Abstract
Purpose of review
Cough is one of the most common reasons why patients visit physicians. The opioid codeine has been a mainstay in the treatment of cough for decades and this drug is widely regarded as the ‘gold standard’ cough suppressant.
Recent findings
Recent placebo-controlled studies have shown that codeine is no more effective than placebo in suppressing cough caused by either upper respiratory disorders or chronic obstructive pulmonary disease. These recent reports are not consistent with several older placebo-controlled studies that demonstrated the efficacy of codeine. The reasons for this difference are not fully understood.
Summary
We propose that these differences, as well as results from animal models, can be explained by the existence of a complex hierarchical control system that regulates the expression of coughing. This system, known as a holarchy, is composed of regulatory elements known as ‘holons’ that interact with one another to regulate cough. Based on work in animal models, codeine is proposed to act on an intermediate order holon that may not be critical for coughing under some situations in humans. Testing of this hypothesis and further elucidation of the control system for cough will represent an important direction for future research in this area.
Keywords: antitussive, codeine, cough, cough suppressant
Introduction
This review is intended to highlight recent work on the efficacy of codeine to suppress cough in humans. To put these more recent studies in context, older literature in the area is cited and a unified model is presented that accounts for many observations in humans and animal models regarding the effects of codeine as well as recent advances in our understanding of the neural mechanisms of cough. The model represents the application of a novel hypothesis regarding hierarchical control systems theory to our knowledge of the mechanisms of cough.
Significance of cough and questionable efficacy of codeine as an antitussive
Patients often suffer from chronic cough in excess of 4 years [1,2]. Therefore, there is a strong need for strategies to reduce the severity of cough. Future advances in this area will be incremental without a more detailed understanding of the reflex pathway under normal conditions, during pathological changes in lung function, and the mechanisms by which antitussive drugs suppress cough.
Chronic coughing typically represents the repeated occurrence of this airway defensive behavior in excess of what is required to maintain a patent airway. In some cases, cough can be manifest as paroxysms, or intense repetitive bouts of coughing [3]. The excitability of cough can be elevated in various airway disorders and successful treatment of the underlying cause of cough will return the enhanced excitability of this behavior back to the normal range [4]. It is widely thought that enhanced coughing associated with airway disorders is beneficial and that suppressive treatment will increase the risk of morbidities resulting from impaired clearance mechanisms. Evidence that currently available treatments for chronic cough or suppressive therapies such as codeine will prevent patients from coughing or interfere with cough clearance does not exist.
Codeine is widely regarded as the ‘gold standard’ cough suppressant drug. The perception has arisen from its efficacy in animal models [5–8] and in several older studies in humans [9,10]. These older studies were placebo controlled and involved patients with various airway disorders such as chronic bronchitis or chronic obstructive pulmonary disease [9,10]. Dextromethorphan, the most common over-the-counter antitussive in the US, has also been shown to be effective in patients with chronic bronchitis in a placebo-controlled study [11]. These studies, as well as many decades of clinical experience, have led to the widespread perception of codeine, and to a lesser extent dextromethorphan, as effective and safe cough suppressants.
More recent studies have raised issues regarding the efficacy of these drugs. Codeine has been used in several double-blind placebo-controlled studies that failed to demonstrate a significant effect of this drug in suppressing cough compared with placebo [12,13,14••]. The dosage ranges of codeine used in these recent studies were similar to those administered in the older reports (30–60 mg). Dextromethorphan has been found to be weakly effective in several studies of cough caused by upper airway disorders [15,16] but other studies did not find that the drug was effective [17,18]. The questionable efficacy of codeine on coughing is contrary to the intended purpose of this drug – namely, to provide symptomatic relief of coughing regardless of etiology. As such, the widespread perception of codeine as the ‘gold standard’ cough suppressant drug must be reevaluated. We suggest that although codeine is probably the best available cough suppressant, no current drug fits the classification of ‘gold standard’.
