Abstract
Objectives
To describe first dose and steady state antiretroviral drug exposure in the female genital tract.
Design
Non-blinded, single center, open-label pharmacokinetic study in HIV-infected women.
Method
Twenty-seven women initiating combination antiretroviral therapy underwent comprehensive blood plasma and cervicovaginal fluid sampling for drug concentrations during the first dose of antiretroviral therapy and at steady-state. Drug concentrations were measured by validated HPLC/UV or HPLC-MS/MS methods. Pharmacokinetic parameters were estimated for 11 drugs by non-compartmental analysis. Descriptive statistics and 95% confidence intervals were generated using Intercooled STATA Release 8.0 (Stata Corporation, College Station, Texas, USA).
Results
For all antiretroviral drugs, genital tract concentrations were detected rapidly after the first dose. Drugs were stratified according to the genital tract concentrations achieved relative to blood plasma. Median rank order of highest to lowest genital tract concentrations relative to blood plasma at steady state were: lamivudine (concentrations achieved were 411% greater than blood plasma), emtricitabine (395%), zidovudine (235%) tenofovir (75%), ritonavir (26%), didanosine (21%), atazanavir (18%), lopinavir (8%), abacavir (8%), stavudine (5%), and efavirenz (0.4%).
Conclusions
This is the first study to comprehensively evaluate antiretroviral drug exposure in the female genital tract. These findings support the use of lamivudine, zidovudine, tenofovir and emtricitabine as excellent pre-exposure/post-exposure prophylaxis (PrEP/PEP) candidates. Atazanavir and lopinavir might be useful agents for these applications due to favorable therapeutic indices, despite lower genital tract concentrations. Agents such as stavudine, abacavir, and efavirenz that achieve genital tract exposures less than 10% of blood plasma are less attractive PrEP/PEP candidates.
Keywords: antiretroviral therapy, genital tract, HIV, pharmacokinetics, prophylaxis, sexual transmission, women
Introduction
Approximately 50% of adults living with HIV/AIDS are women [1]. Heterosexual HIV transmission is the most prominent mode of transmission in the developing world [2]. In Africa, where women account for an estimated 76% of HIV-infected persons aged 15–24 years [2], alternative methods to prevent sexual transmission are urgently needed. The oral administration of antiretroviral drugs to block HIV transmission is an attractive, minimally invasive approach to prevention [3]. This concept is already in practice domestically, as evidenced in the published United States guidelines for use in non-occupational post-exposure prophylaxis (nPEP) [4]. The increasing availability and use of generic antiretroviral drugs to treat HIV internationally would allow for the use of antiretroviral drugs for the prevention of HIV transmission in developing countries.
Early treatment with an effective antiretroviral drug regimen is most critical to preventing successful HIV transmission, as demonstrated by experience with nPEP and experimental animal models [5–10]. Employing antiretroviral drugs that concentrate quickly at the site of inoculation [i.e. genital tract (GT)] for pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) is a logical extension of this evidence. Therefore, we hypothesize that to prevent sexual transmission of HIV in women, oral antiretroviral drugs that achieve high concentrations quickly in the GT after a first dose would be optimal candidates for PEP or PrEP regimens. No human pharmacology data have, however, been generated in either healthy volunteers or HIV-infected cohorts that allow rational selection of drugs used for this purpose. To this end, we comprehensively evaluated antiretroviral drug exposure in the female GTof HIV-infected women for 11 commonly used antiretroviral medications in the three drug classes: nucleoside/tide analogue reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), and protease inhibitors (PI).
Methods
Study design and population
HIV-infected, non-pregnant adult women were enrolled in a non-blinded, observational, open-label prospective, pharmacokinetic study to assess first dose and steady-state antiretroviral drug exposure in cervicovaginal fluid (CVF) relative to blood plasma (BP) from November 2002 to May 2006. Subjects were recruited from the Infectious Diseases Clinic at the University of North Carolina at Chapel Hill. Exclusion criteria included the following: ≤ 17 years of age; active bacterial, fungal, or opportunistic GT or systemic infection at the time of enrollment; hysterectomy; pregnancy; hematocrit less than 30%; unable to abstain from sexual intercourse or douching for 48 h prior to study visits; unable to complete a dosing card; and an estimated drug adherence rate of <80%, as determined by dosing card and patient self-report.
The antiretroviral drug regimen was selected by the patient’s primary HIV provider. Women could be antiretroviral naive and initiating antiretroviral drugs, changing a failing regimen, or re-initiating antiretroviral drugs after a therapy interruption. All patients gave informed consent prior to study participation. This protocol was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill.
Before being enrolled into the study, patients underwent a routine pelvic examination, physical examination, extensive sexually transmitted infection (STI) screening, and routine safety laboratory tests, including a complete blood count, serum chemistries, liver function tests, and serum pregnancy testing. STI screening included testing for chlamydia and gonorrhea by cervical polymerase chain reaction (PCR), testing for trichomonas by urine culture and PCR [11], and testing for syphilis by serum rapid plasma reagin (RPR) with confirmatory treponemal testing if positive. STI testing was performed in the University of North Carolina Sexually Transmitted Diseases Cooperative Research Center Microbiology Core Lab or the McLendon Laboratories of the UNC Hospitals. Any patient with a STI at screening was referred to her primary provider for treatment, and then offered re-screening after successfully completing STI therapy.
