Abstract
Holocarboxylase synthetase (HCS) catalyzes the covalent binding of biotin to carboxylases and histones. In mammals, the expression of HCS depends on biotin, but the mechanism of regulation is unknown. Here we tested the hypothesis that microRNA (miR) plays a role in the regulation of the HCS gene. Human embryonic kidney cells were used as the primary model, but cell lines from other tissues and primary human cells were also tested. In silico searches revealed an evolutionary conserved binding site for miR-539 in the 3 prime -untranslated region (3 prime -UTR) of HCS mRNA. Transgenic cells and reporter gene constructs were used to demonstrate that miR-539 decreases the expression of HCS at the level of transcription rather than translation; these findings were corroborated in nontransgenic cells. When miR-539 was overexpressed in transgenic cells, the abundance of both HCS and biotinylated histones decreased. The abundance of miR-539 was tissue dependent: fibroblasts gt kidney cells gt intestinal cells gt lymphoid cells. Dose-response studies revealed that the abundance of miR-539 was significantly higher at physiological concentrations of biotin than both biotin-deficient and biotin-supplemented media in all cell lines tested. In kidney cells, the expression of HCS was lower in cells in physiological medium than in deficient and supplemented medium. In contrast, in fibroblasts, lymphoid cells, and intestinal cells, there was no apparent link between miR-539 abundance and HCS expression, suggesting that factors other than miR-539 also contribute to the regulation of HCS expression in some tissues. Collectively, the results of this study suggest that miR-539 is among the factors sensing biotin and regulating HCS.
Introduction
Biotin serves as essential coenzyme for carboxylases and is also covalently attached to histones (1). Mammalian cells cannot synthesize biotin and, therefore, depend on a constant supply of exogenous biotin to maintain normal levels of protein biotinylation. Insufficient biotin supply is associated with decreased levels of biotinylated holocarboxylases (2, 3), leading to signs of biotin deficiency, such as abnormal production of small organic acids (4) and fatty acids (5), and impaired immune function (6). Likewise, biotin deficiency causes abnormally low biotinylation of histones at distinct genomic loci (7, 8). Low concentrations of histone biotinylation are associated with aberrant gene regulation (7, 8), e.g., derepression of retrotransposons leading to chromosomal instability (7).
The binding of biotin to both carboxylases and histones is catalyzed by holocarboxylase synthetase (HCS)5 (9–12). Because of its crucial role in protein biotinylation, HCS plays an essential role in the homeostasis of biotin-dependent pathways. Not surprisingly, HCS knockdown causes phenotypes such as decreased life span and decreased heat survival in Drosophila melanogaster (12). Consistent with the importance of HCS-dependent biotinylation reactions, no living HCS null humans have ever been identified. Individuals with mutations in the HCS gene require lifelong treatment with pharmacological doses of biotin for normal health (13, 14).
The role of HCS in biotin homeostasis goes beyond its role as a biotin protein ligase. Two models have been proposed to provide a mechanistic link among HCS, gene regulation, and biotin homeostasis; these 2 models are not mutually exclusive. In one model, HCS produces the intermediate biotinyl-5′-AMP, which then regulates the expression of genes coding for biotin-dependent carboxylases (15). In the other model, biotin increases expression and nuclear translocation of HCS, which then biotinylates histone H4 in promoter 1 of the biotin transporter gene SMVT to mediate local enrichment of K12-biotinylated histone H4 (H4K12bio) (8). H4K12bio is a known gene repression mark in chromatin (16) and causes decreased expression of the biotin transporter SMVT in biotin-supplemented cells (8).
The mechanisms leading to increased HCS expression in biotin-supplemented cells are unknown. Here, we tested the hypothesis that microRNA (miR) binding sites in the 3′-untranslated region (3′-UTR) of HCS mRNA play roles in the regulation of HCS expression, thereby participating in the maintenance of biotin-dependent metabolic pathways in human cells. mir are encoded by endogenous genes and, after processing in the nucleus and cytoplasm, are #126 22 nucleotides in length (17–21). They decrease gene expression by targeting mRNA for degradation or by blocking translation via base-pairing to complementary sites in the 3′-UTR of the target mRNA. The 3′-UTR in the human HCS gene is >3,000 bp in length (13) and was investigated here as a potential target for regulation by mir.
Materials and Methods
Cell culture.