The concept of codeine as a ‘gold standard’ has several important implications. First, it is likely that codeine is frequently prescribed in clinical situations in which it is ineffective. Presumably, this occurs because of the now questionable perception that codeine should be effective in most clinical circumstances. Also, few other cough suppressant drugs are available. Second, the questionable perception of codeine as a ‘gold standard’ antitussive agent hinders the drug discovery and development process. Although codeine has a significant side-effect profile, the fact that it is perceived to be broadly effective could present an obstacle to the adoption of discovery programs in the pharmaceutical industry aimed at novel antitussive agents. Moreover, the clinical development of novel antitussive agents can be impaired by adherence to approaches that require comparisons to a ‘gold standard’ that is actually ineffective. A common approach is to establish a human model in which a gold standard drug is effective. The novel drug can then be tested in that model for efficacy relative to the gold standard. Furthermore, the risk of an outcome in which the novel antitussive drug is ineffective is reduced because the human model was already shown to be sensitive to a cough suppressant drug. The requirement that a human model be established in which a ‘gold standard’ is effective before testing with a novel drug can be implemented is not met when using codeine. For example, codeine was ineffective relative to placebo in patients with chronic obstructive pulmonary disease during irritant induced cough challenge as well as in a battery of other assessments of this behavior [14••]. These observations imply that clinical development of a novel antitussive agent is likely to represent a challenging endeavor for a pharmaceutical company.
The reasons for the apparently conflicting data regarding the efficacy of codeine are unclear. We have recently proposed [19•] that the difference between these studies was related to upper or lower airway involvement of the disorder causing cough, with coughing due to lower airway disorders being sensitive to codeine and that due to upper airway pathologies being insensitive to the drug. Nevertheless, the results of Smith et al. [14••] do not support this hypothesis. As noted above, they studied patients with chronic obstructive pulmonary disease and reported that codeine was ineffective for cough suppression under ambulatory conditions. Smith et al. [14••] reported that the previous studies were largely conducted under controlled conditions, such as in research laboratories. This approach contrasts with the trend for similar studies in recent years to be performed under ambulatory conditions [14••]. Furthermore, Smith et al. [14••] suggested that the patients in the older studies may have been able to taste the codeine, resulting in an unblinded protocol. While this is possible, we believe that it is unlikely that it would be a problem spanning several studies in different laboratories. The suggestion that different results may be explained by the conditions under which the studies were conducted may mesh with recent work on perceptual factors associated with cough and the central control of the behavior.
The production of cough is associated with quantifiable sensations in humans [20]. An example of this is the ‘urge to cough’ sensation produced by inhalation of capsaicin in humans with no airway pathology [20]. This sensation increases in direct proportion with the dose of capsaicin and precedes the production of cough by this irritant [20]. The presence of sensations associated with cough in humans indicates that suprapontine pathways, such as the cortex, can be involved in the regulation of coughing. Indeed, humans are capable of voluntarily coughing and can voluntarily suppress cough by a non-opioid mechanism [13,21,22]. The extent to which suprapontine mechanisms can contribute to or modify the production of cough in humans with airway pathology is unknown. Furthermore, the role of suprapontine pathways in mediating or modifying the efficacy of antitussive agents in humans is unknown. From work in animal models, codeine and other centrally acting antitussives suppress cough by an action in the brainstem [7,23]. These findings in animal models do not rule out an important role for suprapontine mechanisms in the actions of cough suppressant drugs. Given these issues, it is plausible that mechanisms associated with consciousness play a far greater role in the efficacy of antitussive drugs than has previously been appreciated. As such, it would not be surprising for the conditions under which clinical studies are implemented to have a profound impact on the efficacy of a cough suppressant drug.
Functional control mechanisms for cough
Clarification of these matters will certainly be facilitated by a greater understanding of the central organization of the cough production mechanism. Considerable detail on the central mechanisms that control coughing is available from animal models [24–28]. The magnitude and timing of coughing is controlled by a cough pattern generator located in the brainstem [24–29] which is a complex network of brainstem respiratory neurons that undergo changes in discharge pattern during cough. The current model is based on the hypothesis that a single pattern generator produces both cough and breathing. This hypothesis holds that this pattern generator normally controls the respiratory muscles to produce breathing but can undergo a rapid reorganization known as reconfiguration to produce the cough motor pattern. This hypothesis does not completely explain how cough suppressant drugs act in animal models. To account for the specific effects of antitussive drugs in animal models, we have proposed modification of the current model of the cough pattern generation system to include an additional element, known as a gate [29]. The gating mechanism is proposed to control the excitability of the rest of the cough pattern generation system such that the duration of time over which this gating mechanism is actuated by an airway stimulus determines whether single or repetitive coughs are produced. Furthermore, we proposed that cough suppressants such as codeine act by specific inhibition of this gating mechanism [6,29].
