Abstract
Objective
A hallmark of rheumatoid arthritis (RA) is invasion of the synovial pannus into cartilage and this step requires degradation of the collagen matrix. The aim of this study was to explore the role of one of the collagen-degrading matrix metalloproteinases (MMPs), membrane-type 1 MMP (MT1-MMP), in synovial pannus invasiveness.
Methods
Expression and localization of MT1-MMP in human RA pannus were investigated by Western blot analysis of primary synovial cells and immunohistochemistry of RA joints specimens. The functional role of MT1-MMP was analyzed by 3D collagen invasion assays and a cartilage invasion assay in the presence or absence of tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, or GM6001. The effect of adenoviral expression of a dominant negative MT1-MMP construct lacking a catalytic domain was also examined.
Results
MT1-MMP was highly expressed at the pannus-cartilage junction of RA joints. Freshly isolated rheumatoid synovial tissues and isolated RA synovial fibroblasts invaded into a 3D collagen matrix in an MT1-MMP-dependent manner. Invasion was blocked by TIMP-2 and GM6001, but not by TIMP-1. It was also inhibited by the over-expression of a dominant negative MT1-MMP which inhibits collagenolytic activity and proMMP-2 activation by MT1-MMP on the cell surface. Synovial fibroblasts also invaded into cartilage in an MT1-MMP-dependent manner. This process was further enhanced by removing aggrecan from the cartilage matrix.
Conclusion
MT1-MMP is an essential collagen-degrading proteinase during pannus invasion in human RA. Specific inhibition of MT1-MMP-dependent invasion may form a novel therapeutic strategy for RA.
Keywords: MT1-MMP, synovial pannus, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease, and one of its key pathological features is the destruction of joint tissues, including cartilage, by inflamed synovial pannus leading to a loss of joint function. Invading synovial pannus tissue produces proteinases that degrade components of the joint extracellular matrix (ECM) (1). Cartilage is a unique tissue comprised of a small number of resident chondrocytes and their abundant surrounding ECM, and its function is heavily dependent on the nature of the ECM (2, 3). Cartilage ECM is predominantly composed of type II collagen and aggrecan with other minor components including small leucine rich proteoglycans and type IX and XI collagens (2, 3). Aggrecan is considered to be lost first in the early stages of RA, followed by collagen degradation. Aggrecan loss is primarily due to two types of metalloproteinases; aggrecanases that belong to the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family and matrix metalloproteinases (MMPs)(1, 4). As aggrecan maintains water in the cartilage, it is an important ECM component endowing cartilage with compressive resistance properties. Loss of aggrecan thus abrogates cartilage function. The proteinases responsible for collagen degradation are members of the MMP family including collagenases (MMPs-1, -8, -13), gelatinase A (MMP-2) and membrane-type 1 MMP (MT1-MMP/MMP14) (5). Type II collagen is the major collagen in cartilage, accounting for 90 % of the total collagen (2). Monomeric type II collagen is composed of three alpha 1(II) chains in a triple helical structure which form a fibrillar meshwork by cross-linking with each other and with other minor collagens. The collagen meshwork provides the tissue with structure and tensile strength. Thus, degradation of collagen results in irreversible structural and functional damage to the cartilage.
Fibrillar collagens including type II collagen are resistant to most proteinases due to their triple helical structure, but five MMPs, namely MMP-1, MMP-2, MMP-8, MMP-13, and MT1-MMP, can degrade type II collagen (5). Among them, MT1-MMP has been shown to be an essential collagenolytic enzyme during development (6, 7). MT1-MMP null mice show skeletal defects and general fibrosis which are thought to be caused by a lack of cellular collagenolytic activity (6). Among the above collagenases MT1-MMP is the only one that can promote cellular invasion into a collagen matrix (8). MT1-MMP, as a cellular collagenase, has also been shown to play an essential role in angiogenesis (7, 9), tumour invasion (10–12) and growth (13) and smooth muscle cell migration in the arterial wall (14). Besides collagen II, MT1-MMP degrades collagens I and III, fibronectin, laminins 1 and 5, fibrin, and aggrecan core protein (15–17). MT1-MMP also activates proMMP-2 (11) and proMMP-13 (18) and it processes ECM receptors including CD44 (19) and syndecan 1 (20), promoting cell migration. MT1-MMP was reported to be expressed by the lining cells of human RA synovium (21, 22) and cells at the cartilage pannus junction (22), and potential involvement of MT1-MMP in cartilage invasion by RA synovial cells by activation of proMMP-2 or by itself has been reported (21–23). However, the exact role of MT1-MMP in RA pathogenesis and the mechanism of synovial pannus invasion are not clear at present.
We have previously shown that homo-dimer formation of MT1-MMP is essential for cell surface collagen degradation (24) and proMMP-2 activation (25), and that disruption of homo-dimerization by over-expressing a catalytic domain deletion mutant of MT1-MMP (MT1-ΔCat) effectively inhibits both collagenolytic activity and proMMP-2 activation (24, 25). Thus, MT1-ΔCat is a useful tool to examine the biological significance of MT1-MMP. In this study, we found that MT1-MMP is highly expressed in synovial cells from patients with RA, particularly in the cells actively invading into cartilage. Using an adenoviral MT1-ΔCat over-expression system, we demonstrated that MT1-MMP is a key enzyme in synovial invasion in rheumatoid arthritis.
Materials and Methods
Human RA joint specimens
Specimens of RA synovial tissue were obtained with consent from patients undergoing joint replacement surgery at Charing Cross Hospital, London and Bath United Hospitals. Ethical approval was granted from the Riverside Research Ethics Committee (RREC 2064, 1752). All patients met the American College of Rheumatology revised criteria for RA (26). RA joint synovia were identified from their anatomically distinct areas and harvested directly into RPMI containing 5% fetal bovine serum (FBS)/1% penicillin/streptomycin.
Synovial cell isolation and culture
Fresh RA synovial tissue specimens were subjected to collagenase digestion. Briefly, the diced synovium was incubated in 1 % bacterial collagenase in RPMI for 90 minutes with gentle shaking. Cells isolated were then washed, counted and frozen in DMEM containing 10 % DMSO and 50 % FBS in liquid nitrogen until required for experiments. For Western blot analysis of MT1-MMP, cells were directly seeded onto culture plates without further passage. Synovial cells were cultured in 10 % FBS/1% penicillin/streptomycin in DMEM at 37 °C in a humidified chamber. For invasion assays, synovial cells passaged between 3–4 times were used. After the third passage, the majority of the cell population was fibroblast-like.
