Abstract
Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) activase (RCA) in the thylakoid membrane (TM) has been shown to play a role in protection and regulation of photosynthesis under moderate heat stress. However, the physiological significance of RCA bound to the TM (TM–RCA) without heat stress remains unknown. In this study, it is first shown, using experiments in vivo, that the TM–RCA varies in rice leaves at different development stages, under different environmental conditions, and in a rice mutant. Furthermore, it is shown that the amount of TM–RCA always increased when the Rubisco activation state and the pH gradient across the TM (ΔpH) decreased. It was then demonstrated in vitro that the RCA bound dynamically to TM and the amount of TM–RCA increased during Rubisco activation. A high level of ATP and a high pH value promoted the dissociation of RCA from the TM. Both the RCA association with and dissociation from the TM showed conformational changes related to the ATP level or pH as indicated by the changes in fluorescence intensity of 1-anilinonaphthalene-8-sulphonic acid (ANS) binding to RCA. These results suggest that the reversible association of RCA with the TM is ATP and pH (or ΔpH) dependent; it might be involved in the RCA activation of Rubisco, in addition to the previously discovered role in the protection and regulation of photosynthesis under heat stress.
Keywords: Activation, ATP, ΔpH, Rubisco, Rubisco activase, thylakoid membrane
Introduction
Ribulose-1, 5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco) activase (RCA) is a member of the AAA+ (ATPases associated with a variety of cellular activities) family (Neuwald et al., 1999). It activates Rubisco that catalyses the first reaction in photosynthetic CO2 assimilation (Spreitzer and Salvucci, 2002). Recently, enhanced thermostability of RCA has been shown to improve photosynthesis and growth under moderate heat stress (Kurek et al., 2007). Sage et al. (2008) proposed that RCA may be an important factor determining the response of boreal plants to global warming based on studies with the dominant species in the boreal forest of North America. Thus, engineering crops with RCA of high resistance to stresses represents an important option to increase photosynthetic efficiency.
In some plant species, RCA has two isoforms of 41–43 kDa and 45–46 kDa, both of which are encoded by the same nuclear gene that produces two transcripts via alternative splicing (Salvucci et al., 1987; Werneke et al., 1988). However, barley has another gene encoding only a single and divergent 41 kDa isoform (Rundle and Zielinski, 1991) and cotton contains multiple genes (Law et al., 2001). The larger isoform of RCA has a unique extension containing cysteine residues at the C-terminus. Usually, RCA is a soluble protein localized in the chloroplast stroma of algae and higher plants, and requires ATP hydrolysis to remove inhibitory sugar phosphates from the active site of Rubisco so as to cause Rubisco to exhibit its maximal catalytic activity in vivo (Portis, 1990). On the other hand, RCA is inhibited by ADP. Thus, RCA is sensitive to the stromal ATP/ADP level (Robinson and Portis, 1988; Kallis et al., 2000). According to the model of RCA activation of Rubisco (Portis, 2003), ATP provides energy for both movement of RCA subunits to Rubisco and formation of a RCA–Rubisco supercomplex, and Rubisco is encircled by a ring containing 16 (or possibly eight) RCA subunits. However, the stromal ATP/ADP ratio under steady-state conditions does not vary greatly with light intensity (Brooks et al., 1988). Moreover, light and photosynthetic electron transport are required for full activation of Rubisco by RCA in lysed chloroplasts even when ATP is supplied exogenously at saturating concentrations (Campbell and Ogren, 1990a). Recently, more evidence indicates that light modulation of RCA is controlled by the redox state of thioredoxin-f via the critical cysteine residues of the C-terminal extension in the larger RCA isoform (Zhang and Portis, 1999; Portis et al., 2008). However, this regulatory mechanism is unable to explain the dependence of Rubisco activation on light intensity in some plant species, such as tobacco, in which the larger isoform of RCA is absent. Thus, the basic molecular mechanism of how RCA is regulated in the activation of Rubisco in vivo is not completely clear.
Several reports demonstrated that both electron transport and a pH gradient (ΔpH) across the thylakoid membrane (TM) are required for light activation of Rubisco, and the TM may be directly involved in the light activation of Rubisco by RCA (Campbell and Ogren, 1990a, b). Moreover, an increase in the amount of RCA bound to the TM (TM–RCA) was detected under moderate heat treatment (42 °C) which reduced the activity of Rubisco (Rokka et al., 2001; Yang et al., 2005; Feng et al., 2007). The physiological significance of RCA bound to the TM is suggested to be important in protecting the thylakoid-associated translation machinery against heat inactivation (Rokka et al., 2001). However, Salvucci et al. (2001) suggested that the appearance of RCA in the TM fraction is due to the self-aggregation of thermally denatured RCA. Whether the association of RCA with the TM exists is not yet very clear. Moreover the existence of RCA in the TM fraction of control leaves (Rokka et al., 2001) may suggest another possible role for TM–RCA in non-heat-stressed leaves if it is not caused by a partial insolubilization of RCA.
In this study, the amount of RCA in the TM fraction was examined in a variety of leaves without moderate heat stress and it was shown that the amount of TM–RCA varied dramatically under different environment conditions and at different leaf developmental stages. Using both in vivo and in vitro analyses, it was further demonstrated that the association of RCA with the TM without heat stress is reversible, and is ATP and pH (or ΔpH) dependent. In addition to the previously discovered role in the protection and regulation of photosynthesis under heat stress we propose a model is proposed to describe the potential role of reversible association of RCA with TM in regulation of Rubisco activation.
Materials and methods
Plant growth
Pot-grown rice (Oryza sativa L. cultivar japonica 9522), Δlut, a rice mutant in which the gene encoding carotenoid isomerase was mutated (Fang et al., 2008), and tobacco (Nicotiana tabacum) plants were grown at a photosynthetic photon flux density (PPDF) of 500 μmol m−2 s−1 with a 12 h light/12 h dark cycle, 28 °C/20 °C (day/night), and 60–70% humidity in a phytotron. Spinach (Spinacia oleracea L.) and chili pepper (Capsicum frutescens L.) plants were grown in the field.
Sampling and treatment of rice plant
For experiments on different developmental stages, all rice leaves were collected at 10 am in the light. One-, 2- and >4-week-old leaves were collected from rice at the booting, heading, and filling stages, respectively.
For all the experiments examining different environmental conditions and genotypes, the 4-week-old flag leaves at the filling stages were used. For the experiment involving different sampling times, leaves were collected in the daytime (10 am in the light) and at night (10 pm in the dark); for the experiments involving high CO2 (1000 μmol mol−1) and very low light (30 μmol m−2 s−1, near the light compensation point) treatments, the rice grown in pots was transferred into a NK system Biotron (NC type, Nippon medical and chemical instruments Co. Ltd. Osaka, Japan) at 28 °C and 60–70% humidity for ∼12 h, then the flag leaves were collected in the Biotron in the light, and leaves of rice grown in pots in the phytotron were used as the control.
Leaves of rice plants for each experiment were collected from at least three pots to minimize the influence of different growth conditions among pots.
Measurements of leaf photosynthesis
The measurements of net photosynthetic rate (Pn) were made in situ using a portable LI-6400 photosynthetic gas analysis system (LI-COR, USA). For the measurements, CO2 concentration and light intensity were controlled under their growth conditions with the LI-COR CO2 injection system and by a LI-COR LED irradiation source. To produce the curve of light-saturated Pn versus the intercellular CO2 concentration (Ci), Pn values were measured at CO2 concentrations of 240, 180, 120, 60, 380, 580, 750, 900, 1050, and 1200 μmol CO2 mol−1 in turn, and the PPFD was kept at 1200 μmol m−2 s−1 during the measurement. Both the maximum carboxylation rate (Vcmax) and the maximum electron transport rate (Jmax) values were calculated from Pn/Ci curve data according to Farquhar et al. (1980) and von Caemmerer and Farquhar (1981).