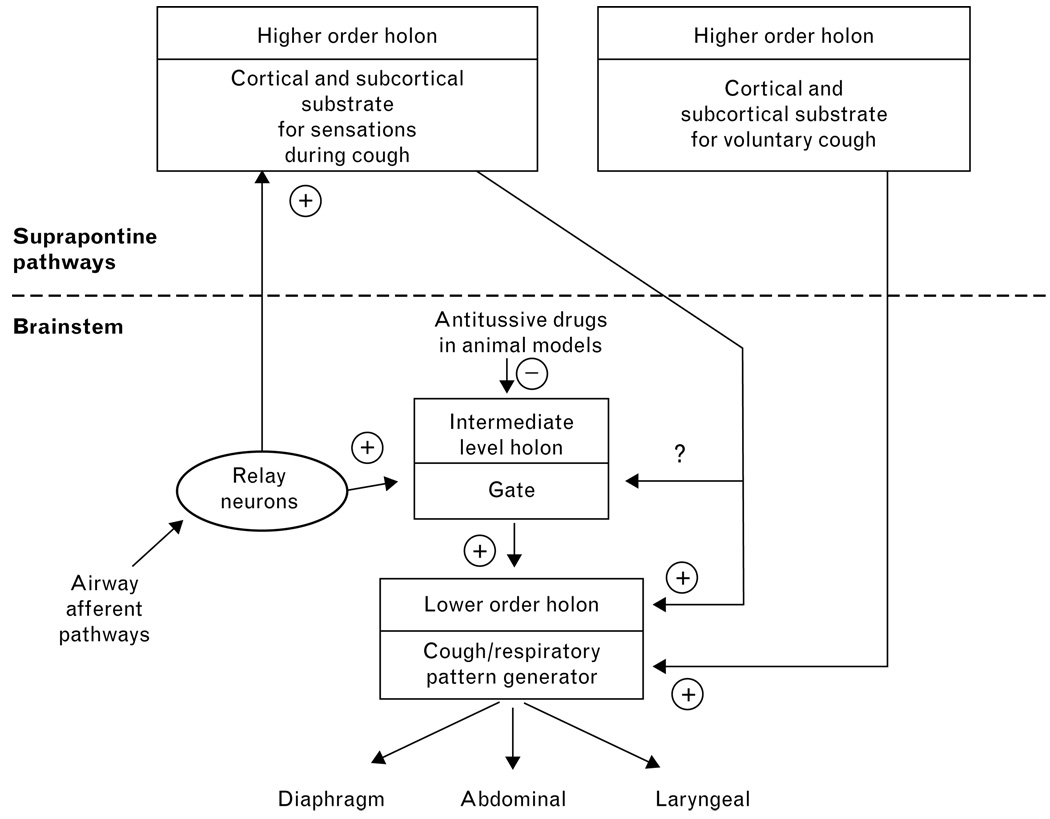
The gating mechanism is an example of a functional control element in the cough network. This element is also proposed to account for the permissive effects of slowly adapting pulmonary stretch receptors on cough, an action that is very different than their effects on breathing [30,31]. The presence of one or more control elements that change the regulation of the controlled system is consistent with a hierarchical control theory first proposed by Koestler [32]. His theory was based on the existence of control elements known as holons that could change the regulation of the subsystems that they controlled. A unique feature of holons is that their influence is not easily predictable by study of the lower level systems that they control [32]. He also proposed that although a holon was a control element that regulated a lower level subsystem, it could itself be regulated by higher level holons. Koestler called the entire system a holarchy. We have recently proposed that the central regulatory system for cough is a holarchy and the gating mechanism is a holon [33].