Antibodies
Mouse monoclonal anti-MT1-MMP hemopexin (Hpx) domain antibody, 222-1D8, was generated by injecting recombinant MT1-MMP Hpx domain expressed in E.coli as described previously (25). Goat polyclonal anti-actin antibody was from Santa Cruz (CA, USA) and anti-FLAG M2 antibody was from Sigma-Aldrich (Dorset, UK). Mouse monoclonal anti-human CD68 antibody was from DAKO (Cambridge, UK).
Histology of RA specimens
Fresh RA metacarpal joints were fixed in 4 % formaldehyde in PBS followed by decalcification in 10 % EDTA in PBS. Decalcification was monitored by radiography and took about 4 weeks to complete. Decalcified tissues were then embedded in paraffin wax for sectioning at 5 µm using a microtome. Sections were stained for MT1-MMP with 222-1D8 antibody (0.2 µg/ml) with hematoxiline counter staining; and for collagens and aggrecan with Fast Green FCS and Safranin O, respectively. Some specimens were also subjected to staining with anti-CD68 antibody (1:250 dilution) and Masson’s Trichrome staining. Cartilage invasion assay samples (see below) were fixed in 4 % formaldehyde in PBS and then embedded in paraffin wax for sectioning at 5 µm using a microtome. Sections were stained for MT1-MMP with 222-1D8 (0.2 µg/ml) with hematoxiline counter staining and for aggrecan with Safranin O. Images were taken using a CCD-equipped microscope with a 10× objective lens (Leica, Milton Keynes, UK).
Adenoviral vector
FLAG-tagged MT1-MMP (MT1F) (FLAG tag of DYKDDDDK was inserted immediately down stream of RRKR111) and its catalytic domain deletion mutant (MT1F-ΔCat) lacking Tyr112 to Pro312 were constructed using the PCR-extension method and the sequence confirmed by DNA sequencing as described previously (24, 25). For expression in human synovial cells, adenoviral vectors were constructed using the AdEasy™ system (Q-BIOgene, CA, USA) according to the manufacture’s instructions. The transgene is expressed under the Cytomegalovirus promotor. In addition to the recombinant adenoviruses expressing MT1F (Ad-MT1F) and MT1F-ΔCat (Ad-MT1F-ΔCat), mock virus was also made (Ad-Mock). High titer virus stocks were prepared by discontinuous cesium chloride gradient ultracentrifugation, and their titer measured using the Adeno-X™ titer kit (BD Biosciences, UK).
Ex vivo invasion assay
For our 3-D ex vivo invasion assay, we used acid-soluble type I collagen without pepsin treatment (3 mg/ml, Cellmatrix type 1-A, Nitta gelatin, Osaka, Japan). Nine parts collagen gel was mixed with one part of 10× RPMI (Sigma- Aldrich) and the pH adjusted to 8.0 by addition of 1 M NaOH on ice. The collagen was further diluted using DMEM adjusting its concentration to 2 mg/ml. Synovial tissues were diced to small pieces around 2 mm3 in size and embedded within the collagen gel. Tissues in the collagen gel were cultured in 10 % FBS/DMEM for 5 days and images were taken with a CCD-equipped microscope (Nikon TE-2000, Nikon, Surrey, UK).
In situ collagen degradation assay
An in situ collagen degradation assay was carried out as described previously (24). Six-well culture plates were coated with a thin-layer of chilled neutralised PureCol™ collagen (Nutacon, Leimuiden, Nederland) at 2.7 mg/ml in 1 ×RPMI medium (typically 100 µl /well) and incubated for 60 min at 37 °C to enable fiber formation. Isolated RA synovial fibroblasts were seeded on the collagen film (1 ×105/well) and cultured for 4 days in the absence of serum at 37 °C. At the end of culture period, the remaining collagen film was exposed by removing cells using repeated treatment with PBS containing 0.5 mg/ml trypsin and 1 mM EDTA. The collagen film was then fixed with 3 % para-formaldehyde for 20 min at room temperature. Collagen was visualized by staining with Coomassie Brilliant Blue R250 and the images captured using a 20× objective lens and a CCD camera-equipped microscope (Nikon TE-2000). The degraded area was visualized as a clear unstained zone.
Microcarrier beads invasion assay
Isolated RA synovial fibroblasts were attached to Cytodex 3™ microcarrier beads (Sigma-Aldrich) by incubating trypsinized cells with sterile beads at 37 °C overnight with gentle agitation. The beads and attached cells were suspended in a 2 mg/ml type I collagen gel of Cellmatrix type 1-A (Nitta gelatin), and cultured for up to 72 h. At the end of the culture period, images were taken using the 10× objective lens on a CCD camera-equipped microscope (Nikon TE-2000). The distance cells had migrated from the bead surface was measured using Openlab software (Improvision, Coventry, UK). Median values were calculated and statistical comparisons made between groups with the Mann-Whitney U test (GraphPad Prism, GraphPad Software, San Diego, USA).
Cartilage invasion assay
Fresh bovine nasal septum cartilage was diced to a size of approximately 5 × 5 × 3 mm and treated with or without retinoic acid for 10 days in a serum-free DMEM. Pieces were then frozen and thawed three-times to kill chondrocytes. Retinoic acid-treated cartilage was further incubated in an excess volume of serum free DMEM to remove retinoic acid remaining in the tissue. In some experiments, fresh cartilage was immediately frozen. Isolated RA synovial cells were cultured on top of the cartilage in the presence or absence of inhibitors for 2 or 4 weeks in DMEM supplemented with 2 % FBS and antibiotics.