Measurements of chlorophyll (Chl) content
Chl contents of leaves were determined spectrophotometrically according to Arnon (1949).
Measurements of Rubisco activity
Total soluble protein was rapidly extracted from liquid N2-frozen leaves with a CO2-free buffer containing 50 mM TRIS-HCl (pH 7.8), 1 mM EDTA, 50 mM NaCl, and 2 mM β-mercaptoethanol. The extract was then centrifuged at 12 000 g and 4 °C for 6 min, and the obtained supernatant was used immediately for Rubisco activity measurements.
Rubisco initial activity was measured immediately using the supernatant mentioned above, while its total activity was determined after full activation. In the present study the oxygenase activity of Rubisco was determined as the indicator of Rubisco activity. The oxygenase activity of Rubisco was measured with a Clarke-type O2 electrode according to the method described by Cox et al. (1999).
Measurements of Chl fluorescence
Chl fluorescence was monitored with a modified PAM-2000 fluorometer (Walz, Effeltrich, Germany) as described by Gray and Lewis (2006). After a 30 min dark adaptation, continuous actinic light (growth light intensity) was applied. Fo and Fm are defined as the minimum photosystem II (PSII) fluorescence yield in dark-adapted leaves and the maximum PSII fluorescence yield reached during a saturating pulse of white light, respectively. PSII potential or maximal photochemical efficiency is the ratio Fv/Fm, where Fv is the variable part of the fluorescence emission and is equal to Fm–Fo. The effective photochemical efficiency of PSII (ΦPSII) is defined as (Fm′–F)/Fm′ as proposed by Genty et al. (1989). Non-photochemical quenching (NPQ) is quantified by Fm/Fm′–1 (van Kooten and Snel, 1990).
The redox change of P700 was monitored by absorbance at 810 nm minus that at 830 nm, using a Dual-PAM-100 fluorometer (Walz, Effeltrich, Germany), and the initial rate of P700+ reduction following far-red light (>705 nm, 5.2 μmol m−2 s−1) was calculated after a 40 s illumination that allowed the oxidation of P700 to a steady state (Klughammer and Schreiber, 1998).
Measurements of millisecond-delayed light emission of Chl fluorescence (ms-DLE)
Measurements of ms-DLE were carried out using a phosphoroscope according to the method described by Wang et al. (2006). The leaf discs were collected in the light and dark adapted at the same temperature, and were immediately inserted into the sample cell to measure the ms-DLE. The light intensity was controlled at their growth conditions.
Extraction and purification of RCA and Rubisco
The leaves of rice and spinach detached from the plants in the light were immediately frozen with liquid N2. RCA was purified from the frozen leaves according to Robinson et al. (1988). The obtained RCA was then filtrated through a column of antibody against Rubisco from tobacco to remove the contaminating trace amount of rice Rubisco. The antibody column was prepared using protein A–Sepharose CL-4B (Sigma, USA) according to the method described by Gupta and Tan (1981). Rubisco was purified according to the methods described by Kung et al. (1980). The purified RCA was preserved in liquid N2, while the purified Rubisco was preserved in a suspension containing 65% saturated (NH4)2SO4. The concentration of proteins was determined according to Bradford (1976).
Isolation of thylakoids
Thylakoids were isolated from rice leaves according to the method described by Chen and Xu (2006). All extract solutions contained 1 mM phenylmethylsulphonyl fluoride (PMSF; Sigma, USA), and all steps were performed at 4 °C. The isolated thylakoids were stored at –40 °C.
Blue-Native-polyacrylamide gel electrophoresis (BN-PAGE)
BN-PAGE was performed as described by Schagger et al. (1994) and all steps were carried out on ice. Briefly, the TM (0.5 mg Chl ml−1) from rice leaves was suspended in BTH buffer (25 mM BIS-TRIS-HCl, pH 7.0, 20% glycerol). Subsequently, the TM was collected by centrifugation (at 18 000 g and 4 °C for 2 min), and the obtained pellets were dissolved in BTH buffer containing 1% (w/v, final concentration) dodecylmaltoside (DM) with gentle shaking for 30 min. Non-solubilized membranes and aggregates were removed by centrifugation (at 20 000 g and 4 °C for 30 min), the supernatant was transferred to a new tube, mixed with BN sample buffer, and loaded onto a BN-gel (BN: 5–13.5% acrylamide) using a Protean II electrophoresis system (Bio-Rad, USA). BN-polyacrylamide gels contained a 4% acrylamide stacking gel. The electrophoresis unit was chilled to 4 °C.
For electrophoresis in the second dimension, a slice of the BN-gel lane was cut and treated with SDS–PAGE loading buffer at room temperature for <10 min without shaking. The treated gel slice was used for the second dimensional SDS–PAGE using a 10% running gel at room temperature. After electrophoresis, the gel was stained with Coomassie Brilliant Blue or analysed by western blotting.
Assay for determining the proportion of TM–RCA
Total and TM proteins of rice leaves were extracted from the frozen leaves and isolated thylakoids with extraction buffer B [25 mM TRIS-HCl, pH 7.8, 1 mM EDTA, 5 mM MgCl2, 1% (w/v) SDS, 2 mM β-mercaptoethanol] by incubating at 100 °C for 3–5 min. The extracts were centrifuged at 12 000 g and 4 °C for 10 min, and the obtained supernatant was preserved for SDS–PAGE.
Total and TM proteins were separated by 10% SDS–PAGE as described by Kim et al. (1993) in the absence and presence of 4 M urea, respectively. For western blot, proteins separated electrophoretically were transferred to a nitrocellulose membrane (Amersham Pharmacia, Milton Keynes, UK) within a semi-dry transfer cell (Amersham Pharmacia) and detected with antibodies raised against RCA from tobacco leaves. For quantification of Rubisco and RCA, the bands from SDS–PAGE (Rubisco) and western blots (RCA) were analysed by Labworks 4.6 software (USA). Rubisco and RCA amounts were expressed on a leaf area basis and each loaded sample contained 1–2 μg of Chl. The quantification of the TM–RCA/total RCA ratio was obtained through a western blot of TM–RCA and a series of dilutions of total RCA on one gel. The absolute value of TM–RCA and total RCA in control leaves (4-week-old flag leaves) was quantified through a western blot of TM–RCA (or total RCA) and a series of dilutions of purified RCA from spinach on one gel (Supplementary Figure S1 available at JXB online).
Treatments of thylakoids with different levels of ATP and different pH values
Thylakoids isolated from rice leaves (<2 weeks old) were incubated at 25 °C for 20 min in buffer A (25 mM Tricine, pH 7.8, 10 mM NaCl, 5 mM MgCl2, 10 mM NaF, 1 mM PMSF, 0.4 M sucrose) containing different concentrations of ATP. Subsequently, the TM was collected by centrifugation (at 10 000 g and 4 °C for 10 min), and the obtained pellets were washed and dissolved twice in buffer A. The TM was also incubated buffer A with a series of different pH values at 25 °C for 20 min. Then the TM was collected again according to the method described above. The sample of TM with a Chl content of 0.4 mg ml−1 was preserved, and the amount of TM–RCA in the sample was analysed by SDS–PAGE and western blot.
Rubisco activation in the presence of TM
Rice Rubisco was deactivated by adding excess RuBP in a CO2-free assay, and incubated with the mixture of RCA and the TM of rice in buffer A containing 1 mM ATP at 25 °C for 20 min. Activated Rubisco, rather than deactivated Rubisco, from rice leaves was used as control. In this assay 0.1 mg ml−1 RCA, 0.2 mg ml−1 Rubisco, 0.1 mM RuBP, and TM containing 0.2 mg Chl ml−1 were used. Thereafter the TM was collected according to the method described above, and the amount of TM–RCA was analysed by SDS–PAGE and Western blot.
