Abstract
Aging is associated with obesity and cancer. Calorie restriction both slows down aging and delays cancer. Evidence has emerged that the nutrient-sensing mammalian target of rapamycin (mTOR) pathway is involved in cellular and organismal aging. Here we show that the mTOR inhibitor rapamycin prevents age-related weight gain, decreases rate of aging, increases lifespan, and suppresses carcinogenesis in transgenic HER-2/neu cancer-prone mice. Rapamycin dramatically delayed tumor onset as well as decreased the number of tumors per animal and tumor size. We suggest that, by slowing down organismal aging, rapamycin delays cancer.
Astonishing discoveries in model organisms indicate that lifespan is genetically controlled.1 In particular, the nutrient-sensing target of rapamycin (TOR) pathway is involved in both mammalian cell senescence2 and aging in diverse organisms from worms to mammals.3,4,5,6 In mammals, TOR (mTOR) controls cell growth and metabolism in response to nutrients (eg, amino acids), insulin, and growth factors such as IGF-1.7 Calorie restriction (CR) deactivates mTOR in mice.8 Not surprisingly, CR extends lifespan in most species including rodents and primates.9,10 Furthermore, the TOR inhibitor rapamycin decelerates senescence in both yeast11 and mammalian cells.12 Based on these findings it was suggested that rapamycin, a clinically approved drug, is an antiaging drug.13 Recently it has been demonstrated that rapamycin in fact extends lifespan in mice.14 However, its effect on longevity of cancer-prone mice has not been addressed. There are several lines of evidence that suggest that suppression of organismal aging may delay carcinogenesis. Thus, cancer is an age-related disease, and the incidence of cancer increases with age in both humans and animals.15,16 Consistently, carcinogenesis is delayed in slowly aging Ames dwarf mice.17,18 Cancer is often associated with age-related obesity and metabolic syndrome,19 and calorie restriction affects both the process of aging (by slowing it down) and carcinogenesis (by delaying the tumor onset in normal and cancer-prone mice20,21). Interestingly, centenarians, people who age slowly, are endowed with a peculiar resistance to cancer.22 Therefore it is reasonable to hypothesize that by slowing down aging rapamycin could delay cancer. Our data demonstrate that rapamycin not only extends lifespan but also significantly delays the onset of spontaneous carcinogenesis in cancer-prone HER-2/neu transgenic mice.
Materials and Methods
Animals and Experimental Design
Homozygous FVB/N HER-2/neu transgenic mice originally obtained from Charles River (Hollister, CA) by the Italian National Research Center for Aging (INRCA) were housed and bred in the Department of Carcinogenesis and Oncogerontology, N.N. Petrov Research Institute of Oncology. Mice received standard laboratory chow and tap water ad libitum.23,24 All studies were conducted in accordance with the ethical standards and according to national and international guidelines and have been approved by the authors’ institutional review board.
Longevity Study
Fifty-eight 2-month-old female FVB/N HER-2/neu mice were randomly divided into two groups. The first group of animals received 1.5 mg/kg rapamycin (LC Laboratories, Woburn, MA) subcutaneously (s.c.) 3 times a week for a period of 2 weeks followed by 2-week intervals without rapamycin. Mice in the second group received s.c. 0.1 ml of solvent without rapamycin and served as a control. Rapamycin was dissolved in 95% ethanol and then diluted with apyrogenic sterile water to a final concentration of 38 μg in 0.1 ml of 2% ethanol.
