activated in response to agents such as curcumin which induced apoptosis in cisplatin-resistant ovarian cancer cells [206]. Thus, the activation of p38 MAPK regardless of the upstream signaling pathway seems to be important in mediating cisplatin-induced cytotoxicity. Winograd-Katz and Levitzki identified EGFR as a substrate for p38 MAPK and cisplatin-induced receptor internalization was triggered by p38-mediated phosphorylation of the receptor [207]. p38 MAPK has been shown to mediate its effect via p18(Hamlet), a p38 MAPK-regulated protein, which interacts with p53 and stimulates the transcription of proapoptotic genes PUMA and NOXA to induce apoptosis [208]. Like JNK, p38 MAPK has also been implicated in contributing to nephrotoxicity possibly via TNFα [209, 210]. Thus, the p38 MAPK pathway plays a critical role in regulating cisplatin-induced apoptosis.
7.5. Cisplatin and Akt
Akt belongs to a family of serine/threonine kinases which act downstream of phosphoinositide 3-kinase (PI3K) and plays a critical role in cell survival [211]. Several studies have established the involvement of Akt in contributing to the acquired resistance to cisplatin in several cancers, including ovarian [174, 212], uterine [213], small-cell lung cancer [214], nonsmall-cell lung cancer [215] and hepatoblastoma [216]. Hayakawa et al. first demonstrated that cisplatin-induced DNA damage caused phosphorylation of BAD at Ser136 via Akt and inhibition of Akt sensitized ovarian cancer cells to cisplatin [174]. Asselin et al. provided evidence that the X-linked inhibitor of apoptosis (XIAP) inhibits cisplatin-mediated cell death in cisplatin-sensitive A2780 ovarian cancer cells via phosphorylation and activation of Akt [217]. On the other hand, Dan et al. demonstrated that XIAP is a substrate for Akt and phosphorylation of XIAP by Akt prevents its ubiquitination and degradation in response to cisplatin, suggesting that XIAP promotes cell survival acting downstream of Akt [218]. In small-cell lung cancer cells, the antiapoptotic protein survivin appears to mediate the effect of Akt in protecting against cisplatin-induced cell death [214].
PI3K/Akt inhibitor not only sensitized ovarian cancer cells to cisplatin in vitro but also enhanced the antitumor activity of cisplatin in nude mice implanted with Caov-3 human ovarian cancer xenograft [212]. Cisplatin increased p53 and decreased XIAP in cisplatin-sensitive ovarian cancer 2008 cells but not in cisplatin-resistant variant 2008/C13* cells unless Akt was inhibited. The status of p53 also influenced the ability of Akt inhibitors to potentiate cisplatin sensitivity. Ectopic expression of the tumor suppressor PTEN which inhibits PI3K/Akt pathway sensitized cisplatin-resistant ovarian cancer 2008/C13* cells containing wild-type p53 but not in A2780/CP cells containing mutant p53 [105]. It has been suggested that Akt promotes chemoresistance by decreasing p53 phosphorylation and PUMA upregulation [219]. Heat shock protein, HSP27 which is often overexpressed in cisplatin-resistant cells enhanced cisplatin-induced Akt phosphorylation, suggesting that HSP27 may contribute to chemoresistance via the Akt pathway [220]. Among the Akt isoforms, Akt2 has been associated with chemoresistance of ovarian and uterine cancers [205, 213, 221]. However, acquisition of resistance by human lung cancer cells was associated with Akt1 overexpression and gene amplification [222]. Abedini et al. demonstrated that Akt confers cisplatin resistance via inhibition of p53-dependent ubiquitination and degradation of FLIP in response to cisplatin [223]. Claerhout et al. raised the possibility that autophagy plays an important role in contributing to cisplatin resistance [224]. In a progressive model of cutaneous squamous cell carcinoma cell lines, inhibition of autophagy by 3-methyladenine or by ATG5 knockdown, along with inhibition of Akt enhanced the cytotoxicity of cisplatin [224]. Recently, it has been reported that several microRNAs are deregulated in ovarian cancer and miR-214 promotes cell survival and cisplatin resistance by downregulating PTEN and activating Akt [225]. Thus, Akt/PTEN pathway plays an important role in cisplatin resistance and could be intervened to reverse the resistant phenotype.
8. Conclusion
Despite significant advancements in drug development and molecular-targeted therapy, traditional chemotherapy continues to be the major treatment option. For more than thirty years, cisplatin serves as one of the most important anticancer drugs used clinically. However, cisplatin resistance continues to be the major hurdle in cancer chemotherapy. As depicted in Figure 1, cellular sensitivity to cisplatin is not only regulated by its uptake, efflux or interaction with its target DNA but cellular responses to cisplatin-induced DNA damage also play a major role in deciding the ultimate cell fate. Cells can activate protective responses to inhibit cell cycle progression and repair cisplatin-induced DNA damage. Although extensive DNA damage can induce cell death by apoptosis, several signaling pathways, including Akt, PKC, and MAPKs (e.g., ERK, JNK, and p38 MAPK) can regulate cisplatin-induced apoptosis. The tumor suppressor protein p53 play a critical role in regulating cell cycle arrest, DNA repair and apoptosis. The nonreceptor tyrosine kinase c-Abl can also participate in DNA damage response by activating various MAPKs and interacting with p53 and p73. Recent evidence suggests that microRNAs can also regulate cisplatin sensitivity. Since various signaling pathways regulate cisplatin sensitivity, one way to improve the efficacy of cisplatin is to use it in combination with agents that target the signaling pathways and contribute to cisplatin resistance. Additionally, combining cisplatin with molecular-targeted therapy should lower the dosage of cisplatin currently employed and thus help in alleviating its side effects such as nephrotoxicity. As discussed in this paper, the cellular context has significant impact in deciding the ultimate response to cisplatin and may vary from one patient to another. Thus, the major challenge is to develop individualized therapy options that will be tailor-made to benefit a particular patient. Given the uncertainty with the success of any newly developed drug and the success of cisplatin as a chemotherapeutic agent, this approach may be more feasible and should be actively pursued.
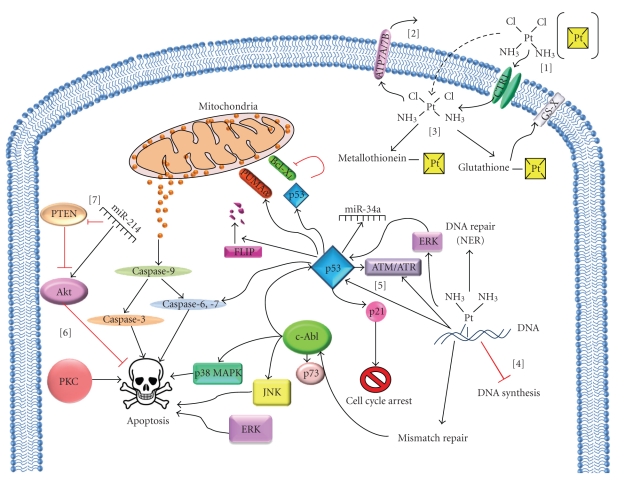
Figure 1.