Human Embryonic Kidney-293 (HEK-293) cells, Jurkat human lymphoblast cells, Caco-2 human colon cells, and IMR-90 primary human lung fibroblasts were purchased from ATCC. In some experiments, transgenic HEK-293 cells that overexpress HCS fused to green fluorescent protein (GFP) were used. Cells were cultured using routine procedures as recommended by ATCC (22). IMR-90 primary cells were used before senescence. For most experiments, cells were cultured in biotin-defined media containing 25 pmol/L biotin (“deficient”), 250 pmol/L biotin (“physiological”), or 10,000 pmol/L biotin (“pharmacological”). These concentrations represent levels typically seen in serum from biotin-deficient, biotin-normal, and biotin-supplemented adults (23, 24). Biotin-defined media were prepared as described previously (2). Carboxylase-bound biotin was routinely monitored using streptavidin blots as marker for cellular biotin (2).
Ecd-miR539-CMV-Luc-HCS3UTR.
The Ecd-miR539-CMV-Luc-HCS3UTR plasmid and other plasmids were built using standard molecular biology techniques (Supplemental Methods) (25–27).
Identification of miR targets in the HCS 3prime-UTR.
The 3′-UTR of human HCS was screened in silico for miR target sequences by using TargetScan (28) and microRNA.org (29). Both softwares predicted a miR-539 target site 1763–1785 bp downstream from the HCS stop codon. The importance of this site in HCS regulation was tested using plasmid CMV-miR539-CMV-Luc-539T (Supplemental Methods).
Luciferase assay.
Luciferase activity was assayed in triplicate, using independent samples; empty vectors and β-galactosidase vectors were used as baseline and transfection controls, respectively (30).
Overexpression of miR-539 in HEK-293 cells.
A miR-539 overexpression plasmid (CMV-miR539-CMV-GFP) was built to determine whether miR-539 decreases the expression of endogenous HCS (Supplemental Methods).
miR-539 promoter activity.
miR-539 promoter activity was quantified using plasmid miR539Pro-GFP in stably transformed HEK-293 cells (Supplemental Methods).
Quantitative real-time PCR (qRT-PCR).
Total RNA was isolated from cells using RNA Spin Mini isolation kits (GE Health). The abundance of miR-539 was quantified by stem-loop qRT-PCR (31). Briefly, primer 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACACAC-3′ was used for reverse transcription of miR-539 by using the ImProm-II Reverse Transcription System (Promega). miR-539 was quantified by qRT-PCR, using forward primer 5′-CGGCGGGGAGAAATTATCCT-3′ and reverse primer 5′-GTGCAGGGTCCGAGGT-3′. The relative expression of miR-539 was normalized using U6 snRNA as control; U6 snRNA was reverse transcribed using 5′- GTCAGGCAGCGTGCAGGGTCCGAGGTATTCGCACGCTGCCTGACAAAAAT-3′, and PCR amplified using forward primer 5′-CGCAAGGATGACACGCAAATT-3′and reverse primer 5′-GTGCAGGGTCCGAGGT-3′. All reverse transcriptions were conducted using 2 μg of total RNA. For qRT-PCR, the thermocycler was set at 95°C for 15 s and 55°C for 60 s per cycle for a total 40 cycles. Relative changes in mRNA abundances were quantified by using the Ct method; glyceraldehyde-5-phosphate dehydrogenase (GAPDH) mRNA and U6 RNA were used as reference amplicons for data normalization (32, 33).
RNA Spin Mini Isolation kits (GE Health) were used for analysis of luciferase, GFP, and HCS; 1.5 μg of total RNA was reverse transcribed using random hexamer primers (Fermentas). In qRT-PCR, the cDNA was amplified using forward primer 5′-ATGGAAGATAGACTCCACAT-3′ and reverse primer 5′-TGAGACCTGATCCTTAACTTCC-3′ for HCS analysis; forward primer 5′-CGGATTACCAGGGATTTCAGTC-3′ and reverse primer 5′-AAACCGGGAGGTAGATGAGATG-3′ for luciferase analysis; and forward primer 5′-GACCACTACCAGCAGAACAC-3′ and reverse primer 5′- GAACTCCAGCAGGACCATG-3′ for GFP analysis. GAPDH mRNA abundance was used for data normalization; GAPDH cDNA was amplified using forward primer 5′-TCCACTGGCGTCTTCACC-3′ and reverse primer: 5′- GGCAGAGATGATGACCCTTT-3′. All qRT-PCR reactions were performed at 95°C for 15 s and 52°C for 60 s per cycle for a total of 40 cycles.
Northern blots.
Total RNA was isolated from cells using the mirPremier miRNA isolation kit (Sigma) and miR was visualized by using the MicroRNA Northern Blot assay kit (Signosis) according to the manufacturers’ instructions. Biotinylated probes for miR-539 and U6 snRNA (control) were purchased from Signosis.
Western blots.