Study visits
Following acceptable screening results, patients were admitted for an overnight stay in the Verne S. Caviness General Clinical Research Center. The first dose of the new antiretroviral drug regimen was observed, and six paired BP and CVF samples were obtained (‘first dose’ conditions). At least 3 weeks later, after continuing the regimen (‘steady-state’ conditions), patients returned to the research center whereby the first-dose BP and CVF sampling strategy was repeated after an observed dose. Patients were also followed monthly for an additional 6 months, with BP and GT samples obtained at a single time points 2 to 24 h postdose.
Sample collection and processing
Under both first dose and steady-state conditions, CVF and BP samples were obtained at 0 (immediately predose), 2, 4, 6, 12, and 24 h after observed doses of the antiretroviral drug regimen. All CVF samples were obtained via direct aspiration with a volumetric aspiration device. Subjects were instructed to remain supine for approximately 10 min prior to collection in order to pool CVF in the posterior fornix of the vagina for optimal collection volume. Samples at 0 and 24 h were obtained during a pelvic examination, and the remaining samples at 2, 4, 6, and 12 h post-dose were self-collected. CVF volumes typically ranged from 0.1 to 0.4 ml per sample. A previously conducted pilot study [12] demonstrated this method to be a reproducible and robust approach to specimen collection. To minimize any HIV RNA and drug concentration variability, all visits were scheduled in the 7 to 10 days following the end of menses.
After collection, directly aspirated CVF was transferred to labeled cryovials and stored at −80°C until analysis. Whole blood was obtained using K3EDTA-containing collection tubes (Becton Dickinson Diagnostics, Franklin Lakes, New Jersey, USA) and centrifuged at 1300 g (2600 rpm) at 4°C for 15 min. The resulting plasma was aliquoted into labeled cryovials and stored at −80°C until analysis. Drug concentrations in BP were measured using validated high performance liquid chromatography (HPLC)/UV methods [13–15], and concentrations in CVF were quantified using a validated HPLC-mass spectrometry (MS)/MS method [16].
Briefly, CVF concentrations were measured using a simultaneous assay for 17 antiretroviral drugs. After thawing, samples were centrifuged and the resultant supernatant underwent solid phase extraction using BOND ELUT C-18 columns (Varian, Harbor City, California, USA) as previously described [15]. Cimetidine (in acetate buffer, pH 5.0) was used as internal standard, and was applied directly to the conditioned column prior to CVF introduction. A Shimadzu solvent delivery system (Columbia, Maryland, USA) and a LEAP HTC Pal thermostatted (6°C) autosampler (Carrboro, North Carolina, USA) connected to an Applied Biosystems API4000 triple quandruple mass spectrometer and Turbospray ion source (Applied Biosystems, Foster City, California, USA) with an Aquasil C18 column (Thermo-Electron, San Jose, California, USA) was used for the analysis. Multiple reaction monitoring and positive-to-negative polarity switching were used. Assay sensitivity was 1 ng/ml for abacavir (ABC); 5 ng/ml for lamivudine (3TC), zidovudine (ZDV), emtricitabine (FTC), lopinavir (LPV), atazanavir(ATV) and efavirenz (EFV); and 10 ng/ml for tenofovir (TDF), didanosine (ddI), stavudine (d4T) and ritonavir (RTV). Overall assay precision was 2.0–14.3 CV%, and accuracy was 88–113%. Recovery for the drugs studied ranged from 80% for RTV and LPV to 99% for 3TC, ddI and ABC.
All analytical work was performed by the UNC Center for AIDS Research (CFAR) Clinical Pharmacology and Analytical Chemistry Core, which participates in quarterly national and international external proficiency testing [17,18]. These results consistently demonstrate high levels of accuracy and precision for our antiretroviral assays.
Data analysis methods
Pharmacokinetic parameters, including the area under the time–concentration curve (AUC0−τ), were estimated for both CVF and BP using WinNonlin (version 4.0.1, Pharsight, Inc. Mountain View, California, USA). For these computations, concentration measurements below the lower limit of detection were imputed as zero and those below the lower limit of quantitation (LLQ) were imputed as ½ LLQ. For each antiretroviral agent, the GT: BP AUC0−τ ratios were calculated, and multiplied by 100 to represent penetration of drug into the GT relative to BP [19]. Descriptive statistical methods, particularly medians and the interquartile range (IQR), were used in the primary analyses of these AUC ratios. The 95% confidence intervals of the median AUC ratios for each drug at each visit were calculated using Intercooled STATA Release 8.0 (Stata Corporation, College Station, Texas, USA).
Results
Demographics
Demographic data for the 27 women enrolled are presented in Table 1. The study population was predominantly African American (70%), middle-aged (median: 35 years; IQR: 31–42 years), with a BP HIV RNA of 4.7 (IQR, 4.0–5.0) log10 copies/ml and a CD4+T-cell count of 307 (220–372) cells/μl at study entry. As most subjects were antiretroviral experienced (67%), the provider-selected regimens varied widely. Seventy percent (19/27) of the regimens contained 3TC. TDF was found in 56% of the regimens, ABC in 30%, and ZDV in 24%. Thirty-three percent of the women were on EFV-containing therapy, and 52% were on a PI-containing regimen (57% ATV/r, 36% LPV/r, and 7% ATV); one woman (4%) received EFV with LPV/r. Two individuals (7%) were on triple nucleoside therapy, and one (4%) received nevirapine. (Nevirapine data not presented.)