Rice RCA, rice TM, and Rubisco from rice, spinach, tobacco, and chili pepper were used in this experiment.
Measurements of the fluorescence intensity of 1-anilinonaphthalene-8-sulphonic acid (ANS) binding to RCA
Samples of purified RCA (80 μg ml−1) and Rubisco (80 μg ml−1) from spinach were mixed well in the presence of 2 mM or 25 μM RuBP, then the mixture was incubated with different levels of ATP for 30 min. Afterwards, ANS solution mixed well with 0.1 M phosphate buffer (pH 7.4) was added (ANS final concentration 40 μM). After 15 min in the dark, the fluorescence intensity of ANS binding to RCA was measured with a 970 CRT fluorescence spectrophotometer (Shanghai Analytical Instrumental Plant, China) at room temperature according to the method described by Wang and Portis (1991). Fluorescence emission spectra excited by light with a wavelength of 380 nm were recorded between 400 nm and 600 nm.
Statistical analysis
Statistical analysis of all data was performed by the software SPSS 10.0 (SPSS Inc., USA). A least significant difference (LSD) test of one-way analysis of variance (ANOVA) was performed in a post-hoc comparison of means among the data of rice leaf gas exchange and Chl fluorescence measurements. Differences were considered significant only at P <0.05. All graphs presented herein were produced using the software SigmaPlot 9.0 (SPSS Inc., USA).
Results
Existence of RCA on the TM from rice leaves without moderate heat stress
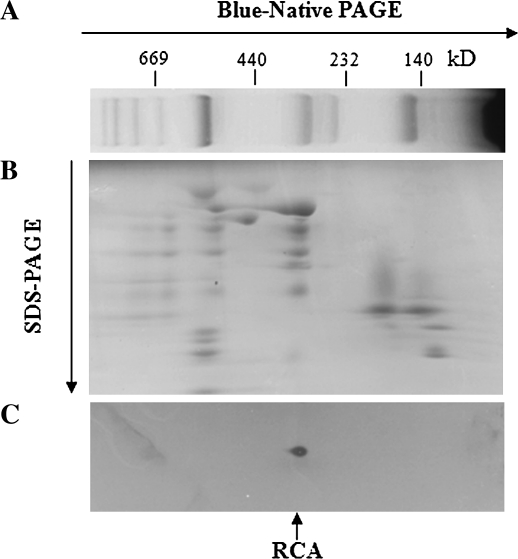
In order to confirm the existence of RCA on the TM without moderate heat stress, RCA on the TM was investigated using western blots of two-dimensional BN-PAGE/SDS–PAGE (Fig. 1). In order to reduce the self-aggregation of thermal denatured RCA, all steps, including the preparation of TM and solubilization of TM proteins, were performed on ice or at 4 °C. The results showed that the spot of TM–RCA was in a position identical to that of some other protein having a molecular weight of ∼320–340 kDa. Only one spot (<41 kDa) was observed in Fig. 1C, which may be due to the degradation of RCA during the solubilization of TM proteins. The above result indicates that some RCAs are bound to the TM in rice in vivo.
Fig. 1.
BN-PAGE and western blot analysis of the TM–RCA from rice leaves. (A) BN-PAGE, (B) second dimension electrophoresis, (C) western blot analysis.
Variation in the amount of TM–RCA in rice leaves without moderate heat stress
To examine variations in the amount of RCA on the TM without moderate heat stress, the TM–RCA in rice leaves was first examined at different developmental stages and under various environmental conditions using western blots with a specific antibody against RCA from tobacco. Consistent with previous reports (To et al., 1999), two bands of 41 kDa and 45 kDa were detected in rice leaves (Figs 2, 3), and rice was dominated by the smaller isoform in the total protein extract. Importantly, variations in TM–RCA were observed among all the cases analysed (Figs 2, 3), but the TM–RCA seemed to be composed more of the larger isoform.
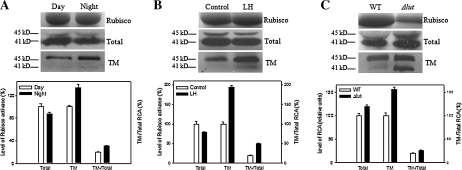
Fig. 2.
Changes in the amount of Rubisco, total RCA, and TM–RCA in rice leaves at different leaf ages (A) and leaf positions (B). 1w, 2w, and 4w represent 1-, 2-, and 4-week-old flag leaves, respectively, while 1st, 2nd, and 3rd represent the first (flag, 4-week-old) leaf, second leaf (∼6-week-old), and third leaf (∼8-week-old) (counted from the top of the plant), respectively. RCA amounts are all expressed on a leaf area basis. Each value is the mean of more than three independent experiments with the SE expressed as a vertical bar. The amount of total RCA (∼2.93 μg cm−2) and TM–RCA (∼0.58 μg cm−2) in 4-week-old flag leaves in the day was defined as 100%, respectively.
Fig. 3.
Changes in the amount of Rubisco, total RCA, and TM–RCA of rice leaves collected at different times of the day (A), treated with high CO2 and low light (B), and in Δlut (C). Day and Night represent the rice leaves collected in the daytime and at night. LH represents the rice leaves treated with high CO2 and low light. Four-week-old flag leaves at the filling stage were used in this figure.
The amounts of Rubisco in 1-, 2-, 6-, and 8-week-old leaves were ∼57, 105, 93, and 70% of that in 4-week-old leaves (∼0.24 mg cm−2), respectively. The amounts of total RCA in 1- and 8-week-old leaves were only ∼60% of that in 4-week-old leaves (∼2.93 μg cm−2), and there was a slight difference detected in other leaves; the amounts of TM–RCA in 1-, 2-, 6-, and 8-week-old leaves were ∼33, 113, 49, and 24% of that in 4-week-old leaves (∼0.58 μg cm−2), respectively; and the proportions of RCA in 1-, 2-, 6-, and 8-week-old leaves were 48, 116, 49, and 37% of that in 4-week-old leaves (∼20% of total RCA), respectively (Fig. 2). It seems that the proportions of TM–RCA were higher in the leaves that showed more Rubisco and total RCA contents. In addition, the ratio of smaller to larger isoforms of TM–RCA was higher in 1- and 2-week-old leaves compared with that in leaves >4 weeks old.
In contrast, changes in Rubisco and total RCA are different from the change in the proportion of TM–RCA under different environmental conditions (Fig. 3). The amounts of Rubisco and total RCA in the flag leaves in the daytime were slightly higher than those at night, but the amount of TM–RCA and its proportion at night were notably higher than those in the daytime (Fig. 3A). The treatment with high CO2 (1000 μmol mol−1) and very low light intensity (30 μmol m−2 s−1) led to a slight change in Rubisco and a decrease in total RCA while the amount of TM–RCA and its proportion were much higher compared with the control (Fig. 3B).
Additionally, TM–RCA and its proportion as well as total RCA increased in a rice mutant (Δlut), while Rubisco decreased a lot. Moreover, the ratio of smaller to larger isoforms of TM–RCA was higher in Δlut than in the control (Fig. 3C).
It seems that there is no direct influence of Rubisco and total RCA contents on the variation of the proportion of TM–RCA.