Whole cell proteins were extracted from HEK-293 cells and resolved by gel electrophoresis using NuPage 4–12% Bis-Tris gels (3). Transblots were probed using the following antibodies: goat anti-luciferase (Promega), rabbit anti-human HCS (C-terminus) (8), and mouse anti-GFP (Roche Applied Science). Equal loading was confirmed using goat anti-GAPDH (Santa Cruz). Histones were extracted from HEK-293 cells by using 1 mol/L HCl and resolved by gel electrophoresis (34). Transblots were probed using goat anti-biotin (Abcam). Equal loading was confirmed using goat anti-human histone H3, C-terminus (Santa Cruz). Proteins were visualized using fluorophore-labeled secondary antibodies and an Odyssey Infrared Imaging system (LICOR).
Chromatin immunoprecipitation (ChIP) assays.
ChIP assays were conducted in HCS-GFP-transformed cells as described (7, 16), with the following minor modifications. Anti-GFP was used for immunoprecipitations and the relative abundance of miR-539 in immunoprecipitated DNA and input DNA was quantified by qRT-PCR using primers 5′ GGTTTTATGACTAAAATCTCTCCATGGG -3′ (forward) and 5′ CCATTGTAAACACCGTGTATGCTC -3′ (reverse), and 100 ng of DNA template. The relative enrichments were normalized for nucleosomal occupancy at the miR-539 locus by precipitating chromatin with rabbit antiserum to the C-terminus in human histone H3 (Abcam). Data are expressed in units of percent of input DNA that was precipitated with antibodies.
Statistics.
Variances among groups were homogenous, as judged by Bartlett‘s test (35). When more than 2 groups were compared, significance of differences among groups was tested by 1-way ANOVA. Fisher's protected least significant difference procedure was used for post hoc testing. When 2 groups were compared, significance of differences was tested by paired t test. StatView 5.0.1 (SAS Institute) was used for statistical analyses. Differences were considered significant if P < 0.05. Data are reported as means ± SD.
Results
Regulation of HCS expression by miR-539.
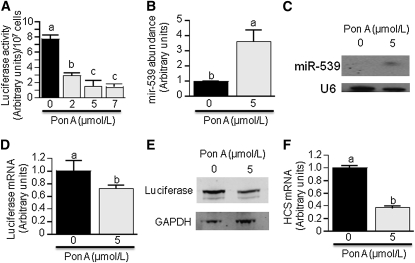
In silico analysis by TargetScan and microRna.org predicted a miR-539 binding site (5′-AACGAUGUAGGUUUCAUUUCUCA-3′) 1763–1785 basepairs downstream from the stop codon in human HCS mRNA. This prediction was confirmed experimentally by using pVgRXR-transformed HEK-293 cells. Ecdysone receptor and retinoid X receptor (RXR) positive clones were selected with zeocin before transfection with Ecd-mir539-CMV-Luc-HCS3UTR and selection with G418. The activity of luciferase was greater in ponasterone A (PonA)-treated cells (48 h) than in PonA-free controls (Fig. 1A). The observed effect was dose-dependent; inhibition of luciferase activity reached a plateau when the concentration of PonA was 5 μmol/L (Fig. 1A). All subsequent experiments were conducted using 5 μmol/L PonA. In control experiments, we confirmed that treatment with PonA increased the abundance of miR-539 in transformed HEK-293 cells by using qRT-PCR (Fig. 1B), and northern blot analysis (Fig. 1C). qRT-PCR data revealed that miR-539 concentrations in PonA-treated cells were 2.6-fold greater than in PonA-free controls. The low luciferase activity (= HCS reporter) observed in PonA-treated HEK-293 cells was paralleled by a low abundance of both luciferase mRNA (Fig. 1D) and protein (Fig. 1E). Importantly, the abundance of endogenous HCS mRNA was 63% lower in PonA-treated (5 μmol/L) HEK-293 cells than in PonA-free controls (Fig. 1F).
FIGURE 1.
miR-539 represses HCS expression by binding to the HCS 3′-UTR in HEK-293 cells. (A) Cells were stably transformed with Ecd-mir539-CMV-Luc-HCS3UTR and pVgRXR, and miR-539 was induced with PonA for 48 h. (B) Cells from panel A were used to quantify miR-539 by stem-loop qRT-PCR. (C) Same as panel B, but miR-539 was visualized by northern blot analysis. (D) Same as panel B, but luciferase mRNA was quantified by qRT-PCR. (E) Same as panel B, but luciferase protein was visualized by western blot analysis; GAPDH served as loading control. (F) Same as panel B, but the abundance of endogenous HCS mRNA was quantified by qRT-PCR. Values are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
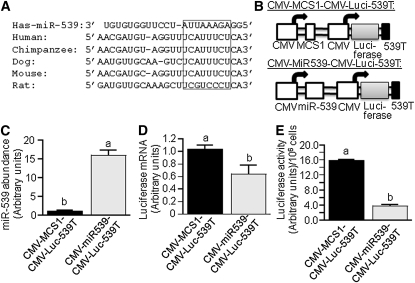
Gene repression by miRNA at the mRNA level is initiated by the formation of a miR-mRNA duplex in the 3′-UTR of mRNA (36). The miR-539 target site in human HCS is evolutionary conserved in mammals (Fig. 2A). The binding site perfectly matches to the 5′ end of the miR-539 with the exception of 2 mismatches in rat HCS.