Once a week all mice were palpated for detection of mammary tumors appearance. The localization and the size of tumors were registered. The neoplastic masses were measured with a caliper, and progressively growing masses of >3 mm in mean diameter were regarded as tumors. The mean number of palpable mammary carcinomas per mouse was calculated as the cumulative number of tumors per total number of tumor-bearing mice. Animals were weighed once a month and were observed throughout their lifespan.23,24
Pathomorphological Examination
All animals were autopsied. All tumors, as well as the tissues and organs with suspected tumor development, were excised, fixed in 10% buffered formalin, and embedded into paraffin. Five-μm histological sections were stained with hematoxylin and eosin and were microscopically examined. Tumors were classified according to International Agency for Research on Cancer recommendations as described.23,24
Statistics
Experimental results were statistically processed by the methods of variation statistics with the use of STATGRAPH statistic program kit as previously described.23,24 The significance of the discrepancies was defined according to the Student t criterion, Fischer exact method, χ2, nonparametric Wilcoxon–Mann–Whitney, and Friedman RM ANOVA on Ranks. Student–Newman–Keuls Method was used for all pairwise multiple comparisons. Correlation coefficient was estimated by Spearman method.23,24 Differences in tumor incidence were evaluated by the Mantel–Haenszel log-rank test.
Parameters of Gompertz model were estimated using maximum likelihood method, nonlinear optimization procedure, and self-written code in ‘Matlab’; confidence intervals for the parameters were obtained using the bootstrap method (see Anisimov et al23,24).
For experimental group the Cox regression model was used to estimate relative risk of death and tumor development under the treatment compared with the control group: h(t,z) = h0(t) exp(zβ), where h(t,z) and h0(t) denote the conditional hazard and baseline hazard rates, respectively, β is the unknown parameter for treatment group, and z takes values 0 and 1, being an indicator variable for two samples—the control and treatment group.
Semiparametric model of heterogeneous mortality23,24 was used to estimate the influence of the treatment on frailty distribution and baseline hazard.
Mathematical Modeling of the Results
For the rapamycin-treated group (rapamycin group) the Cox regression model was used to estimate relative risk of death and tumor development under the treatment compared with the control group: h(t,z) = h0(t) exp(zβ), where h(t,z) and h0(t) denote the conditional hazard and baseline hazard rates, respectively, β is the unknown parameter for treatment group, and z takes values 0 and 1, being an indicator variable for two samples—the control and treatment group. For rapamycin group the relative risk of death and the first tumor development is lower (exp(β)<1) compared with control group (see supplemental Table S1 at http://ajp.amjpathol.org). All estimated changes in risks were significant, except for the mice with tumors without metastases.
Semiparametric model of heterogeneous mortality was used to compare the control and rapamycin groups to the group of the female mice treated with rapamycin in terms of frailty distribution and baseline hazard.
Survival and mortality rate functions are given by the formulas:
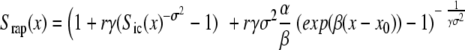 |
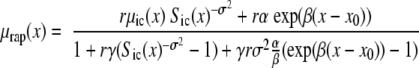 |
Parameter σ2 indicates the presence of heterogeneity in the control population.
Differences in the baseline hazard are controlled by parameters α and β. Parameter α reflects permanent (constant) decrease or increase of the baseline hazard compared with the control group, depending on whether α is greater or less than zero. Parameter β describes the amplification or disappearance of the α-effect, according to whether β is greater or less than zero.
Differences in the frailty distribution are controlled by parameters r and γ. Parameter r < 1 shows an increase in the average robustness, whereas r > 1 indicates an accumulation of frail individuals in the population compared with the control group. Parameter γ ≠ 1 shows an increase (γ > 1) or decrease (γ < 1) in the population heterogeneity.
To compare the survival function for rapamycin-treated female mice to the control group, three specifications of the model were considered. The first one deals only with the differences in the average frailties of the populations (α = 0, r ≠ 1, γ = 1). With the second specification, differences in the mean of the frailty distributions are accompanied by differences in the baseline hazards (α ≠ 0, β = 0, r ≠ 1, γ = 1).
The third specification describes differences in survival patterns between the group of interest and the control group as a combination of differences in the baseline hazard and both parameters of the frailty distribution (α ≠ 0, β ≠ 0, r ≠ 1, γ ≠ 1). Because these specifications of the model are nested, the likelihood ratio statistics were used to determine which one gives the best fit to the data.
The parameter estimates were obtained using maximum likelihood method, nonlinear optimization procedure, and self-written code in ‘Matlab’; confidence intervals for the parameters were obtained using the bootstrap method.