Cellular responses to cisplatin-induced DNA damage. [1] Entry of cisplatin into cells by passive diffusion (indicated by dotted arrows), carrier-mediated transport, employing copper transporter-1 (CTR1). [2] Efflux of cisplatin from the cells by the ATP-dependent transporters, ATP7A and ATP7B. [3] Cisplatin binds to cellular thiols, such as glutathione and metallothionein. The glutathione-cisplatin conjugates are further transported from the cells by the ATP-dependent, GS-X pumps. [4] Once cisplatin interacts with DNA, it stalls cell proliferation by inhibiting DNA synthesis, followed by activation of DNA damage response. [5] Cisplatin-DNA adducts is primarily repaired via the nucleotide excision repair (NER) system and also induces cell-cycle arrest. The DNA damage response is transduced mainly via p53 and c-Abl. Cisplatin-induced DNA damage activates p53, leading to the induction of p21, GADD45, proapoptotic PUMAα, caspase-6, -7, and microRNAs such as miR-34a. p53 also promotes cisplatin-induced apoptosis by binding and inhibiting the antiapoptotic Bcl-xL and also by degradation of FLIP. Cisplatin-DNA adducts activates the mismatch repair system which further activates c-Abl, leading to the activation of JNK and p38 MAPK and stabilization of p73 resulting in apoptosis. [6] Kinases such as PKC, ERK, and Akt are also involved in the regulation of cisplatin-induced cell death. [7] miR-214 promotes cisplatin resistance by downregulating PTEN and activating Akt.
Acknowledgments
The authors sincerely apologize if they inadvertently left out any major contribution in this field. This paper was supported by the Grant CA071727 (AB) from the National Cancer Institute. S. Krishnamurthy is supported by the Predoctoral Traineeship Award BC083099 from DOD BCRP.
References
- 1.Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985;55(10):2303–2316. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg B, Van Camp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205(4972):698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222(5191):385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 4.Sherman SE, Gibson D, Wang AH-J, Lippard SJ. X-ray structure of the major adduct of the anticancer drug cisplatin with DNA: cis-[Pt(NH3)2{d(pGpG)}] Science. 1985;230(4724):412–417. doi: 10.1126/science.4048939. [DOI] [PubMed] [Google Scholar]
- 5.Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990;2(8-9):275–280. [PubMed] [Google Scholar]
- 6.Kerr JFR, Winterford CM, Harmon BV. Apoptosis: its significance in cancer and cancer therapy. Cancer. 1994;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nature Reviews Cancer. 2007;7(8):573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 8.Todd RC, Lippard SJ. Inhibition of transcription by platinum antitumor compounds. Metallomics. 2009;1(4):280–291. doi: 10.1039/b907567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebillard A, Lagadic-Gossmann D, Dimanche-Boitrel M-T. Cisplatin cytotoxicity: DNA and plasma membrane targets. Current Medicinal Chemistry. 2008;15(26):2656–2663. doi: 10.2174/092986708786242903. [DOI] [PubMed] [Google Scholar]
- 10.Zorbas H, Keppler BK. Cisplatin damage: are DNA repair proteins saviors or traitors to the cell? ChemBioChem. 2005;6(7):1157–1166. doi: 10.1002/cbic.200400427. [DOI] [PubMed] [Google Scholar]
- 11.Hall MD, Okabe M, Shen D-W, Liang X-J, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annual Review of Pharmacology and Toxicology. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- 12.Kartalou M, Essigmann JM. Recognition of cisplatin adducts by cellular proteins. Mutation Research. 2001;478(1-2):1–21. doi: 10.1016/s0027-5107(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 13.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Molecular Pharmacology. 2010;77(6):887–894. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature Reviews Drug Discovery. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 15.Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutation Research. 2001;478(1-2):23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 16.Zamble DB, Lippard SJ. Cisplatin and DNA repair in cancer chemotherapy. Trends in Biochemical Sciences. 1995;20(10):435–439. doi: 10.1016/s0968-0004(00)89095-7. [DOI] [PubMed] [Google Scholar]
- 17.Sedletska Y, Giraud-Panis M-J, Malinge J-M. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Current Medicinal Chemistry: Anti-Cancer Agents. 2005;5(3):251–265. doi: 10.2174/1568011053765967. [DOI] [PubMed] [Google Scholar]
- 18.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Molecular Pharmacology. 2006;70(4):1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 19.Holzer AK, Katano K, Klomp LWJ, Howell SB. Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clinical Cancer Research. 2004;10(19):6744–6749. doi: 10.1158/1078-0432.CCR-04-0748. [DOI] [PubMed] [Google Scholar]
- 20.Safaei R, Holzer AK, Katano K, Samimi G, Howell SB. The role of copper transporters in the development of resistance to Pt drugs. Journal of Inorganic Biochemistry. 2004;98(10):1607–1613. doi: 10.1016/j.jinorgbio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Byfield JE, Calabro-Jones PM. Carrier-dependent and carrier-independent transport of anti-cancer alkylating agents. Nature. 1981;294(5838):281–283. doi: 10.1038/294281a0. [DOI] [PubMed] [Google Scholar]
- 22.Andrews PA, Mann SC, Huynh HH, Albright KD. Role of the Na+, K+-adenosine triphosphatase in the accumulation of cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells. Cancer Research. 1991;51(14):3677–3681. [PubMed] [Google Scholar]
- 23.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(22):14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safaei R, Howell SB. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Critical Reviews in Oncology/Hematology. 2005;53(1):13–23. doi: 10.1016/j.critrevonc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Blair BG, Larson C, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clinical Cancer Research. 2009;15(13):4312–4321. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai K, Kamatani N, Georges E, Ling V. Identification of a membrane glycoprotein overexpressed in murine lymphoma sublines resistant to cis-diamminedichloroplatinum(II) Journal of Biological Chemistry. 1990;265(22):13137–13142. [PubMed] [Google Scholar]
- 27.Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. British Journal of Cancer. 1993;67(6):1171–1176. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chemical Reviews. 1999;99(9):2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 29.Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacology and Therapeutics. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- 30.McKay BC, Becerril C, Ljungman M. P53 plays a protective role against UV- and cisplatin-induced apoptosis in transcription-coupled repair proficient fibroblasts. Oncogene. 2001;20(46):6805–6808. doi: 10.1038/sj.onc.1204901. [DOI] [PubMed] [Google Scholar]
- 31.Welsh C, Day R, McGurk C, Masters JRW, Wood RD, Köberle B. Reduced levels of XPA, ERCC1 and XPF DNA repair proteins in testis tumor cell lines. International Journal of Cancer. 2004;110(3):352–361. doi: 10.1002/ijc.20134. [DOI] [PubMed] [Google Scholar]
- 32.Köberle B, Masters JRW, Hartley JA, Wood RD. Defective repair of cisplatin-induced DNA damage caused by reduced XPA protein in testicular germ cell tumours. Current Biology. 1999;9(5):273–276. doi: 10.1016/s0960-9822(99)80118-3. [DOI] [PubMed] [Google Scholar]