FIGURE 2.
The 3′UTR of HCS mRNA contains a miR-593 binding site. (A) Sequence alignment between miR-593 and its putative binding site in the 3′UTR of human HCS mRNA and other species. The box denotes the most highly conserved region. (B) Schematic presentation of plasmids CMV-MCS1-CMV-Luci-539T (upper panel) and CMV-miR539-CMV-Luci-539T (lower panel). HEK-293 cells were stably transformed with CMV-MCS1-CMV-Luci-539T or CMV-miR539-CMV-Luci-539T, and the abundance of miR-539 (C), luciferase mRNA (D), and luciferase activity (E) were quantified by stem-loop qRT-PCR, qRT-PCR, and chemiluminescence, respectively. Values are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
Plasmid CMV-miR539-CMV-Luc-539T mediates constitutive expression of both miR-539 and luciferase fused to the putative 22-bp miR-539 binding site from human HCS (Fig. 2B). When HEK-293 cells were transfected with CMV-miR539-CMV-Luc-539T, the abundance of miR-539 was 13-fold greater than in cells transfected with the control plasmid CMV-multiple cloning site (MCS)1-CMV-Luc-539T (Fig. 2C). Luciferase mRNA and activity were 39% and 76% lower, respectively, in cells transfected with CMV-miR539-CMV-Luc-539T compared with CMV-MCS1-CMV-Luc-539T (Fig. 2D,E).
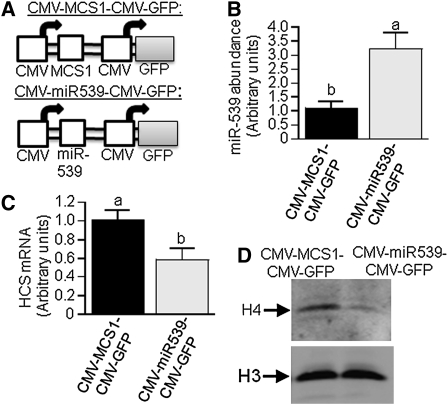
miR-539-mediated downregulation of HCS impairs histone biotinylation.
HCS is known to mediate biotinylation of histones (11, 12). Here, we demonstrated that miR-539 may decrease the expression of HCS to an extent that affects histone biotinylation. HEK-293 cells were stably transformed CMV-miR539-CMV-GFP to overexpress miR-539; controls were transformed with the empty vector CMV-MCS1-CMV-GFP (Fig. 3A). When cells were transformed with CMV-miR539-CMV-GFP, the expression of miR-593 was 2.1-fold greater than in controls (Fig. 3B); the high expression of miR-539 coincided with a mRNA abundance of endogenous HCS that was 43% lower in transformed cells than in controls (Fig. 3C). The low expression of HCS in miR-539 overexpression cells caused substantially lower levels of biotinylated histone H4, as judged by western blot analysis and anti-biotin antibody. Similar loading was confirmed by anti-histone H3 (Fig. 3D).
FIGURE 3.
Overexpression of miR-539 in HEK-293 cells causes low HCS expression and low abundance of biotinylated histones. (A) Schematic presentation of plasmids CMV-MCS1-CMV-GFP (upper panel) and CMV-miR539-CMV-GFP (lower panel). HEK-293 cells were stably transformed with CMV-MCS1-CMV-GFP or CMV-miR539-CMV-GFP, and the abundance of miR-539 (B), HCS mRNA (panel C), and biotinylated histone H4 (D, upper gel) were quantified. In Western blots, equal loading was confirmed by using anti-H3 (D, lower gel). Values are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
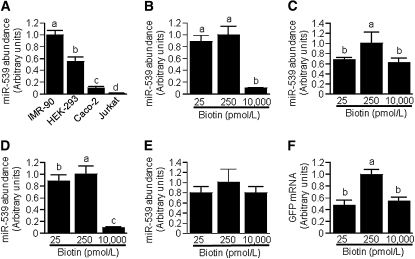
Repression of HCS expression is sensitive to biotin.