Correlation of gas exchange and Chl content with TM–RCA
To elucidate the possible physiological role of TM–RCA in photosynthesis, the linkage between RCA localization and net photosynthetic parameters, that is leaf Pn, Vcmax, Jmax, and Chl content was examined. A higher proportion of TM–RCA showed a higher Pn in all leaves at different developmental stages (Table 1). However, the proportion of TM–RCA was higher while the Pn was much lower (∼2.6 μmol m−2 s−1, measured at 1000 μmol CO2 mol−1 and 30 μmol photons m−2 s−1) in leaves treated with high CO2 and low light intensity (Fig. 3B). Moreover, the higher proportion of TM–RCA was also observed at night (Fig. 3A) while the Pn was less than zero. Except for the case of Δlut, a higher proportion of TM–RCA was also linked to a higher Vcmax and Jmax (Table 1).
Table 1.
Pn, Vcmax, Jmax, and Chl content in rice leaves
| Pn (μmol m−2 s−1) | Vcmax (μmol m−2 s−1) | Jmax (μmol m−2 s−1) | Chl (mg dm−2) | ||
| Leaf age (position) | 1 week | 10.8±0.4 a | 95.2±6.7 a,b | 142.0±8.1 b | 1.55±0.27 a |
| 2 weeks | 15.4±0.7 b | 129.3±5.7 c | 174.7±4.6 c | 3.26±0.17 b | |
| 4 weeks (first) | 15.7±0.6 b | 126.3±4.1 c | 171.7±5.0 c | 4.33±0.11 c | |
| 6 weeks (second) | 12.6±0.7 a,b | 83.8±6.4 a | 111.3±7.0 a | 4.07±0.40 c | |
| 8 weeks (third) | 10.4±0.8 a | 80.3±4.5 a | 110.8±5.7 a | 2.77±0.11 b | |
| Genotype | Wild type | 15.2±0.9 b | 88.2±2.2 b | 134.5±5.2 b | 4.33±0.10 b |
| Δlut | 11.4±1.4 a | 57.4±3.4 a | 89.0±5.4 a | 2.24±0.10 a |
Leaf Pn measurements were made at their growth light intensity (500 μmol photons m−2 s−1) and CO2 concentration (380 μmol CO2 mol−1). Vcmax and Jmax were calculated from the data of the photosynthetic response to CO2 measured at 1200 μmol photons m−2 s−1. Each value is the mean±SE of 6–7 leaves. Different lower case letters indicate significant (P <0.05) differences between the compared values.
In contrast to the changes in proportions of TM–RCA, the results of Chl measurement showed that Chl contents were higher in 4- and 6-week-old leaves (∼4.3 mg dm−2) than in 1-, 2-, and 8-week-old leaves. The Chl content was decreased by ∼48% in Δlut while the proportion of TM–RCA was increased (Table 1).
The results of correlation analysis showed that there was no significant correlation among gas exchange, Chl content, and proportion of TM–RCA (Fig. 5A).
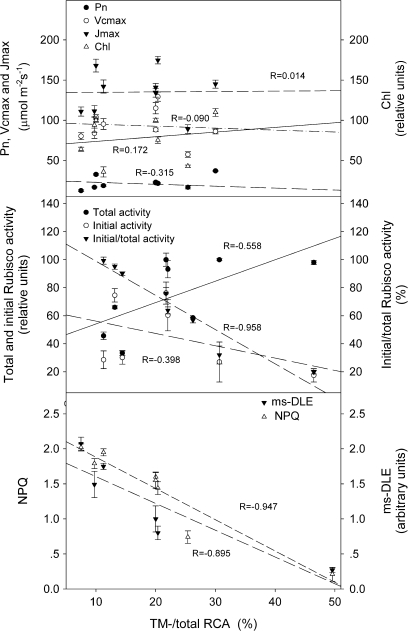
Fig. 5.
Correlation analysis of the TM–RCA/total RCA ratio to photosynthetic parameters (A), Rubisco activity (B), and Chl fluorescence (C). Correlation analysis of the data in Tables 1–3, and Fig.4 was performed using the software SPSS 10.0 (SPSS Inc., USA). R: Pearson correlation coefficients (two tailed).
Increase in the proportion of TM–RCA at a lower initial/total Rubisco activity ratio
Total and initial Rubisco activities were measured to explore the relationship of Rubisco activity to the proportion of TM–RCA in vivo. The results in Table 2 show that the change in Rubisco total activity was different from that in the proportion of TM–RCA. Similar to the proportion of TM–RCA, total activities in 2- and 4-week-old leaves (∼0.06 μmol O2 mg−1 protein min−1) were higher than those in 1-, 6-, and 8-week-old leaves. However, changes of total activities under different environment conditions and in Δlut were not consistent with those of the proportions of TM–RCA. Compared with their control, total activities in the leaves at night, in leaves treated with high CO2 and low light, and in Δlut decreased by only 7.9, 6.3, and 50%, respectively.
Table 2.
Rubisco activity, the initial/total activity ratio, and the TM–RCA/total RCA ratio in rice leaves
| Total activity (μmol mg−1 min−1) | Initial activity (μmol mg−1 min−1) | Initial/total activity (%) | TM–RCA/total RCA (%) | ||
| Leaf age (position) | 1 week | 0.021±0.002 a | 0.019±0.003 a | 90.1±1.3 b | 11.3±2.6 b |
| 2 weeks | 0.059±0.008 c | 0.038±0.007 b | 63.8±2.5 a | 20.4±2.0 c | |
| 4 weeks (first) | 0.063±0.006 c | 0.048±0.005 b | 75.9±4.3 a | 20.0±1.2 c | |
| 6 weeks (second) | 0.049±0.001 b | 0.047±0.003 b | 95.1±1.9 c | 9.7±1.0 b | |
| 8 weeks (third) | 0.019±0.003 a | 0.018±0.004 a | 99.6±2.5 c | 7.5±1.7 a | |
| Time of day | Day | 0.063±0.006 | 0.048±0.005 b | 75.9±4.3 b | 20.0±0.6 a |
| Night | 0.058±0.005 | 0.017±0.009 a | 29.8±9.8 a | 30.6±1.2 b | |
| CO2 and light treatment | Control | 0.063±0.006 | 0.048±0.006 b | 75.9±4.3 b | 20.0±0.9 a |
| LH | 0.059±0.009 | 0.011±0.003 a | 19.8±1.4 a | 49.5±2.5 b | |
| Genotype | Wild type | 0.063±0.002 b | 0.048±0.002 b | 75.9±0.9 b | 20.0±1.2 a |
| Δlut | 0.027±0.002 a | 0.015±0.001 a | 56.8±1.9 a | 25.4±0.9 b |
The Rubisco activity was expressed as its oxygenase activity (μmol O2 mg−1 total protein min−1) measured by a Clarke-type O2 electrode, and the rice leaves were collected at the heading stage. LH, rice was treated under high CO2 (1000 μmol mol−1) and very low light (30 μmol m−2 s−1) for ∼12 h and each value (±SE) is the average of 3–4 measurements. Different lower case letters indicate significant (P <0.05) differences between the compared values.
Unlike the change in the proportion of TM–RCA, initial activities of Rubisco in 4- and 6-week-old leaves (∼0.048 μmol O2 mg−1 protein min−1) were higher than those in 1-, 2-, and 8-week-old leaves, respectively. Initial activity in the leaves at night, in leaves treated with high CO2 and low light, and in Δlut were only ∼35, 25, and 42% of those of their control, respectively (Table 2).
Interestingly, it was found that leaves with a higher proportion of TM–RCA had a lower initial/total Rubisco activity ratio. As shown in Table 2, a decrease in the initial/total Rubisco activity ratio was closely linked to an increase in the proportion of TM–RCA. When rice plants were treated with high CO2 and low light, the proportion of TM–RCA increased greatly while the initial/total Rubisco activity ratio decreased from ∼75% to 20% (Table 2). Moreover, the results of correlation analysis showed that the correlation of the proportion of TM–RCA to the initial/total Rubisco activity ratio reached a significant level (the Pearson correlation coefficients was –0.958; Fig. 5B).