RESULTS
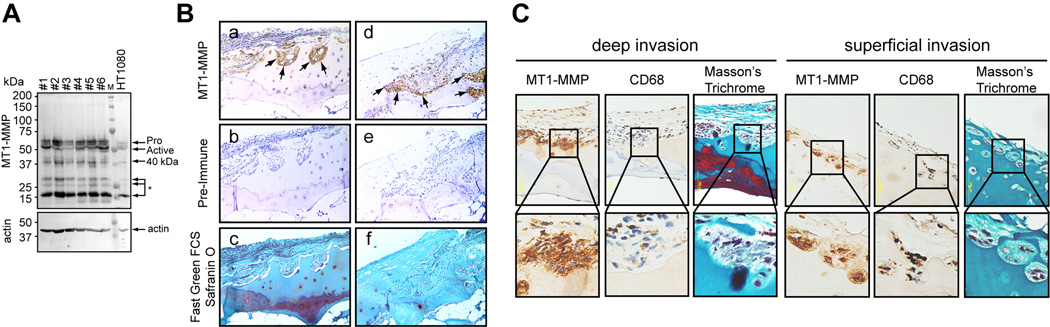
Cartilage invasion by pannus requires type II collagen degradation (1). Since one of the membrane-bound MMPs, MT1-MMP has been shown to promote cellular invasion into a collagen matrix (8–10, 27), we examined whether human rheumatoid synovial cells from patients with RA express MT1-MMP by Western blot analysis using a specific anti-MT1-MMP antibody. As shown in Figure 1A, all of the tested primary synovial cells expressed high levels of MT1-MMP (n=6). HT1080, human fibrosarcoma cells, were taken as a positive control as they are well known to express MT1-MMP (11, 28). Band patterns seen in synovial cells are the same as in HT1080, with the active form of MT1-MMP (50 kDa), a catalytic domain-processed form of around 40 kDa, and further degraded forms of lower molecular weights being visible. It is noteworthy that the level of MT1-MMP expressed by the synovial cells is equivalent to or higher than that in HT1080, an invasive cancer cell line. Next, we tested if MT1-MMP expression correlates with cartilage invasion by immunohistochemical analysis of rheumatoid joint specimens stained with the same anti-MT1-MMP antibody. As shown in Figure 1B, MT1-MMP was highly expressed in pannus tissue, particularly at the pannus-cartilage junction, i.e. at the invasion edge of the pannus (see Figure 1B a, d arrows). In these specimens, cartilage completely lacks aggrecan as indicated by the lack of Safranin O staining (Figure 1B c, f). We have analysed joint specimens from 24 different RA patients and similar results were obtained with all specimens (data not shown). Some RA joint specimens were also stained with anti-MT1-MMP and anti-CD68 antibodies. Most cells invading into cartilage, especially those invading deeply into the tissue, were CD68-negative and spindle shaped (Figure 1C, deep invasion). However, in some regions, CD68 positive cells were present in the pits formed on the cartilage surface (Figure 1C, superficial invasion). Nevertheless, both cell types highly express MT1-MMP at the invasion front.
Figure 1. Expression of MT1-MMP in RA pannus.
A. Primary synovial cells were isolated from the RA pannus of different patients and plated into 6 well plates and grown until they became confluent. Cell lysates were then subjected to Western blot analysis using a mouse monoclonal anti-MT1-MMP hemopexin domain antibody and a polyclonal anti-actin antibody. #1 to #6 indicate samples from different patients. HT1080 cell lysate was applied as a positive control. Annotations are: Pro, proMT1-MMP; Active, active form; 40 kDa, 40 kDa processed form; *, further degraded form; actin, actin. B. RA joint sections were stained with anti-MT1-MMP antibody (a, d), pre-immune mouse IgG (b, e), and Fast Green FCS with Safranin O (c, f). Three matching representative areas are shown (a–c and d–f). Arrows indicate the cartilage-pannus junction where MT1-MMP is highly expressed. Note that Safranin O staining is almost negative throughout the specimens. C. RA joint sections were stained for MT1-MMP, CD68 macrophage marker and Masson’s Trichrome, and images captured using 40× objective lens. Regions marked with a square box are enlarged and shown in the bottom of each image. A representative deep invasion area (left) and superficial invasion area (right) are shown.
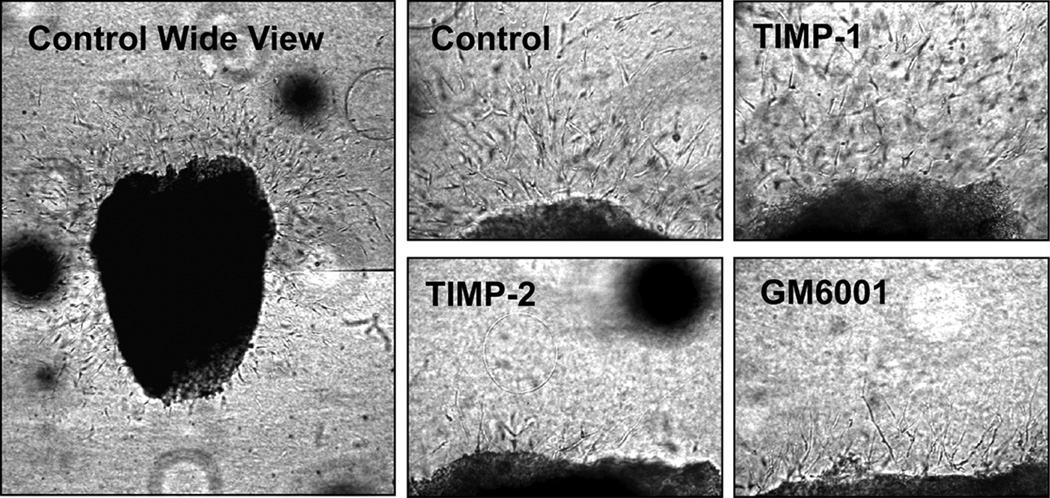
To test the functional contribution of MT1-MMP to synovial pannus invasion, we developed an ex vivo invasion model. Freshly isolated RA synovial tissues were diced into small pieces about 2 mm3, embedded into a 3-D collagen gel and cultivated for 5 days. In the initial 24 h, small T-cell-like cells migrated into the collagen matrix, but they were undetectable after 48 h. Numerous large spindle-shaped fibroblastic cells started to invade into collagen after 48–72 h (Figure 2). Because the invasion edge of synovial tissue consists of large fibroblastic cells (Figure 1C), the fibroblastic cell invasion into collagen gel observed in this ex vivo model is likely to represent in vivo pannus invasion. Next, we performed this invasion assay in the presence of tissue inhibitor of metalloproteinases (TIMP)-1 (0.5 µM), TIMP-2 (0.5 µM), or GM6001 (10 µM). It is known that the activity of transmembrane-type MT-MMPs including MT1-, MT2-, MT3-, and MT5-MMP cannot be inhibited by TIMP-1 but is inhibited by TIMP-2 and GM6001 (29, 30). As shown in Figure 2, invasion was completely inhibited by TIMP-2 and GM6001, but not by TIMP-1. We have performed this invasion assay using joint synovial tissues from 5 different patients, and the results were identical (data not shown). Among TIMP-1-insensitive MT-MMPs, MT1-MMP and MT2-MMP have been shown to promote cell invasion into a type I collagen matrix (8). However, we could not detect MT2-MMP in any of the synovial cells we tested by Western Blotting (data not shown) which supports previous reports (21, 31) showing either no expression or inconsistent low expression of MT2-MMP compared to MT1-MMP. Therefore, these data suggest that MT1-MMP may be a key collagenolytic enzyme promoting synovial invasion.
Figure 2. Ex vivo invasion assay of RA pannus tissue.
Fresh RA pannus tissues were subjected to an ex vivo invasion assay as described in the Methods section, and invasion was monitored after 5 days. “Control wide view” represents a typical pattern of invasion from a single piece of tissue, and this image was created by combining two fields of view that were obtained using 10× objective lens. Tissues were cultured in the presence or absence of TIMP-1 (0.5 µM), TIMP-2 (0.5 µM) or GM6001 (10 µM). These images were taken using a 20 × objective lens.