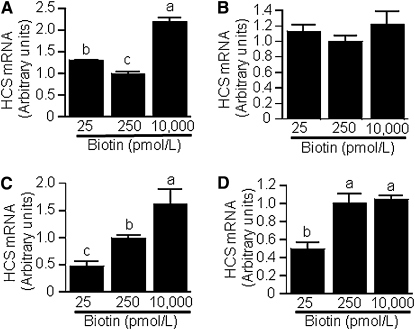
The expression of HCS depends on biotin in Jurkat human lymphoblastoma cells (8). Here, we provide evidence that the biotin-dependent expression of HCS in human cells is mediated, at least in part, by miR-539. First, we screened 4 human cell lines cultured in regular commercial media for the relative abundance of miR-539 and observed large differences (Fig. 4A): IMR-90 primary fibroblasts > HEK-293 embryonic kidney > Caco-2 colon carcinoma > Jurkat cells. In subsequent experiments, we determined whether miR-539 abundance depends on the concentration of biotin in culture media. When HEK-293 cells, Caco-2 cells, Jurkat cells, and IMR-90 fibroblasts were cultured in biotin-defined media (Fig. 4BndashE), the abundance of miR-539 was greater in physiological medium compared with other media in HEK-293 and Jurkat cells (Fig. 4C,D) but not in IMR-90 and Caco-2 cells (Fig. 4B,E); we confirmed this observation in multiple independent repeats and consistently obtained the same trends and significance levels (not shown). We were puzzled by the lack of a linear dose-response curve of miR-539 in biotin-defined cells and conducted additional tests to confirm that miR-539 expression is truly greater in cells cultured in physiological media than in deficient and pharmacological media. In these experiments, HEK-293 cells were stably transformed with plasmid miR539Pro-GFP and cultured in biotin-defined media. GFP mRNA abundance was significantly greater in cells cultured in physiological medium than in cells cultured in deficient or pharmacological media (Fig. 4F), consistent with the patterns in HEK-293 wild-type cells (Fig. 4C). Next, we quantified HCS mRNA levels in biotin-defined cells. In HEK-293 cells, high miR-539 levels coincided with low HCS mRNA levels; i.e., HCS mRNA was lower in physiological medium than in other media (Fig. 5A). A similar trend was observed for IMR-90 fibroblasts but those trends were not statistically significant (P = 0.20) (Fig. 5B). In Jurkat (Fig. 5C) and Caco-2 cells (Fig. 5D), HCS mRNA levels increased with increasing biotin supply. These observations suggest that the abundance of miR-539 depends on the availability of biotin in all the tissues tested here, but that in some tissues alternative mechanisms of HCS regulation override possible effects of miR-539 (see Discussion).
FIGURE 4.
The abundance of miR-539 depends on tissue (A) and biotin concentration (B–F) in IMR-90 fibroblasts, HEK-293 cells, Caco-2 cells, and Jurkat cells. Cells were cultured in biotin-defined media and miR-539 was quantified in IMR-90 fibroblasts (B), HEK-293 (C), Jurkat cells (D), and Caco-2 cells (E); in control experiments, HEK-293 cells were stably transformed with plasmid miR539Pro-GFP and GFP mRNA was quantified (F). Values are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
FIGURE 5.
The expression of HCS depends on biotin concentration in HEK-293 cells, Jurkat cells, and Caco-2 cells. Cells were cultured in biotin-defined media and HCS mRNA was quantified in HEK-293 cells (A), IMR-90 fibroblasts (B), Jurkat cells (C), and Caco-2 cells (D). Values are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
The expression of miR-539 depends on HCS.
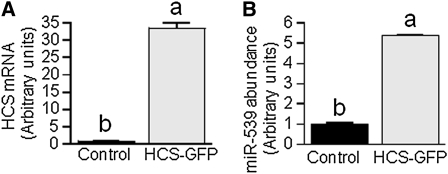
The abundance of HCS mRNA was 33-fold greater in HEK-293 cells transformed with HCS-GFP compared with controls (Fig. 6A), whereas the abundance of miR-539 mRNA was 439% greater in HCS-GFP-transformed cells compared with controls (Fig. 6B). The enrichment of HCS at the miR-539 locus was not significantly altered by overexpression of HCS, as judged by ChIP assay (data not shown), suggesting that biotin-dependent chromatin remodeling processes play no role in this feedback loop.
FIGURE 6.
The abundance of miR-539 depends on HCS in HEK-293. Cells were stably transformed with plasmid HCS-GFP; wild-type HEK-293 cells were used as controls. HCS mRNA (A) and miR-539 mRNA (B) were quantified by qRT-PCR. Values are means ± SD, n = 3. Means without a common letter differ, P < 0.05.