- 33.Rosell R, Taron M, Barnadas A, Scagliotti G, Sarries C, Roig B. Nucleotide excision repair pathways involved in cisplatin resistance in non-small-cell lung cancer. Cancer Control. 2003;10(4):297–305. doi: 10.1177/107327480301000404. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Fan W, Xu S, Zhou Y. Sensitization to the cytotoxicity of cisplatin by transfection with nucleotide excision repair gene xeroderma pigmentosun group A antisense RNA in human lung adenocarcinoma cells. Clinical Cancer Research. 2003;9(16, part 1):5874–5879. [PubMed] [Google Scholar]
- 35.Sun Y, Li T, Ma K, et al. The impacts of ercc1 gene exon VIII alternative splicing on cisplatin-resistance in ovarian cancer cells. Cancer Investigation. 2009;27(9):891–897. doi: 10.3109/07357900902744536. [DOI] [PubMed] [Google Scholar]
- 36.Chen H-Y, Shao C-J, Chen F-R, Kwan A-L, Chen Z-P. Role of ERCCl promoter hypermethylation in drug resistance to cisplatin in human gliomas. International Journal of Cancer. 2010;126(8):1944–1954. doi: 10.1002/ijc.24772. [DOI] [PubMed] [Google Scholar]
- 37.Orelli B, McClendon TB, Tsodikov OV, Ellenberger T, Niedernhofer LJ, Schärer OD. The XPA-binding domain of ERCC1 is required for nucleotide excision repair but not other DNA repair pathways. Journal of Biological Chemistry. 2010;285(6):3705–3712. doi: 10.1074/jbc.M109.067538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arora S, Kothandapani A, Tillison K, Kalman-Maltese V, Patrick SM. Downregulation of XPF-ERCC1 enhances cisplatin efficacy in cancer cells. DNA Repair. 2010;9(7):745–753. doi: 10.1016/j.dnarep.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guthrie OW, Li-Korotky H-S, Durrant JD, Balaban C. Cisplatin induces cytoplasmic to nuclear translocation of nucleotide excision repair factors among spiral ganglion neurons. Hearing Research. 2008;239(1-2):79–91. doi: 10.1016/j.heares.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Kang T-H, Lindsey-Boltz LA, Reardon JT, Sancar A. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(11):4890–4895. doi: 10.1073/pnas.0915085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colton SL, Xu XS, Wang YA, Wang G. The involvement of ataxia-telangiectasia mutated protein activation in nucleotide excision repair-facilitated cell survival with cisplatin treatment. Journal of Biological Chemistry. 2006;281(37):27117–27125. doi: 10.1074/jbc.M602826200. [DOI] [PubMed] [Google Scholar]
- 42.Bhana S, Lloyd DR. The role of p53 in DNA damage-mediated cytotoxicity overrides its ability to regulate nucleotide excision repair in human fibroblasts. Mutagenesis. 2008;23(1):43–50. doi: 10.1093/mutage/gem041. [DOI] [PubMed] [Google Scholar]
- 43.Lomonaco SL, Xu XS, Wang G. The role of Bcl-x(L) protein in nucleotide excision repair-facilitated cell protection against cisplatin-induced apoptosis. DNA and Cell Biology. 2009;28(6):285–294. doi: 10.1089/dna.2008.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damsma GE, Alt A, Brueckner F, Carell T, Cramer P. Mechanism of transcriptional stalling at cisplatin-damaged DNA. Nature Structural and Molecular Biology. 2007;14(12):1127–1133. doi: 10.1038/nsmb1314. [DOI] [PubMed] [Google Scholar]
- 45.Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7(10):1353–1359. doi: 10.4161/cc.7.10.5930. [DOI] [PubMed] [Google Scholar]
- 46.Alt A, Lammens K, Chiocchini C, et al. Bypass of DNA lesions generated during anticancer treatment with cisplatin by DNA polymerase η . Science. 2007;318(5852):967–970. doi: 10.1126/science.1148242. [DOI] [PubMed] [Google Scholar]
- 47.Sedletska Y, Fourrier L, Malinge J-M. Modulation of MutS ATP-dependent functional activities by DNA containing a cisplatin compound lesion (base damage and mismatch) Journal of Molecular Biology. 2007;369(1):27–40. doi: 10.1016/j.jmb.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 48.Yamada M, O’Regan E, Brown R, Karran P. Selective recognition of a cisplatin-DNA adduct by human mismatch repair proteins. Nucleic Acids Research. 1997;25(3):491–496. doi: 10.1093/nar/25.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fourrier L, Brooks P, Malinge J-M. Binding discrimination of MutS to a set of lesions and compound lesions (base damage and mismatch) reveals its potential role as a cisplatin-damaged DNA sensing protein. Journal of Biological Chemistry. 2003;278(23):21267–21275. doi: 10.1074/jbc.M301390200. [DOI] [PubMed] [Google Scholar]
- 50.Papouli E, Cejka P, Jiricny J. Dependence of the cytotoxicity of DNA-damaging agents on the mismatch repair status of human cells. Cancer Research. 2004;64(10):3391–3394. doi: 10.1158/0008-5472.CAN-04-0513. [DOI] [PubMed] [Google Scholar]
- 51.Drummond JT, Genschel J, Wolf E, Modrich P. DHFR/MSH3 amplification in methotrexate-resistant cells alters the hMutSα/hMutSβ ratio and reduces the efficiency of base-base mismatch repair. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(19):10144–10149. doi: 10.1073/pnas.94.19.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown R, Hirst GL, Gallagher WM, et al. hMLH1 expression and cellular responses of ovarian tumour cells to treatment with cytotoxic anticancer agents. Oncogene. 1997;15(1):45–52. doi: 10.1038/sj.onc.1201167. [DOI] [PubMed] [Google Scholar]
- 53.Aebi S, Kurdi-Haidar B, Gordon R, et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Research. 1996;56(13):3087–3090. [PubMed] [Google Scholar]
- 54.Topping RP, Wilkinson JC, Scarpinato D. Mismatch repair protein deficiency compromises cisplatin-induced apoptotic signaling. Journal of Biological Chemistry. 2009;284(21):14029–14039. doi: 10.1074/jbc.M809303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding X, Mohd AB, Huang Z, et al. MLH1 expression sensitises ovarian cancer cells to cell death mediated by XIAP inhibition. British Journal of Cancer. 2009;101(2):269–277. doi: 10.1038/sj.bjc.6605180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avdievich E, Reiss C, Scherer SJ, et al. Distinct effects of the recurrent Mlh1G67R mutation on MMR functions, cancer, and meiosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4247–4252. doi: 10.1073/pnas.0800276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin DP, Wang Y, Scherer SJ, et al. An Msh2 point mutation uncouples DNA mismatch repair and apoptosis. Cancer Research. 2004;64(2):517–522. doi: 10.1158/0008-5472.can-03-2957. [DOI] [PubMed] [Google Scholar]
- 58.Yang G, Scherer SJ, Shell SS, et al. Dominant effects of an Msh6 missense mutation on DNA repair and cancer susceptibility. Cancer Cell. 2004;6(2):139–150. doi: 10.1016/j.ccr.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 59.Lin X, Howell SB. DNA mismatch repair and p53 function are major determinants of the rate of development of cisplatin resistance. Molecular Cancer Therapeutics. 2006;5(5):1239–1247. doi: 10.1158/1535-7163.MCT-05-0491. [DOI] [PubMed] [Google Scholar]
- 60.Shimodaira H, Yoshioka-Yamashita A, Kolodner RD, Wang JYJ. Interaction of mismatch repair protein PMS2 and the p53-related transcription factor p73 in apoptosis response to cisplatin. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2420–2425. doi: 10.1073/pnas.0438031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin X, Ramamurthi K, Mishima M, Kondo A, Christen RD, Howell SB. p53 modulates the effect of loss of DNA mismatch repair on the sensitivity of human colon cancer cells to the cytotoxic and mutagenic effects of cisplatin. Cancer Research. 2001;61(4):1508–1516. [PubMed] [Google Scholar]