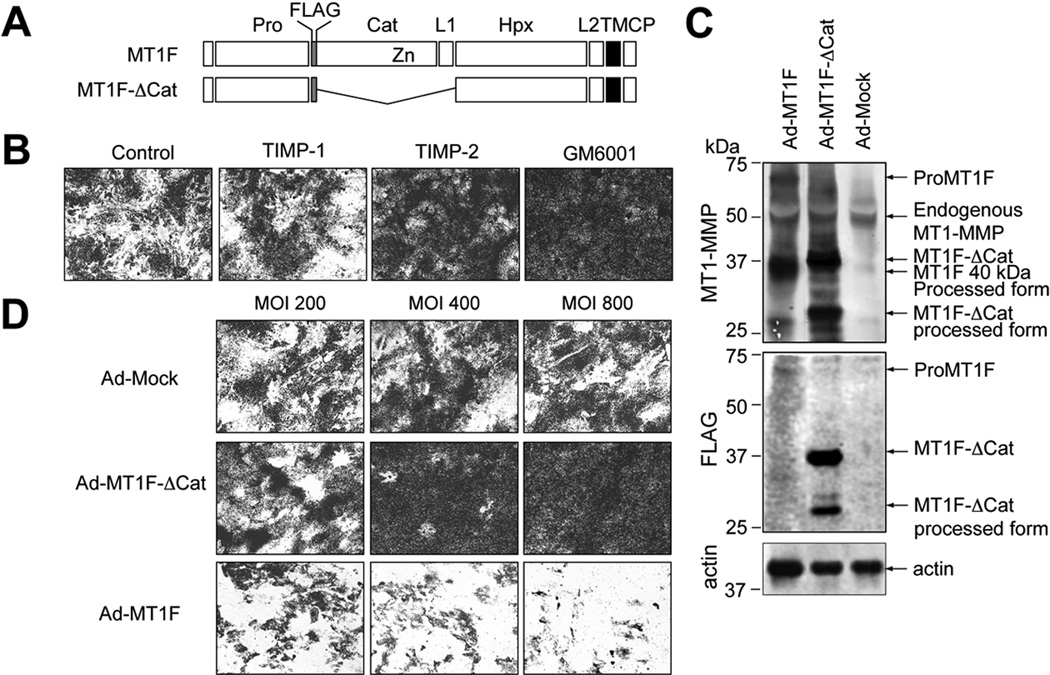
We have recently reported that MT1-MMP requires homo-dimerization to degrade collagen, and inhibition of this interaction by expression of a catalytic domain deletion mutant of MT1-MMP (MT1F-ΔCat) effectively inhibited collagenolytic activity on the cell surface (24). Therefore, we constructed an adenoviral vector for expression of MT1F-ΔCat (Ad-MT1F-ΔCat) to test the role of MT1-MMP in synovial fibroblast invasiveness (Figure 3A). We also made Ad-MT1F to over-express the enzyme as well (Figure 3A). When isolated RA synovial fibroblasts were cultured on a collagen film, they degraded collagen leaving unstained clear areas, and this activity was inhibited by TIMP-2 and GM6001 but not by TIMP-1 (Figure 3B). This suggests that collagen degradation by these cells was caused by MT1-MMP, and not by any soluble MMPs including MMP-1, MMP-2, MMP-8 and MMP-13. We then tested the effect of adenoviral expression of MT1F and MT1F-ΔCat. As shown in Figure 3C, these exogenous genes were overexpressed in synovial fibroblasts. It was noted that most of MT1F expressed in the cells processed to a 40 kDa species and lost its catalytic domain containing FLAG tag (Figure 3C). Such processing has been reported previously and suggests that functional activity is high in these cells (30, 32, 33). The collagen-degrading activity was not affected by infection of the cells with different MOI of mock viruses (Ad-Mock), but could be inhibited in a dose-dependent manner by Ad-MT1F-ΔCat (Figure 3D). Degradation was further accelerated in a dose-dependent manner by infection with Ad-MT1F. Taken together, the data suggest that MT1-MMP is the cellular collagenase in human synovial cells.
Figure 3. MT1-MMP is the cellular collagenase in RA synovial cells.
Passaged synovial cells were subjected to an in situ collagen degrading assay for 3 days as described in the Methods section.
A. Schematic representation of MT1-MMP constructs. Pro, propeptide; FLAG, FLAG-tag; Cat, catalytic domain; L1, linker-1 region; Hpx, hemopexin domain; L2, linker-2 region; TM, transmembrane domain; CP, cytoplasmic tail; and Zn, catalytic zinc atom. B. Synovial cells were subjected to an in situ collagen degradation assay in the presence or absence of TIMP-1 (0.5 µM), TIMP-2 (0.5 µM) or GM6001 (10 µM) as described in the Methods section. Dark stained areas represent remaining collagen and clear areas represent the area where collagen has been degraded. C. Synovial cells were infected with adenovirus constructs of Mock (Ad-Mock), MT1F- ΔCat (Ad- MT1F- ΔCat) or MT1-F (Ad-MT1F), and cell lysates were subjected to Western blot analysis for MT1-MMP, FLAG tag and actin. D. Synovial cells infected with the above viruses at different multiplicity of infection (MOI) were subjected to the in situ collagen degradation assay.