Discussion
Gene regulatory networks that interface with dietary bioactive food compounds and miR are only beginning to emerge (37, 38). Here we provide novel mechanistic insights into the modulation of miR-539 by the water-soluble vitamin biotin in humans, and the regulation of HCS by miR-539 and biotin. Our studies revealed that the miR-539 promoter is a biotin sensor in human cells; that miR-539 levels are greater at physiological biotin concentrations than at deficient or pharmacological concentrations; that the miR-539 binding site in the HCS 3′-UTR predicted by in silico search engines is real; that effects of miR-539 on the expression of HCS occur at the transcriptional rather than translational level; that the expression of miR-539 depends on HCS in a regulatory feedback loop that does not involve biotinylation of histones; and that in lymphoid and intestinal cell lineages, and perhaps some other tissues, factors other than miR-539 override HCS regulation by miR-539.
Our observations are of particular importance, given the key role of HCS in macronutrient metabolism, epigenetics and gene regulation, and biotin homeostasis. The roles of HCS in macronutrient metabolism are mediated by HCS-dependent biotinylation of acetyl-CoA carboxylases 1 and 2 (fatty acid synthesis and oxidation, respctively), pyruvate carboxylase (gluconeogenesis), propionyl-CoA carboxylase (odd-chain fatty acids), and 3-methylcrotonyl-CoA carboxylase (leucine metabolism) (1, 9, 10). The roles of HCS in epigenetics and gene regulation are mediated by HCS-dependent biotinylation of histones (11, 12, 39–41) and HCS-dependent generation of the intermediate biotinyl-AMP (15, 42). Decreased biotinylation of histones causes abnormal phenotypes and gene expression patterns (12) that are distinct from those seen in carboxylase deficiency (43). The roles of HCS in biotin homeostasis are mediated by HCS-dependent regulation of the biotin transporter SMVT (8) and by HCS-dependent biotinylation of acetyl-CoA carboxylase, which acts as a biotin reservoir (44). Consistent with the crucial role of HCS in cell biology, individuals with HCS mutations depend on lifelong treatment with pharmacological doses of biotin and no living HCS null person has yet be identified (14, 45).
Although our studies provide unambiguous evidence that miR-539 participates in HCS regulation, the biotin-sensing mechanisms in the putative miR-539 promoter are not entirely clear. We propose that multiple, sometimes counteracting, biotin-dependent factors feed into miR-539 regulation. First, in our in silico search we identified possible binding sites for miR-153 and miR-379 in the human HCS 3′-UTR. It is currently unknown whether these miR respond to biotin and play a role in the regulation of HCS expression. Second, in previous studies, we and others demonstrated that gene expression may be altered by biotin-dependent transcription factors such as NF-κB (46), jun and fos (3), and Sp1 and Sp3 (47), which also might affect miR-539 expression. Third, our miR-539 reporter gene construct (miR539Pro-GFP) included 2,500 basepairs upstream from the miR-539 transcription start site. We cannot formally exclude the possibility that sequences further upstream, enhancers residing in introns, position effect variegation, or chromosome:chromosome interactions contributed in meaningful ways to reporter gene activity. Fourth, miR-539 likely affects the expression of a fairly large number of genes, and altered expression of these genes in biotin-defined cells might contribute to the trans regulation of miR-539. Fifth, previous studies suggest that biotin metabolites have biotin-sparing effects (48). We cannot formally exclude the possibility that these metabolites participate in the regulation of HCS by miR-539. Collectively, a combination of diverse biotin-dependent factors from the list above might contribute to miR-539 regulation and explain our observation that throughout all cell lines tested miR-539 expression was consistently greater in physiological medium compared with other media. Some of these possibilities are currently being investigated in our laboratory and are likely to broaden the knowledge base in nutrition, epigenetics, miR, and gene regulation.
Supplementary Material
Acknowledgments
B.B. designed and conducted research, and analyzed data; R.R.-M. conducted research; S.S.K.W. conducted research; J.Z. designed research, analyzed data, wrote the paper, and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported in part by funds provided through the Hatch Act. Additional support was provided by NIH grants DK063945, DK077816, DK082476 and ES015206, USDA CSREES grant 2006-35200-17138, and by NSF grant EPS 0701892.
Supplemental Figure 1 and Methods are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: ChIP, chromatin immunoprecipitation; GFP, green fluorescent protein; GAPDH, glyceraldehyde-5-phosphate dehydrogenase; H4K12bio, K12-biotinylated histone H4; HCS, holocarboxylase synthetase; HEK-293, human embryonic kidney-293; miR, microRNA; MCS, multiple cloning site; PonA, ponasterone A; qRT-PCR, quantitative real-time PCR; RXR, retinoid X receptor; 3 prime -UTR, 3 prime -untranslated region.