The third specification of the model corresponds to the data better than the others (see supplemental Table S2 at http://ajp.amjpathol.org). The estimated parameter values of this specification are presented in supplemental Table S3 (at http://ajp.amjpathol.org). The control group is heterogeneous (parameter σ2 ≠ 0). The group of rapamycin-treated mice has slightly increased baseline hazard compared with the control group (parameters α > 0, β > 0). The experimental group is frailer on average (r > 1) and more heterogeneous (γ > 1), compared with the control group.
Results
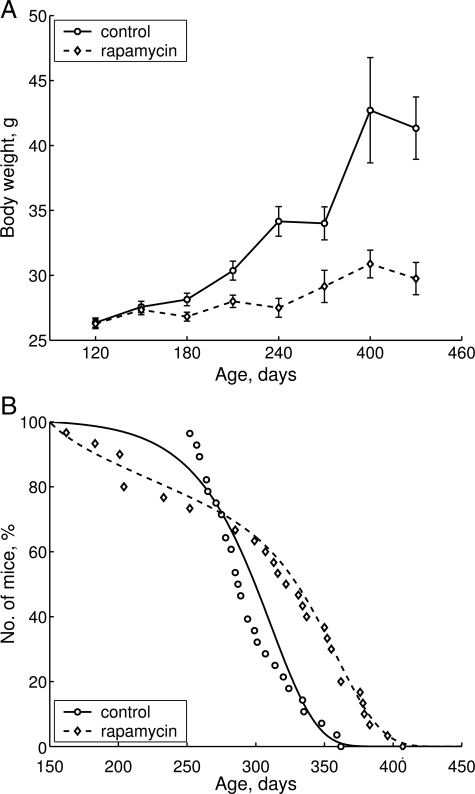
Treatment with rapamycin significantly inhibited age-related weight gain (Figure 1A). Whereas control mice constantly gained weight during their lifespan, mice that received rapamycin demonstrated a very modest weight increase. As a result, for the period between 4 and 10 months, the weight of control animals increased by 62% and only by 17.5% in the group treated with rapamycin (P > 0.05; Figure 1A). Most importantly, in control group only 4 mice survived until 11 months (14.3%) compared with 13 animals (43.3%) in rapamycin-treated group (P < 0.001; Figure 1B and Table 1). Rapamycin treatment increased mean (+4.1%) and maximal lifespan (+12.4%; Table 2). Notably, the increase in mean lifespan was relatively modest because it was blunted by aging-independent mortality (Figure 1B, days 150 to 250). Mean lifespan of long-living animals (last 10% of survivors) was significantly greater in the group receiving rapamycin (+11%) compared with control. Parameter α of the Gompertz model, which is interpreted as the rate of aging, was 1.8 times lower in the group subjected to rapamycin treatment than in control. All differences between control and experimental groups were statistically significant (Table 2).
Figure 1.
Effects of rapamycin on age-related weight gain and lifespan of female transgenic HER-2/neu mice. A: Effect of rapamycin on body weight in female transgenic HER-2/neu mice. The rapamycin group of animals received 1.5 mg/kg rapamycin three times a week for a period of 2 weeks followed by 2-week intervals without rapamycin. Mice in control group received solvent without rapamycin. Mice were weighed once a month. B: Effect of rapamycin on mice survival. Mice were observed throughout their lifespan, and all animals were autopsied. Distributions of lifespan in control and experimental groups were significantly different (log-rank test, P = 0.00588). Survival dynamics showed significant differences.
Table 1.
Effect of Rapamycin on Survival Distribution in Female HER-2.neu Mice
| Group | Number of survivors at the age of
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 mo | 6 mo | 7 mo | 8 mo | 9 mo | 10 mo | 11 mo | 12 mo | 13 mo | 14 mo | |
| Control | 28 | 28 | 28 | 28 | 21 | 9 | 4 | 0 | 0 | 0 |
| Rapamycin | 30 | 29 | 24 | 23 | 22 | 19* | 13* | 6* | 2 | 0 |
The difference with the corresponding age in the control group is significant:
P < 0.01 (Fischer exact test).