Increase in the proportion of TM–RCA at the lower level of NPQ and ms-DLE
To explore the role of electron transport in changes of TM–RCA, some Chl fluorescence parameters of photosynthetic electron transport were measured. The results in Table 3 showed that there was no significant difference in Fv/Fm among the different rice leaves mentioned above. ΦPSII represents the effective photochemical efficiency of PSII, which is related to the rate of linear electron transport (Genty et al., 1989; Maxwell and Johnson, 2000), and the initial rate of dark reduction of P700+ (P700+) after termination of far-red illumination reflects the rate of cyclic electron flow around PSI (Maxwell and Biggins, 1976; Havaux, 1996). ΦPSII and P700+ in mature (2- and 4-week-old) leaves increased while TM–RCA proportions also increased compared with those in young and old leaves. A similar result was also observed in the leaves of rice treated with high CO2 and low light (Table 3). However, the proportion of TM–RCA increased at night (Fig. 3A) when no non-cyclic electron flow exists, and the proportion of TM–RCA increased in Δlut while the P700+ decreased by >60%.
Table 3.
Chl fluorescence parameters Fv/Fm, ΦPSII, NPQ, and the initial rate of P700+ reduction in rice leaves
| Fv/Fm | ΦPSII | NPQ | P700+ (s−1) | ||
| Leaf age (position) | 1 week | 0.813±0.002 | 0.40±0.01 b,c | 1.95±0.05 c | 0.80±0.06 a |
| 2 weeks | 0.813±0.002 | 0.45±0.01 c | 1.44±0.09 a | 1.23±0.08 c | |
| 4 weeks (first) | 0.806±0.002 | 0.43±0.01 c | 1.60±0.06 a | 1.06±0.08 b | |
| 6 weeks (second) | 0.811±0.002 | 0.37±0.03 b | 1.79±0.07 b | 0.78±0.08 a | |
| 8 weeks (third) | 0.808±0.002 | 0.29±0.01 a | 2.02±0.05 c | ND | |
| CO2 and light treatment | Control (4 weeks) | 0.806±0.002 | 0.43±0.01 a | 1.60±0.06 b | 0.94±0.08 a |
| LH | 0.812±0.002 | 0.66±0.01 b | 0.21±0.10 a | 1.72±0.12 b | |
| Genotype | Wild type (4 weeks) | 0.806±0.001 b | 0.43±0.01 | 1.60±0.06 b | 1.06±0.08 b |
| Δlut | 0.711±0.024 a | 0.39±0.02 | 0.72±0.09 a | 0.41±0.07 a |
Each value (±SE) is the average of 4–6 measurements. Different lower case letters indicate significant (P <0.05) differences between the compared values.
ND, not determined.
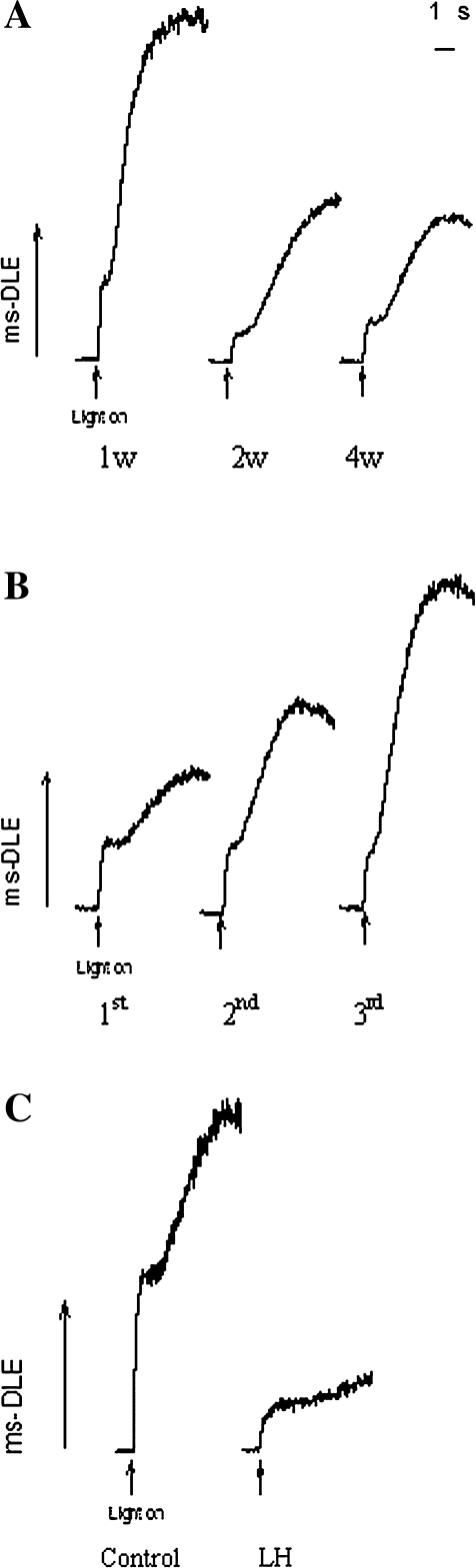
The intensity of ms-DLE in mature (2- and 4-week-old) leaves was ∼50% and 60% lower than that in young (1-week-old leaves) and old (>6-week-old) leaves, respectively (Fig. 4A, B). Moreover, the intensity of ms-DLE in leaves treated with high CO2 and low light decreased to ∼26% of that of control (Fig. 4C). The change in NPQ was almost the same as that in ms-DLE (Table 3). The correlation coefficients of the proportion of TM–RCA to ms-DLE and NPQ were –0.895 and –0.947, respectively (Fig. 5C).
Fig. 4.
The intensity of ms-DLE of rice leaves at different leaf ages (A), leaf positions (B), and treated with high CO2 and low light (C). ms-DLE was measured as described in the Materials and methods, and measurements were made at the heading and filling stages. 1w, 2w, 4w, 1st, 2nd, 3rd, and LH are as defined in the legends of Figs 2 and 3.
Increase in the amount of TM–RCA during Rubisco activation in vitro
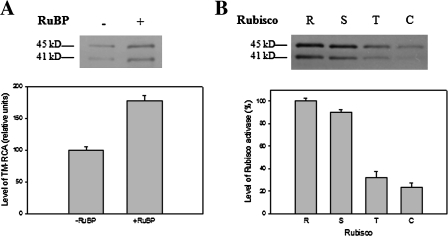
To explore further the relationship between TM–RCA and Rubisco activation, TM–RCA was quantified when RCA was incubated with the TM and Rubisco deactivated by excessive RuBP in a CO2-free assay. The results showed that the amount of TM–RCA was significantly higher in the assay with the deactivated Rubisco than in the control (Fig. 6A).
Fig. 6.
The binding of RCA to the TM during activation of Rubisco from different species by rice RCA. A thylakoid sample containing 1–2 μg of Chl was loaded in each line. (A) RCA and TMs from rice leaves were incubated with activated (–RuBP) and deactivated (+RuBP) Rubisco from rice leaves in an assay system containing 1 mM ATP. (B) RCA and TMs from rice were incubated with deactivated Rubisco (+RuBP) from non-Solanaceae plants (rice and spinach) and Solanaceae plants (tobacco and chili pepper). R, S, T, and C represent Rubisco from rice, spinach, tobacco, and chili pepper, respectively.
The amount of TM–RCA after incubation of rice RCA and TM with deactivated Rubisco from rice, spinach, tobacco, and chili pepper was also quantified. The result showed that TM–RCA was increased in the presence of the Rubisco from rice and spinach but not that from tobacco and chili pepper (Fig. 6B).