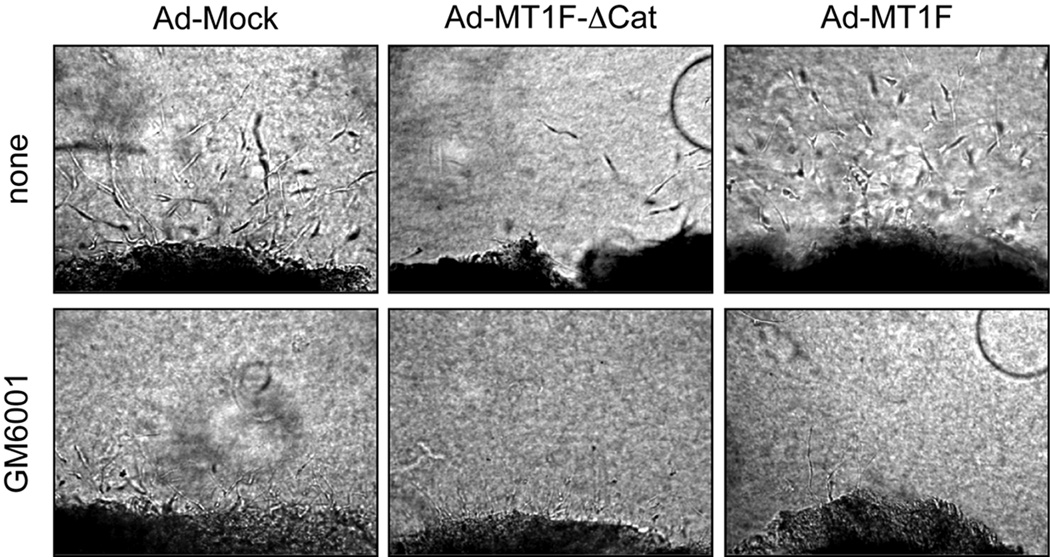
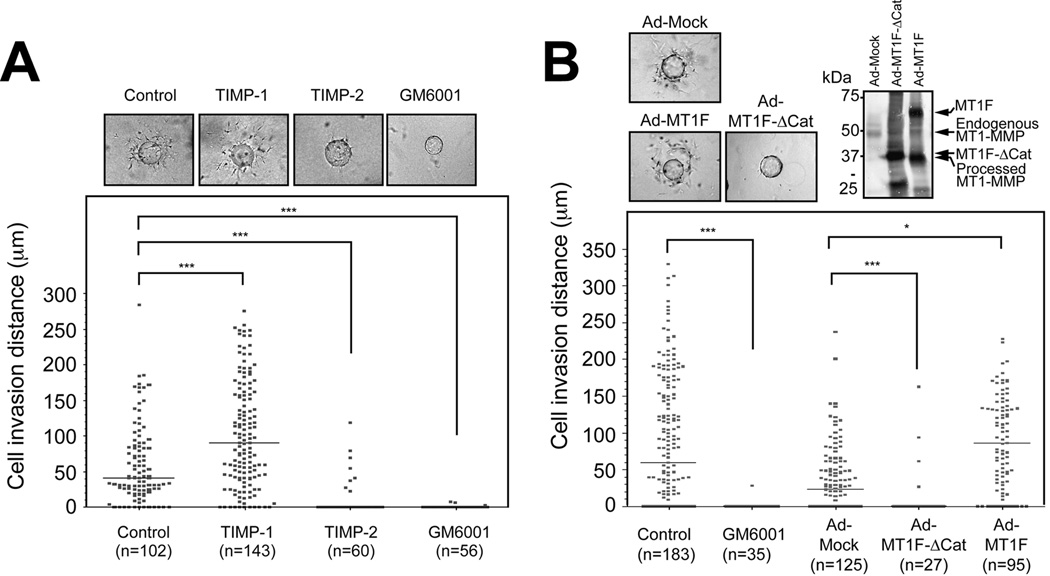
We next examined whether MT1F-ΔCat can inhibit synovial cell invasion using an ex vivo collagen invasion assay. To establish an effective adenoviral infection of human RA synovial tissue, we carried out dose-dependent infection with a recombinant adenovirus that expresses green fluorescent protein and found that a virus titre of 108 pfu/ml was minimally toxic and maximally effective for expression of the gene, although, in this system, it is inevitable that there will be some non-infected cells especially in deeper areas of the tissue. As shown in Figure 4, cells from RA synovial tissue infected with Mock viruses invaded into the collagen gel, and this invasion was largely suppressed by GM6001. In contrast, pannus infected with Ad-MT1F-ΔCat showed significantly reduced invasion, and addition of GM6001 further inhibited invasion. Infection with Ad-MT1-F slightly enhanced invasiveness in terms of invasion distance compared to Ad-Mock infected cells, and GM6001 again strongly inhibited invasion. In order to obtain more quantitative measurement of synovial cell invasion, we carried out microcarrier bead invasion assays using isolated synovial fibroblasts. As shown in Figure 5, synovial cells attached to microcarrier beads invaded from the beads into the collagen gel very efficiently. This invasion was inhibited by TIMP-2 and GM6001 but not by TIMP-1 (Figure 5A). TIMP-1 rather enhanced invasion in this particular experiment, but this effect was observed in one out of three experiments using different synovial cells. When these cells were infected with Ad-MT1F-ΔCat prior to the assay, invasion was almost completely inhibited, whereas the expression of MT1-F enhanced the invasion (Figure 5B). These data suggest that MT1-MMP is indeed a crucial collagenolytic proteinase enabling these synovial cells to invade into a collagen matrix.
Figure 4. Effect of MT1F-ΔCat expression on pannus cell invasion.
Fresh synovial pannus tissues were infected with adenovirus constructs for Mock (Ad-Mock), MT1F- ΔCat (Ad- MT1F- ΔCat) or MT1-F (Ad-MT1F) at 108 pfu/ml, and subjected to the ex vivo invasion assay for five days in the presence or absence of GM6001 (10 µM).
Figure 5. Synovial cell invasion assay using microcarrier beads.
Isolated synovial cells were subjected to a microcarrier bead invasion assay as described in the Methods. The effects of TIMP-1, TIMP-2 and GM6001 are shown in A, and the effect of adenoviral infection with Ad-Mock, Ad- MT1F-ΔCat or Ad-MT1F are shown in B. A. Scatter plot representation of the migration distance from the bead surface. The number of cells analyzed is shown for each treatment. ***p<0.0001. Representative pictures of the assayed bead in each treatment are shown on the top. Control represents cells without any inhibitors. B. Scatter plot representation of the migration distance from the bead surface of cells treated or infected as indicated. Number of the cells analyzed is shown for each treatment. Control represents cells without any inhibitors or adenoviral infection. *p<0.0199; and ***p<0.0001. Representative pictures of the assayed beads and Western blot analysis of cell lysates from infected cells with Ad-Mock, Ad- MT1F- ΔCat or Ad-MT1F using 222-1D8 (MT1-MMP) are shown on the top. Control represents cells without any inhibitors or virus infection.