Table 2.
Effect of Rapamycin on Parameters of Lifespan in Female HER-2/neu Mice
| Parameters | Control | Rapamycin |
|---|---|---|
| Number of mice | 28 | 30 |
| Mean lifespan (M ± SE), days | 296 ± 5.9 | 308 ± 12.9 (+4.1%) |
| Median | 288 | 327 (+13.6%) |
| Mean lifespan of last 10% survivors, days | 356 ± 4.3 | 395 ± 6.9 (+11.0%)† |
| Maximum lifespan, days | 362 | 407 (+12.4%) |
| Aging rate α (days−2)‡ | 3.02 (3.01; 3.21) | 1.67* (1.64; 1.76) –1.8 times |
| MRDT, days§ | 23.0 (21.6; 23.0) | 41.6* (39.4; 42.3) +1.8 times |
The difference with controls is significant,
P < 0.05;
P < 0.01.
Parameter α in the Gompertz equation R = R0(exp) αt, where R0 = mortality at t0 = 150.
MRDT, mortality rate doubling time, days (95% confidence limits are given in parentheses).
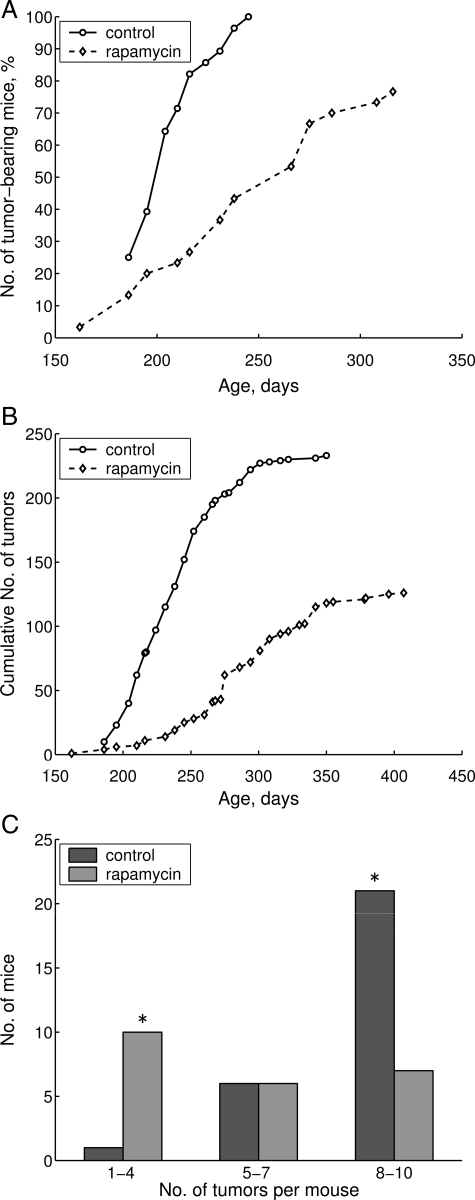
The kinetic of tumor incidence in rapamycin-treated mice was significantly slower than the kinetic in control animals (Figure 2, A and B). Thus, 50% of control mice developed mammary adenocarcinoma by day 206, whereas in rapamycin-treated group this period was extended to 240 days. Remarkably, rapamycin decreased the mean number of tumors per tumor-bearing mouse by 33.7% and the mean size of mammary adenocarcinoma by 23.5% (Table 3).
Figure 2.
Suppression of carcinogenesis by rapamycin. A: Effect of rapamycin on tumor yield curves in female transgenic HER-2/neu mice. B: Effect of rapamycin on cumulative number of tumors in female transgenic HER-2/neu mice. C: Effect of rapamycin on distribution of mice with multiple mammary tumors in female transgenic HER-2/neu mice. Asterisks mean that according to the Fisher exact test for count data the distributions of the number of mice bearing 1 to 4, 5 to 7, and 8 to 10 tumors is significant with P = 0.0006959.