The holarchy hypothesis may help account for the inconsistencies in the literature regarding codeine. A holarchical model that is consistent with available data is shown in Fig. 1. One or more suprapontine pathways may represent higher order holons in the system. Their influence would not be manifest in animal models that are anesthetized or decerebrated. Indeed, the extent to which animals can experience sensations during cough is unknown. On the other hand, awake guinea pigs can be conditioned to cough in response to innocuous odors [34] which supports the concept that complex suprapontine control mechanisms exist in animals. The gating mechanism represents an intermediate level holon that is restricted to the brainstem and controls the expression of coughing during anesthesia and/or when suprapontine pathways have been eliminated by decerebration. This holon is sensitive to codeine. During consciousness in humans, suprapontine holons may control the brainstem holon for cough (the gate) and thus change its responsiveness to codeine. We have proposed the existence of a suprapontine holon (Fig. 1) that can actuate voluntary cough by controlling the lower order holon that is represented by the cough/respiratory pattern generator. The cough/respiratory pattern generator determines the distribution of motor drive to the respiratory muscles during cough and the temporal features of the behavior (the duration of the inspiratory, compressive, and expulsive phases). This hypothesis can be tested. For example, an antitussive drug that is effective in humans should have specific actions on coughing induced by airway pathology but have no effect on voluntary cough. Such a finding would support the existence of a holarchical control system consisting of at least one lower order holon that is sensitive to an antitussive and at least one higher order holon that is not affected by the drug. Unfortunately, testing the hypothesis in this manner will require discovery of an antitussive that is effective in humans. Further testing of this control mechanism is possible. If separate holons exist for cough associated with sensations and voluntary behaviors, as shown in Fig. 1, the ability of a subject to produce voluntary cough should not be changed during capsaicin inhalation sufficient to elicit an urge to cough sensation.
Figure 1. Proposed holarchical organization of the central cough generation mechanism.
The model shows multiple control elements for cough (holons) that are organized in a hierarchical manner. Holons are functional entities made up of neural substrates (axonal pathways, groups of neurons, and nuclei) that mediate a particular control mechanism. The model can account for sensations during cough, voluntary cough, the actions of codeine in animal models, and the recent difficulties in demonstrating the efficacy of codeine in humans. We propose that codeine acts primarily on an intermediate order holon that is either not a dominant controller of cough in awake humans or controls cough in humans only in specific situations. The model can be tested (see text).
Conclusion
Clearly the widespread notion that codeine is an effective cough suppressant is not supported by the available evidence. Codeine may in fact have efficacy to suppress cough in humans only in specific situations. The reasons for this may be related to the fact that the control mechanisms for cough appear to be far more complex than has been appreciated. As these complex control mechanisms may involve neural substrates that are only manifest during consciousness in humans, many current animal models of cough may require reevaluation. As such, the elucidation of the control of cough in humans as well as the discovery of novel and effective antitussive agents is likely to be a challenging process.
Acknowledgements
The author was supported by HL 70125, NS 54025, and NS 50699 from the National Institutes of Health.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 112).
- 1.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123(4 Pt 1):413–417. doi: 10.1164/arrd.1981.123.4.413. [DOI] [PubMed] [Google Scholar]
- 2.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640–647. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 3.Talbert DG. Paroxysmal cough injury, vascular rupture and ‘shaken baby syndrome’. Med Hypotheses. 2005;64:8–13. doi: 10.1016/j.mehy.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell F, Thomas VE, Pride NB, Fuller RW. Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med. 1994;150:374–380. doi: 10.1164/ajrccm.150.2.8049818. [DOI] [PubMed] [Google Scholar]
- 5.May AJ, Widdicombe JG. Depression of the cough reflex by pentobarbitone and some opium derivatives. Br J Pharmacol Chemother. 1954;9:335–340. doi: 10.1111/j.1476-5381.1954.tb01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 7.Chou D, Wang SC. Studies on the localization of the central cough mechanism: site of action of antitussive drugs. J Pharmacol Exp Ther. 1975;194:499–505. [PubMed] [Google Scholar]
- 8.Korpas J, Tomori Z. Cough and other respiratory reflexes. Basel; New York: S. Karger; 1979. [Google Scholar]
- 9.Sevelius H, McCoy JF, Colmore JP. Dose response to codeine in patients with chronic cough. Clin Pharmacol Ther. 1971;12:449–455. doi: 10.1002/cpt1971123449. [DOI] [PubMed] [Google Scholar]
- 10.Sevelius H, Colmore JP. Objective assessment of antitussive agents in patients with chronic cough. J New Drugs. 1966;6:216–223. [PubMed] [Google Scholar]
- 11.Aylward M, Maddock J, Davies DE, et al. Dextromethorphan and codeine: comparison of plasma kinetics and antitussive effects. Eur J Respir Dis. 1984;65:283–291. [PubMed] [Google Scholar]
- 12.Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol. 1997;49:1045–1049. doi: 10.1111/j.2042-7158.1997.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 13.Hutchings HA, Eccles R. The opioid agonist codeine and antagonist naltrexone do not affect voluntary suppression of capsaicin induced cough in healthy subjects. Eur Respir J. 1994;7:715–719. doi: 10.1183/09031936.94.07040715. [DOI] [PubMed] [Google Scholar]
- 14. Smith J, Owen E, Earis J, Woodcock A. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117:831–835. doi: 10.1016/j.jaci.2005.09.055.. This comprehensive study evaluated the efficacy of codeine in a double-blind, placebo-controlled crossover paradigm in patients with chronic obstructive pulmonary disease. The cough responses were measured objectively which is an important component of the study.