Table 1.
Baseline demographics and antiretroviral regimen components of 27 study women.
| Age (years) | 35 (31–42) |
| Race/ethnicity | |
| African American | 19 (70%) |
| Caucasian | 5 (19%) |
| Hispanic | 2 (7%) |
| American Indian | 1 (4%) |
| Antiretroviral-experienced | 18 (67%) |
| Baseline HIV RNA (log10 copies/ml) | 4.7 (4.0–5.0) |
| Baseline CD4+ T cells (cells/μl) | 307 (220–372) |
| NRTI Components of antiretroviral regimens | |
| 3TC | 19 (70%) |
| TDF | 15 (56%) |
| ABC | 8 (30%) |
| ZDV | 6 (24%) |
| ddI | 5 (20%) |
| FTC | 4 (16%) |
| d4T | 4 (16%) |
| NNRTI components of antiretroviral regimens | |
| EFV | 9 (33%) |
| NVP | 1 (4%) |
| PI components of antiretroviral regimens | |
| LPV/r | 5 (18%) |
| ATV | 1 (4%) |
| ATV/r | 8 (30%) |
| Other antiretroviral regimens | |
| Triple NRTI | 2 (7%) |
| EFV/LPV/r | 1 (4%) |
Data presented as median (interquartile range) or number (percentage). ABC, abacavir; ATV, atazanavir; ATV/r, atazanavir/ritonavir; ddI, didanosine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; LPV/r, lopinavir/ritonavir; NNRTI, non- nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; TDF, tenofovir; ZDV, zidovudine; 3TC, lamivudine.
Antiretroviral pharmacokinetics in blood plasma and genital tract
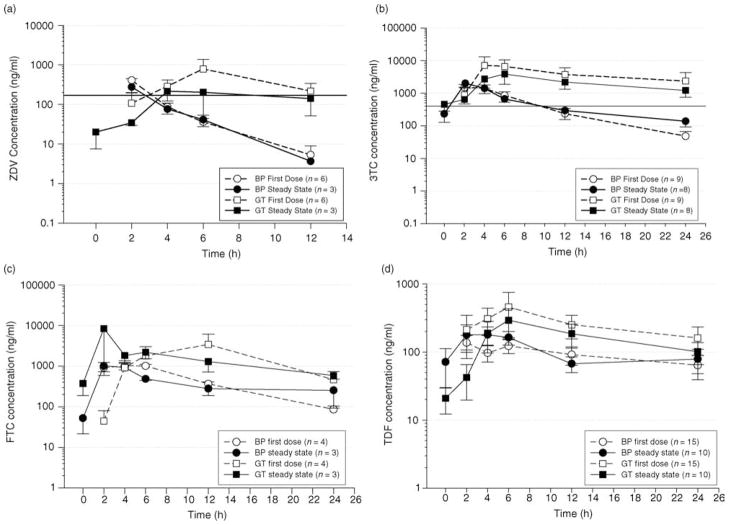
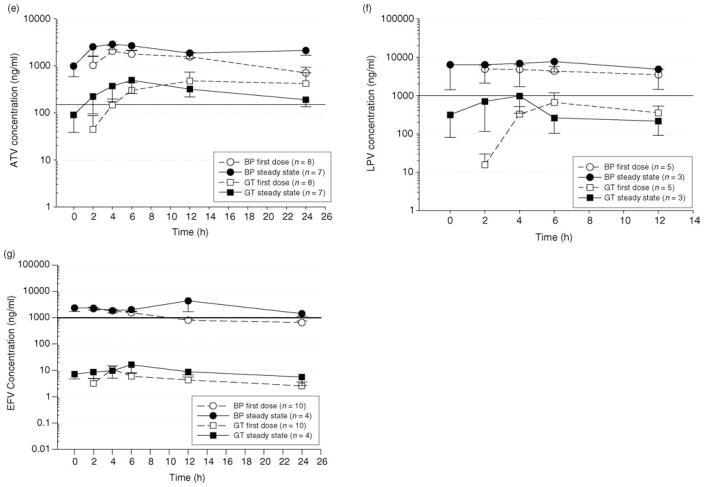
Using the direct cervicovaginal aspirate sampling method, all drugs were detected in the GT 2–4 h after the first dose, and reached peak concentrations for the dosing interval by 4–6 h. Figure 1a–g depict individual pharmacokinetic profiles of selected antiretroviral drugs over their respective dosing intervals in BP and GTafter a first dose and at steady state. These drugs were selected as achieving the highest concentrations in their respective drug classes, using doses and/or dosing regimens most commonly prescribed.
Fig. 1.