Table 3.
Effect of Rapamycin on Development of Mammary Adenocarcinomas (MAC) in Transgenic HER-2/neu Mice
| Parameters | Control | Rapamycin |
|---|---|---|
| Number of mice | 28 | 30 |
| Number of tumor-bearing mice, % | 28 (100%) | 23* (76.7%) |
| Mean latency of the first mammary adenocarcinoma, days | 206 ± 3.3 | 240 ± 8.9† (+16.5%) |
| Total number of mammary adenocarcinomas | 233 | 126 |
| Number of tumors per tumor-bearing mouse | 8.3 ± 0.3 | 5.5 ± 0.6† (−33.7%) |
| Mean size of MAC, cm3 | 1.7 ± 0.04 | 1.3 ± 0.08† (−23.5%) |
| Nos. of mice with MAC metastases into lungs, % | 10 (37.0%) | 12 (+52.2%) |
| Mean time of death of metastases-bearing mice | 301 ± 10.9 | 357 ± 7.9† (−18.6%) |
| Mean size of MAC, cm | 0.5 ± 0.05 | 0.5 ± 0.06 |
The difference with controls is significant,
P < 0.01;
P < 0.001.
The number of tumors per animal was also significantly decreased by rapamycin. Thus, 75% of control mice were bearing from 8 to 10 tumors per mouse, whereas in the group treated with rapamycin only 30.4% of animals were bearing this number of mammary adenocarcinoma per animal (Figure 2C). In turn, only one control mouse developed fewer than four tumors, whereas in rapamycin-treated group, 10 of 23 mice (43.5%) developed one to four tumors (Figure 2C). These data demonstrate that rapamycin not only increased lifespan, but also reduced tumorigenesis in cancer-prone mice.
Discussion
Rapamycin delays tumor onset and progression in cancer-prone and carcinogen-treated rodents.25,26,27,28,29 In particular, five MMTV-c-Neu mice bearing tumors were treated with 150 μg rapamycin (a dose approximately 3 times higher than the dose used in our study) for 32 days and tumor regression was observed.29 However, the effect of rapamycin on longevity of cancer-prone mice has not been previously investigated. Theoretical considerations predict that rapamycin would slow down organismal aging.13,30 Here we show for the first time that rapamycin prolongs lifespan and decreases rate of aging in cancer-prone mice. Noteworthy, rapamycin failed to increase lifespan when given to mice with already established tumors, even though it decelerated tumor growth (data not shown). This suggests that rapamycin decreases tumorigenesis by slowing down aging rather than increases lifespan by decelerating cancer. In fact, effects of rapamycin are reminiscent of effects of CR, which both slows down the aging process and delays cancer.16 This resemblance is not accidental because both CR and rapamycin deactivate the nutrient-sensing mTOR pathway.8 There are several lines of evidence supporting the indirect mechanism of action of rapamycin on tumorigenesis. First, as we show here, rapamycin prevented age-related weight gain suggesting that, like CR, it may delay cancer by indirect mechanism. This prevention of weight gain was not attributable to toxic effects of rapamycin, because a short-term (32 days) administration of approximately threefold higher doses of rapamycin did not cause weight loss.29 Second, consistent with indirect mechanism of action, rapamycin inhibited cancer growth in mice more profoundly than in cell culture.27,31 And third, similar to CR, rapamycin extends lifespan in a heterogeneous group of mice, which die from various diseases not necessarily related to cancer.14 In fact, rapamycin delays other age-related diseases such as atherosclerosis, metabolic disorders and neurodegeneration, organ fibrosis, age-related macular degeneration, and osteoarthritis.32,33,34,35 Taken together, these data support the notion that rapamycin decelerates age-related diseases by slowing down organismal aging. Similarly, CR and fasting can delay tumorigenesis in cancer-prone mice, including p53-deficient mice.36 In addition, metformin, which mimics CR and inhibits mTOR via AMPK, prolongs lifespan24 and delays cancer in cancer-prone mice.37
Importantly, as we demonstrate here, rapamycin extends maximal lifespan even when administrated intermittently (two consecutive weeks followed by a two-week break). This is a reminiscence of life extension and cancer prevention caused by intermittent CR. For example, fasting delays cancer in p53−/− mice even if applied once a week and started late in life.36 We suggest that slowing down the aging process caused by intermittent administration of rapamycin would be beneficial for humans with high risk of cancer. This is especially important given that existing chemopreventive modalities have not demonstrated life extension.