A decrease in the amount of TM–RCA induced by higher ATP and alkaline pH in vitro
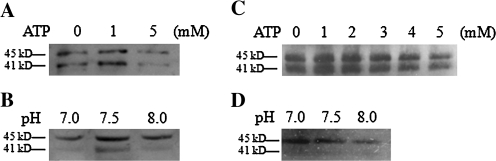
The above results showed that Rubisco activation leads to an increase in TM–RCA. Therefore, TM–RCA during Rubisco activation at different ATP levels and pH values was also examined. The results showed that TM–RCA increased when ATP increased from 0 mM to 1 mM, then it decreased when ATP increased from 1 mM to 5 mM (Fig. 7A). Similarly, TM–RCA increased when the pH was adjusted from 7 to 7.5, then it decreased when the pH was adjusted from 7.5 to 8.0 (Fig. 7B). It seems that the binding of RCA to the TM during Rubisco activation is reversible.
Fig. 7.
Effects of ATP level or pH on the amount of RCA on the TM in vitro. RCA and TMs were incubated with Rubisco (+RuBP) in the assay at different ATP levels (A) and different pH values (B) at 25 °C for 20 min. TMs from fresh rice leaves incubated at different ATP levels (C) or different pH values (D) at 25 °C for 20 min. The amount of TM–RCA was detected by western blot.
After the incubation of TM from fresh rice leaves at different levels of ATP and different pH values, the TM–RCA was quantified. The results showed that the amount of TM–RCA gradually increased and reached a maximum at 1–2 mM ATP, but it decreased when ATP exceeded 2 mM (Fig. 7C). Furthermore, the amount of TM–RCA was maximal at pH 7.0, while it was minimal at pH 8.0 in the range of pH 6.5–8.0 (Fig. 7D). It seems that the dissociation of RCA from the TM is ATP and pH dependent.
Reversible change in the fluorescence emission spectra of ANS binding to RCA during Rubisco activation at different ATP levels
ANS has a low fluorescence intensity in aqueous solution; however, an increase in fluorescence intensity can be observed upon binding to accessible hydrophobic regions of proteins (Nooshin and Li-Chan, 2000). The change in the fluorescence of ANS binding to protein offers a probe to detect the effects of a conformational alteration in RCA on its reversible association with the TM.
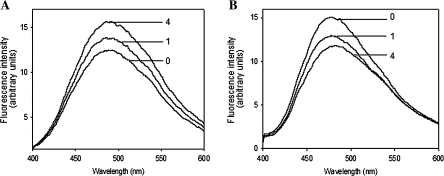
The fluorescence of ANS binding to protein was examined during Rubisco activation in the presence of 2 mM or 25 μM RuBP. The results showed that the fluorescence intensity of ANS binding to Rubisco and RCA increased dramatically when ATP was increased from 0 nm to 1 nm and 4 mM in the presence an excess of RuBP (2 mM) (Fig. 8A). The change in fluorescence intensity was mainly from the ANS binding to RCA, since Rubisco remains inactive in the presence of excessive RuBP, and there were almost no changes in fluorescence of ANS binding to Rubisco and RCA at different ATP levels (data not shown). Therefore, the above result indicated the enhanced structural alteration in RCA by the accelerated Rubisco activation at the higher ATP level. In contrast, in the presence of a low RuBP concentration (25 μM), the fluorescence intensity of ANS binding to protein was decreased when ATP was increased (Fig. 8B). Since small amounts of RuBP (25 μM) would be used up and Rubisco could be maintained in an activated state in the assay, the increase in fluorescence intensity of the ANS binding RCA induced by Rubisco activation is similar between different assays. Thus, the result in Fig. 8B showed that the change in fluorescence intensity was determined by the recovery state of the RCA conformation. The above results indicate that the conformational alteration of RCA induced by activation is reversible at higher ATP concentrations.
Fig. 8.
Effects of ATP on the fluorescence of ANS binding to RCA during Rubisco activation. The mixture of Rubisco and RCA in the presence of 2 mM (A) and 25 μM RuBP (B) was incubated with 0, 1, and 4 mM ATP at room temperature for 30 min, then ANS was added and the fluorescence intensity was measured after 15 min in the dark according to the method described in the Materials and methods. The numbers in the figure indicate the ATP concentration (mM).
Similarly, an additional experiment showed that the conformational alteration of RCA induced by moderate heat stress is also reversible, as shown by the fluorescence emission spectra of ANS binding to RCA at a different level of ATP and different pH (Supplementary Fig. S3 at JXB online).
Discussion
The increase in TM–RCA is a universal phenomenon and it is not limited to moderate heat stress
It has been reported previously that RCA was observed on the TM, and its amount increased under moderate heat stress of 42 °C (Rokka et al., 2001; Yang et al., 2005; Feng et al., 2007). Consistent with these results, the existence of TM–RCA was also observed in rice leaves without moderate heat stress (Figs 2, 3). The present results also showed that increases in TM–RCA in vivo were observed in rice leaves under different environment conditions, at different developmental stages, and in the mutant Δlut without moderate heat stress. Moreover, TM–RCA increased in leaves not only in vivo (Figs 2, 3) but also in the process of Rubisco activation by RCA in vitro (Fig. 6). Obviously, this means that the increase in TM–RCA is a universal phenomenon which is not limited to heat stress.
Salvucci et al. (2001) suggested that RCA in the TM fraction is the result of self-aggregation of thermally denatured RCA. However, in contrast to the thermally denatured RCA, the RCA washed out from the TM can activate Rubisco (Supplementary Fig. S2 at JXB online). This indicates that RCA in the TM fraction without heat stress may be different from that under heat stress. It has been reported that no aggregation of RCA was observed in chloroplasts of spinach and tobacco plants incubated at the growth temperature, and the smaller isoform aggregated first under heat treatment because of its sensitivity to high temperature (Crafts-Brandner et al., 1997; Salvucci et al., 2001). In the experiments described here, the preparation of TM was always performed on ice or at 4 °C, and more of the larger isoform was detected in TM–RCA (Figs 2, 3). This means that TM–RCA is not mainly due to the aggregation of RCA. Moreover, the results from BN-PAGE (Fig. 1) imply that the association of some RCA with the TM occurs. Additionally, Salvucci et al. (2001) had reported that no unfolding/aggregation of tobacco RCA was observed at 35 °C in the presence of 0.75 mM ATP in vitro. Thus, it seems that the increased in TM–RCA during Rubisco activation in vitro (Fig. 6) is also not due to the aggregation of RCA.
Reversible association of RCA with the TM is related to the ATP level and pH
It has been proposed that RCA is likely to act as a chaperone protecting the thylakoid-associated protein synthesis machinery against heat inactivation under heat stress (Rokka et al., 2001). However, Salvucci et al. (2001) suggested that non-specific binding to the TM may stabilize RCA during periods of heat stress and promote self-aggregation of RCA. The increase in TM–RCA under conditions without heat stress in the present experiments indicates that TM–RCA may have other unrecognized functions besides protection.
A strong negative correlation between the proportion of TM–RCA and the Rubisco activation state indicates that TM–RCA may be related to light activation of Rubisco in vivo (Fig. 5B). Similar results were also observed in some previous publications under moderate heat stress (Yang et al., 2005; Feng et al., 2007). The slow phase of ms-DLE reflects the state of ΔpH and the related photophosphorylation (Wraight and Crofts, 1971; Li and Shen, 1994), and NPQ is induced by the formation of ΔpH across the TM (Lavaud et al., 2004). Moreover, it is reported that ΔpH appears necessary for maximum light activation of Rubisco (Campbell and Ogren, 1990b), and Makino and Sage (2007) also suggested that a high NPQ is generally associated with a high activation state of Rubisco in vivo. Thus, the above deduction is also supported by the increase in TM–RCA at low ΔpH as shown by changes in ms-DLE and NPQ (Fig. 5C).