(a) Mean (SE) pharmacokinetic profile of zidovudine (ZDV) over a 12 h dosing interval in blood plasma (BP) and genital tract (GT) at first dose and steady state. Subjects received 300 mg every 12 h. The solid line represents an extracellular target concentration previously suggested as efficacious in clinical studies [20,21]. (b) Mean (SE) pharmacokinetic profile of lamivudine (3TC) over a 24 h dosing interval in BP and GT at first dose and steady state. Subjects received 300 mg every 24 h. The solid line represents an extracellular target concentration previously suggested as efficacious in clinical studies [20,21]. (c) Mean (SE) pharmacokinetic profile of emtricitabine (FTC) over a 24 h dosing interval in BP and GT at first dose and steady state. Subjects received 200 mg every 24 h. No target concentration for clinical efficacy has been defined. (d) Mean (SE) pharmacokinetic profiles of tenofovir (TDF) over a 24 h dosing interval in BP and GT at first dose and steady state. Patients received 300 mg of TDF every 24 h. No target concentration for clinical efficacy has been defined. (e) Mean (SE) pharmacokinetic profile of atazanavir (ATV) over a 24 h dosing interval in BP and GT at first dose and steady state. Patient received 300 mg of ATV and 100 mg of ritonavir (RTV) every 24 h. The solid line represents the suggested clinical target concentration for ATV [22]. (f) Mean (SE) pharmacokinetic profile of lopinavir (LPV) over a 12 h dosing interval in BP and GT at first dose and steady state. Subjects received 400 mg of LPV and 100 mg of RTV every 12 h. The solid line represents a suggested clinical target concentration for LPV [22]. (g) Mean (SE) pharmacokinetic profile of efavirenz (EFV) over a 24 h dosing interval in BP and GT at first dose and steady state. Subjects received 600 mg of EFV every 24 h. The solid line represents a suggested clinical target concentration for EFV [22].
Nucleoside/tide reverse transcriptase inhibitors
Figure 1a depicts extracellular ZDV concentrations over a 12 h dosing interval. After a first dose, ZDV concentrations were detectable in both BP and GT secretions 2 h after a dose. Although BP concentrations steadily declined from that time point, GT concentrations continued to increase and reached a maximum concentration at 6 h post-dose. At steady state, concentrations were detectable in the GT, but not in BP, prior to dosing. The GTand BP concentrations were similar 4 h postdose; after this point, GT concentrations declined minimally, while BP concentrations declined significantly. An average extracellular BP concentration (Cav) previously suggested for efficacy [20,21], is indicated in the figure as a black horizontal line. GT concentrations were at or near this target for a longer period of time than BP concentrations.
Lamivudine exposure after once-daily dosing can be seen in Fig. 1b. Similar results were seen for subjects receiving twice daily dosing (not shown). GT concentrations were approximately 1-log higher than BP concentrations throughout the dosing interval after a single dose and under steady-state conditions. The GT concentrations exceeded the suggested average extracellular target concentration (Cav) previously suggested for efficacy [20,21]. Similarly, GT concentrations of FTC were higher than BP concentrations from 6–24 h after a single dose, and remained higher than BP concentrations during the entire dosing interval under steady-state conditions (Fig. 1c). For TDF (Fig. 1d), the GT concentrations were at or above BP concentrations 4 h after a dose, and remained higher throughout the dosing interval.
Protease inhibitors
Pharmacokinetic profiles for ATV and LPV are shown in Fig. 1e and f. In contrast to the NRTIs, ATV GT concentrations (when given as 300 mg ATV/100 mg RTV once daily) were lower than BP throughout the dosing interval both after first dose and at steady-state. These concentrations still, however, exceeded a suggested BP trough concentration of 150 ng/ml for efficacy against wild-type virus [22]: the mean (±SD) ATV trough concentration in the GT after a first dose was 417 ± 818 ng/ml, and at steady state was 169 ± 148 ng/ml. The GT concentrations for LPV were also lower than BP at both first dose and steady-state. A suggested LPV trough concentration for efficacy of 1000 ng/ml is depicted in Fig. 1f [22]. The GT concentrations approached this target over a dosing interval, and briefly exceed this concentration at the 4-h time point (mean ± SD = 1522 ± 1659 ng/ml). At the end of the dosing interval, mean ±SD GT concentrations were 437 ± 631 ng/ml after a single dose and 354 ± 384 ng/ml at steady-state.
Nonnucleoside reverse transcriptase inhibitors
The summary EFV pharmacokinetic profiles are shown in Fig. 1g. The BP and GTexposures are clearly distinct. Whereas EFV was detected in the GT 2 h after a single dose, concentrations were approximately 10 ng/ml: 2 logs lower than BP. The suggested target trough concentration for EFV is 1000 ng/ml [22].
Pharmacokinetic parameters
Table 2 lists steady-state pharmacokinetic parameter estimates in BP and GT for each drug. BP concentrations at the end of the dosing interval (Cτ) and the AUC over 24 h (AUC0–24h) for each drug were within the expected range. On average, GT time to maximal concentration (Tmax) was delayed in comparison with BP. All drugs were, however, detectable in GT secretions within 4 h of antiretroviral drug ingestion.
Table 2.