- 62.Branch P, Masson M, Aquilina G, Bignami M, Karran P. Spontaneous development of drug resistance: mismatch repair and p53 defects in resistance to cisplatin in human tumor cells. Oncogene. 2000;19(28):3138–3145. doi: 10.1038/sj.onc.1203668. [DOI] [PubMed] [Google Scholar]
- 63.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anti-Cancer Agents in Medicinal Chemistry. 2007;7(1):3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. Journal of Biological Chemistry. 1993;268(27):20116–20125. [PubMed] [Google Scholar]
- 65.Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(7):3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boubakari, Bracht K, Neumann C, Grünert R, Bednarski PJ. No correlation between GSH levels in human cancer cell lines and the cell growth inhibitory activities of platinum diamine complexes. Archiv der Pharmazie. 2004;337(12):668–671. doi: 10.1002/ardp.200400620. [DOI] [PubMed] [Google Scholar]
- 67.Bracht K, Boubakari, Grünert R, Bednarski PJ. Correlations between the activities of 19 anti-tumor agents and the intracellular glutathione concentrations in a panel of 14 human cancer cell lines: comparisons with the National Cancer Institute data. Anti-Cancer Drugs. 2006;17(1):41–51. doi: 10.1097/01.cad.0000190280.60005.05. [DOI] [PubMed] [Google Scholar]
- 68.Kasherman Y, Sturup S, Gibson D. Is glutathione the major cellular target of cisplatin? A study of the interactions of cisplatin with cancer cell extracts. Journal of Medicinal Chemistry. 2009;52(14):4319–4328. doi: 10.1021/jm900138u. [DOI] [PubMed] [Google Scholar]
- 69.Chen HHW, Song I-S, Hossain A, et al. Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Molecular Pharmacology. 2008;74(3):697–704. doi: 10.1124/mol.108.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ban N, Takahashi Y, Takayama T, et al. Transfection of glutathione S-transferase (GST)-π antisense complementary DNA increases the sensitivity of a colon cancer cell line to adriamycin, cisplatin, melphalan, and etoposide. Cancer Research. 1996;56(15):3577–3582. [PubMed] [Google Scholar]
- 71.Miyazaki M, Kohno K, Saburi Y, et al. Drug resistance to cis-diamminedichloroplatinum(II) in Chinese hamster ovary cell lines transfected with glutathione S-transferase pi gene. Biochemical and Biophysical Research Communications. 1990;166(3):1358–1364. doi: 10.1016/0006-291x(90)91016-l. [DOI] [PubMed] [Google Scholar]
- 72.Pasello M, Michelacci F, Scionti I, et al. Overcoming glutathione S-transferase P1-related cisplatin resistance in osteosarcoma. Cancer Research. 2008;68(16):6661–6668. doi: 10.1158/0008-5472.CAN-07-5840. [DOI] [PubMed] [Google Scholar]
- 73.Peklak-Scott C, Smitherman PK, Townsend AJ, Morrow CS. Role of glutathione S-transferase P1-1 in the cellular detoxification of cisplatin. Molecular Cancer Therapeutics. 2008;7(10):3247–3255. doi: 10.1158/1535-7163.MCT-08-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LaPensee EW, Schwemberger SJ, LaPensee CR, Bahassi EM, Afton SE, Ben-Jonathan N. Prolactin confers resistance against cisplatin in breast cancer cells by activating glutathione-S-transferase. Carcinogenesis. 2009;30(8):1298–1304. doi: 10.1093/carcin/bgp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piaggi S, Raggi C, Corti A, et al. Glutathione transferase omega 1-1 (GSTO1-1) plays an anti-apoptotic role in cell resistance to cisplatin toxicity. Carcinogenesis. 2010;31(5):804–811. doi: 10.1093/carcin/bgq031. [DOI] [PubMed] [Google Scholar]
- 76.Basu A, Lazo JS. A hypothesis regarding the protective role of metallothioneins against the toxicity of DNA interactive anticancer drugs. Toxicology Letters. 1990;50(2-3):123–135. doi: 10.1016/0378-4274(90)90002-4. [DOI] [PubMed] [Google Scholar]
- 77.Andrews PA, Murphy MP, Howell SB. Metallothionein-mediated cisplatin resistance in human ovarian carcinoma cells. Cancer Chemotherapy and Pharmacology. 1987;19(2):149–154. doi: 10.1007/BF00254568. [DOI] [PubMed] [Google Scholar]
- 78.Kelley SL, Basu A, Teicher BA, Hacker MP, Hamer DH, Lazo JS. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988;241(4874):1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- 79.Surowiak P, Materna V, Maciejczyk A, et al. Nuclear metallothionein expression correlates with cisplatin resistance of ovarian cancer cells and poor clinical outcome. Virchows Archiv. 2007;450(3):279–285. doi: 10.1007/s00428-006-0362-7. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki T, Ohata S, Togawa T, Himeno S, Tanabe S. Arsenic accumulation decreased in metallothionein null cisplatin-resistant cell lines. Journal of Toxicological Sciences. 2007;32(3):321–328. doi: 10.2131/jts.32.321. [DOI] [PubMed] [Google Scholar]
- 81.Karotki AV, Vašák M. Reaction of human metallothionein-3 with cisplatin and transplatin. Journal of Biological Inorganic Chemistry. 2009;14(7):1129–1138. doi: 10.1007/s00775-009-0557-x. [DOI] [PubMed] [Google Scholar]
- 82.Hidalgo J, Aschner M, Zatta P, Vašák M. Roles of the metallothionein family of proteins in the central nervous system. Brain Research Bulletin. 2001;55(2):133–145. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 83.Chu G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. Journal of Biological Chemistry. 1994;269(2):787–790. [PubMed] [Google Scholar]
- 84.Hagrman D, Goodisman J, Dabrowiak JC, Souid A-K. Kinetic study on the reaction of cisplatin with metallothionein. Drug Metabolism and Disposition. 2003;31(7):916–923. doi: 10.1124/dmd.31.7.916. [DOI] [PubMed] [Google Scholar]
- 85.Woo ES, Monks A, Watkins SC, Wang AS, Lazo JS. Diversity of metallothionein content and subcellular localization in the National Cancer Institute tumor panel. Cancer Chemotherapy and Pharmacology. 1997;41(1):61–68. doi: 10.1007/s002800050708. [DOI] [PubMed] [Google Scholar]
- 86.Cherian MG, Jayasurya A, Bay B-H. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutation Research. 2003;533(1-2):201–209. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Theocharis SE, Margeli AP, Klijanienko JT, Kouraklis GP. Metallothionein expression in human neoplasia. Histopathology. 2004;45(2):103–118. doi: 10.1111/j.1365-2559.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 88.Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6(9):1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 89.De Laurenzi V, Melino G. Evolution of functions within the p53/p63/p73 family. Annals of the New York Academy of Sciences. 2000;926:90–100. doi: 10.1111/j.1749-6632.2000.tb05602.x. [DOI] [PubMed] [Google Scholar]
- 90.Fujiwara T, Grimm EA, Mukhopadhyay T, Zhang W-W, Owen-Schaub LB, Roth JA. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Research. 1994;54(9):2287–2291. [PubMed] [Google Scholar]
- 91.Horowitz J. Adenovirus-mediated p53 gene therapy: overview of preclinical studies and potential clinical applications. Current Opinion in Molecular Therapeutics. 1999;1(4):500–509. [PubMed] [Google Scholar]
- 92.Kigawa J, Sato S, Shimada M, et al. p53 gene status and chemosensitivity in ovarian cancer. Human Cell. 2001;14(3):165–171. [PubMed] [Google Scholar]
- 93.Song K, Cowan KH, Sinha BK. In vivo studies of adenovirus-mediated p53 gene therapy for cis-platinum-resistant human ovarian tumor xenografts. Oncology Research. 1999;11(3):153–159. [PubMed] [Google Scholar]
- 94.Pabla N, Huang S, Mi Q-S, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. Journal of Biological Chemistry. 2008;283(10):6572–6583. doi: 10.1074/jbc.M707568200. [DOI] [PubMed] [Google Scholar]
