Abstract
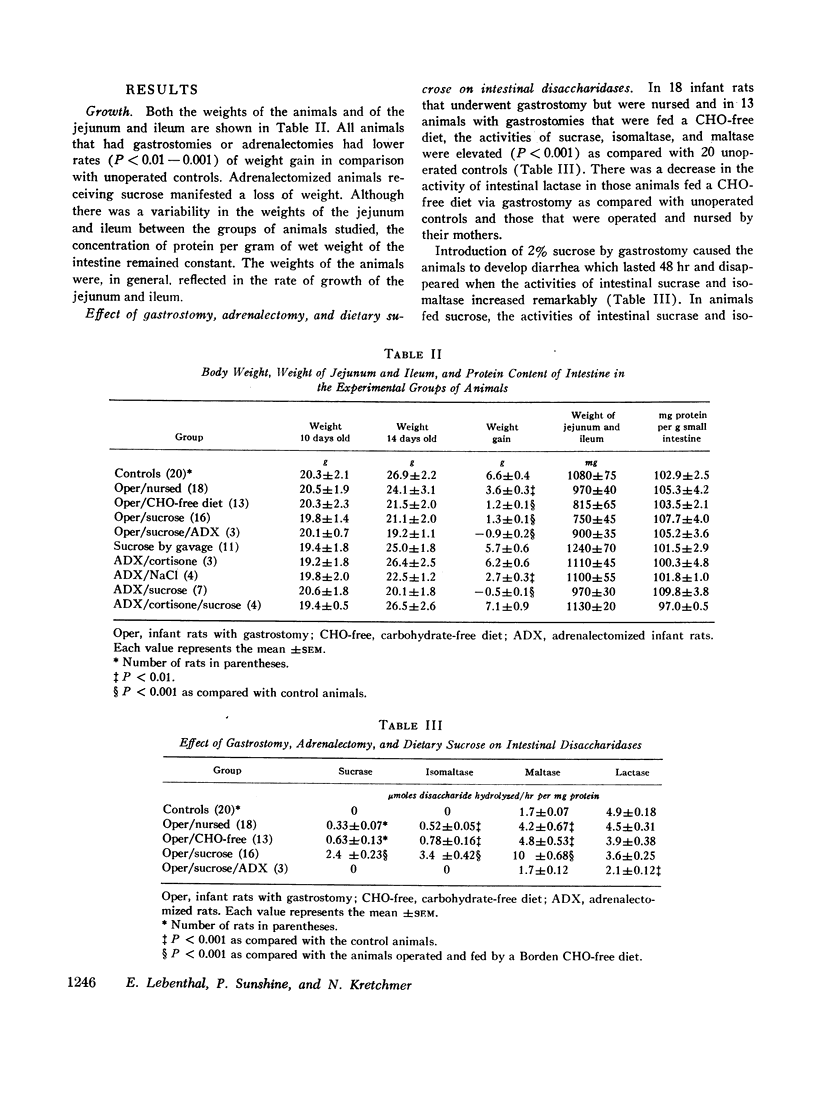
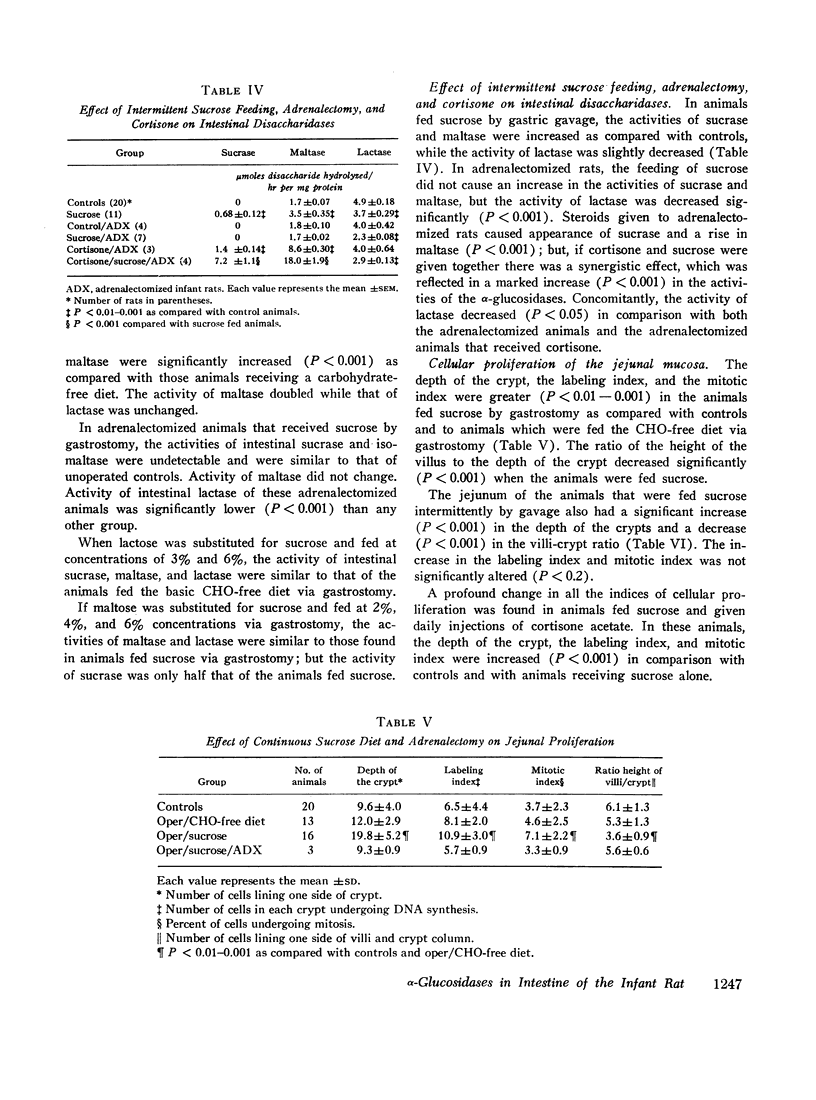
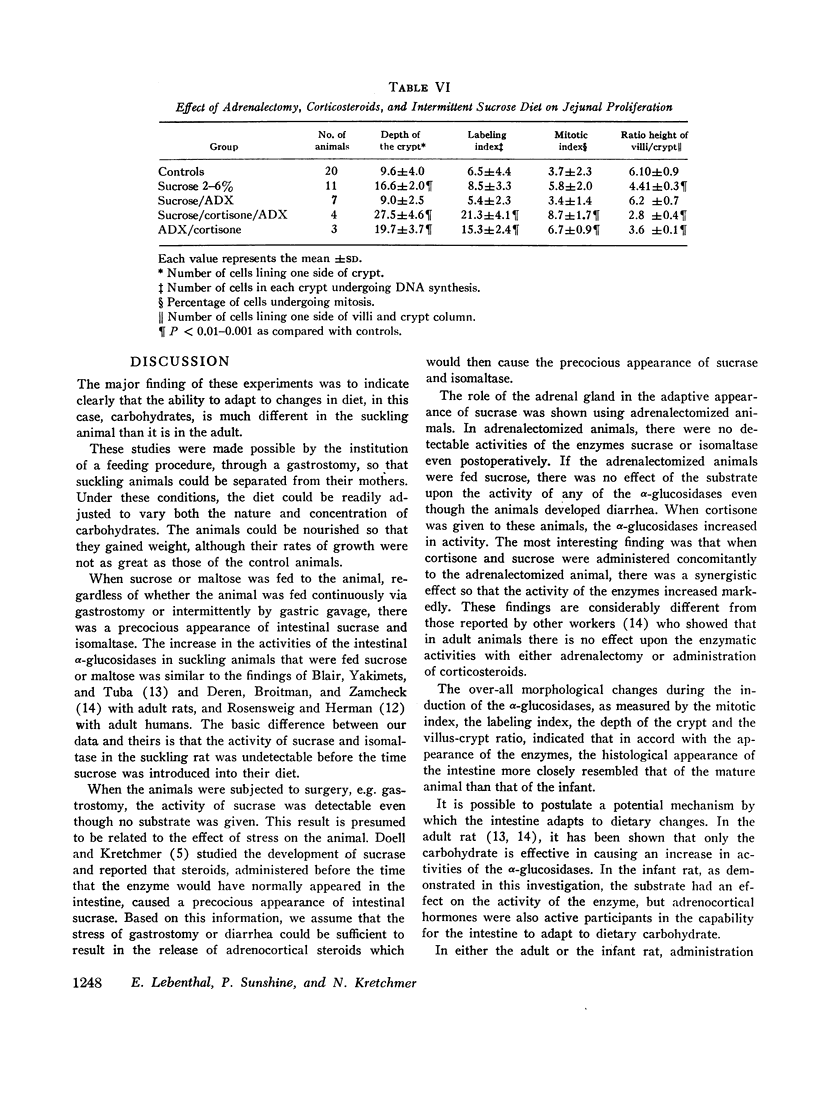
The activities of intestinal sucrase and isomaltase are not detectable in rats before 15-16 days of age, but administration of corticosteroids precociously induces the activities of these two α-glucosidases. 9-day old rats were removed from their mothers, warmed in an incubator, and fed by constant infusion through gastrostomies. The basic diet was a soya preparation to which various sugars were added. When the diet contained 2% sucrose, diarrhea ensued for 48 hr, but subsided when intestinal sucrase and isomaltase appeared precociously. In animals fed sucrose, the activities of sucrase and isomaltase were markedly increased as compared to animals on carbohydrate-free diets (sucrase 2.41±0.23 vs. 0.63±0.13 U, isomaltase 3.43±0.42 vs. 0.78±0.18 U). Maltase activity was doubled, while lactase was not altered significantly. The mitotic index of crypt cells, the depth of crypts, and incorporation of thymidine-3H into DNA were increased. In adrenalectomized rats, activities of sucrase and isomaltase were not detected nor induced by sucrose. Steroids given to adrenalectomized rats caused appearance of the enzymes; but if cortisone and sucrose were given together, there was synergism evidenced by a marked increase in activities (sucrase 7.2±1.1 vs. 0.68±0.12 U). In contrast to observations in adult animals, the effect of sucrose on α-glucosidases in developing animals demands the participation of the adrenal gland.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURICCHIO S., RUBINO A., MUERSET G. INTESTINAL GLYCOSIDASE ACTIVITIES IN THE HUMAN EMBRYO, FETUS, AND NEWBORN. Pediatrics. 1965 Jun;35:944–954. [PubMed] [Google Scholar]