Steady state pharmacokinetic (PK) parameters by drug, in blood (BP) and genital tract (GT).
| NRTIs |
PIs |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PK parameter | Matrix | ABC n =5 | 3TC n = 13 | TDF n = 10 | ZDV n = 3 | ddI n = 2 | FTC n = 3 | d4T n = 3 | LPV n = 4 | ATV n = 8 | RTV n = 12 | NNRTI EFV n = 6 |
| AUC0–24h(μg*h/ml) | BP | 8.54 (7.99, 9.65) | 8.79 (6.88, 12.2) | 1.69 (1.03, 3.56) | 1.45 (1.28, 1.87) | 5.82 (4.32, 7.32) | 10.9 (9.37, 11.0) | 3.28 (3.23, 3.31) | 89.3 (68.5, 166.0) | 46.6 (15.3, 69.1) | 9.07 (4.87, 19.5) | 48.6 (36.3, 61.8) |
| GT | 0.36 (0.035, 1.07) | 26.3 (1.12, 49.0) | 1.75 (0.60, 3.13) | 2.29 (1.31, 2.44) | 0.21 (0.16, 0.26) | 30.8 (25.6, 52.6) | 0.16 (0.078, 0.28) | 7.43 (3.24, 45.6) | 6.41 (3.19, 10.4) | 3.04 (1.07, 13.7) | 0.15 (0.087, 0.21) | |
| Cτ (ng/ml) | BP | 43.8 (22.8, 60.5) | 109.6 (70.9, 216.7) | 40.5 (21.6, 53.4) | 16.1 (13.6, 29.7) | 74.7 (60.4, 89.1) | 96.6 (92.4, 113.1) | 50 (40, 55) | 2526 (2089, 4984) | 1394 (507.3, 2 256) | 99.3 (66.1, 223.9) | 1712 (1276, 2 153) |
| GT | 31.1 (14.2, 89.8) | 1731 (1083, 3265) | 68.4 (28.2, 112.6) | 173 (128, 1683) | 154.7 (79.9, 229.6) | 596 (537.3, 644.2) | 1.8 (0.9, 2.4) | 111.3 (93.9, 1208) | 242.3 (145.2, 427.9) | 109.7 (50, 643) | 5.6 (3.5, 9.1) | |
All values presented are median (interquartile range); τ represents the end of the dosing interval (either 12 h or 24 h). Area under the curve (AUC) values represent 24 h intervals; for drugs dosed every 12 h, the calculated AUC0–12h was doubled and then used to perform summary measures. ABC, abacavir; ATV, atazanavir; ddI, didanosine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; LPV, lopinavir; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RTV, ritonavir; TDF, tenofovir; ZDV, zidovudine; 3TC, lamivudine.
Table 3 lists median (IQR) GT: BP AUC ratios in rank order from the highest exposure to lowest exposure after single and multiple doses of antiretroviral drugs. A ratio equal to 100% indicates drug exposure in the GT that is equivalent to BP. Therefore, ratios greater than 100% indicate greater GT drug exposures than BP, and those less than 100% indicate lower GT exposures than BP.
Table 3.
Rank order by genital tract (GT): blood plasma (BP) area under the curve (AUC) ratio (%) at first dose.
| Rank order by first dose AUC ratio (%) | Median (IQR)AUC ratio (%) | 95% confidence interval (%) | Rank order by steady-state AUC ratio (%) | Median (IQR) AUC ratio (%) | 95% confidence interval (%) |
|---|---|---|---|---|---|
| ZDV | 371 (113, 604) | 21, 2051 | 3TC | 411 (230, 594) | 161, 701 |
| 3TC | 241 (109, 1548) | 117, 792 | FTC* | 395 (187, 671) | 187, 671 |
| TDF | 135 (0.23, 447) | 25, 408 | ZDV* | 235 (121, 2115) | 121, 2120 |
| FTC* | 111 (91, 1100) | 83, 1100 | TDF | 75 (37, 645) | 18, 699 |
| ABC | 21 (8, 70) | 2.5, 238 | RTV | 26 (11, 169) | 10, 185 |
| RTV | 18 (0.5, 42) | 0.5, 41 | ddI* | 21 (1, 40) | 1, 40 |
| LPV | 17 (0.3, 30) | 0.1, 162 | ATV | 18 (5, 66) | 4, 74 |
| ATV | 16 (10, 79) | 4, 163 | LPV* | 8 (3, 128) | 0.4, 245 |
| ddI* | 6 (6, 131) | 0, 243 | ABC* | 8 (8, 13) | 4, 134 |
| d4T* | 4 (0.7, 35) | 0, 64 | d4T* | 5 (0, 12) | 0, 12 |
| EFV | 0.5 (0.1, 0.8) | 0.05, 0.8 | EFV | 0.4 (0.1, 0.6) | 0.01, 0.9 |
Data presented as median [interquartile range (IQR)], with a 95% confidence interval of the median. A value of 100% indicates equivalent in exposure in BP and GT.
Confidence interval held at the minimum and maximum values of the sample.
ABC, abacavir; ATV, atazanavir; ddI, didanosine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; LPV, lopinavir; RTV, ritonavir; TDF, tenofovir; ZDV, zidovudine; 3TC, lamivudine.
ZDV, 3TC, and FTC achieved greater GTexposures than BP both after a single dose and under steady-state conditions. The median GT exposures for these drugs ranged from 111 to 371% that of BP after a single dose and 235 to 411% after multiple doses. TDF GT exposure at first dose achieved a median of 135%, and 75% of BP at steady state. In contrast, ATV, stavudine (d4T), EFV, and RTVachieved lower GTexposures than BP, ranging from 0.5 to 21% that of BP after a first dose, and 0.4 to 26% after multiple doses.