Since the ΔpH is utilized to synthesize ATP in the chloroplast, the requirement for ΔpH in light activation of Rubisco appeared to be identical to the requirement for ATP and alkaline pH (Streusand and Portis, 1987). Therefore, the negative correlation of TM–RCA with the ΔpH as shown by the changes of ms-DLE and NPQ (Fig. 5C) indicates that TM–RCA is related to the level ATP and pH in vivo.
Furthermore, the results also showed that the reversible association of RCA is ATP and pH dependent in vitro. On the one hand, the association of soluble RCA with the TM without heat stress seems to be a result of Rubisco activation by RCA. It is supported by the finding of a considerable increase in TM–RCA when deactivated Rubisco was activated by RCA (Fig. 6A). Similarly, it is also supported by the increase in TM–RCA only when deactivated Rubisco from rice and spinach (non-Solanaceous species) is included in the assay (Fig. 6B), since Rubisco from tobacco and chili pepper (Solanaceous family plants) cannot be activated by RCA from non-Solanaceous species (Wang et al., 1992). The results also showed that the change in RCA conformation during Rubisco activation (Fig. 8A) was similar to that in RCA treated by moderate heat stress (Supplementary Fig. S3 at JXB online), and Rokka et al. (2001) suggested that the first step of the association of RCA with the TM is the structural changes of RCA induced by heat treatment. In addition, the above deduction is also consistent with the model described by Portis (2003); the RCA-ADP resulting from Rubisco activation has no activity until ADP is replaced by ATP. TM–RCA also had no Rubisco-activating activity in the presence of 1 mM ATP until it was washed from the TM (data not shown).
On the other hand, binding of RCA to the TM during Rubisco activation is reversible. Although the RCA cannot be washed from the TM even at 2 M NaCl (NaBr) or 1% Triton X-100 (Rokka et al., 2001), the higher ATP and alkaline pH could induce the dissociation of TM–RCA in vitro (Fig. 7C, D), consistent with the finding that the activity of RCA disengaged from the TM is ATP and pH dependent (Supplementary Fig. S2 at JXB online). Furthermore, the results of the present study also showed that a reversible conformational change of RCA induced by Rubisco activation or heat stress depends on the level of ATP and the pH in the assay (Fig. 8, Supplementary Fig. S3).
Consequently, the amount of TM–RCA seems to be the result of reversible association of RCA with the TM, which is related to the ATP level and the pH. Since TM–RCA is not the active form of RCA, this is the reason why the proportion of TM–RCA is negatively related to the activation state of Rubisco in vivo.
A potential new role for TM–RCA in Rubisco activation
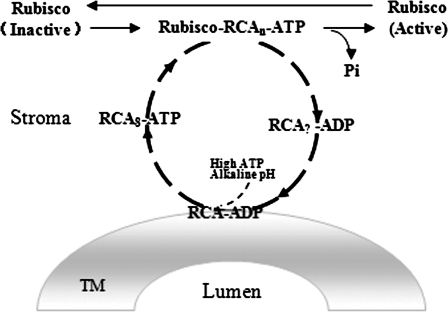
Portis (2003) proposed a model whereby inactive Rubisco is surrounded by 16 (or eight) RCA subunits forming a Rubisco–RCA complex with a ring structure. In this model, ATP hydrolysis and Pi release in this complex cause conformational changes in the ring structure, resulting in activation of inactive Rubisco and the dissociation of the Rubisco–RCA (ring structure). When ADP in RCA-ADP is subsequently replaced by ATP, a new round of RCA oligomerization and binding of RCA to Rubisco begins. In the present study, TM–RCA increased during Rubisco activation (Fig. 6), suggesting that the dissociated RCA from the Rubisco–RCA complex might associate with the TM. The experiments also showed that the dissociation of the RCA polymer from the TM depends upon a high ATP level and alkaline pH in the stroma (Fig. 7). This process of Rubisco activation including TM–RCA is summarized in a new model (Fig. 9).
Fig. 9.
Hypothetical model of Rubisco activation by reversible association of RCA with the TM. In the chloroplast stroma, inactive Rubisco (due to binding inhibition or another reason) is surrounded by RCA in a ring structure (RCAn, n=16 or n=8). ATP hydrolysis in the RCA–Rubisco supercomplex releases Pi and causes conformational changes in the Rubisco and RCA ring structure, resulting in activation of inactive Rubisco. Then some of the dissociated RCAs from the complex bind to the TM. Subsequently, the TM–RCA complex leaves the TM at higher ATP concentrations and alkaline pH, and forms the ring structure again on Rubisco (based on the work of Portis, 2003).
In the present experiment in vivo, there is more of the smaller isoform in total RCA, but more of the larger isoform on the TM (Figs 2, 3). Previous experiments suggested that the smaller RCA isoform bound to the TM first (Rokka et al., 2001). This leads to a hypothesis that the binding of the smaller isoform of RCA to the TM precedes its redox regulation by the larger isoform. This hypothesis is consistent with the observation that in a rice mutant (Δlut), where the cyclic electron transport around PSI (Table 3) and correspondingly the redox state of the larger isoform of RCA are severely influenced, there is more of the smaller isoform of RCA bound to the TM (Fig. 3). Certainly, more experimental evidence is needed to test this hypothesis.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Western blot analysis of total and TM–RCA from 4-week-old rice leaves.
Fig. S2. The activity of RCA dissociated from the TM under different ATP levels and pH in vitro.
Fig. S3. Effects of different ATP levels and pH on the fluorescence intensity of ANS binding to heat-treated RCA.
Supplementary Material
Acknowledgments
This work was supported by National Basic Research Program of China (grant no. 2009CB118504) and the Chinese Academy of Sciences (grant nos KZCX2-YW-N-59 and KZCX3-SW-440). We also thank Professors Archie R Portis Jr, Ji-Rong Huang, Xin-Guang Zhu, and Kang-Cheng Ruan for their kind revision and critical comments.