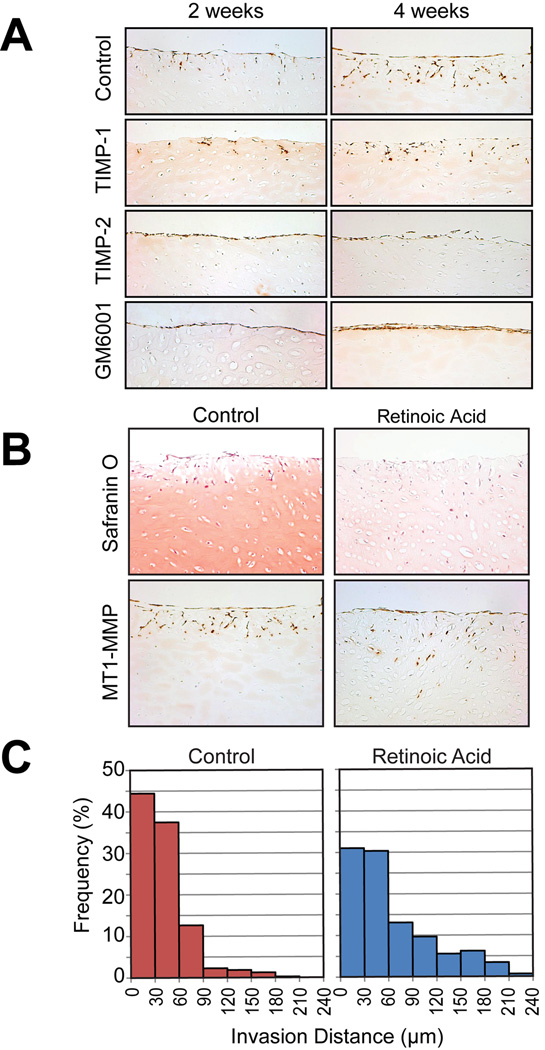
We next examined the invasion of synovial fibroblasts into the cartilage matrix. For this assay, we chose bovine nasal cartilage as it is possible to dissect out pieces of reproducible size and quantity for different treatments. Fresh bovine nasal cartilage was frozen and thawed three times to kill the chondrocytes and diced into 3 × 5 × 5 mm pieces. Human synovial cells were then cultured on the cartilage surface for up to 4 weeks. As shown in Figure 6A, synovial cells invaded into the cartilage in a time-dependent manner. This invasion was not inhibited by TIMP-1, but was completely inhibited by TIMP-2 and GM6001. We have attempted adenoviral infection in this experimental system, but were hampered by the 4-week length of the assay since adenoviral transgene expression lasts only up to 10 days.
Figure 6. Invasion of synovial cells into cartilage.
A. Fresh bovine nasal cartilage was frozen and thawed 3 times to kill the chondrocytes, and cut into pices of about 5 × 5 × 3 mm. Isolated human synovial cells were then cultured on the cartilage pieces in the presence or absence of TIMP-1 (0.5 µM), TIMP-2 (0.5 µM), or GM6001 (10 µM) for 2 weeks or 4 weeks. Cartilage pieces were then fixed with 4 % formaldehyde in PBS, and paraffin sections were stained with anti-MT1-MMP antibody, 222-1D8. B. Fresh bovine nasal cartilage pieces were treated with or without retinoic acid (10 µM) to remove aggrecan by inducing aggrecanases for 7 days prior to freezing and thawing. Synovial cells were then cultured on the cartilage pieces for 4 weeks, and paraffin sections were stained for Safranin O or with the 222-1D8 antibody (MT1-MMP). C. Number of cells at various depths were counted and expressed as a histogram. Note that synovial cells invaded much deeper into retinoic acid-treated cartilage compared to non-treated cartilage.
In RA cartilage, aggrecan is thought to be lost in the early stages of the disease, and indeed there is not much aggrecan left at the late stage of the disease, as shown in Figure 1B. Pratta et al. (34) reported that aggrecan protects collagen against degradation by MMP-1 in vitro. Therefore we next examined whether removal of aggrecan from the cartilage would affect synovial cell invasion. To remove aggrecan, live cartilage was treated with 10 µM retinoic acid for 7 days to stimulate aggrecanase production and aggrecan removal. The cartilage was then subjected to freezing and thawing three times to kill the chondrocytes. As shown in Figure 6B, this treatment depleted aggrecan from the cartilage as indicated by the lack of Safranin O staining. Untreated cartilage maintained staining although some loss of staining was noted at the edge of the tissue. In both treatments, synovial cells clearly invaded into the cartilage in 4 weeks. However, synovial fibroblasts invaded much deeper into retinoic acid-treated cartilage. To analyze this quantitatively, we counted a number of the cells at different depths from the surface of the cartilage. As shown in Figure 6C, in untreated cartilage, most synovial cells invaded up to 90 µm from the cartilage surface, whereas in retinoic acid-treated cartilage more cells were present in deeper areas. The proportion of cells that had invaded deeper than 90 µm was 17 % for non-treated cartilage and 39 % for retinoic acid-treated cartilage. These data suggest that aggrecan indeed protects collagen in cartilage, and aggrecan loss may be an important initial step in promoting synovial invasion into cartilage in RA.
DISCUSSION
The synovial pannus is formed as a result of inflammation, which causes proliferation of synovial lining cells and infiltration of monocytes and lymphocytes that produce high levels of inflammatory mediators such as TNFα and IL-1 (35). It is thought that the major cause of sustained inflammation is over-production of cytokines, and indeed anti-TNFα therapy has been proven to be a very effective treatment for RA (36). However, expression of MT1-MMP does not correlate with the area where inflammatory cells reside but rather with the invasive edge, suggesting that inflammatory cytokines may not stimulate expression of MT1-MMP. In fact, IL-1 and TNFα treatment did not modify MT1-MMP expression in isolated synovial fibroblasts (M-C. Miller and Y. Itoh, unpublished data). This is not surprising, since the promotor region of MT1-MMP lacks AP-1 and NFκB responsible elements and a TATA box, which are present in many inflammatory cytokine-inducible genes (37, 38). It has been shown that concanavalin A (39), cytochalasin D (40), collagen (41) and v-src transformation of epithelial cells (42) induce MT1-MMP expression. However, it is not known what regulates MT1-MMP gene expression under pathological conditions such as in RA. Nevertheless, there appeared to be a strong correlation between high levels of MT1-MMP expression and synovial invasion in RA.
To investigate whether there is a functional link between MT1-MMP expression and pannus invasion, we examined invasion of freshly isolated synovial tissue or isolated synovial fibroblasts into collagen gels or cartilage. In every assay, our results indicate that synovial cells utilize MT1-MMP as a collagenolytic enzyme to invade into the matrix. Invasion was almost completely inhibited by TIMP-2 and GM6001 but not by TIMP-1. The concentration of these inhibitors used in the experiments were over excess to achieve almost complete inhibition of the enzymes (0.5 µM for TIMPs and 10 µM for GM6001). Thus soluble collagenases, including MMP-1, MMP-2, MMP-8 and MMP-13, are unlikely to be involved. These results agree with the previous report by Hotary et al.(8) that over-expression of these soluble collagenases does not enhance cell invasion. Furthermore, collagen invasion was effectively inhibited by adenoviral expression of MT1F-ΔCat, suggesting that MT1-MMP is the responsible enzyme. However, a number of reports in the past have shown that MMP-1 and MMP-13 are highly up regulated in RA synovial fibroblasts with cytokine treatment and in RA joint tissues (43–50). So what are they doing? These soluble collagenases are likely to degrade collagens and other matrix components in cartilage, such as aggrecan (51), in a wider area, which may in turn compromise the integrity of the tissue and promote pannus invasion indirectly. Nonetheless, further work is required to understand the exact roles of these soluble collagenases in the pathogenesis of RA.