Acknowledgments
We thank three anonymous reviewers for helpful comments and suggestions.
Footnotes
Address reprint requests to Vladimir N. Anisimov, M.D., Ph.D., DSc., Department of Carcinogenesis and Oncogerontology, N.N. Petrov Research Institute of Oncology, Leningradskaya Str. 68, Pesochny-2, St. Petersburg 197758 Russia, or Mikhail V. Blagosklonny, M.D., Ph.D., Department of Cell Stress Biology, Roswell Park Cancer Institute, BLSC, L3–312, Elm and Carlton Streets, Buffalo, NY 14263. E-mail: aging@mail.ru or blagosklonny@oncotarget.com.
Supported in part by NIH grant CA102522 (to M.P.A.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab. 2006;17:33–35. doi: 10.1016/j.tem.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase. Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–5499. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction. SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RWr KKS, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Disc Today. 2007;12:218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandezr E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature. 2009;460:392–396. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- Anisimov VN. Biology of aging and cancer. Cancer Control. 2007;14:23–31. doi: 10.1177/107327480701400104. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Perkins SN, Brown CC, Haines DC, Phang JM. Calorie restriction induces a p53-independent delay of spontaneous carcinogenesis in p53-deficient and wild-type mice. Cancer Res. 1997;57:2843–2846. [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Popovich IG, Zabezhinski MA. Methods of evaluating the effect of pharmacological drugs on aging and life span in mice. Methods Mol Biol. 2007;371:227–236. doi: 10.1007/978-1-59745-361-5_17. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, Hensley HH, Hamilton TC, Testa JR. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–2413. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, Veenstra TD, Issaq HJ, Linnoila RI, Dennis PA. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res. 2007;13:2281–2289. doi: 10.1158/1078-0432.CCR-06-2570. [DOI] [PubMed] [Google Scholar]
- Namba R, Young LJ, Abbey CK, Kim L, Damonte P, Borowsky AD, Qi J, Tepper CG, MacLeod CL, Cardiff RD, Gregg JP. Rapamycin inhibits growth of premalignant and malignant mammary lesions in a mouse model of ductal carcinoma in situ. Clin Cancer Res. 2006;12:2613–2621. doi: 10.1158/1078-0432.CCR-05-2170. [DOI] [PubMed] [Google Scholar]
- Robinson J, Lai C, Martin A, Nye E, Tomlinson I, Silver A. Oral rapamycin reduces tumour burden and vascularization in Lkb1(+/−) mice. J Pathol. 2009;219:35–40. doi: 10.1002/path.2562. [DOI] [PubMed] [Google Scholar]
- Mosley JD, Poirier JT, Seachrist DD, Landis MD, Keri RA. Rapamycin inhibits multiple stages of c-Neu/ErbB2 induced tumor progression in a transgenic mouse model of HER2-positive breast cancer. Mol Cancer Ther. 2007;6:2188–2197. doi: 10.1158/1535-7163.MCT-07-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther. 2008;7:1520–1524. doi: 10.4161/cbt.7.10.6663. [DOI] [PubMed] [Google Scholar]
- Liu M, Howes A, Lesperance J, Stallcup WB, Hauser CA, Kadoya K, Oshima RG, Abraham RT. Antitumor activity of rapamycin in a transgenic mouse model of ErbB2-dependent human breast cancer. Cancer Res. 2005;65:5325–5336. doi: 10.1158/0008-5472.CAN-04-4589. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Qi H, Liu LF, Zheng XFS. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Disc Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging. 2009;1:281–288. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]