- 95.Kondo S, Barna BP, Kondo Y, et al. WAF1/CIP1 increases the susceptibility of p53 non-functional malignant glioma cells to cisplatin-induced apoptosis. Oncogene. 1996;13(6):1279–1285. [PubMed] [Google Scholar]
- 96.Zamble DB, Jacks T, Lippard SJ. p53-dependent and -independent responses to cisplatin in mouse testicular teratocarcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6163–6168. doi: 10.1073/pnas.95.11.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Research. 2006;66(6):3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 98.Kanamori Y, Kigawa J, Minagawa Y, et al. A newly developed adenovirus-mediated transfer of a wild-type p53 gene increases sensitivity to cis-diamminedichloroplatinum (II) in p53-deleted ovarian cancer cells. European Journal of Cancer. 1998;34(11):1802–1806. doi: 10.1016/s0959-8049(98)00199-3. [DOI] [PubMed] [Google Scholar]
- 99.Kigawa J, Sato S, Shimada M, Kanamori Y, Itamochi H, Terakawa N. Effect of p53 gene transfer and cisplatin in a peritonitis carcinomatosa model with p53-deficient ovarian cancer cells. Gynecologic Oncology. 2002;84(2):210–215. doi: 10.1006/gyno.2001.6488. [DOI] [PubMed] [Google Scholar]
- 100.Wetzel CC, Berberich SJ. p53 binds to cisplatin-damaged DNA. Biochimica et Biophysica Acta. 2001;1517(3):392–397. doi: 10.1016/s0167-4781(00)00305-5. [DOI] [PubMed] [Google Scholar]
- 101.Hernlund E, Kutuk O, Basaga H, Linder S, Panaretakis T, Shoshan M. Cisplatin-induced nitrosylation of p53 prevents its mitochondrial translocation. Free Radical Biology and Medicine. 2009;46(12):1607–1613. doi: 10.1016/j.freeradbiomed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 102.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. Journal of Biological Chemistry. 2000;275(46):35778–35785. doi: 10.1074/jbc.M004267200. [DOI] [PubMed] [Google Scholar]
- 103.Abedini MR, Muller EJ, Brun J, Bergeron R, Gray DA, Tsang BK. Cisplatin induces p53-dependent FLICE-like inhibitory protein ubiquitination in ovarian cancer cells. Cancer Research. 2008;68(12):4511–4517. doi: 10.1158/0008-5472.CAN-08-0673. [DOI] [PubMed] [Google Scholar]
- 104.Kutuk O, Arisan ED, Tezil T, Shoshan MC, Basaga H. Cisplatin overcomes Bcl-2-mediated resistance to apoptosis via preferential engagement of Bak: critical role of Noxa-mediated lipid peroxidation. Carcinogenesis. 2009;30(9):1517–1527. doi: 10.1093/carcin/bgp165. [DOI] [PubMed] [Google Scholar]
- 105.Yan X, Fraser M, Qiu Q, Tsang BK. Over-expression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis in a p53-dependent manner. Gynecologic Oncology. 2006;102(2):348–355. doi: 10.1016/j.ygyno.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 106.Kim H-S, Hwang J-T, Yun H, et al. Inhibition of AMP-activated protein kinase sensitizes cancer cells to cisplatin-induced apoptosis via hyper-induction of p53. Journal of Biological Chemistry. 2008;283(7):3731–3742. doi: 10.1074/jbc.M704432200. [DOI] [PubMed] [Google Scholar]
- 107.Jiang M, Wei Q, Wang J, et al. Regulation of PUMA-α by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25(29):4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- 108.Yang C, Kaushal V, Haun RS, Seth R, Shah SV, Kaushal GP. Transcriptional activation of caspase-6 and -7 genes by cisplatin-induced p53 and its functional significance in cisplatin nephrotoxicity. Cell Death and Differentiation. 2008;15(3):530–544. doi: 10.1038/sj.cdd.4402287. [DOI] [PubMed] [Google Scholar]
- 109.Bhatt K, Zhou L, Mi QS, et al. microRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. doi: 10.2119/molmed.2010.00002. Molecular Medicine, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan S, Smith ML, Rivet DJ, II, et al. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Research. 1995;55(8):1649–1654. [PubMed] [Google Scholar]
- 111.Brown R, Clugston C, Burns P, et al. Increased accumulation of p53 protein in cisplatin-resistant ovarian cell lines. International Journal of Cancer. 1993;55(4):678–684. doi: 10.1002/ijc.2910550428. [DOI] [PubMed] [Google Scholar]
- 112.Johnson CL, Lu D, Huang J, Basu A. Regulation of p53 stabilization by DNA damage and protein kinase C. Molecular Cancer Therapeutics. 2002;1(10):861–867. [PubMed] [Google Scholar]
- 113.Perego P, Giarola M, Righetti SC, et al. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Research. 1996;56(3):556–562. [PubMed] [Google Scholar]
- 114.Righetti SC, Della Torre G, Pilotti S, et al. A comparative study of p53 gene mutations, protein accumulation, and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Research. 1996;56(4):689–693. [PubMed] [Google Scholar]
- 115.Vekris A, Meynard D, Haaz M-C, Bayssas M, Bonnet J, Robert J. Molecular determinants of the cytotoxicity of platinum compounds: the contribution of in silico research. Cancer Research. 2004;64(1):356–362. doi: 10.1158/0008-5472.can-03-2258. [DOI] [PubMed] [Google Scholar]
- 116.Piovesan B, Pennell N, Berinstein NL. Human lymphoblastoid cell lines expressing mutant p53 exhibit decreased sensitivity to cisplatin-induced cytotoxicity. Oncogene. 1998;17(18):2339–2350. doi: 10.1038/sj.onc.1202147. [DOI] [PubMed] [Google Scholar]
- 117.Preyer M, Shu C-W, Wang JYJ. Delayed activation of Bax by DNA damage in embryonic stem cells with knock-in mutations of the Abl nuclear localization signals. Cell Death and Differentiation. 2007;14(6):1139–1148. doi: 10.1038/sj.cdd.4402119. [DOI] [PubMed] [Google Scholar]
- 118.Kharbanda S, Ren R, Pandey P, et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376(6543):785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 119.Kharbanda S, Pandey P, Yamauchi T, et al. Activation of MEK kinase 1 by the c-Abl protein tyrosine kinase in response to DNA damage. Molecular and Cellular Biology. 2000;20(14):4979–4989. doi: 10.1128/mcb.20.14.4979-4989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nehmé A, Baskaran R, Nebel S, et al. Induction of JNK and c-Abl signalling by cisplatin and oxaliplatin in mismatch repair-proficient and -deficient cells. British Journal of Cancer. 1999;79(7-8):1104–1110. doi: 10.1038/sj.bjc.6690176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nehmé A, Baskaran R, Aebi S, et al. Differential induction of c-Jun NH2-terminal kinase and c-Abl kinase in DNA mismatch repair-proficient and -deficient cells exposed to cisplatin. Cancer Research. 1997;57(15):3253–3257. [PubMed] [Google Scholar]
- 122.Gong J, Costanzo A, Yang H-Q, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399(6738):806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 123.Tsai KKC, Yuan Z-M. c-Abl stabilizes p73 by a phosphorylation-augmented interaction. Cancer Research. 2003;63(12):3418–3424. [PubMed] [Google Scholar]
- 124.Truong T, Sun G, Doorly M, Wang JYJ, Schwartz MA. Modulation of DNA damage-induced apoptosis by cell adhesion is independently mediated by p53 and c-Abl. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10281–10286. doi: 10.1073/pnas.1635435100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leong C-O, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. Journal of Clinical Investigation. 2007;117(5):1370–1380. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toh WH, Siddique MM, Boominathan L, Lin KW, Sabapathy K. c-Jun regulates the stability and activity of the p53 homologue, p73. Journal of Biological Chemistry. 2004;279(43):44713–44722. doi: 10.1074/jbc.M407672200. [DOI] [PubMed] [Google Scholar]