MT1-MMP plays an important role in postnatal development, as evidenced by the phenotype of MT1-MMP-null mice (6, 7). Lack of the MT1-MMP gene causes skeletal dysplasia accompanied by severe runting, osteopenia and fibrosis of soft tissue, and the mice die around 7–12 weeks after birth (6, 7). An interesting additional phenotype is the development of severe generalised arthritis accompanied by the presence of hyper-cellular and vascularised synovial tissues degrading articular cartilage in all joints (6, 7). This apparently contrasts with our finding that MT1-MMP promotes synovial invasion into cartilage in RA. However, the cell types involved in cartilage degradation appear to be different in each case. In the MT1-MMP-null mice, cells degrading cartilage are tartrate-resistant acidic phosphatase (TRAP)-positive osteoclast-like giant cells, which dominate the inflamed synovium (6, 7). We found no apparent TRAP-positive osteoclast-like cells at the cartilage-pannus junction in the human RA joints we examined. We did occasionally find CD68-positive macrophages in pits on the cartilage surface, but cells invading deeply into the cartilage were Factor VIII- (endothelial marker), CD3- (T-cell marker) and CD68-negative (Figure 1C and unpublished observations). Thus it is likely that fibroblast-like cells are the major cartilage-invading cell type in human RA (52). Taken together, the arthritic phenotype of the MT1-MMP null mice is likely to be caused by a different mechanism.
Besides type II collagen, aggrecan is the other major component of cartilage, endowing cartilage with compressive resistance properties by retaining water molecules in the tissue (3). In RA and osteoarthritis (OA), aggrecan is depleted from cartilage by the action of aggrecanases belonging to the ADAMTS family (1, 4). It has been reported that deletion of the ADAMTS-5 gene in mice protects against aggrecan depletion and cartilage degradation in experimental OA and inflammatory arthritis models (53, 54). Pratta et al. (34) have reported that removing aggrecan makes cartilage more susceptible to collagen degradation by MMP-1 in vitro, and Little et al (55) have recently shown that mutation of the aggrecanase cleavage sequence in the interglobular domain of aggrecan by gene knock-in prevents aggrecan loss and cartilage erosion. These reports suggest that aggrecan may protect against collagen degradation. In this study, we confirmed this protective effect of aggrecan. Removing aggrecan by pre-treating cartilage with retinoic acid made cartilage more susceptible to synovial cell invasion. As aggrecan is completely lost in advanced RA, this may be one of the mechanisms promoting synovial pannus invasion. The mechanism by which aggrecan protects against collagenolytic action remains unclear. It is possible that the negatively charged sulfated polysaccharide chains of aggrecan that fill the collagen fibril gaps may prevent enzyme-collagen interactions. Without retinoic acid treatment, synovial cells were still able to invade into cartilage, but they appeared to be unable to migrate further than 60 µm from the cartilage surface. During the course of the experiments, we found that safranin O staining near the cartilage surface became weaker during incubation period of 2–4 weeks, even in the absence of viable cells (data not shown). This is presumably due to diffusion of aggrecan into culture media. It is thus possible that the loss of aggrecan up to 60 µm might have been sufficient for synovial cells to be able to invade.
In summary, we have provided evidence which supports that MT1-MMP is a key proteinase that promotes pannus invasion into cartilage. MT1-MMP also plays an important role in monocyte transmigration through the endothelium (56) and in angiogenesis (7, 9, 57), both of which contribute to RA progression. We thus believe that MT1-MMP can be a target molecule for potential therapeutic intervention for RA.
Acknowledgments
Funding:
This work was supported by an Arthritis Research Campaign Core grant to the Kennedy Institute of Rheumatology, A Royal College of Surgeons of England fellowship (M-C. Miller), Engineering and Physical Science Research Council studentship (H. Manning), Cancer Research UK project grant C1507/A5541 (Y. Itoh) and the NIH grant AR40994 (H. Nagase).
References
- 1.Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4(3):128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- 2.Sledge C, Reddi A, Walsh D, Blake D. Biology of the Normal Joint. In: S R, Harris EJ, CB S, editors. Kelly's Textbook of Rheumatology. 6 ed. Philadelphia: W.B: Saunders Company; 2001. pp. 1–26. [Google Scholar]
- 3.Heinegård D, Lorenzo P, Saxne T. Matrix Glycoproteins, Proteoglycans, and Cartilage. In: S R, Harris EJ, CB S, editors. Kelly's Textbook of Rheumatology. 6 ed. Philadelphia: W.B: Saunders Company; 2001. pp. 41–53. [Google Scholar]
- 4.Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5(2):94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 6.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97(8):4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000;149(6):1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95(3):365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 10.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167(4):769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 12.Ueda J, Kajita M, Suenaga N, Fujii K, Seiki M. Sequence-specific silencing of MT1-MMP expression suppresses tumor cell migration and invasion: importance of MT1-MMP as a therapeutic target for invasive tumors. Oncogene. 2003;22(54):8716–8722. doi: 10.1038/sj.onc.1206962. [DOI] [PubMed] [Google Scholar]
- 13.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 14.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, et al. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202(5):663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272(4):2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 16.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148(3):615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh Y, Nagase H. Matrix metalloproteinases in cancer. Essays Biochem. 2002;38:21–36. doi: 10.1042/bse0380021. [DOI] [PubMed] [Google Scholar]
- 18.Knäuper V, Will H, López-Otín C, Smith B, Atkinson SJ, Stanton H, et al. Cellular mechanisms for human procollagenase 3 (mmp 13) activation: evidence that mt1 mmp (mmp 14) and gelatinase a (mmp 2) are able to generate active enzyme. J Biol Chem. 1996;271(29):17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 19.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153(5):893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, et al. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278(42):40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka H, Makino K, Takizawa M, Nakamura H, Fujimoto N, Moriya H, et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in rheumatoid synovium. Lab Invest. 2000;80(5):677–687. doi: 10.1038/labinvest.3780071. [DOI] [PubMed] [Google Scholar]