References
- 1.Zempleni J, Wijeratne SS, Hassan YI. Biotin. Biofactors. 2009;35:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manthey KC, Griffin JB, Zempleni J. Biotin supply affects expression of biotin transporters, biotinylation of carboxylases, and metabolism of interleukin-2 in Jurkat cells. J Nutr. 2002;132:887–92 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Melendez R, Griffin JB, Sarath G, Zempleni J. High-throughput immunoblotting identifies biotin-dependent signaling proteins in HepG2 hepatocarcinoma cells. J Nutr. 2005;135:1659–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr. 2002;75:295–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mock DM, Johnson SB, Holman RT. Effects of biotin deficiency on serum fatty acid composition: Evidence for abnormalities in humans. J Nutr. 1988;118:342–8 [DOI] [PubMed] [Google Scholar]
- 6.Kuroishi T, Kinbara M, Sato N, Tanaka Y, Nagai Y, Iwakura Y, Endo Y, Sugawara S. Biotin status affects nickel allergy via regulation of interleukin-1beta production in mice. J Nutr. 2009;139:1031–6 [DOI] [PubMed] [Google Scholar]
- 7.Chew YC, West JT, Kratzer SJ, Ilvarsonn AM, Eissenberg JC, Dave BJ, Klinkebiel D, Christman JK, Zempleni J. Biotinylation of histones represses transposable elements in human and mouse cells and cell lines, and in Drosophila melanogaster. J Nutr. 2008;138:2316–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gralla M, Camporeale G, Zempleni J. Holocarboxylase synthetase regulates expression of biotin transporters by chromatin remodeling events at the SMVT locus. J Nutr Biochem. 2008;19:400–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dakshinamurti K, Chauhan J. Biotin-binding proteins. : Dakshinamurti K, editor Vitamin receptors: Vitamins as ligands in cell communication. Cambridge, UK: Cambridge University Press; 1994. p. 200–49 [Google Scholar]
- 10.Leon-Del-Rio A, Leclerc D, Akerman B, Wakamatsu N, Gravel RA. Isolation of a cDNA encoding human holocarboxylase synthetase by functional complementation of a biotin auxotroph of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:4626–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narang MA, Dumas R, Ayer LM, Gravel RA. Reduced histone biotinylation in multiple carboxylase deficiency patients: a nuclear role for holocarboxylase synthetase. Hum Mol Genet. 2004;13:15–23 [DOI] [PubMed] [Google Scholar]
- 12.Camporeale G, Giordano E, Rendina R, Zempleni J, Eissenberg JC. Drosophila holocarboxylase synthetase is a chromosomal protein required for normal histone biotinylation, gene transcription patterns, lifespan and heat tolerance. J Nutr. 2006;136:2735–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y, Aoki Y, Ishida Y, Chiba Y, Iwamatsu A, Kishino T, Niikawa N, Matsubara Y, Narisawa K. Isolation and characterization of mutations in the human holocarboxylase synthetase cDNA. Nat Genet. 1994;8:122–8 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Yang X, Aoki Y, Kure S, Matsubara Y. Mutations in the holocarboxylase synthetase gene HLCS. Hum Mutat. 2005;26:285–90 [DOI] [PubMed] [Google Scholar]
- 15.Solorzano-Vargas RS, Pacheco-Alvarez D, Leon-Del-Rio A. Holocarboxylase synthetase is an obligate participant in biotin-mediated regulation of its own expression and of biotin-dependent carboxylases mRNA levels in human cells. Proc Natl Acad Sci USA. 2002;99:5325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camporeale G, Oommen AM, Griffin JB, Sarath G, Zempleni J. K12-biotinylated histone H4 marks heterochromatin in human lymphoblastoma cells. J Nutr Biochem. 2007;18:760–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54 [DOI] [PubMed] [Google Scholar]
- 18.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8 [DOI] [PubMed] [Google Scholar]
- 19.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97 [DOI] [PubMed] [Google Scholar]
- 20.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5 [DOI] [PubMed] [Google Scholar]
- 21.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205 [DOI] [PubMed] [Google Scholar]
- 22.American Type Culture Collection Manassas (VA): ATCC; [cited 2009 Aug 15]. Available from: www.atcc.org [Google Scholar]
- 23.Mock DM, Lankford GL, Mock NI. Biotin accounts for only half of the total avidin-binding substances in human serum. J Nutr. 1995;125:941–6 [DOI] [PubMed] [Google Scholar]
- 24.Zempleni J, Helm RM, Mock DM. In vivo biotin supplementation at a pharmacologic dose decreases proliferation rates of human peripheral blood mononuclear cells and cytokine release. J Nutr. 2001;131:1479–84 [DOI] [PubMed] [Google Scholar]
- 25.Vogel MJ, Guelen L, de Wit E, Peric-Hupkes D, Loden M, Talhout W, Feenstra M, Abbas B, Classen AK, van Steensel B. Human heterochromatin proteins form large domains containing KRAB-ZNF genes. Genome Res. 2006;16:1493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–6 [DOI] [PubMed] [Google Scholar]
- 27.Rangasamy D, Tremethick DJ, Greaves IK. Gene knockdown by ecdysone-based inducible RNAi in stable mammalian cell lines. Nat Protoc. 2008;3:79–88 [DOI] [PubMed] [Google Scholar]
- 28.TargetScan. Whitehead Institute for Biomedical Research. [cited 2009 May 8]. Available from: www.targetscan.org/index.html.