Discussion
This is the first study to comprehensively evaluate antiretroviral drug exposure in the GT after single and multiple dosing. In this study of 27 HIV-infected women, GT concentrations of all 11 antiretroviral drugs reported here were detected rapidly after single doses. Differing GTexposures between the drugs relative to BP, however, suggest that only certain drugs may be preferable for PrEP or PEP regimens.
Of the nucleoside analogs evaluated, 3TC, ZDV, FTC, and TDF achieved GT concentrations similar to, or higher than, BP, and therefore would be excellent PEP and PrEP candidates for further study. Mean 3TC and ZDV concentrations in the GT were near or above extracellular concentrations suggested for clinical efficacy [20,21]. The median GT AUC of FTC at steady state was approximately three times higher than the suggested BP AUC of 10 ug*h/ml for clinical efficacy [23].
Of the other compounds studied, LPVand ATVachieved low to moderate GT concentrations. Since these may be similar to the recommended BP trough concentrations necessary for efficacy in patients with wild-type HIV-1 virus [22], further study of these agents is warranted for use in PrEP and PEP either given alone or in combination with NRTIs. EFV had very low GT concentrations relative to BP, and is unlikely to be useful in PEP/PrEP regimens.
Low drug exposures in the GT may contribute to higher viral shedding in the GT and the development of viral resistance. Recently, receipt of NNRTI-containing therapy was found to be independently associated with HIV shedding at the cervix (odds ratio = 2.24 compared to a PI-based regimen) in 107 women (31 on NVP and 76 on EFV) [24]. Additionally, data presented from the ACTG study A5077 suggested an increased rate of NNRTI mutations in the female GT [25] in individuals who were highly antiretroviral drug-experienced. Additionally, a recent study of 14 women found three with partial or complete compartmentalization of HIV in the GT compared to BP [26]. In our study, the low exposures of EFV (0.5% GT: BP AUC) observed in this study suggest NNRTI drug resistance to be biologically plausible [27,28]. As drug concentrations in the GT are seldom measured, however, a direct relationship between drug exposure, viral burden, and viral resistance patterns in the GT has not been established.
It is currently unclear which specific mechanisms and physicochemical properties of drugs dictate passage into the female GT. However, drug penetration appears to be related to the protein-binding capacity of each drug. Highly protein-bound drugs such as LPV (98–99% protein bound) [29] and ATV (86% protein bound) [30] had lower GT exposures (8 and 18%, respectively, at steady state), whereas drugs with low protein-binding such as the nucleoside/tide analogs ZDV, 3TC, and FTC (<4% to <38% protein bound) [31–33], generally had higher GTexposures (ranging from 235–411%). d4Tand ddI were exceptions to this trend, as GT exposures were low (5 and 21%, respectively) despite <5% protein binding [34,35]. For d4T, peak plasma concentrations occur at approximately 2 h post-dose. Since our first sampling time was 2 h post-dose, it is possible that the true peak concentration was not captured, and complete exposure information was not obtained.
To further this initial pharmacokinetic evaluation, a number of investigations are desirable. Linking drug exposure to biologic response (e.g., decrease in HIV RNA) in the female GT is critical to confirm that higher extracellular drug concentrations are predictive of a more potent response and guide drug selection for PrEP and PEP. Pharmacokinetic/pharmacodynamic modeling analyses are ongoing in our laboratory to evaluate the correlation between differential GT drug exposure and virologic response. Additionally, our previous NRTI work in male GT mononuclear cells suggests similar or lower concentrations of intracellular triphosphate active metabolites compared to BP despite higher GT extracellular drug exposures [36]. Therefore, research characterizing the intracellular active triphosphorylated metabolites for NRTIs in the female GT is necessary. Our study observed an extracellular half-life of TDF in the GT secretions twice as long as the extracellular half-life of TDF in BP, suggesting that TDF might be dosed at extended intervals for PEP or PrEP. Under first dose conditions, the median half-life was 14.5 h (95% CI: 3.6–29.2 h) in the GT, and 7.4 h (95% CI: 4.8–12.4 h) in BP (data not shown). However, characterizing intracellular concentrations of tenofovir diphosphate in GT mononuclear cells would be most important to move this application forward.
Although for most drugs, no differences in plasma drug exposure have been found between HIV-infected subjects and healthy volunteers [28–32], it may also be beneficial to evaluate the GT exposures of these drugs in healthy women, as they will be the primary recipients of PrEP and PEP. Additionally, no data exist on the influence of local infection and inflammation on drug concentrations in the female GT. As 130–1800% increases in drug concentrations have been documented with the inflammation present in meningitis and prostatitis, [37–39], GT infections may also increase antiretroviral drug concentrations in addition to increasing susceptibility to HIV infection [40–42].
Finally, understanding antiretroviral protein binding in the GT is necessary. This investigation measured total (protein unbound drug + protein bound drug) drug concentrations in both BP and GT. However, it has been suggested that concentrations of drug binding proteins (specifically albumin and alpha-1 acid glycoprotein) in the female GT may be lower than in BP [43]. If this is true, despite differences in total drug concentrations between BP and GT, free drug concentrations in the GT may be similar to, or greater than, BP. Hence, free drug concentrations may be a more important predictor of efficacy. Although these types of investigations are technically difficult and lend themselves better to in-vitro study, this work will be required for a more thorough understanding of antiretroviral behavior in the GT.