Glossary
Abbreviations
- ANS
1-anilinonaphthalene-8-sulphonic acid
- BN
blue native
- NPQ
non-photochemical quenching
- ΔpH
pH gradient across the thylakoid membrane
- RCA
Rubisco activase
- Rubisco
ribulose-1, 5-bisphosphate carboxylase/oxygenase
- RuBP
ribulose-1, 5-bisphosphate
- TM
thylakoid membrane
References
- Arnon DJ. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks A, Portis AR, Jr, Sharkey T. Effects of irradiance and methyl viologen treatment on ATP, ADP, and activation of ribulose bisphosphate carboxylase in spinach leaves. Plant Physiology. 1988;88:850–853. doi: 10.1104/pp.88.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. A novel role for light in the activation of ribulosebisphosphate carboxylase/oxygenase. Plant Physiology. 1990a;92:110–115. doi: 10.1104/pp.92.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. Electron transport through photosystem I stimulates light activation of ribulose bisphosphate carboxylase/oxygenase (Rubisco) by Rubisco activase. Plant Physiology. 1990b;94:479–484. doi: 10.1104/pp.94.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xu DQ. Two patterns of leaf photosynthetic response to irradiance transition from saturating to limiting one in some plant species. New Phytologist. 2006;169:789–798. doi: 10.1111/j.1469-8137.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- Cox SD, Lilley R Mc C, Andrews TJ. Chemiluminescence of Mn2+-activated Rubisco: temperature and pH responses differ between L2 and L8S8 forms, and inhibitors provide no evidence for involvement of active oxygen species. Australian Journal of Plant Physiology. 1999;26:475–484. [Google Scholar]
- Crafts-Brandner SJ, van de Loo FJ, Salvucci ME. The two forms of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiology. 1997;114:439–444. doi: 10.1104/pp.114.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Chai CJ, Qian Q, et al. Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. The Plant Journal. 2008;54:177–189. doi: 10.1111/j.1365-313X.2008.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Feng LL, Wang K, Li Y, Tan YP, Kong J, Li H, Li YS, Zhu YG. Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Reports. 2007;26:1635–1646. doi: 10.1007/s00299-006-0299-y. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker N. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gray DW, Lewis LA. Simultaneous collection of rapid chlorophyll fluorescence induction kinetics, fluorescence quenching parameters, and environmental data using an automated PAM-2000/CR10X data logging system. Photosynthesis Research. 2006;87:295–301. doi: 10.1007/s11120-005-9010-3. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Tan EM. Isolation of circulating immune complexes by conglutinin and separation of antigen from dissociated complexes by immobilized protein A. Clinical and Experimental Immunology. 1981;46:9–19. [PMC free article] [PubMed] [Google Scholar]
- Havaux M. Short-term responses of photosystem I to heat stress: induction of a PSΠ-independent electron transport through PSI fed by stromal components. Photosynthesis Research. 1996;47:85–97. doi: 10.1007/BF00017756. [DOI] [PubMed] [Google Scholar]
- Kallis RP, Ewy RG, Portis AR., Jr Alteration of the adenine nucleotide response and increased Rubisco activation activity of Arabidopsis Rubisco activase by site-directed mutagenesis. Plant Physiology. 2000;123:1077–1086. doi: 10.1104/pp.123.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Nemson JA, Melis A. Photosystem II reaction center damage and repair in Dunaliella salina (green alga) Plant Physiology. 1993;103:181–189. doi: 10.1104/pp.103.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. Measuring P700 absorbance changes in the near infrared spectral region with a dual wavelength pulse modulation system. In: Grab G, editor. Photosynthesis: mechanisms and effects. Dordrecht: Kluwer Academic Publishers; 1998. pp. 4357–4360. [Google Scholar]
- Kung SD, Chollet R, Marsho TV. Crystallization and assay procedures of tobacco ribulose-1, 5-bisphosphate carboxylase-oxygenase. Methods in Enzymology. 1980;69:326–336. [Google Scholar]
- Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu GH. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rate under moderate heat stress. The Plant Cell. 2007;19:3230–3241. doi: 10.1105/tpc.107.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavaud J, Rousseau B, Etienne AL. General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae) Journal of Phycology. 2004;40:130–137. [Google Scholar]
- Law RD, Crafts-Brandner SJ, Salvucci ME. Heat stress induces the synthesis of a new form of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase in cotton leaves. Planta. 2001;214:117–125. doi: 10.1007/s004250100592. [DOI] [PubMed] [Google Scholar]
- Li DY, Shen YK. The relation between components of proton motive force and photosynthesis. Chinese Science Bulletin. 1994;39:1712–1715. [Google Scholar]
- Makino A, Sage RF. Temperature response of photosynthesis in transgenic rice transformed with ‘sense’ or ‘antisense’ rbcS. Plant and Cell Physiology. 2007;48:1472–1483. doi: 10.1093/pcp/pcm118. [DOI] [PubMed] [Google Scholar]
- Maxwell PC, Biggins J. Role of cyclic electron transport in photosynthesis as measured by the photoinduced turnover of P700 in vivo. Biochemistry. 1976;15:3975–3981. doi: 10.1021/bi00663a011. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Research. 1999;9:27–43. [PubMed] [Google Scholar]
- Nooshin AP, Li-Chan ECY. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescence probes. Journal of Agricultural and Food Chemistry. 2000;48:328–334. doi: 10.1021/jf990393p. [DOI] [PubMed] [Google Scholar]
- Portis AR., Jr Rubisco activase. Biochimica et Biophysica Acta. 1990;1015:15–28. doi: 10.1016/0005-2728(90)90211-l. [DOI] [PubMed] [Google Scholar]
- Portis AR., Jr Rubisco activase—Rubisco's catalytic chaperone. Photosynthesis Research. 2003;75:11–27. doi: 10.1023/A:1022458108678. [DOI] [PubMed] [Google Scholar]
- Portis AR, Jr, Li CS, Wang DF, Salvucci ME. Regulation of Rubisco activase and its interaction with Rubisco. Journal of Experimental Botany. 2008;59:1597–1604. doi: 10.1093/jxb/erm240. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Portis AR., Jr Involvement of stromal ATP in the light activation of ribulose-1, 5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiology. 1988;86:293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SP, Streusand VJ, Chatfield JM, Portis AR., Jr Purification and assay of Rubisco activase from leaves. Plant Physiology. 1988;88:1008–1014. doi: 10.1104/pp.88.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokka A, Zhang L, Aro EM. Rubisco activase: an enzyme with a temperature-dependent dual function. The Plant Journal. 2001;25:463–471. doi: 10.1046/j.1365-313x.2001.00981.x. [DOI] [PubMed] [Google Scholar]
- Rundle SJ, Zielinski RE. Organization and expression of two tandemly oriented genes encoding ribulosebisphosphate carboxylase/oxygenase activase in barley. Journal of Biological Chemistry. 1991;266:4677–4685. [PubMed] [Google Scholar]
- Sage FR, Way DA, Kubien DS. Rubisco, Rubisco activase, and global climate change. Journal of Experimental Botany. 2008;59:1581–1595. doi: 10.1093/jxb/ern053. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E. Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiology. 2001;127:1053–1064. [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Werneke JM, Ogren WL, Portis AR., Jr Purification and species distribution of Rubisco activase. Plant Physiology. 1987;84:930–936. doi: 10.1104/pp.84.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H, Cramer WA, von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Analytical Biochemistry. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- Spreitzer RJ, Salvucci ME. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annual Review of Plant Biology. 2002;53:449–475. doi: 10.1146/annurev.arplant.53.100301.135233. [DOI] [PubMed] [Google Scholar]
- Streusand VJ, Portis AR., Jr Rubisco activase mediates ATP-dependent activation of ribulose bisphosphate carboxylase. Plant Physiology. 1987;85:152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KY, Suen DF, Chen SCG. Molecular characterization of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase in rice leaves. Planta. 1999;209:66–76. doi: 10.1007/s004250050607. [DOI] [PubMed] [Google Scholar]
- van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynthesis Research. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi HL. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiology. 2006;141:465–474. doi: 10.1104/pp.105.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Portis AR., Jr A flurometric study with 1-anilinonaphthalene-8-sulfonic acid (ANS) of the interactions of ATP and ADP with rubisco activase. Biochimica et Biophysica Acta. 1991;1079:263–267. doi: 10.1016/0167-4838(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Snyder GW, Esau BD, Portis AR, Jr, Ogren WL. Species-dependent variation in the interaction of substrate-bound ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) and Rubisco activase. Plant Physiology. 1992;100:1858–1862. doi: 10.1104/pp.100.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke JM, Zielinski RE, Ogren WL. Structure and expression of spinach leaf cDNA encoding ribulosebisphosphate carboxylase/oxygenase activase. Proceedings of the National Academy of Sciences, USA. 1988;85:787–791. doi: 10.1073/pnas.85.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraight CA, Crofts AR. Delayed fluorescence and the high-energy state of chloroplasts. European Journal of Biochemistry. 1971;19:386–397. doi: 10.1111/j.1432-1033.1971.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Yang XH, Zheng L, Lu CM. Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiology. 2005;138:2299–2390. doi: 10.1104/pp.105.063164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Portis AR., Jr Mechanism of light regulation of Rubisco: a specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proceedings of the National Academy of Sciences, USA. 1999;96:9438–9443. doi: 10.1073/pnas.96.16.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.