- 127.Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Molecular Cell. 2008;29(3):350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 128.Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nature Medicine. 2009;15(10):1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 129.Machuy N, Rajalingam K, Rudel T. Requirement of caspase-mediated cleavage of c-Abl during stress-induced apoptosis. Cell Death and Differentiation. 2004;11(3):290–300. doi: 10.1038/sj.cdd.4401336. [DOI] [PubMed] [Google Scholar]
- 130.Galan-Moya EM, Hernandez-Losa J, Aceves Luquero CI, et al. c-Abl activates p38 MAPK independently of its tyrosine kinase activity: implications in cisplatin-based therapy. International Journal of Cancer. 2008;122(2):289–297. doi: 10.1002/ijc.23063. [DOI] [PubMed] [Google Scholar]
- 131.Nuñez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17(25):3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 132.Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO Journal. 1995;14(22):5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Basu A, Sivaprasad U. Protein kinase Cε makes the life and death decision. Cellular Signalling. 2007;19(8):1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochemical Journal. 2003;370(2):361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacology and Therapeutics. 1993;59(3):257–280. doi: 10.1016/0163-7258(93)90070-t. [DOI] [PubMed] [Google Scholar]
- 136.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 137.Hofmann J, Doppler W, Jakob A, et al. Enhancement of the antiproliferative effect of cis-diamminedichloroplatinum(II) and nitrogen mustard by inhibitors of protein kinase C. International Journal of Cancer. 1988;42(3):382–388. doi: 10.1002/ijc.2910420313. [DOI] [PubMed] [Google Scholar]
- 138.Isonishi S, Andrews PA, Howell SB. Increased sensitivity to cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells in response to treatment with 12-O-tetradecanoylphorbol 13-acetate. Journal of Biological Chemistry. 1990;265(7):3623–3627. [PubMed] [Google Scholar]
- 139.Basu A, Teicher BA, Lazo JS. Involvement of protein kinase C in phorbol ester-induced sensitization of HeLa cells to cis-diamminedichloroplatinum(II) Journal of Biological Chemistry. 1990;265(15):8451–8457. [PubMed] [Google Scholar]
- 140.Hirata J, Kikuchi Y, Kita T, et al. Modulation of sensitivity of human ovarian cancer cells to cis-diamminedichloroplatinum(II) by 12-O-tetradecanoylphorbol-13-acetate and D,L-buthionine-S,R-sulphoximine. International Journal of Cancer. 1993;55(3):521–527. doi: 10.1002/ijc.2910550332. [DOI] [PubMed] [Google Scholar]
- 141.Basu A, Lazo JS. Sensitization of human cervical carcinoma cells to cis-diamminedichloroplatinum(II) by bryostatin 1. Cancer Research. 1992;52(11):3119–3124. [PubMed] [Google Scholar]
- 142.Nezhat F, Wadler S, Muggia F, et al. Phase II trial of the combination of bryostatin-1 and cisplatin in advanced or recurrent carcinoma of the cervix: a New York Gynecologic Oncology Group study. Gynecologic Oncology. 2004;93(1):144–148. doi: 10.1016/j.ygyno.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 143.Pavlick AC, Wu J, Roberts J, et al. Phase i study of bryostatin 1, a protein kinase C modulator, preceding cisplatin in patients with refractory non-hematologic tumors. Cancer Chemotherapy and Pharmacology. 2009;64(4):803–810. doi: 10.1007/s00280-009-0931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Basu A, Kozikowski AP, Sato K, Lazo JS. Cellular sensitization to cis-diamminedichloroplatinum(II) by novel analogues of the protein kinase C activator lyngbyatoxin A. Cancer Research. 1991;51(10):2511–2514. [PubMed] [Google Scholar]
- 145.Basu A, Weixel KM. Comparison of protein kinase C activity and isoform expression in cisplatin-sensitive and -resistant ovarian carcinoma cells. International Journal of Cancer. 1995;62(4):457–460. doi: 10.1002/ijc.2910620416. [DOI] [PubMed] [Google Scholar]
- 146.Mohanty S, Huang J, Basu A. Enhancement of cisplatin sensitivity of cisplatin-resistant human cervical carcinoma cells by bryostatin 1. Clinical Cancer Research. 2005;11(18):6730–6737. doi: 10.1158/1078-0432.CCR-05-0450. [DOI] [PubMed] [Google Scholar]
- 147.Geiger T, Müller M, Dean NM, Fabbro D. Antitumor activity of a PKC-α antisense oligonucleotide in combination with standard chemotherapeutic agents against various human tumors transplanted into nude mice. Anti-Cancer Drug Design. 1998;13(1):35–45. [PubMed] [Google Scholar]
- 148.Tortora G, Ciardiello F. Antisense strategies targeting protein kinase C: preclinical and clinical development. Seminars in Oncology. 2003;30(4):26–31. doi: 10.1016/s0093-7754(03)00282-3. [DOI] [PubMed] [Google Scholar]
- 149.Righetti SC, Perego P, Carenini N, et al. Molecular alterations of cells resistant to platinum drugs: role of PKCα . Biochimica et Biophysica Acta. 2006;1763(1):93–100. doi: 10.1016/j.bbamcr.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 150.Lee K-W, Sang GK, Kim H-P, et al. Enzastaurin, a protein kinase Cβ inhibitor, suppresses signaling through the ribosomal S6 kinase and bad pathways and induces apoptosis in human gastric cancer cells. Cancer Research. 2008;68(6):1916–1926. doi: 10.1158/0008-5472.CAN-07-3195. [DOI] [PubMed] [Google Scholar]
- 151.Ghayur T, Hugunin M, Talanian RV, et al. Proteolytic activation of protein kinase C δ by an ICE/CED 3-like protease induces characteristics of apoptosis. Journal of Experimental Medicine. 1996;184(6):2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Segal-Bendirdjian E, Jacquemin-Sablon A. Cisplatin resistance in a murine leukemia cell line is associated with a defective apoptotic process. Experimental Cell Research. 1995;218(1):201–212. doi: 10.1006/excr.1995.1148. [DOI] [PubMed] [Google Scholar]
- 153.Huang J, Mohanty S, Basu A. Cisplatin resistance is associated with deregulation in protein kinase C-δ . Biochemical and Biophysical Research Communications. 2004;316(4):1002–1008. doi: 10.1016/j.bbrc.2004.02.149. [DOI] [PubMed] [Google Scholar]
- 154.Basu A, Akkaraju GR. Regulation of caspase activation and cis-diamminedichloroplatinum(II)- induced cell death by protein kinase C. Biochemistry. 1999;38(14):4245–4251. doi: 10.1021/bi982854q. [DOI] [PubMed] [Google Scholar]
- 155.Basu A, Woolard MD, Johnson CL. Involvement of protein kinase C-δ in DNA damage-induced apoptosis. Cell Death and Differentiation. 2001;8(9):899–908. doi: 10.1038/sj.cdd.4400885. [DOI] [PubMed] [Google Scholar]
- 156.Basu A, Adkins B, Basu C. Down-regulation of caspase-2 by rottlerin via protein kinase C-δ-independent pathway. Cancer Research. 2008;68(8):2795–2802. doi: 10.1158/0008-5472.CAN-07-6244. [DOI] [PubMed] [Google Scholar]
- 157.Iioka Y, Mishima K, Azuma N, et al. Overexpression of protein kinase Cδ enhances cisplatin-induced cytotoxicity correlated with p53 in gastric cancer cell line. Pathobiology. 2005;72(3):152–159. doi: 10.1159/000084119. [DOI] [PubMed] [Google Scholar]
- 158.Persaud SD, Hoang V, Huang J, Basu A. Involvement of proteolytic activation of PKCdelta in cisplatin-induced apoptosis in human small cell lung cancer H69 cells. International Journal of Oncology. 2005;27(1):149–154. [PubMed] [Google Scholar]
- 159.Muscella A, Urso L, Calabriso N, et al. Anti-apoptotic effects of protein kinase C-δ and c-fos in cisplatin-treated thyroid cells. British Journal of Pharmacology. 2009;156(5):751–763. doi: 10.1111/j.1476-5381.2008.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Basu A, Cline JS. Oncogenic transformation alters cisplatin-induced apoptosis in rat embryo fibroblasts. International Journal of Cancer. 1995;63(4):597–603. doi: 10.1002/ijc.2910630422. [DOI] [PubMed] [Google Scholar]