- 22.Konttinen YT, Ceponis A, Takagi M, Ainola M, Sorsa T, Sutinen M, et al. New collagenolytic enzymes/cascade identified at the pannus-hard tissue junction in rheumatoid arthritis: destruction from above. Matrix Biol. 1998;17(8–9):585–601. doi: 10.1016/s0945-053x(98)90110-x. [DOI] [PubMed] [Google Scholar]
- 23.Rutkauskaite E, Volkmer D, Shigeyama Y, Schedel J, Pap G, Muller-Ladner U, et al. Retroviral gene transfer of an antisense construct against membrane type 1 matrix metalloproteinase reduces the invasiveness of rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2005;52(7):2010–2014. doi: 10.1002/art.21156. [DOI] [PubMed] [Google Scholar]
- 24.Itoh Y, Ito N, Nagase H, Evans RD, Bird SA, Seiki M. Cell surface collagenolysis requires homodimerization of the membrane-bound collagenase MT1-MMP. Mol Biol Cell. 2006;17(12):5390–5399. doi: 10.1091/mbc.E06-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20(17):4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 27.Itoh Y. MT1-MMP: a key regulator of cell migration in tissue. IUBMB Life. 2006;58(10):589–596. doi: 10.1080/15216540600962818. [DOI] [PubMed] [Google Scholar]
- 28.Strongin AY, Marmer BL, Grant GA, Goldberg GI. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J. Biol. Chem. 1993;268(19):14033–14039. [PubMed] [Google Scholar]
- 29.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271(29):17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 30.Itoh Y, Seiki M. MT1-MMP: A potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206(1):1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 31.Pap T, Shigeyama Y, Kuchen S, Fernihough JK, Simmen B, Gay RE, et al. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000;43(6):1226–1232. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Stanton H, Gavrilovic J, Atkinson SJ, d'Ortho MP, Yamada KM, Zardi L, et al. The activation of ProMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of MT1-MMP (MMP-14) to a 45 kDa form. J Cell Sci. 1998;111(Pt 18):2789–2798. doi: 10.1242/jcs.111.18.2789. [DOI] [PubMed] [Google Scholar]
- 33.Lehti K, Lohi J, Valtanen H, Keski-Oja J. Proteolytic processing of membrane-type-1 matrix metalloproteinase is associated with gelatinase A activation at the cell surface. Biochem J. 1998;334(Pt 2):345–353. doi: 10.1042/bj3340345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278(46):45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 35.Goldring SR. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology (Oxford) 2003;42 Suppl 2:ii11–ii16. doi: 10.1093/rheumatology/keg327. [DOI] [PubMed] [Google Scholar]
- 36.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 37.Lohi J, Lehti K, Valtanen H, Parks WC, Keski-Oja J. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000;242(1–2):75–86. doi: 10.1016/s0378-1119(99)00549-1. [DOI] [PubMed] [Google Scholar]
- 38.Cha HJ, Okada A, Kim KW, Sato H, Seiki M. Identification of cis-acting promoter elements that support expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) in v-src transformed Madin-Darby canine kidney cells. Clin Exp Metastasis. 2000;18(8):675–681. doi: 10.1023/a:1013190118556. [DOI] [PubMed] [Google Scholar]
- 39.Overall CM, Sodek J. Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. Induction and endogenous activation of collagenase, 72-kDa gelatinase, and Pump-1 is accompanied by the suppression of the tissue inhibitor of matrix metalloproteinases. J. Biol. Chem. 1990;265(34):21141–21151. [PubMed] [Google Scholar]
- 40.Ailenberg M, Silverman M. Cellular activation of mesangial gelatinase A by cytochalasin D is accompanied by enhanced mRNA expression of both gelatinase A and its membrane-associated gelatinase A activator (MT-MMP) Biochem J. 1996;313(Pt 3):879–884. doi: 10.1042/bj3130879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellerbroek SM, Fishman DA, Kearns AS, Bafetti LM, Stack MS. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through beta1 integrin. Cancer Res. 1999;59(7):1635–1641. [PubMed] [Google Scholar]
- 42.Kadono Y, Okada Y, Namiki M, Seiki M, Sato H. Transformation of epithelial Madin-Darby canine kidney cells with p60(v- src) induces expression of membrane-type 1 matrix metalloproteinase and invasiveness [In Process Citation] Cancer Res. 1998;58(10):2240–2244. [PubMed] [Google Scholar]
- 43.Okada Y, Nagase H, Harris ED., Jr Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J Rheumatol. 1987;14 Spec No:41-2. [PubMed] [Google Scholar]
- 44.Ito A, Itoh Y, Sasaguri Y, Morimatsu M, Mori Y. Effects of interleukin-6 on the metabolism of connective tissue components in rheumatoid synovial fibroblasts. Arthritis Rheum. 1992;35:1197–1201. doi: 10.1002/art.1780351012. [DOI] [PubMed] [Google Scholar]
- 45.Vincenti MP, Coon CI, White LA, Barchowsky A, Brinckerhoff CE. src-related tyrosine kinases regulate transcriptional activation of the interstitial collagenase gene, MMP-1, in interleukin-1-stimulated synovial fibroblasts. Arthritis Rheum. 1996;39(4):574–582. doi: 10.1002/art.1780390406. [DOI] [PubMed] [Google Scholar]
- 46.Lindy O, Konttinen YT, Sorsa T, Ding Y, Santavirta S, Ceponis A, et al. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997;40(8):1391–1399. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- 47.Langdon C, Leith J, Smith F, Richards CD. Oncostatin M stimulates monocyte chemoattractant protein-1- and interleukin-1-induced matrix metalloproteinase-1 production by human synovial fibroblasts in vitro. Arthritis Rheum. 1997;40(12):2139–2146. doi: 10.1002/art.1780401207. [DOI] [PubMed] [Google Scholar]
- 48.Tetlow LC, Woolley DE. Comparative immunolocalization studies of collagenase 1 and collagenase 3 production in the rheumatoid lesion, and by human chondrocytes and synoviocytes in vitro. Br J Rheumatol. 1998;37(1):64–70. doi: 10.1093/rheumatology/37.1.64. [DOI] [PubMed] [Google Scholar]
- 49.Moore BA, Aznavoorian S, Engler JA, Windsor LJ. Induction of collagenase-3 (MMP-13) in rheumatoid arthritis synovial fibroblasts. Biochim Biophys Acta. 2000;1502(2):307–318. doi: 10.1016/s0925-4439(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 50.Hui W, Rowan AD, Richards CD, Cawston TE. Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 2003;48(12):3404–3418. doi: 10.1002/art.11333. [DOI] [PubMed] [Google Scholar]
- 51.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380(1–2):17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 52.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39(11):1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 53.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 54.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 55.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, et al. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117(6):1627–1636. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sithu SD, English WR, Olson P, Krubasik D, Baker AH, Murphy G, et al. Membrane-type 1-matrix metalloproteinase regulates intracellular adhesion molecule-1 (ICAM-1)-mediated monocyte transmigration. J Biol Chem. 2007;282(34):25010–25019. doi: 10.1074/jbc.M611273200. [DOI] [PubMed] [Google Scholar]
- 57.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, et al. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167(4):757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]