In conclusion, this investigation is the first to comprehensively evaluate antiretroviral drug exposure in the female GT. Regardless of drug, GT concentrations were detected 2–4 h after a single antiretroviral dose. Additionally, GT exposures were similar after a first dose and at steady state. The results of this investigation support the use of 3TC, ZDV, TDF, and potentially FTC as excellent PrEP/PEP candidates. ATVand LPV/r may prove useful agents due to favorable GT concentrations in relation to HIV-1 wild-type susceptibility. We believe agents that achieve less than 10% of BP exposure, such as EFV and d4T, are less likely PrEP/PEP candidates.
Acknowledgments
We are indebted to the women volunteers, and the staff of the UNC Infectious Diseases Clinic, the Verne S. Caviness General Clinical Research Center, and the UNC CFAR Clinical Pharmacology and Analytical Chemistry Core.
Sponsorship: This work was supported by the National Institute of Allergy and Infectious Diseases (AI54980; A.D.M.K.), the UNC Center for AIDS Research/National Institute of Allergy and Infectious Disease (AI50410; N.L.R., A.S.B.), the UNC General Clinical Research Center/National Institutes of Health (RR00046), and the UNC Building Interdisciplinary Research Careers in Women’s Health program (HD001441; A.D.M.K., K.B.P.).
Footnotes
This work was presented in part at the 13th Conference on Retroviruses and Opportunistic Infections.
References
- 1.UNAIDS. UNAIDS Global Fact Sheet. 24–27/07/2005. THird IAS Conference on HIV Pathogenesis and Treatment; Rio de Janeiro. [Google Scholar]
- 2.UNAIDS. UNAIDS women and AIDS fact sheet. 23/11/2004. UNAIDS Epidemic Update. Geneva: UNAIDS; 2004. [Google Scholar]
- 3.Hosseinipour M, Cohen MS, Vernazza PL, Kashuba AD. Can antiretroviral therapy be used to prevent sexual transmission of human immunodeficiency virus type 1? Clin Infect Disease. 2002;34:1391–1395. doi: 10.1086/340403. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Recommendations from the US Department of Health and Human Services (DHHS) [Accessed: 27 June 2007];Management of possible sexual, infection-drug-use, or other nonoccupational exposure to hiv, including considerations related to antiretroviral therapy. 2005 January 21; www.AIDSinfo.nih.gov.
- 5.Otten RA, Smith DK, Adams DR. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2) J Virol. 2000;74:9771–9775. doi: 10.1128/jvi.74.20.9771-9775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai C-C, Follis KE. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 7.Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, Bischofberger N, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Rompay KK, Berardi CJ, Aguirre NL, Bischofberger N, Lietman PS, Pedersen NC, et al. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS. 1998;12:F79–F83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Van Rompay KK, Schmidt KA, Lawson JR, Singh R, Bischofberger N, Marthas ML. Topical administration of low-dose tenofovir disoproxil fumarate to protect infant macaques against multiple oral exposures of low doses of simian immunodeficiency virus. J Infect Dis. 2002;186:1508–1513. doi: 10.1086/344360. [DOI] [PubMed] [Google Scholar]
- 10.Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:874–876. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 11.Kaydos SC, Swygard H, Wise SL, Sena AC, Leone PL, Miller WC, et al. Development and validation of a PCR-based enzyme-linked immunosorbent assay with urine for use in clinical research for the detection of Trichomonas vaginalis in women. J Clin Microbiol. 2002;40:89–95. doi: 10.1128/JCM.40.1.89-95.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min SS, Corbett AH, Rezk N, Cu-Uvin S, Fiscus SA, Petch L, et al. Protease inhibitor and nonnucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1 infected women. J AIDS. 2004;37:1577–1580. doi: 10.1097/00126334-200412150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Rezk NL, Tidwell RR, Kashuba ADM. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet detection. J Chromatogr B. 2003;791:137–147. doi: 10.1016/s1570-0232(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 14.Rezk NL, Crutchley RD, Yeh RF, Kashuba AD. Full Validation of an Analytical Method for the HIV-Protease Inhibitor Atazanavir in Combination With 8 Other Antiretroviral Agents and its Applicability to Therapeutic Drug Monitoring. Ther Drug Monit. 2006;28:517–525. doi: 10.1097/00007691-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Rezk NL, Crutchley DR, Kashuba ADM. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. J Chromatogr B. 2005;822:201–208. doi: 10.1016/j.jchromb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Jung BH, Rezk NL, Bridges AS, Kashuba ADM. Simultaneous determination of 16 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomedl Chromatogr. 2007 doi: 10.1002/bmc.865. in press. [DOI] [PubMed] [Google Scholar]
- 17.Holland DT, DiFrancesco R, Connor JD, Morse GD. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004. Ther Drug Monit. 2006;28:367–374. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- 18.Droste JAH, Aarnoutse RE, Koopmans PP, Hekster YA, Burger DM. Evaluation of Antiretroviral Drug Measurements by an Interlaboratory Quality Control Program. J Acq Immun Def Syn. 2003;32:287–291. doi: 10.1097/00126334-200303010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Reddy S, Troiani L, Pereira A, Kim J, van Kempen A, Cohen M, Kashuba A. Zidovudine and lamivudine seminal plasma:blood plasma concentration ratios do not predict total seminal plasma exposure. Tenth Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2003. [poster 4553] [Google Scholar]
- 20.Fletcher CV, Acosta EP, Henry K, Page LM, Gross CR, Kawle SP, et al. Concentration-controlled zidovudine therapy. Clin Pharmacol Ther. 1998;64:331–338. doi: 10.1016/S0009-9236(98)90182-5. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher CV, Anderson PL, Kakuda TN, Schacker TW, Henry K, Gross CR, Brundage RC. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS. 2002;16:551–560. doi: 10.1097/00002030-200203080-00006. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council. [Accessed 27 June 2007];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2006 May 4; www.aidsinfo.nih.gov.