- 161.Zhang L, Huang J, Yang N, et al. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCt as a biomarker and potential oncogene in ovarian carcinoma. Cancer Research. 2006;66(9):4627–4635. doi: 10.1158/0008-5472.CAN-05-4527. [DOI] [PubMed] [Google Scholar]
- 162.Baldwin RM, Garratt-Lalonde M, Parolin DAE, Krzyzanowski PM, Andrade MA, Lorimer IAJ. Protection of glioblastoma cells from cisplatin cytotoxicity via protein kinase Cι-mediated attenuation of p38 MAP kinase signaling. Oncogene. 2006;25(20):2909–2919. doi: 10.1038/sj.onc.1209312. [DOI] [PubMed] [Google Scholar]
- 163.Urso L, Muscella A, Calabriso N, et al. Effects of cisplatin on matrix metalloproteinase-2 in transformed thyroid cells. Biochemical Pharmacology. 2010;79(6):810–816. doi: 10.1016/j.bcp.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 164.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 165.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 166.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 167.Tang MKS, Zhou HY, Yam JWP, Wong AST. c-Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2. Neoplasia. 2010;12(2):128–138. doi: 10.1593/neo.91438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. Journal of Biological Chemistry. 2000;275(50):39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- 169.Yeh PY, Chuang S-E, Yeh K-H, Song YC, Ea C-K, Cheng A-L. Increase of the resistance of human cervical carcinoma cells to cisplatin by inhibition of the MEK to ERK signaling pathway partly via enhancement of anticancer drug-induced NFκB activation. Biochemical Pharmacology. 2002;63(8):1423–1430. doi: 10.1016/s0006-2952(02)00908-5. [DOI] [PubMed] [Google Scholar]
- 170.Nowak G. Protein kinase C-α and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. Journal of Biological Chemistry. 2002;277(45):43377–43388. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hayakawa J, Ohmichi M, Kurachi H, et al. Inhibition of extracellular signal-regulated protein kinase or c-Jun N- terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. Journal of Biological Chemistry. 1999;274(44):31648–31654. doi: 10.1074/jbc.274.44.31648. [DOI] [PubMed] [Google Scholar]
- 172.Persons DL, Yazlovitskaya EM, Cui W, Pelling JC. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: Inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clinical Cancer Research. 1999;5(5):1007–1014. [PubMed] [Google Scholar]
- 173.Basu A, Tu H. Activation of ERK during DNA damage-induced apoptosis involves protein kinase Cδ . Biochemical and Biophysical Research Communications. 2005;334(4):1068–1073. doi: 10.1016/j.bbrc.2005.06.199. [DOI] [PubMed] [Google Scholar]
- 174.Hayakawa J, Ohmichi M, Kurachi H, et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Research. 2000;60(21):5988–5994. [PubMed] [Google Scholar]
- 175.Mandic A, Viktorsson K, Heiden T, Hansson J, Shoshan MC. The MEK1 inhibitor PD98059 sensitizes C8161 melanoma cells to cisplatin-induced apoptosis. Melanoma Research. 2001;11(1):11–19. doi: 10.1097/00008390-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 176.Yeh PY, Yeh K-H, Chuang S-E, Song YC, Cheng A-L. Suppression of MEK/ERK signaling pathway enhances cisplatin-induced NF-κB activation by protein phosphatase 4-mediated NF-κB p 65 Thr dephosphorylation. Journal of Biological Chemistry. 2004;279(25):26143–26148. doi: 10.1074/jbc.M402362200. [DOI] [PubMed] [Google Scholar]
- 177.Amrán D, Sancho P, Fernández C, et al. Pharmacological inhibitors of extracellular signal-regulated protein kinases attenuate the apoptotic action of cisplatin in human myeloid leukemia cells via glutathione-independent reduction in intracellular drug accumulation. Biochimica et Biophysica Acta. 2005;1743(3):269–279. doi: 10.1016/j.bbamcr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Y, Qu X, Jing W, et al. GSTP1 determines cis-platinum cytotoxicity in gastric adenocarcinoma MGC803 cells: regulation by promoter methylation and extracellular regulated kinase signaling. Anti-Cancer Drugs. 2009;20(3):208–214. doi: 10.1097/CAD.0b013e328322fbaa. [DOI] [PubMed] [Google Scholar]
- 179.Lee S, Yoon S, Kim D-H. A high nuclear basal level of ERK2 phosphorylation contributes to the resistance of cisplatin-resistant human ovarian cancer cells. Gynecologic Oncology. 2007;104(2):338–344. doi: 10.1016/j.ygyno.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 180.Basu S, Harfouche R, Soni S, Chimote G, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(19):7957–7961. doi: 10.1073/pnas.0902857106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.DeHaan RD, Yazlovitskaya EM, Persons DL. Regulation of p53 target gene expression by cisplatin-induced extracellular signal-regulated kinase. Cancer Chemotherapy and Pharmacology. 2001;48(5):383–388. doi: 10.1007/s002800100318. [DOI] [PubMed] [Google Scholar]
- 182.Nishioka T, Guo J, Yamamoto D, Chen L, Huppi P, Chen CY. Nicotine, through upregulating pro-survival signaling, cooperates with NNK to promote transformation. Journal of Cellular Biochemistry. 2010;109(1):152–161. doi: 10.1002/jcb.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Woessmann W, Chen X, Borkhardt A. Ras-mediated activation of ERK by cisplatin induces cell death independently of p53 in osteosarcoma and neuroblastoma cell lines. Cancer Chemotherapy and Pharmacology. 2002;50(5):397–404. doi: 10.1007/s00280-002-0502-y. [DOI] [PubMed] [Google Scholar]
- 184.Schweyer S, Soruri A, Meschter O, et al. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. British Journal of Cancer. 2004;91(3):589–598. doi: 10.1038/sj.bjc.6601919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Choi BK, Choi CH, Oh HL, Kim YK. Role of ERK activation in cisplatin-induced apoptosis in A172 human glioma cells. NeuroToxicology. 2004;25(6):915–924. doi: 10.1016/j.neuro.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 186.Kim YK, Kim HJ, Kwon CH, et al. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. Journal of Applied Toxicology. 2005;25(5):374–382. doi: 10.1002/jat.1081. [DOI] [PubMed] [Google Scholar]
- 187.Sheu LF, Young ZH, Lee WC, Chen YF, Kao WY, Chen A. STI571 sensitizes nasopharyngeal carcinoma cells to cisplatin: sustained activation of ERK with improved growth inhibition. International Journal of Oncology. 2007;30(2):403–411. [PubMed] [Google Scholar]
- 188.Dhar R, Basu A. Constitutive activation of p70 S6 kinase is associated with intrinsic resistance to cisplatin. International Journal of Oncology. 2008;32(5):1133–1137. [PubMed] [Google Scholar]
- 189.Park SA, Park HJ, Lee BI, Ahn YH, Kim SU, Choi KS. Bcl-2 blocks cisplatin-induced apoptosis by suppression of ERK-mediated p53 accumulation in B104 cells. Molecular Brain Research. 2001;93(1):18–26. doi: 10.1016/s0169-328x(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 190.Jo S-K, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney International. 2005;67(2):458–466. doi: 10.1111/j.1523-1755.2005.67102.x. [DOI] [PubMed] [Google Scholar]