- 15.Parvez L, Vaidya M, Sakhardande A, et al. Evaluation of antitussive agents in man. Pulm Pharmacol. 1996;9(5–6):299–308. doi: 10.1006/pulp.1996.0039. [DOI] [PubMed] [Google Scholar]
- 16.Pavesi L, Subburaj S, Porter-Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta-analysis. Chest. 2001;120:1121–1128. doi: 10.1378/chest.120.4.1121. [DOI] [PubMed] [Google Scholar]
- 17.Lee PCL, Jawad MS, Eccles R. Antitussive efficacy of dextromethorphan in cough associated with acute upper respiratory tract infection. J Pharm Pharmacol. 2000;52:1137–1142. doi: 10.1211/0022357001774903. [DOI] [PubMed] [Google Scholar]
- 18.Tukiainen H, Karttunen P, Silvasti M, et al. The treatment of acute transient cough: a placebo-controlled comparison of dextromethorphan and dextromethorphan-beta 2-sympathomimetic combination. Eur J Respir Dis. 1986;69:95–99. [PubMed] [Google Scholar]
- 19. Bolser DC. Cough suppressant and pharmacologic protussive therapy: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):238S–249S. doi: 10.1378/chest.129.1_suppl.238S.. This review discusses evidence regarding the efficacy of cough suppressants in humans with airway disease, their use, as well as the current clinical approach to the treatment of chronic cough.
- 20.Davenport P, Sapienza CM, Bolser DC. Psychophysical assessment of the urge-to-cough. Eur Respir Rev. 2002;85:249–253. [Google Scholar]
- 21.Hutchings HA, Eccles R, Smith AP, Jawad MS. Voluntary cough suppression as an indication of symptom severity in upper respiratory tract infections. Eur Respir J. 1993;6:1449–1454. [PubMed] [Google Scholar]
- 22.Smith Hammond CA, Goldstein LB, Zajac DJ, et al. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 23.Bolser D, DeGennaro FC, O’Reilly S, et al. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol. 1994;113:1344–1348. doi: 10.1111/j.1476-5381.1994.tb17145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baekey DM, Morris KF, Gestreau C, et al. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol. 2001;534(Pt 2):565–581. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon R, Baekey DM, Morris KF, et al. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol. 2000;525(Pt 1):207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon R, Baekey DM, Morris KF, Lindsey BG. Brainstem respiratory networks and cough. Pulm Pharmacol. 1996;9(5–6):343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- 27.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- 28.Shannon R, Baekey DM, Morris KF, et al. Pontine respiratory group neuron discharge is altered during fictive cough in the decerebrate cat. Respir Physiol Neurobiol. 2004;142:43–54. doi: 10.1016/j.resp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther. 2002;15:221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 30.Hanacek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration. 1984;45:161–168. doi: 10.1159/000194614. [DOI] [PubMed] [Google Scholar]
- 31.Sant’Ambrogio G, Sant’Ambrogio FB, Davies A. Airway receptors in cough. Bull Eur Physiopathol Respir. 1984;20:43–47. [PubMed] [Google Scholar]
- 32.Koestler A. The ghost in the machine. New York: The Macmillan Company; 1967. [Google Scholar]
- 33.Bolser DC, Poliacek I, Jakus J, et al. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir Physiol Neurobiol. 2006;152:255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto A, Yanai M, Sekizawa K, et al. Conditioned enhancement of cough response in awake guinea pigs. Int Arch Allergy Immunol. 1995;108:95–98. doi: 10.1159/000237124. [DOI] [PubMed] [Google Scholar]