- 23.Wang LH, Begley J, St Claire RL, III, Harris J, Wakeford C, Roussea FS. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;11:1173–1182. doi: 10.1089/aid.2004.20.1173. [DOI] [PubMed] [Google Scholar]
- 24.Neely MN, Benning L, Xu J, Strickler HD, Greenblatt RM, Minkoff H, et al. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;1:38–42. doi: 10.1097/01.qai.0000248352.18007.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzenstein D, Winters M, Fiscus S, Bettendorf D, Kantor R, Wantman M, et al. for the AIDS Clinical Trials Group 5077 Team. Drug resistance in plasma and genital compartments among viremic, multidrug-experienced men and women. 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006. [poster 618] [Google Scholar]
- 26.Kemal KS, Burger H, Mayers D, Anastos K, Foley B, Kitchen C, et al. HIV-1 drug resistance in variants from the female genital tract and plasma. J Infect Diseases. 2007;195:535–545. doi: 10.1086/510855. [DOI] [PubMed] [Google Scholar]
- 27.Winslow DL, Garber S, Reid C, Scarnati H, Baker D, Rayner MM, et al. Selection conditions affect the evolution of specific mutations in the reverse transcriptase gene associated with resistance to DMP 266. AIDS. 1996;10:1205–1209. doi: 10.1097/00002030-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sustiva U.S. prescribing information. Bristol-Princeton, NJ: Meyers Squibb Company; Mar, 2006. revision. www.sustiva.com. [Google Scholar]
- 29.Full U.S. prescribing information. North Chicago, IL: Abbott Laboratories; Oct, 2005. Kaletra (lopinavir/ritonavir) tablets and oral solution. [Google Scholar]
- 30.Full U.S. prescribing information. Princeton, NJ: Bristol-Myers-Squibb Company; Jun, 2006. Reyataz (atazanavir) capsules. [Google Scholar]
- 31.Full U.S. prescribing information. Research Triangle Park, NC: GlaxoSmithKline; Mar, 2006. Epivir (lamivudine) tablets and oral solution. [Google Scholar]
- 32.Full U.S. prescribing information. Foster City, CA: Gilead Sciences; Sep, 2005. Emtriva (emtricitabine) tablets and oral solution. [Google Scholar]
- 33.Full U.S. prescribing information. Research Triangle Park, NC: GlaxoSmithKline; Apr, 2006. Retrovir (zidovudine) tablets. [Google Scholar]
- 34.Videx EC (didanosine) delayed-release capsule enteric-coated beadlets Full U.S. prescribing information. Princeton, NJ: Bristol-Myers-Squibb Company; Jul, 2006. [Google Scholar]
- 35.Full U.S. prescribing information. Princeton, NJ: Bristol-Myers-Squibb Company; Feb, 2006. Zerit (stavudine) capsules and oral solution. [Google Scholar]
- 36.Reddy S, Troiani L, Kim J, et al. Differential phosphorylation of zidovudine and lamivudine (ZDV/3TC) between semen and blood mononuclear cells (MCs) in HIV-1 infected men. Tenth Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2003. [poster 530] [Google Scholar]
- 37.Katoh N, Ono Y, Ohshima S, Miyake K. Diffusion of cefmenoxime and latamoxef into prostatic fluid in the patients with acute bacterial prostatitis. Urol Int. 1992;48:191–194. doi: 10.1159/000282329. [DOI] [PubMed] [Google Scholar]
- 38.Kuhnen E, Pfeifer G, Frenkel C. Penetration of fosfomycin into cerebrospinal fluid across noninflamed and inflamed meninges. Infection. 1987;15:422–424. doi: 10.1007/BF01647220. [DOI] [PubMed] [Google Scholar]
- 39.Moretti MV, Pauluzzi S, Cesana M. Penetration of rufloxacin into the cerebrospinal fluid in patients with inflamed and uninflamed meninges. Antimicrob Agents Chemother. 2000;44:73–77. doi: 10.1128/aac.44.1.73-77.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 41.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 42.McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 43.Salas Herrera IG, Pearson RM, Turner P. Quantitation of albumin and alpha-1-acid glycoprotein in human cervical mucus. Hum Exp Toxicol. 1991;10:137–139. doi: 10.1177/096032719101000209. [DOI] [PubMed] [Google Scholar]