- 29.microRNA. Memorial Sloan-Kettering Cancer Center. [cited 2009 May 8]. Available from: www.microrna.org/microrna/getGeneForm.do.
- 30.Rodriguez-Melendez R, Camporeale G, Griffin JB, Zempleni J. Interleukin-2 receptor γ-dependent endocytosis depends on biotin in Jurkat cells. Am J Physiol Cell Physiol. 2003;284:C415–21 [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods. 2001;25:402–8 [DOI] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8 [DOI] [PubMed] [Google Scholar]
- 34.Camporeale G, Shubert EE, Sarath G, Cerny R, Zempleni J. K8 and K12 are biotinylated in human histone H4. Eur J Biochem. 2004;271:2257–63 [DOI] [PubMed] [Google Scholar]
- 35.Abacus Concepts. StatView Berkeley (CA): Abacus Concepts Inc; 1996 [Google Scholar]
- 36.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–24 [DOI] [PubMed] [Google Scholar]
- 37.Haygood R, Fedrigo O, Hanson B, Yokoyama KD, Wray GA. Promoter regions of many neural- and nutrition-related genes have experienced positive selection during human evolution. Nat Genet. 2007;39:1140–4 [DOI] [PubMed] [Google Scholar]
- 38.Gaedicke S, Zhang X, Schmelzer C, Lou Y, Doering F, Frank J, Rimbach G. Vitamin E dependent microRNA regulation in rat liver. FEBS Lett. 2008;582:3542–6 [DOI] [PubMed] [Google Scholar]
- 39.Hymes J, Wolf B. Human biotinidase isn't just for recycling biotin. J Nutr. 1999;129:485S–9S [DOI] [PubMed] [Google Scholar]
- 40.Stanley JS, Griffin JB, Zempleni J. Biotinylation of histones in human cells: effects of cell proliferation. Eur J Biochem. 2001;268:5424–9 [DOI] [PubMed] [Google Scholar]
- 41.Kobza K, Camporeale G, Rueckert B, Kueh A, Griffin JB, Sarath G, Zempleni J. K4, K9, and K18 in human histone H3 are targets for biotinylation by biotinidase. FEBS J. 2005;272:4249–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Melendez R, Zempleni J. Nitric oxide signaling depends on biotin in Jurkat human lymphoma cells. J Nutr. 2009;139:429–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camporeale G, Zempleni J, Eissenberg JC. Susceptibility to heat stress and aberrant gene expression patterns in holocarboxylase synthetase-deficient Drosophila melanogaster are caused by decreased biotinylation of histones, not of carboxylases. J Nutr. 2007;137:885–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shriver BJ, Roman-Shriver C, Allred JB. Depletion and repletion of biotinyl enzymes in liver of biotin-deficient rats: Evidence of a biotin storage system. J Nutr. 1993;123:1140–9 [DOI] [PubMed] [Google Scholar]
- 45.Aoki Y, Li X, Sakamoto O, Hiratsuka M, Akaishi H, Xu L, Briones P, Suormala T, Baumgartner ER, et al. Identification and characterization of mutations in patients with holocarboxylase synthetase deficiency. Hum Genet. 1999;104:143–8 [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Melendez R, Schwab LD, Zempleni J. Jurkat cells respond to biotin deficiency with increased nuclear translocation of NF-kB, mediating cell survival. Int J Vitam Nutr Res. 2004;74:209–16 [DOI] [PubMed] [Google Scholar]
- 47.Griffin JB, Rodriguez-Melendez R, Zempleni J. The nuclear abundance of transcription factors Sp1 and Sp3 depends on biotin in Jurkat cells. J Nutr. 2003;133:3409–15 [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Melendez R, Lewis B, McMahon RJ, Zempleni J. Diaminobiotin and desthiobiotin have biotin-like activities in Jurkat cells. J Nutr. 2003;133:1259–64 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.