- 191.Zanke BW, Boudreau K, Rubie E, et al. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Current Biology. 1996;6(5):606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 192.Sánchez-Perez I, Murguía JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16(4):533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- 193.Jones EV, Dickman MJ, Whitmarsh AJ. Regulation of p73-mediated apoptosis by c-Jun N-terminal kinase. Biochemical Journal. 2007;405(3):617–623. doi: 10.1042/BJ20061778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang X, Sanmun D, Hu L, Fadeel B, Ernberg I. Epstein-Barr virus-encoded LMP1 promotes cisplatin-induced caspase activation through JNK and NF-κB signaling pathways. Biochemical and Biophysical Research Communications. 2007;360(1):263–268. doi: 10.1016/j.bbrc.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 195.Zhang F, Suarez G, Sha J, Sierra JC, Peterson JW, Chopra AK. Phospholipase A2-activating protein (PLAA) enhances cisplatin-induced apoptosis in HeLa cells. Cellular Signalling. 2009;21(7):1085–1099. doi: 10.1016/j.cellsig.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 196.Zhuo W-L, Wang Y, Zhuo X-L, Zhang Y-S, Chen Z-T. Short interfering RNA directed against TWIST, a novel zinc finger transcription factor, increases A549 cell sensitivity to cisplatin via MAPK/mitochondrial pathway. Biochemical and Biophysical Research Communications. 2008;369(4):1098–1102. doi: 10.1016/j.bbrc.2008.02.143. [DOI] [PubMed] [Google Scholar]
- 197.Zhuo W, Wang Y, Zhuo X, Zhang Y, Ao X, Chen Z. Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway. Lung Cancer. 2008;62(1):8–14. doi: 10.1016/j.lungcan.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 198.Wang Y, He Q-Y, Tsao S-W, Cheung Y-H, Wong A, Chiu J-F. Cytokeratin 8 silencing in human nasopharyngeal carcinoma cells leads to cisplatin sensitization. Cancer Letters. 2008;265(2):188–196. doi: 10.1016/j.canlet.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 199.Pataer A, Swisher SG, Roth JA, Logothetis CJ, Corn P. Inhibition of RNA-dependent protein kinase (PKR) leads to cancer cell death and increases chemosensitivity. Cancer Biology and Therapy. 2009;8(3):245–252. doi: 10.4161/cbt.8.3.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Potapova O, Haghighi A, Bost F, et al. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. Journal of Biological Chemistry. 1997;272(22):14041–14044. doi: 10.1074/jbc.272.22.14041. [DOI] [PubMed] [Google Scholar]
- 201.Francescato HDC, Costa RS, Júnior FB, Coimbra TM. Effect of JNK inhibition on cisplatin-induced renal damage. Nephrology Dialysis Transplantation. 2007;22(8):2138–2148. doi: 10.1093/ndt/gfm144. [DOI] [PubMed] [Google Scholar]
- 202.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. Journal of the American Society of Nephrology. 2008;19(5):923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Losa JH, Cobo CP, Viniegra JG, Sánchez-Arevalo Lobo VJ, Ramón y Cajal S, Sánchez-Prieto R. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene. 2003;22(26):3998–4006. doi: 10.1038/sj.onc.1206608. [DOI] [PubMed] [Google Scholar]
- 204.Mansouri A, Ridgway LD, Korapati AL, et al. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. Journal of Biological Chemistry. 2003;278(21):19245–19256. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- 205.Yuan Z-Q, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. AKT2 inhibition of cisplatin-induced JNK/p38 and bax activation by phosphorylation of ASK1. Implication of AKT2 in chemoresistance. Journal of Biological Chemistry. 2003;278(26):23432–23440. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- 206.Weir NM, Selvendiran K, Kutala VK, et al. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biology and Therapy. 2007;6(2):178–184. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Winograd-Katz SE, Levitzki A. Cisplatin induces PKB/Akt activation and p38MAPK phosphorylation of the EGF receptor. Oncogene. 2006;25(56):7381–7390. doi: 10.1038/sj.onc.1209737. [DOI] [PubMed] [Google Scholar]
- 208.Cuadrado A, Lafarga V, Cheung PCF, et al. A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis. EMBO Journal. 2007;26(8):2115–2126. doi: 10.1038/sj.emboj.7601657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Francescato HDC, Costa RS, da Silva CGA, Coimbra TM. Treatment with a p38 MAPK inhibitor attenuates cisplatin nephrotoxicity starting after the beginning of renal damage. Life Sciences. 2009;84(17-18):590–597. doi: 10.1016/j.lfs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 210.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. American Journal of Physiology. 2005;289(1):F166–F174. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 211.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends in Biochemical Sciences. 2001;26(11):657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 212.Ohta T, Ohmichi M, Hayasaka T, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology. 2006;147(4):1761–1769. doi: 10.1210/en.2005-1450. [DOI] [PubMed] [Google Scholar]
- 213.Gagnon V, Mathieu I, Sexton E, Leblanc K, Asselin E. AKT involvement in cisplatin chemoresistance of human uterine cancer cells. Gynecologic Oncology. 2004;94(3):785–795. doi: 10.1016/j.ygyno.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 214.Belyanskaya LL, Hopkins-Donaldson S, Kurtz S, et al. Cisplatin activates Akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. International Journal of Cancer. 2005;117(5):755–763. doi: 10.1002/ijc.21242. [DOI] [PubMed] [Google Scholar]
- 215.Lee MW, Kim DS, Min NY, Kim HT. Akt1 inhibition by RNA interference sensitizes human non-small cell lung cancer cells to cisplatin. International Journal of Cancer. 2008;122(10):2380–2384. doi: 10.1002/ijc.23371. [DOI] [PubMed] [Google Scholar]
- 216.Hartmann W, Küchler J, Koch A, et al. Activation of phosphatidylinositol-3′-kinase/AKT signaling is essential in hepatoblastoma survival. Clinical Cancer Research. 2009;15(14):4538–4545. doi: 10.1158/1078-0432.CCR-08-2878. [DOI] [PubMed] [Google Scholar]
- 217.Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Research. 2001;61(5):1862–1868. [PubMed] [Google Scholar]
- 218.Dan HC, Sun M, Kaneko S, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) Journal of Biological Chemistry. 2004;279(7):5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 219.Gagnon V, Van Themsche C, Turner S, Leblanc V, Asselin E. Akt and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin, doxorubicin and taxol. Apoptosis. 2008;13(2):259–271. doi: 10.1007/s10495-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 220.Zhang Y, Shen X. Heat shock protein 27 protects L929 cells from cisplatin-lnduced apoptosis by enhancing Akt activation and abating suppression of thioredoxin reductase activity. Clinical Cancer Research. 2007;13(10):2855–2864. doi: 10.1158/1078-0432.CCR-06-2090. [DOI] [PubMed] [Google Scholar]
- 221.Fraser M, Leung BM, Yan X, Dan HC, Cheng JQ, Tsang BK. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Research. 2003;63(21):7081–7088. [PubMed] [Google Scholar]
- 222.Liu L-Z, Zhou X-D, Qian G, Shi X, Fang J, Jiang B-H. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70s6K1 pathway. Cancer Research. 2007;67(13):6325–6332. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- 223.Abedini MR, Muller EJ, Bergeron R, Gray DA, Tsang BK. Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of FLICE-like inhibitory protein. Oncogene. 2010;29(1):11–25. doi: 10.1038/onc.2009.300. [DOI] [PubMed] [Google Scholar]
- 224.Claerhout S, Verschooten L, Van Kelst S, et al. Concomitant inhibition of AKT and autophagy is required for efficient cisplatin-induced apoptosis of metastatic skin carcinoma. doi: 10.1002/ijc.25300. International Journal of Cancer, 2010. [DOI] [PubMed] [Google Scholar]
- 225.Yang H, Kong W, He L, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Research. 2008;68(2):425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]