- BLAIR D. G., YAKIMETS W., TUBA J. Rat intestinal sucrase. II. The effects of rat age and sex and of diet on sucrase activity. Can J Biochem Physiol. 1963 Apr;41:917–929. [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. Studies of small intestine during development. I. Distribution and activity of beta-galactosidase. Biochim Biophys Acta. 1962 Aug 13;62:353–362. doi: 10.1016/0006-3002(62)90097-5. [DOI] [PubMed] [Google Scholar]
- Deren J. J., Broitman S. A., Zamcheck N. Effect of diet upon intestinal disaccharidases and disaccharide absorption. J Clin Invest. 1967 Feb;46(2):186–195. doi: 10.1172/JCI105521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doell R. G., Rosen G., Kretchmer N. Immunochemical studies of intestinal disaccharidases during normal and precocious development. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1268–1273. doi: 10.1073/pnas.54.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOMINA L. S. [Content of certain enzymes in the intestine and other organs of the human fetus]. Vopr Med Khim. 1960 Mar-Apr;6:176–183. [PubMed] [Google Scholar]
- HEILSKOV N. S. C. Studies on animal lactase. II. Distribution in some of the glands of the digestive tract. Acta Physiol Scand. 1951 Oct 9;24(1):84–89. doi: 10.1111/j.1748-1716.1951.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Sunshine P. Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr Res. 1969 Jan;3(1):27–33. doi: 10.1203/00006450-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. Endocrine modification of the developmental formation of ornithine aminotransferase in rat tissues. J Biol Chem. 1969 Sep 25;244(18):4894–4898. [PubMed] [Google Scholar]
- KENNEY F. T. Induction of tyrosine-alpha-ketoglutarate transaminase in rat liver. IV. Evidence for an increase in the rate of enzyme synthesis. J Biol Chem. 1962 Nov;237:3495–3498. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Messer M., Thoman E. B., Galofre A., Dallman T., Dallman P. R. Artificial feeding of infant rats by continuous gastric infusion. J Nutr. 1969 Aug;98(4):404–410. doi: 10.1093/jn/98.4.404. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- RUBINO A., ZIMBALATTI F., AURICCHIO S. INTESTINAL DISACCHARIDASE ACTIVITIES IN ADULT AND SUCKLING RATS. Biochim Biophys Acta. 1964 Nov 22;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- Rosensweig N. S., Herman R. H. Control of jejunal sucrase and maltase activity by dietary sucrose or fructose in man. A model for the study of enzyme regulation in man. J Clin Invest. 1968 Oct;47(10):2253–2262. doi: 10.1172/JCI105910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räihä N. C., Kekomäki M. P. Studies on the development of ornithine-keto acid aminotransferase activity in rat liver. Biochem J. 1968 Jul;108(4):521–525. doi: 10.1042/bj1080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Welsh J. D., Walker A. Intestinal disaccharidase and alkaline phosphatase activity in the dog. Proc Soc Exp Biol Med. 1965 Nov;120(2):525–527. doi: 10.3181/00379727-120-30580. [DOI] [PubMed] [Google Scholar]


